Optimizing Preoperative Anemia in Non-Metastatic Colorectal Cancer: A Systematic Review on Surgical Recovery and Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- M0-Staged Colorectal Carcinoma Patients;
- Pre-operative administration of Iron Supplementation (Oral or IV);
- Peer-reviewed primary studies and reviews addressing the research question;
- Studies published in English;
- Randomized Controlled Trials (RCTs);
- Adults (≥18 years).
2.2.2. Exclusion Criteria
- Metastatic CRC patients;
- Post-operative administration of Iron Supplementation without prior pre-operative administration;
- Editorials, commentaries, opinion pieces, cohort studies, case–control, observational.
- Animal studies;
- Articles without full-text access;
- Non-English Papers;
- Pre-prints.
2.3. Search Strategy
2.4. Study Selection and Data Charting
2.5. Quality Assessment
3. Results
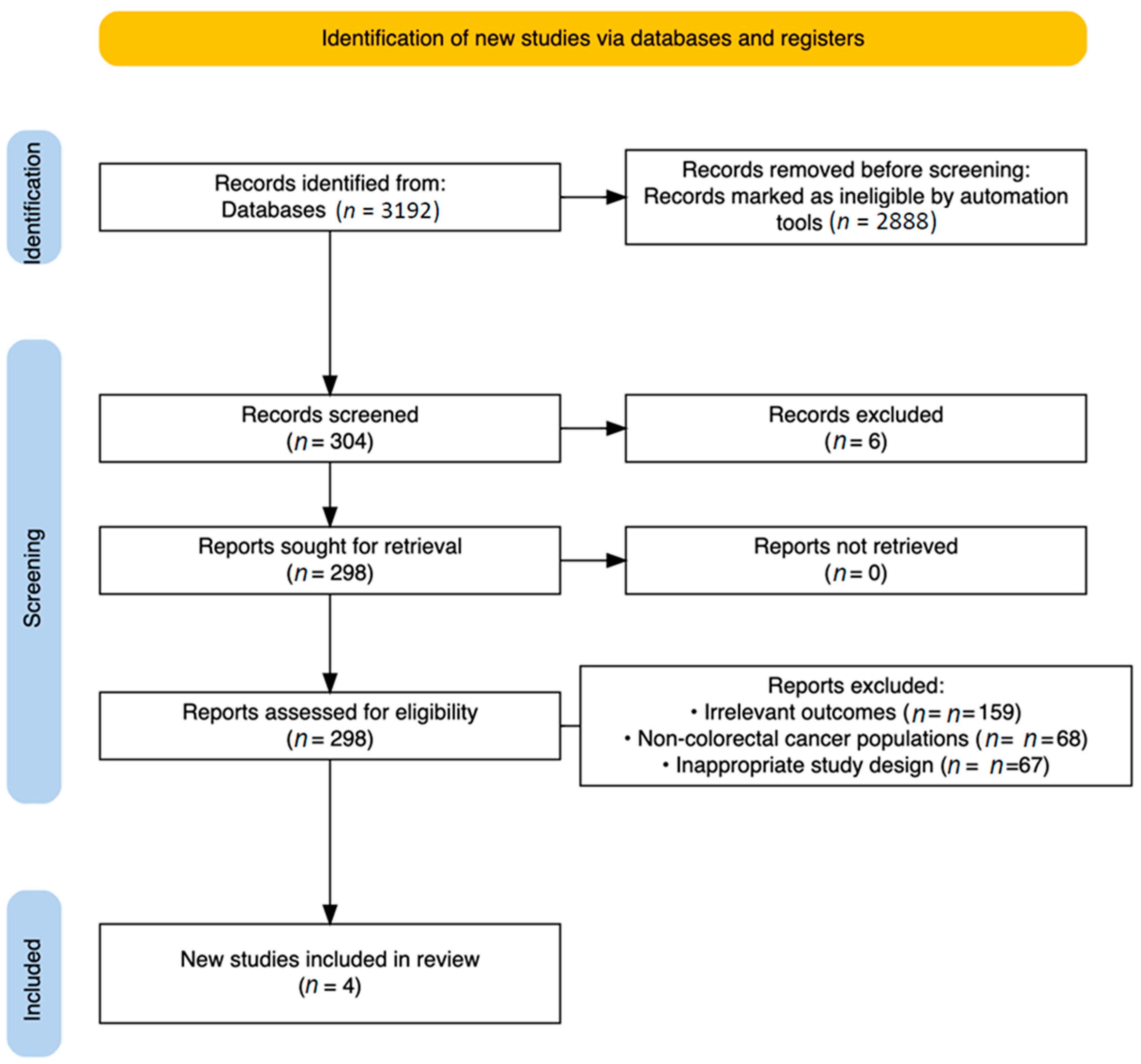
3.1. Study Selection
- Irrelevant outcomes (n = 159);
- Non-colorectal cancer populations (n = 68);
- Inappropriate study design, primarily non-randomized studies (n = 67).
