Complete Mesocolic Excision for Colon Cancer: Insight into Potential Mechanisms of Oncologic Benefit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
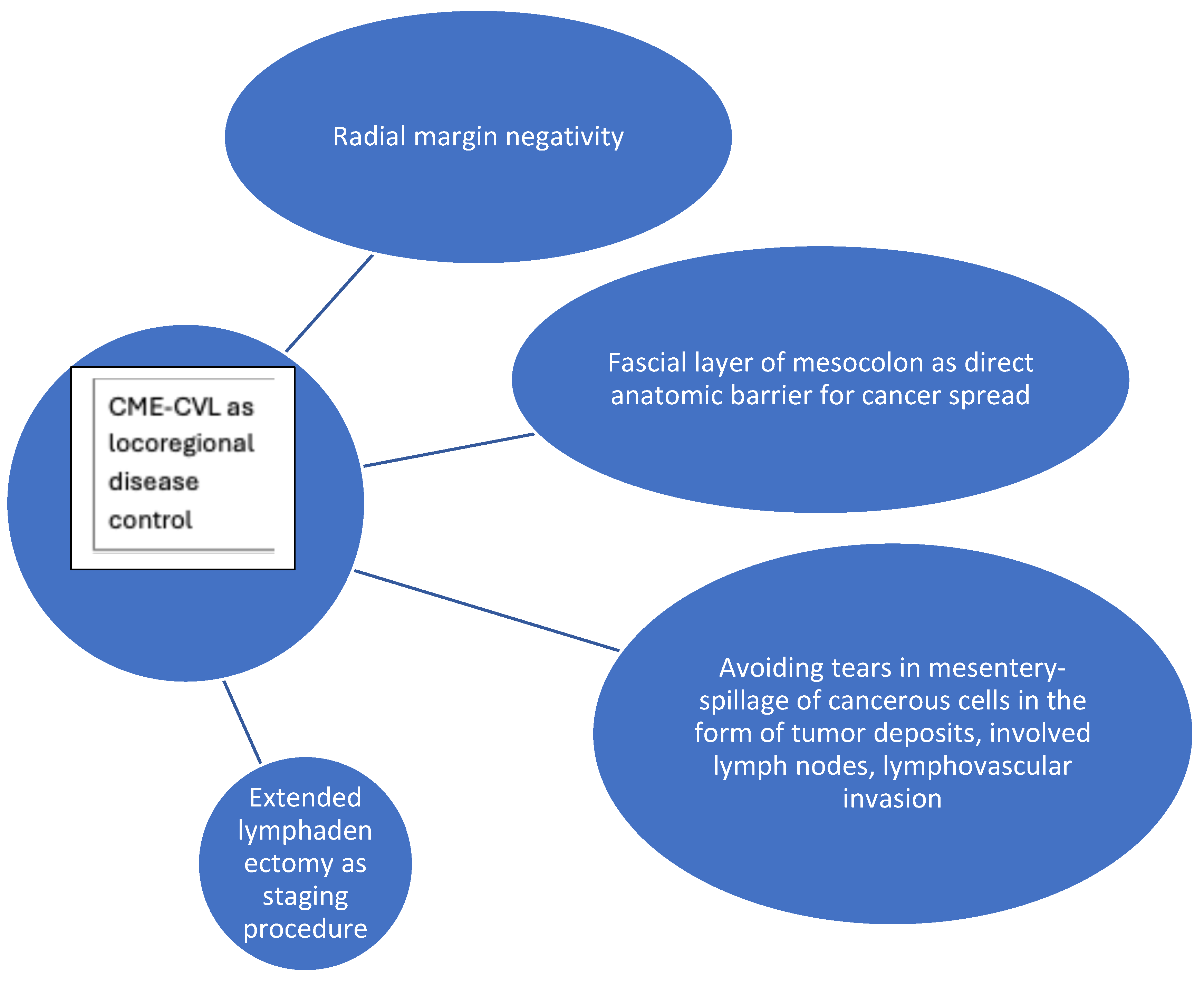
3.1. Anatomic Basis of Complete Mesocolic Excision and Oncologic Implications
3.2. Fascial Layers as Anatomic Landmarks and Borders
3.3. Lymphatic Drainage of Colon Cancer
3.4. Tumor Deposits in Colon Cancer and Complete Mesocolic Excision
3.5. Benefit of Extended Lymphadenectomy in Colon Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CME | Complete mesocolic excision |
| CVL | Central vascular ligation |
| TME | Total mesorectal excision |
References
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique-Past, Present, and Future. Clin. Colon. Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and european complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef]
- Tzanis, A.A.; Carrano, F.M.; Perivoliotis, K.; Kumar, S.S.; Christogiannis, C.; Mavridis, D.; Huo, B.; Bouvy, N.; Christou, N.; Dore, S.; et al. A systematic review, meta-analysis and GRADE assessment of the evidence on complete mesocolic excision for right-sided colon cancer with SAGES and ESCP participation. Surg. Surg. Endosc. 2025, 39, 3466–3473. [Google Scholar] [CrossRef]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef]
- Kobayashi, H.; West, N.P. CME versus D3 Dissection for Colon Cancer. Clin. Colon. Rectal Surg. 2020, 33, 344. [Google Scholar] [CrossRef]
- Coffey, J.C.; Dillon, M.; Sehgal, R.; Dockery, P.; Quondamatteo, F.; Walsh, D.; Walsh, L. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction—A Review. Dig. Surg. 2015, 32, 291–300. [Google Scholar] [CrossRef]
- Sehgal, R.; Coffey, J.C. Standardization of the nomenclature based on contemporary mesocolic anatomy is paramount prior to performing a complete mesocolic excision. Int. J. Color. Dis. 2014, 29, 543–544. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, Y.; Zhang, W.; Shen, D.; Zhong, Y.; Jiang, K.; Yang, X.; Yin, M.; Liang, B.; Tian, L.; et al. An anatomical, histopathological, and molecular biological function study of the fascias posterior to the interperitoneal colon and its associated mesocolon: Their relevance to colonic surgery. J. Anat. 2013, 223, 123–132. [Google Scholar] [CrossRef]
- Wedel, T.; Heimke, M.; Fletcher, J.; Miskovic, D.; Benz, S.; Stelzner, S.; Heinze, T. The retrocolic fascial system revisited for right hemicolectomy with complete mesocolic excision based on anatomical terminology: Do we need the eponyms Toldt, Gerota, Fredet and Treitz? Color. Dis. 2023, 25, 764–774. [Google Scholar] [CrossRef]
- Culligan, K.; Sehgal, R.; Mulligan, D.; Dunne, C.; Walsh, S.; Quondamatteo, F.; Dockery, P.; Coffey, J.C. A detailed appraisal of mesocolic lymphangiology—An immunohistochemical and stereological analysis. J. Anat. 2014, 225, 463–472. [Google Scholar] [CrossRef]
- Liang, J.T.; Huang, J.; Chen, T.C.; Hung, J.S. The Toldt fascia: A historic review and surgical implications in complete mesocolic excision for colon cancer. Asian J. Surg. 2019, 42, 1–5. [Google Scholar] [CrossRef]
