Interplay Between MicroRNAs and Breast Cancer Therapies: Personalized Therapeutic Potential for HER2-Low Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Molecular and Genomic Landscape of HER2-Low Breast Cancer
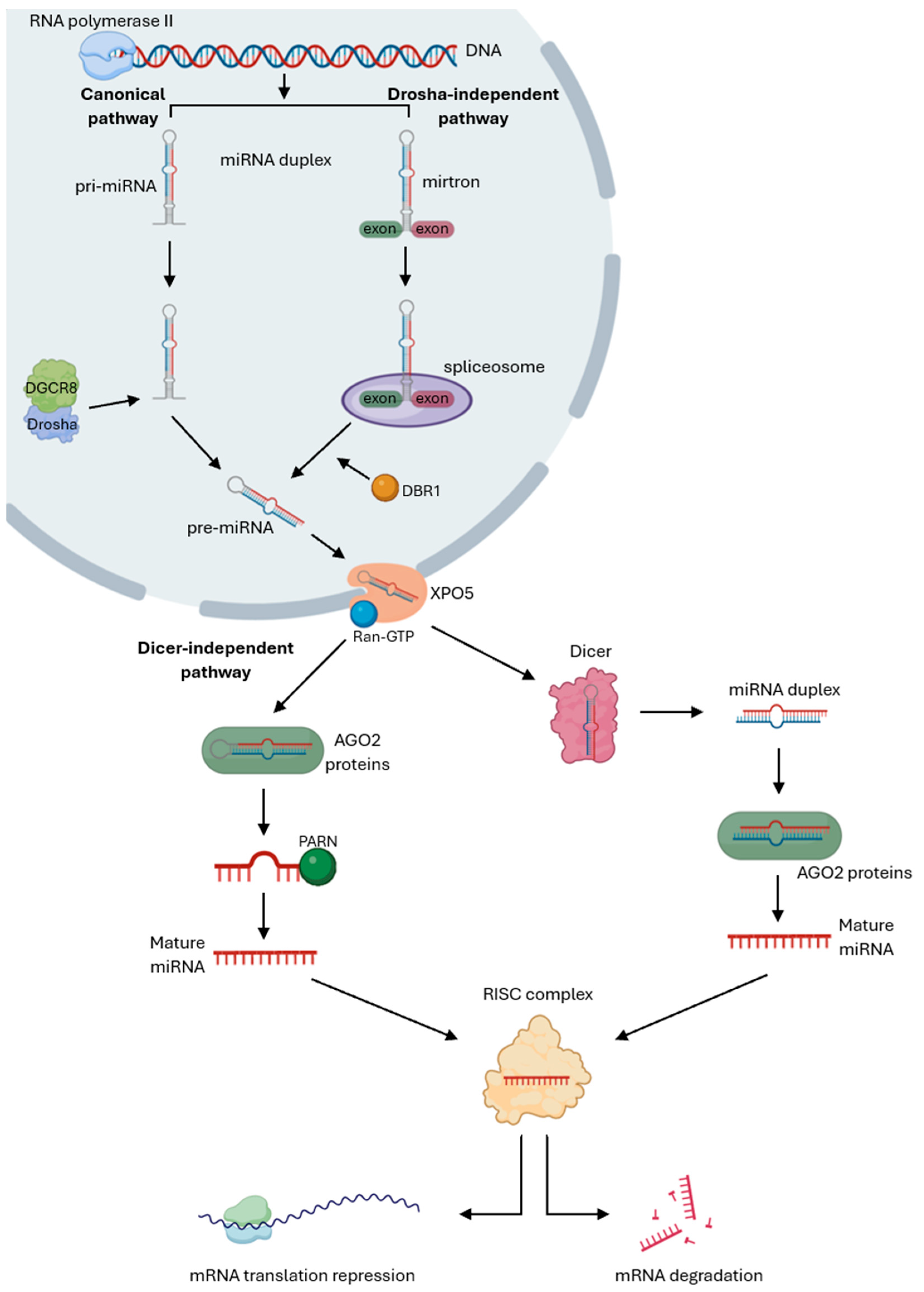
3. MicroRNAs’ Biogenesis and Mechanism of Action
4. MicroRNAs in Cancer
4.1. Function of MicroRNAs in Cancer Hallmarks
4.2. MicroRNAs as Biomarkers in Cancer
5. Targeted Therapies for Breast Cancer
5.1. HER2-Targeted Therapies in Breast Cancer
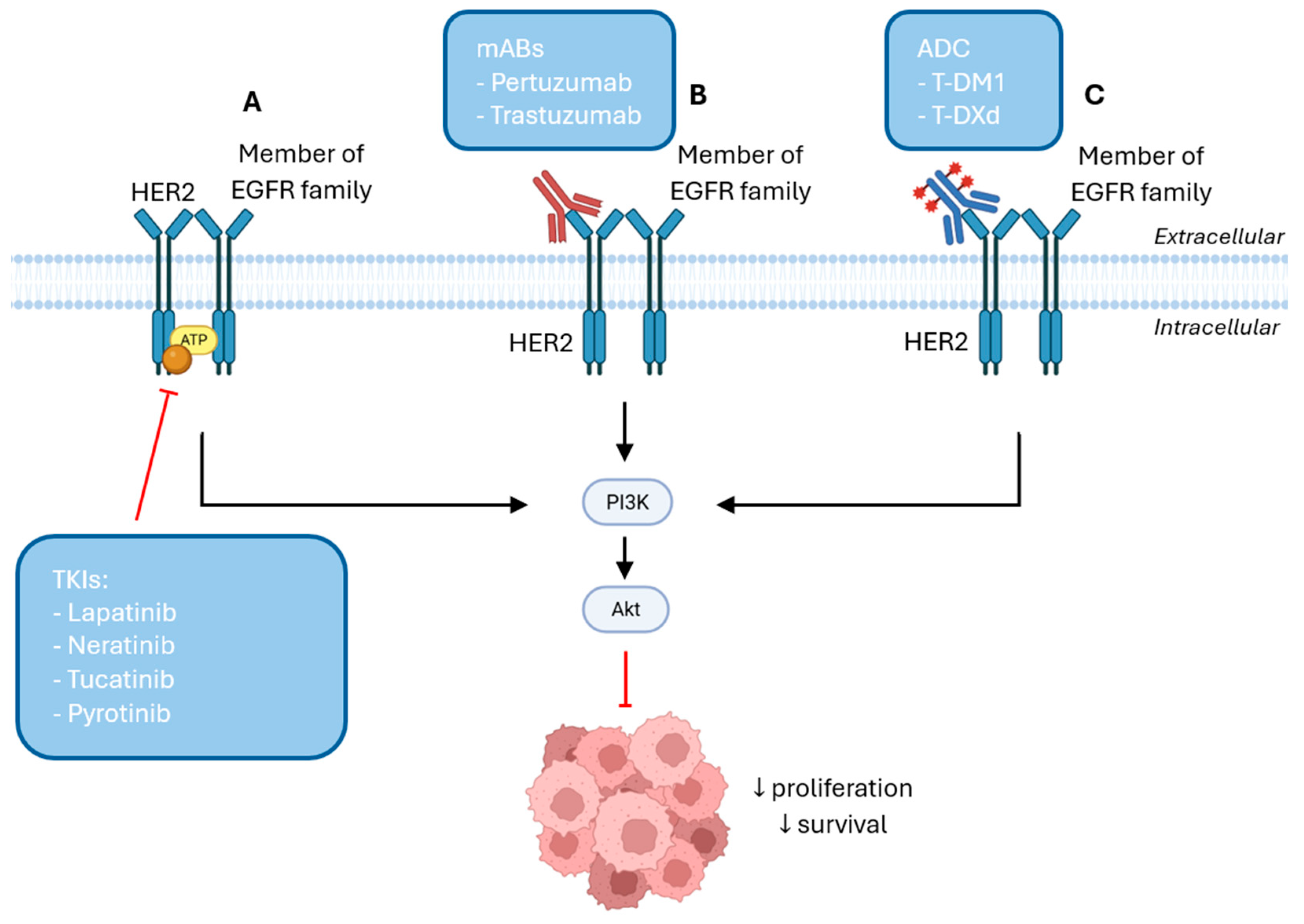
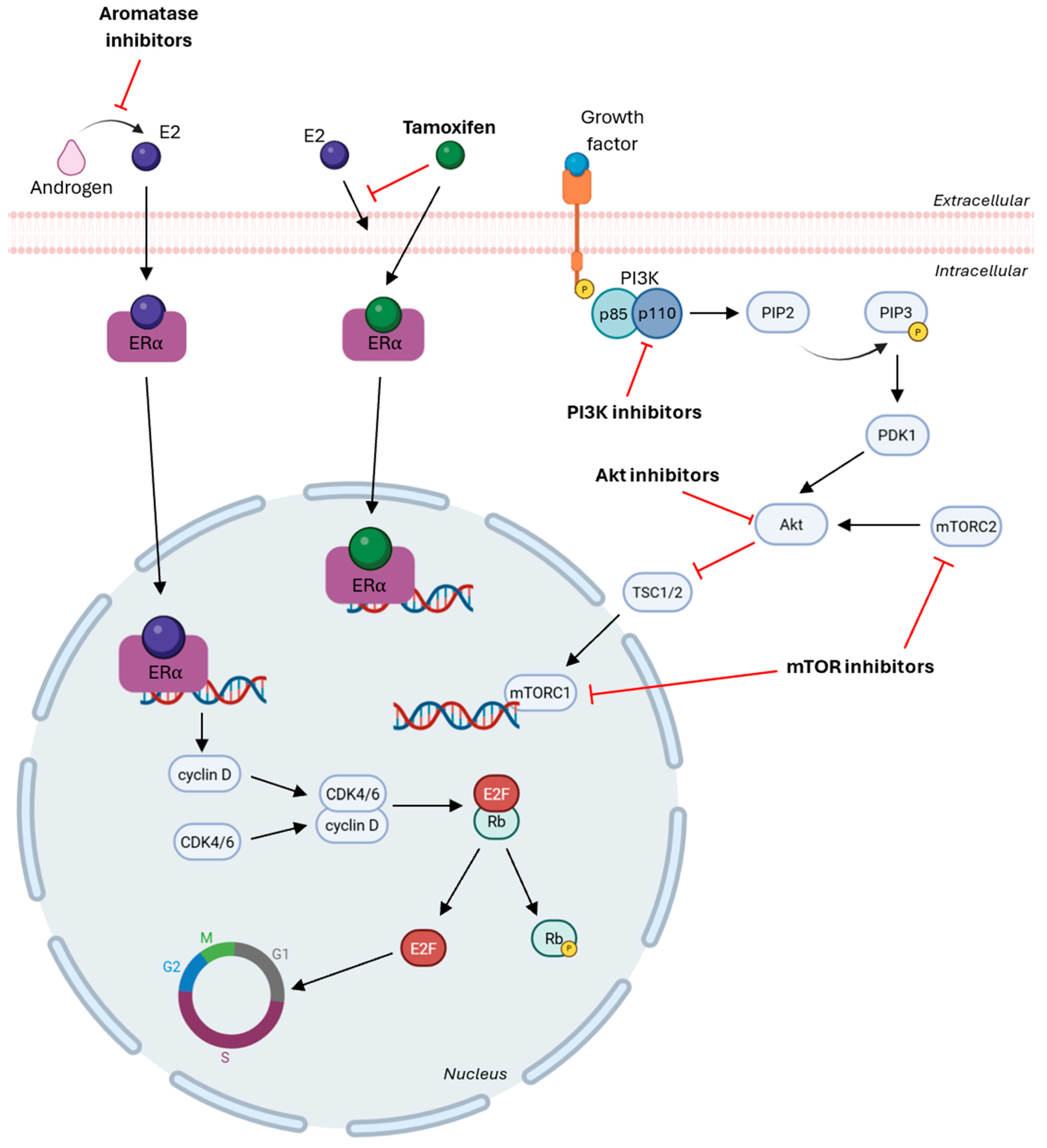
5.2. HR-Targeted Therapies in Breast Cancer
6. Crosstalk Between MicroRNAs and Breast Cancer Therapies
6.1. Advances and Challenges in MicroRNA-Based Therapeutics
6.2. MicroRNAs as a Personalized Therapeutic Strategy for Breast Cancer
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody-Drug Conjugate |
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| AI | Aromatase Inhibitors |