3.2. Quality Assessment Outcomes
3.3. Main Findings
3.4. Specific Resection Sites and Surgical Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Tysarowski, A.; Nasierowska-Guttmejer, A. Quality and practical aspects of pathological and molecular diagnostics in metastatic colorectal cancer (mCRC). Wspolczesna. Onkol. Oncol. 2018, 22, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Wen, L.; Zhang, G.; Huang, C.; Wang, J.; Yao, X. Prognostic impact of tumor budding in rectal cancer after neoadjuvant therapy: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 22. [Google Scholar] [CrossRef]
- Liu, X.-X.; Su, J.; Long, Y.-Y.; He, M.; Zhu, Z.-Q. Perioperative risk factors for survival outcomes in elective colorectal cancer surgery: A retrospective cohort study. BMC. Gastroenterol. 2021, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Tjeertes, E.K.; Ultee, K.H.J.; Stolker, R.J.; Verhagen, H.J.M.; Gonçalves, F.M.B.; Hoofwijk, A.G.M.; Hoeks, S.E. Perioperative Complications are Associated With Adverse Long-Term Prognosis and Affect the Cause of Death After General Surgery. World J. Surg. 2016, 40, 2581–2590. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Du, L.; Li, S.; Zhang, J. Managements for perioperative anxiety in patients with gastrointestinal cancers. Front. Psychiatry. 2024, 15, 1391403. [Google Scholar] [CrossRef]
- Lynch, K.T.; Hassinger, T.E. Preoperative Identification and Management of Anemia in the Colorectal Surgery Patient. Clin. Colon. Rectal. Surg. 2023, 36, 161–166. [Google Scholar] [CrossRef]
- Ploug, M.; Kroijer, R.; Qvist, N.; Lindahl, C.H.; Knudsen, T. Iron deficiency in colorectal cancer patients: A cohort study on prevalence and associations. Color. Dis. 2020, 23, 853–859. [Google Scholar] [CrossRef]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. [Google Scholar] [CrossRef]
- Bangolo, A.; Amoozgar, B.; Habibi, M.; Simms, E.; Nagesh, V.K.; Wadhwani, S.; Wadhwani, N.; Auda, A.; Elias, D.; Mansour, C.; et al. Exploring the gut microbiome’s influence on cancer-associated anemia: Mechanisms, clinical challenges, and innovative therapies. World J. Gastrointest. Pharmacol. Ther. 2025, 16, 105375. [Google Scholar] [CrossRef] [PubMed]
- Chardalias, L.; Papaconstantinou, I.; Gklavas, A.; Politou, M.; Theodosopoulos, T. Iron Deficiency Anemia in Colorectal Cancer Patients: Is Preoperative Intravenous Iron Infusion Indicated? A Narrative Review of the Literature. Cancer. Diagn. Progn. 2023, 3, 163–168. [Google Scholar] [CrossRef]
- Deng, Y.; Weng, M.; Zhang, J. Preoperative anemia and long-term survival in patients undergoing colorectal cancer surgery: A retrospective cohort study. World J. Surg. Oncol. 2023, 21, 122. [Google Scholar] [CrossRef]
- Park, L.J.; Moloo, H.; Ramsay, T.; Thavorn, K.; Presseau, J.; Zwiep, T.; Martel, G.; Devereaux, P.; Talarico, R.; McIsaac, D.I. Associations of preoperative anaemia with healthcare resource use and outcomes after colorectal surgery: A population-based cohort study. Br. J. Anaesth. 2024, 133, 58–66. [Google Scholar] [CrossRef]
- Calu, V.; Piriianu, C.; Miron, A.; Grigorean, V.T. Surgical Site Infections in Colorectal Cancer Surgeries: A Systematic Review and Meta-Analysis of the Impact of Surgical Approach and Associated Risk Factors. Life 2024, 14, 850. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; (WHO/NMH/NHD/MNM/11.1); World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.academia.edu/4215770/WHO_Haemoglobin_concentrations_for_the_diagnosis_of_anaemia_and_assessment_of_severity_Vitamin_and_Mineral_Nutrition_Information_System_Geneva_World_Health_Organization_2011_WHO_NMH_NHD_MNM_11_1_ (accessed on 27 July 2025).