- Garcia-Granero, A.; Pellino, G.; Frasson, M.; Fletcher-Sanfeliu, D.; Bonilla, F.; Sánchez-Guillén, L.; Dolz, A.D.; Romaguera, V.P.; Ortí, L.S.; Martinez-Soriano, F.; et al. The fusion fascia of Fredet: An important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg. Endosc. 2019, 33, 3842–3850. [Google Scholar] [CrossRef]
- Coffey, J.C.; Sehgal, R.; Culligan, K.; Dunne, C.; McGrath, D.; Lawes, N.; Walsh, D. Terminology and nomenclature in colonic surgery: Universal application of a rule-based approach derived from updates on mesenteric anatomy. Tech. Coloproctol. 2014, 18, 789–794. [Google Scholar] [CrossRef]
- Zhu, D.J.; Chen, X.W.; OuYang, M.Z.; Lu, Y. Three surgical planes identified in laparoscopic complete mesocolic excision for right-sided colon cancer. World J. Surg. Oncol. 2016, 14, 7. [Google Scholar] [CrossRef]
- Monsellato, I.; Gatto, T.; Lodin, M.; Panaro, F. Robotic CME in 110 consecutive cases: Feasibility and short-term technical and oncological outcomes. Minerva Surg. 2025, 80, 7–14. [Google Scholar] [CrossRef]
- Munkedal, D.L.E.; Rosenkilde, M.; Nielsen, D.T.; Sommer, T.; West, N.P.; Laurberg, S. Radiological and pathological evaluation of the level of arterial division after colon cancer surgery. Color. Dis. 2017, 19, O238–O245. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Z.; Chi, P.; Huang, Y. Anatomical observation of the right retroperitoneal fascia and its clinical significance in complete mesocolic excision for right colon cancer. Chin. J. Gastrointest. Surg./Zhonghua Wei Chang Wai Ke Za Zhi 2021, 24, 704–710. [Google Scholar] [CrossRef]
- Chemtob, A.; Ignjatovic, D.; Stimec, B.V. Retrocolic Fascia-An Anatomical and Multidetector Computed Tomographic Angiography (MDCTA) Morphometric Analysis in Patients with Right Colon Cancer. Diagnostics 2024, 14, 1952. [Google Scholar] [CrossRef]
- Sumiyama, F.; Hamada, M.; Kobayashi, T.; Matsumi, Y.; Inada, R.; Kurokawa, H.; Uemura, Y. Why did we encounter a pCRM-positive specimen whose preoperative MRI indicates negative mesorectal fascia involvement in middle to low rectal cancer? Tech. Coloproctol. 2025, 29, 81. [Google Scholar] [CrossRef]
- Galvez, A.; Biondo, S.M.; Trenti, L.M.; Espin, E.; Kraft, M.M.; Farres, R.; Codina-Cazador, A.M.; Flor, B.; Garcia-Granero, E.M.; Enriquez-Navascues, J.M.M.; et al. Prognostic Value of the Circumferential Resection Margin After Curative Surgery for Rectal Cancer: A Multicenter Propensity Score-Matched Analysis. Dis. Colon. Rectum. 2023, 66, 887–897. [Google Scholar] [CrossRef]
- Lee, J.M.; Chung, T.; Kim, K.M.; Simon, N.S.M.M.; Han, Y.D.; Cho, M.S.; Hur, H.M.; Lee, K.Y.M.; Kim, N.K.M.; Lee, S.B.M.; et al. Significance of Radial Margin in Patients Undergoing Complete Mesocolic Excision for Colon Cancer. Dis. Colon. Rectum. 2020, 63, 488–496. [Google Scholar] [CrossRef]
- Gundestrup, A.K.; Olsen, A.S.F.; Ingeholm, P.; Bols, B.; Kleif, J.; Bertelsen, C.A. Nonmicroradical Resection Margin as a Predictor of Recurrence in Patients With Stage III Colon Cancer Undergoing Complete Mesocolic Excision: A Prospective Cohort Study. Dis. Colon. Rectum. 2022, 65, 683–691. [Google Scholar] [CrossRef]
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D.L. Association of Radial Margin Positivity With Colon Cancer. JAMA Surg. 2015, 150, 890–898. [Google Scholar] [CrossRef]
- Del Coco, F.; Achilli, P.; Carnevali, P.; Giusti, I.; Giani, A.; Bertoglio, C.L.; Magistro, C.; Origi, M.; Mazzola, M.; Ferrari, G. Long-Term Oncological Outcomes After Complete Mesocolic Excision Versus Standard Resection for Right-Sided Colon Cancer: A Propensity Score Matching Analysis. J. Gastrointest. Cancer 2025, 56, 127. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Neuenschwander, A.U.; Kleif, J. Risk of Local Recurrence after Complete Mesocolic Excision for Right-Sided Colon Cancer: Post-Hoc Sensitivity Analysis of a Population-Based Study. Dis. Colon. Rectum. 2022, 65, 1103–1111. [Google Scholar] [CrossRef]
- Pompeu, B.F.M.; Pasqualotto, E.; Marcolin, P.; Delgado, L.M.; Pigossi, B.D.; de Figueiredo, S.M.P.; Formiga, F.B.M. Complete mesocolic excision versus D2 lymphadenectomy in right hemicolectomy: A meta-analysis of propensity score matched studies and randomized controlled trials. Ann. Med. Surg. 2025, 87, 855–866. [Google Scholar] [CrossRef]
- Lucas, K.; Melling, N.; Giannou, A.D.; Reeh, M.; Mann, O.; Hackert, T.; Izbicki, J.R.; Perez, D.; Grass, J.K. Lymphatic Mapping in Colon Cancer Depending on Injection Time and Tracing Agent: A Systematic Review and Meta-Analysis of Prospective Designed Studies. Cancers 2023, 15, 3196. [Google Scholar] [CrossRef]
- Perrakis, A.; Weber, K.; Merkel, S.; Matzel, K.; Agaimy, A.; Gebbert, C.; Hohenberger, W. Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int. J. Color. Dis. 2014, 29, 1223–1229. [Google Scholar] [CrossRef]
- Karachun, A.; Panaiotti, L.; Chernikovskiy, I.; Achkasov, S.; Gevorkyan, Y.; Savanovich, N.; Sharygin, G.; Markushin, L.; Sushkov, O.; Aleshin, D.; et al. Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). Br. J. Surg. 2020, 107, 499–508. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Kirkegaard-Klitbo, A.; Nielsen, M.; Leotta, S.M.G.; Daisuke, F.; Gögenur, I. Pattern of Colon Cancer Lymph Node Metastases in Patients Undergoing Central Mesocolic Lymph Node Excision: A Systematic Review. Dis. Colon. Rectum. 2016, 59, 1209–1221. [Google Scholar] [CrossRef]
- Sakamoto, T.; Mukai, T.; Noguchi, T.; Matsui, S.; Yamaguchi, T.; Akiyoshi, T.; Kawachi, H.; Fukunaga, Y. Patterns of lymph node metastasis and long-term outcomes of splenic flexure colon cancer: A descriptive study from a Japanese high-volume center. Surg Today. 2025, 55, 1079–1087. [Google Scholar] [CrossRef]
- Cazelles, A.; Cadi, M.; Cossé, C.; Labiad, C.; Lecot, F.; Al Jaafari, B.; Mariani, A.; Karoui, M.; Manceau, G. Preoperative angio-CT colonography improves the quality of lymph node dissection during minimally invasive right hemicolectomy: A propensity score-matched study. Surg. Endosc. 2025, 39, 3247–3258. [Google Scholar] [CrossRef]