- Peña-Rosas, J.P.; WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System; (WHO/NMH/NHD/MNM/11.2); World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.academia.edu/4215786/WHO_Serum_ferritin_concentrations_for_the_assessment_of_iron_status_and_iron_deficiency_in_populations_Vitamin_and_Mineral_Nutrition_Information_System_Geneva_World_Health_Organization_2011_WHO_NMH_NHD_MNM_11_2_ (accessed on 27 July 2025).
- Richardson, D.; Brown, K.; Rusch, J.; Verburgh, E.; Louw, V.; Opie, J. From the marrow to the blood: Optimising the diagnosis of iron deficiency in the setting of inflammation. Pathology 2024, 57, 87–93. [Google Scholar] [CrossRef]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference distributions for serum iron and transferrin saturation: A practical, simple, and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 2002, 16, 237–245. [Google Scholar] [CrossRef]
- Wilson, M.J.; Dekker, J.W.; Bruns, E.; Borstlap, W.; Jeekel, J.; Zwaginga, J.J.; Schipperus, M. Short-term effect of preoperative intravenous iron therapy in colorectal cancer patients with anemia: Results of a cohort study. Transfusion 2018, 58, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.; Keeler, B.D.; Mishra, A.; Simpson, J.A.; Neal, K.; Al-Hassi, H.O.; Brookes, M.J.; Acheson, A.G. Iron therapy for preoperative anaemia. Cochrane Database Syst. Rev. 2019, 12, CD011588. [Google Scholar] [CrossRef] [PubMed]
- Barsballe, K.E.B.; Bundgaard-Nielsen, M.; Ruhnau, B.; Hillingsøe, J.G.; Aasvang, E.K.; Jans, Ø. Efficacy of a pre-operative anaemia clinic in patients undergoing elective abdominal cancer surgery. Acta. Anaesthesiol. Scand. 2024, 68, 1338–1346. [Google Scholar] [CrossRef]
- Fritche, D.; Wensley, F.; Johnson, Y.L.; Robins, C.; Wakatsuki, M.; Fecher-Jones, I.C.; Sheppard, L.; West, M.A.; Aarvold, A.; Edwards, M.R.; et al. Intravenous Iron for Perioperative Anaemia in Colorectal Cancer Surgery: A Nested Cohort Analysis. Cancers 2025, 17, 1877. [Google Scholar] [CrossRef]
- Muñoz, M.; Gómez-Ramírez, S.; Martín-Montañez, E.; Auerbach, M. Perioperative anemia management in colorectal cancer patients: A pragmatic approach. World J. Gastroenterol. 2014, 20, 1972–1985. [Google Scholar] [CrossRef]
- Torrance, H.D.; Brohi, K.; Pearse, R.M.; Mein, C.A.; Wozniak, E.; Prowle, J.R.; Hinds, C.J.; O’Dwyer, M.J. Association Between Gene Expression Biomarkers of Immunosuppression and Blood Transfusion in Severely Injured Polytrauma Patients. Ann. Surg. 2015, 261, 751–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fragkou, P.C.; Torrance, H.D.; Pearse, R.M.; Ackland, G.L.; Prowle, J.R.; Owen, H.C.; Hinds, C.J.; O’dWyer, M.J. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: A prospective cohort study. Crit. Care 2014, 18, 541. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.L.P.; Lau, V.N.M.; Ng, F.F.; Leung, W.W.; Mak, T.W.C.; Lee, A. Perioperative changes in haemoglobin and ferritin concentrations from preoperative intravenous iron isomaltoside for iron deficiency anaemia in patients with colorectal cancer: A pilot randomised controlled trial. PLoS ONE 2022, 17, e0270640. [Google Scholar] [CrossRef] [PubMed]
- Keeler, B.D.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Banerjea, A.; Walter, C.; Maxwell-Armstrong, C.; Williams, J.; et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br. J. Surg. 2017, 104, 214–221. [Google Scholar] [CrossRef]
- Talboom, K.; Borstlap, W.A.A.; Roodbeen, S.X.; Bruns, E.R.J.; Buskens, C.J.; Hompes, R.; Tytgat, K.M.A.J.; Tuynman, J.B.; Consten, E.C.J.; Heuff, G.; et al. Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): A multicentre, open-label, randomised, controlled trial. Lancet Haematol. 2023, 10, e250–e260. [Google Scholar] [CrossRef]
- Christodoulakis, M.; Tsiftsis, D.D. Hellenic Surgical Oncology Perioperative EPO Study Group Preoperative Epoetin Alfa in Colorectal Surgery: A Randomized, Controlled Study. Ann. Surg. Oncol. 2005, 12, 718–725. [Google Scholar] [CrossRef]
- Sydhom, P.; Abdelgalil, M.S.; Al-Quraishi, B.; Shehata, N.; El-Shawaf, M.; Naji, N.; Awwad, N.; Osman, M.T.; Mahmoud, A.; Awad, A.K. Efficacy and safety of preoperative intravenous iron versus standard care in colorectal cancer patients with iron deficiency anemia: A systematic review and meta-analysis. Ann. Med. Surg. 2024, 86, 7105–7119. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, L.; Huang, W.; Wei, Z. Iron Supplementation Effectively Ameliorates Anemia and Reduces the Need for Blood Transfusion in Patients Undergoing Colorectal Cancer Surgery: A Meta-Analysis. Nutr. Cancer 2022, 74, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Borstlap, W.A.A.; Stellingwerf, M.E.; Moolla, Z.; Musters, G.D.; Buskens, C.J.; Tanis, P.J. Iron therapy for the treatment of preoperative anaemia in patients with colorectal carcinoma: A systematic review. Color. Dis. 2015, 17, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; van Haaren, M.; Harlaar, J.; Park, H.C.; Bonjer, H.; Jeekel, J.; Zwaginga, J.; Schipperus, M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 96–104. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Population Group | Non-Anemic | Mild Anemia | Moderate Anemia | Severe Anemia |
|---|---|---|---|---|
| Non-pregnant women (≥15 years) | ≥12 | 11–11.9 | 8–10.9 | <8 |
| Pregnant women | ≥11 | 10–10.9 | 7–9.9 | <7 |
| Men (≥15 years) | ≥13 | 11–12.9 | 8–10.9 | <8 |
| CRP Status | Sex | SF for ID | Sensitivity | Specificity |
|---|---|---|---|---|
| ≤5 mg/L (no inflammation) | WHO cut-off | <12 µg/L (<5 years) <15 µg/L (>5 years) | ||
| Proposed cut-off | <80 µg/L | 93% | 96% | |
| Male | <77 µg/L | 100% | 100% | |
| Female | <68 µg/L | 86% | 91% | |
| >5 mg/L (inflammation present) | Proposed cut-off | <200 µg/L +TSAT <13% | 78% | 92% |
| Male | <373 µg/L | 100% | 68% | |
| Female | <146 µg/L | 80% | 75% |
| TSAT (%) | Interpretation | Clinical Implication |
|---|---|---|
| <20% | Iron Deficiency | May indicate iron deficiency anemia; further evaluation needed |
| 20–50% | Normal Range | Sufficient iron transport and storage |
| >50% | Possible Iron Overload | May suggest conditions like hemochromatosis or iron toxicity |
| 45–50% | Caution Zone (Upper Normal Limit) | Could warrant monitoring in individuals with chronic disease |
| PICO Element | Description |
|---|---|
| Population | Adults (≥18 years) diagnosed with non-metastatic (M0-staged) colorectal cancer, scheduled for elective surgical resection |
| Intervention | Intravenous (IV) iron supplementation |