- Park, H.-M.; Lee, J.; Lee, S.Y.; Heo, S.H.; Jeong, Y.Y.; Kim, H.R.; Kim, C.H. Optimal extent of lymph node dissection in clinical early-stage right colon cancer: A retrospective analysis. Ann. Surg. Treat. Res. 2025, 108, 49–56. [Google Scholar] [CrossRef]
- Kikuchi, R.; Takano, M.; Takagi, K.; Fujimoto, N.; Nozaki, R.; Fujiyoshi, T.; Uchida, Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis. Colon. Rectum. 1995, 38, 1286–1295. [Google Scholar] [CrossRef]
- Arencibia-Pérez, B.; Giner, F.; García-Granero, E.; Roselló-Keränen, S.; Flor-Lorente, B.; Cervantes, A.; Sancho-Muriel, J.; Frasson, M. The Degree of Extramural Spread of T3 Colon Cancer as a Prognostic Factor: Another Appeal to the American Joint Committee on Cancer. Cancer Med. 2025, 14, e70720. [Google Scholar] [CrossRef]
- Riffet, M.; Dupont, B.; Faisant, M.; Cerasuolo, D.; Menahem, B.; Alves, A.; Dubois, F.; Levallet, G.; Bazille, C. New Histoprognostic Factors to Consider for the Staging of Colon Cancers: Tumor Deposits, Invasive Tumor Infiltration and High-Grade Budding. Int. J. Mol. Sci. 2023, 24, 3573. [Google Scholar] [CrossRef]
- Cohen, R.; Shi, Q.; Meyers, J.; Jin, Z.; Svrcek, M.; Fuchs, C.; Couture, F.; Kuebler, P.; Ciombor, K.; Bendell, J.; et al. Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: A post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance)☆. Ann. Oncol. 2021, 32, 1267–1275. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, M.R.; Ha, G.W. Prognostic value of tumor deposits for long-term oncologic outcomes in patients with stage III colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 141–151. [Google Scholar] [CrossRef]
- Hakki, L.; Khan, A.; Do, E.; Gonen, M.; Firat, C.; Vakiani, E.; Shia, J.; Widmar, M.; Wei, I.H.; Smith, J.J.; et al. Tumour deposits are independently associated with recurrence in colon cancer. Color. Dis. 2024, 26, 459–465. [Google Scholar] [CrossRef]
- Jörgren, F.; Agger, E.; Lydrup, M.L.; Buchwald, P. Tumour deposits in colon cancer predict recurrence and reduced survival in a nationwide population-based study. BJS Open 2023, 7, zrad122. [Google Scholar] [CrossRef]
- Wong-Chong, N.; Motl, J.; Hwang, G.; Nassif, G.J.; Albert, M.R.; Monson, J.R.; Lee, L. Impact of Tumor Deposits on Oncologic Outcomes in Stage III Colon Cancer. Dis. Colon. Rectum. 2018, 61, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Peacock, O.; Uppal, A.; Hu, C.; Bednarski, B.K.; Taggart, M.W.; Dasari, A.; Morris, V.K.; Kaur, H.; Kopetz, S.; et al. The prognostic impact of tumor deposits in colorectal cancer: More than just N1c. Cancer 2024, 130, 4052–4060. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, B.B.; Öztürk, İ.; Akyol, C.; Savaş, B.; Utkan, G. Adjuvant Chemotherapy Duration and Disease-Free Survival in Low-Risk Stage III Colon Cancer with N1a-b and N1c Disease: Insights from a Single-Center Retrospective Analysis. J. Gastrointest. Cancer 2024, 56, 14. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Kawasaki, T.; Kaira, K.; Hirano, Y. Pathological Investigation of Grasping-related Mesenteric Injury During Laparoscopic Colorectal Cancer Resection. Anticancer. Res. 2025, 45, 2493–2499. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.W.; Park, J.W.; Ryoo, S.-B.; Oh, H.-K.; Shin, R.; Choi, J.S.; Kim, M.J.; Park, S.-C.; Kim, D.-W.; et al. Tumor Deposits as an Adverse Prognostic Indicator in Stage III Colon Cancer: A Multicenter Database Study. Ann. Surg. Open 2024, 5, e456. [Google Scholar] [CrossRef]