| Comparator | Oral iron supplementation |
| Outcomes | Perioperative and postoperative metrics, including:
|
| Study | Randomization Described | Appropriate Randomization Method | Double-Blinding Described | Appropriate Blinding Method | Withdrawals and Dropouts Reported | Jadad Score |
|---|---|---|---|---|---|---|
| Fung et al., 2022 [28] | Yes (+1) | Yes (+1) | Yes (+1) | Yes (+1) | Yes (+1) | 5/5 |
| Keeler et al., 2017 [29] | Yes (+1) | Yes (+1) | No (0) | Not applicable (0) | Yes (+1) | 3/5 |
| Talboom et al., 2023 [30] | Yes (+1) | Yes (+1) | No (0) | Not applicable (0) | Yes (+1) | 3/5 |
| Christodoulakis et al., 2005 [31] | Yes (+1) | Unclear (0) | No (0) | Not applicable (0) | Yes (+1) | 2/5 |
| Study | RoB2: Randomization Bias | RoB2: Intervention Bias | RoB2: Missing Data Bias | RoB2: Outcome Measurement Bias | RoB2: Reporting Bias | RoB2: Overall Risk |
|---|---|---|---|---|---|---|
| Fung et al., 2022 [28] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Keeler et al., 2017 [29] | Low Risk | Some Concerns | Low Risk | Low Risk | Low Risk | Low Risk |
| Talboom et al., 2023 [30] | Low Risk | Some Concerns | Low Risk | Low Risk | Low Risk | Low Risk |
| Christodoulakis et al., 2005 [31] | Some Concerns | Low Risk | Low Risk | Some Concerns | Low Risk | Some Concerns |
| Study | Design | Country | Population | Groups | Intervention vs. Comparator | Time of Treatment Initiation Before Surgery | Primary Outcome | Secondary Outcomes | Key Findings and Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Keeler et al., 2017 [29] | Multicentre RCT (Superiority Trial) | United Kingdom | 116 Adults with CRC & anemia | IV iron: 55, Oral iron: 61 | IV ferric carboxymaltose vs. Oral ferrous sulfate | Median 14 days before surgery | Mean volume of perioperative blood transfusion | Change in Hb, ferritin, transferrin saturation, anemia status at surgery, complications, length of stay, and mortality | No difference in transfusions. However, IV iron was safe and significantly better at raising Hb, ferritin, and correcting anemia before surgery. |
| Talboom et al., 2023 [30] | Multicentre RCT (Superiority Trial) | Netherlands and Italy | 202 Adults with CRC and iron deficiency anemia | IV iron: 96, Oral iron: 106 | IV ferric carboxymaltose vs. Oral ferrous fumarate | Treatment began a median of 14 days (IQR 11–22) before surgery for intravenous iron and 19 days (IQR 13–27) for Oral iron. | % of patients with normalized Hb before surgery | Hb change over time, iron store restoration (ferritin, TSAT), complications (Clavien-Dindo, CCI), transfusions, ICU admissions, QoL, fatigue, mortality | No difference in Hb normalization before surgery. However, IV iron was safe and led to significantly better Hb normalization 30 days after surgery (60% vs. 21%). |
| Fung et al., 2022 [28] | Pilot RCT (Double-blinded) | Hong Kong SAR | 40 Adults with CRC and iron deficiency anemia | IV iron: 20, Usual care: 20 | IV iron isomaltoside (20 mg/kg, max 1000 mg) vs. no iron therapy | Median 23 days (range 15–31) | Change in Hb and ferritin from baseline to pre-surgery | Transfusions, complications (Clavien-Dindo), QoR-15, DAH30, ICU admission, readmission | IV iron successfully raised Hb and ferritin. Transfusion rates were lower (though not statistically significant in this small study). |