- Willaert, W.; Cosyns, S.; Ceelen, W. Biology-Based Surgery: The Extent of Lymphadenectomy in Cancer of the Colon. Eur. Surg. Res. 2019, 59, 371–379. [Google Scholar] [CrossRef]
- Banerjee, S.; Zhang, X.; Kuang, S.; Wang, J.; Li, L.; Fan, G.; Luo, Y.; Sun, S.; Han, P.; Wu, Q.; et al. Comparative analysis of clonal evolution among patients with right- and left-sided colon and rectal cancer. iScience 2021, 24, 102718. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Tenma, J.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; Jepsen, L.V.; et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: A retrospective, population-based study. Lancet Oncol. 2015, 16, 161–168. [Google Scholar] [CrossRef]
- Nagasaki, T.; Akiyoshi, T.; Fujimoto, Y.; Konishi, T.; Nagayama, S.; Fukunaga, Y.; Arai, M.; Ueno, M. Prognostic Impact of Distribution of Lymph Node Metastases in Stage III Colon Cancer. World J. Surg. 2015, 39, 3008–3015. [Google Scholar] [CrossRef]
- Siani, L.M.; Pulica, C. Laparoscopic complete mesocolic excision with central vascular ligation in right colon cancer: Long-term oncologic outcome between mesocolic and non-mesocolic planes of surgery. Scand. J. Surg. 2015, 104, 219–226. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; Cui, Y.; Shen, Z.; Jiang, K.; Shen, D.; Wang, Y.; Zhan, S.; Guo, P.; Yang, X.; et al. Efficacy and Safety of Complete Mesocolic Excision in Patients with Colon Cancer: Three-year Results from a Prospective, Nonrandomized, Double-blind, Controlled Trial. Ann. Surg. 2020, 271, 519–526. [Google Scholar] [CrossRef]
- Storli, K.E.; Søndenaa, K.; Furnes, B.; Nesvik, I.; Gudlaugsson, E.; Bukholm, I.; Eide, G.E. Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech. Coloproctol. 2014, 18, 557–564. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; De Vita, F.; Ferraraccio, F.; Zamboli, A.; Mabilia, A.; Auricchio, A.; Castellano, P.; Napolitano, V.; Orditura, M. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int. J. Color. Dis. 2014, 29, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.T.; Lai, H.S.; Huang, J.; Sun, C.T. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg. Endosc. 2015, 29, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chen, Q.-X.; Lu, L.-B.; Chen, H.; Wu, J.-X.; Wang, X.-W.; Chen, Y.-Y.; Lin, Q.; Li, J.; Chen, X. Readjustment of nodal staging by integrating tumor deposits and positive lymph nodes in patients with stage III colon cancer: A population-based analysis. Am. J. Cancer Res. 2023, 13, 4976. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10636666/ (accessed on 30 June 2025). [PubMed]
- Turan, B.; Sanli, A.N.; Sanli, D.E.T.; Acar, S. Prognostic impact and independent significance of tumor deposits in early-stage colon cancer: A population-based cohort study. Int. J. Colorectal Dis. 2025, 40, 146. [Google Scholar] [CrossRef]
- Gouvas, N.; Pechlivanides, G.; Zervakis, N.; Kafousi, M.; Xynos, E. Complete mesocolic excision in colon cancer surgery: A comparison between open and laparoscopic approach. Color. Dis. 2012, 14, 1357–1364. [Google Scholar] [CrossRef]
- Zheng, M.H.; Zhang, S.; Feng, B. Complete mesocolic excision: Lessons from anatomy translating to better oncologic outcome. World J. Gastrointest. Oncol. 2016, 8, 235–239. [Google Scholar] [CrossRef]
- Sakjah, S.; Olsen, A.S.F.; Gundestrup, A.K.; Born, P.W.; Bols, B.; Ingeholm, P.; Kleif, J.; Bertelsen, C.A. Plane of mesocolic dissection as predictor of recurrence after complete mesocolic excision for sigmoid colon cancer: A cohort study. Color. Dis. 2022, 24, 943–953. [Google Scholar] [CrossRef]
- Kobayashi, H.; West, N.P.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Quirke, P.; Sugihara, K. Quality of surgery for stage III colon cancer: Comparison between England, Germany, and Japan. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), 398–404. [Google Scholar] [CrossRef]
- Benz, S.R.; Feder, I.S.; Vollmer, S.; Tam, Y.; Reinacher-Schick, A.; Denz, R. Complete mesocolic excision for right colonic cancer: Prospective multicentre study. Br. J. Surg. 2022, 110, 98. [Google Scholar] [CrossRef]
- Noronha, M.M.; Almeida, L.F.C.; Cappellaro, A.P.; da Silva, L.F.L.; da Conceição, L.D.; de Menezes, J.S.A.; Belotto, M.; Peixoto, R.D. Neoadjuvant chemotherapy for colon cancer: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Cancer 2025, 222, 115476. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seretis, F.; Panagaki, A.; Seretis, C.; Sotiropoulou, M.; Psarologos, M.; Mamakos, N.; Polyzois, K.; Drakopoulos, V.; Kapiris, S. Complete Mesocolic Excision for Colon Cancer: Insight into Potential Mechanisms of Oncologic Benefit. Cancers 2025, 17, 2719. https://doi.org/10.3390/cancers17162719
Seretis F, Panagaki A, Seretis C, Sotiropoulou M, Psarologos M, Mamakos N, Polyzois K, Drakopoulos V, Kapiris S. Complete Mesocolic Excision for Colon Cancer: Insight into Potential Mechanisms of Oncologic Benefit. Cancers. 2025; 17(16):2719. https://doi.org/10.3390/cancers17162719
Chicago/Turabian StyleSeretis, Fotios, Antonia Panagaki, Charalambos Seretis, Maria Sotiropoulou, Michail Psarologos, Nikolaos Mamakos, Konstantinos Polyzois, Vasileios Drakopoulos, and Stylianos Kapiris. 2025. "Complete Mesocolic Excision for Colon Cancer: Insight into Potential Mechanisms of Oncologic Benefit" Cancers 17, no. 16: 2719. https://doi.org/10.3390/cancers17162719
APA StyleSeretis, F., Panagaki, A., Seretis, C., Sotiropoulou, M., Psarologos, M., Mamakos, N., Polyzois, K., Drakopoulos, V., & Kapiris, S. (2025). Complete Mesocolic Excision for Colon Cancer: Insight into Potential Mechanisms of Oncologic Benefit. Cancers, 17(16), 2719. https://doi.org/10.3390/cancers17162719