| Christodoulakis et al., 2005 [31] | RCT (Open-label, 3-arm) | Greece | 204 Anemic patients for colorectal surgery | Epoetin 150 IU/kg (69), Epoetin 300 IU/kg (67), Control Iron alone (68) | Epoetin alfa + iron vs. iron alone | 10 days before surgery | Reduction in transfusion requirements | Hb, Hct, ferritin, TSAT, reticulocyte count, adverse events | High-dose Epoetin + Iron significantly reduced transfusions and raised Hb. The lower Epoetin dose was not effective. |
| Study | Groups (n) | Surgery Type (Open/Lap) | Specific Resection Sites |
|---|---|---|---|
| Talboom et al., 2023 [30] | IV Iron (96), Oral Iron (106) | 91% Laparoscopic overall, IV: 86 Lap/10 Open, Oral: 98 Lap/8 Open | - Right Hemicolectomy: IV 64/Oral 72 - Left Hemicolectomy: IV 14/Oral 14 - Low Anterior Resection: IV 11 /Oral 11 - Other (e.g., subtotal colectomy, sigmoid): IV 7/Oral 9 |
| Fung et al., 2022 [28] | IV Iron (20), Usual Care (20) | IV: 14 Lap/6 Open, Control: 18 Lap/2 Open | - Rectosigmoid: IV 11/Control 12 - Non-rectosigmoid: IV 6/Control 7 - Synchronous Tumors: IV 3/Control 1 |
| Keeler et al., 2017 [29] | IV Iron (55), Oral Iron (61) | IV: 26 Lap/22 Open/5 Converted, Oral: 30 Lap/23 Open/4 Converted | - Right Hemicolectomy: IV 29/Oral 35 - Extended RH: IV 5/Oral 5 - Left Hemicolectomy: IV 1/Oral 5 - Sigmoid/Anterior/Subtotal/Hartmann’s/APE: multiple small counts - No Resection: IV 2/Oral 4 |
| Christodoulakis et al., 2005 [31] | EPO150 (69), EPO300 (67), Control (68) | Not specified | - Tumor Location Only: Left: ~30–36% Right: ~28–32% Rectal: ~34–37% Other: ~2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsokkou, S.; Konstantinidis, I.; Papakonstantinou, M.; Chatzikomnitsa, P.; Gkaitatzi, A.D.; Liampou, E.; Fantakis, A.; Kolympa, G.; Toutziari, E.; Alexandrou, D.; et al. Optimizing Preoperative Anemia in Non-Metastatic Colorectal Cancer: A Systematic Review on Surgical Recovery and Outcomes. Cancers 2025, 17, 3689. https://doi.org/10.3390/cancers17223689
Tsokkou S, Konstantinidis I, Papakonstantinou M, Chatzikomnitsa P, Gkaitatzi AD, Liampou E, Fantakis A, Kolympa G, Toutziari E, Alexandrou D, et al. Optimizing Preoperative Anemia in Non-Metastatic Colorectal Cancer: A Systematic Review on Surgical Recovery and Outcomes. Cancers. 2025; 17(22):3689. https://doi.org/10.3390/cancers17223689
Chicago/Turabian StyleTsokkou, Sophia, Ioannis Konstantinidis, Menelaos Papakonstantinou, Paraskevi Chatzikomnitsa, Areti Danai Gkaitatzi, Eftychia Liampou, Antonios Fantakis, Georgia Kolympa, Evdokia Toutziari, Dimitrios Alexandrou, and et al. 2025. "Optimizing Preoperative Anemia in Non-Metastatic Colorectal Cancer: A Systematic Review on Surgical Recovery and Outcomes" Cancers 17, no. 22: 3689. https://doi.org/10.3390/cancers17223689
APA StyleTsokkou, S., Konstantinidis, I., Papakonstantinou, M., Chatzikomnitsa, P., Gkaitatzi, A. D., Liampou, E., Fantakis, A., Kolympa, G., Toutziari, E., Alexandrou, D., Giakoustidis, D., Bangeas, P., Papadopoulos, V. N., & Giakoustidis, A. (2025). Optimizing Preoperative Anemia in Non-Metastatic Colorectal Cancer: A Systematic Review on Surgical Recovery and Outcomes. Cancers, 17(22), 3689. https://doi.org/10.3390/cancers17223689