| Akt | Activated Protein Kinase B |
| AKT3 | Threonine-Protein Kinase |
| ASCO | American Society of Clinical Oncology |
| ASPP2 | Apoptosis-Stimulating Protein 2 |
| CAP | College of American Pathologists |
| CCND1 | Cyclin D1 |
| CDK | Cyclin-Dependent Kinase |
| c-MET | MET Proto-Oncogene |
| CNV | Copy Number Variations |
| DGCR8 | DiGeorge Syndrome Critical Region 8 |
| EBP1 | ErbB3-Binding Protein 1 |
| ECM | Extracellular Matrix |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| ER | Estrogen Receptor |
| ESMO | European Society for Medical Oncology |
| FDF2 | Fibroblast Growth Factor 2 |
| FGF2 | Fibroblast Growth Factor 2 |
| FKBP12 | FK506-binding protein 12 |
| FOXO1 | Forkhead Box O1 |
| HCV | Hepatitis C Virus |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hormone Receptor |
| IGF-1R | Insulin-like Growth Factor 1 Receptor |
| IGF2 | Insulin-like Growth Factor 2 |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin-6 |
| IRS1 | Insulin Receptor Substrate 1 |
| ISH | In Situ Hybridization |
| JAK2 | Janus Kinase 2 |
| MAPK | Mitogen-Activated Protein Kinase |
| MED1 | Mediator Complex Subunit 1 |
| miRISC | miRNA-induced silencing complex |
| miRNAs | MicroRNAs |
| mitomiRs | Mitochondrial MicroRNAs |
| MMP9 | Matrix Metalloproteinase 9 |
| mTOR | mammalian Target of Rapamycin |
| mTORC | mammalian Target of Rapamycin Complex |
| MYB | V-Myb Avian Myeloblastosis Viral Oncogene Homolog |
| OS | Overall Survival |
| PARN | Poly(A)-specific ribonuclease |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PEG | Polyethylene Glycol |
| PEI | Polyethylenimine |
| PFS | Progression-Free Survival |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP2 | Phosphatidylinositol-4, 5-biphosphate |
| PIP3 | Phosphatidylinositol-3, 4, 5-triphosphate |
| PLGA | Poly(Lactic-co-Glycolic Acid) |
| Pre-miRNA | Precursor MicroRNA |
| Pri-miRNA | Primary MicroRNA |
| PTEN | Phosphatase and Tensin Homolog |
| RAF1 | Raf Proto-Oncogene Serine |
| Rb | Retinoblastoma |
| SERM | Selective Estrogen Receptor Modulators |
| SHP2 | Src Homology Region 2-Containing Protein Tyrosine Phosphatase-2 |
| SIRT1 | Sirtuin 1 |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| SOX9 | SRY-Box Transcription Factor 9 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAM | Tumor-Associated Macrophages |
| T-DM1 | Trastuzumab Emtansine |
| T-DXd | Trastuzumab Deruxtecan |
| TERT | Telomerase Reverse Transcriptase |
| TGF-β | Transforming Growth Factor-Beta |
| TKI | Tyrosine Kinase Inhibitors |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| TRL | Toll-like Receptors |
| TSC1/2 | Tuberous Sclerosis Complex 1/2 |
| UTR | Untranslated Region |
| VEGF | Vascular Endothelial Growth Factor |
| XPO5 | Exportin 5 |
| ZEB1 | Zinc Finger E-box-binding Homeobox 1 |
References
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An Update on the Pathological Classification of Breast Cancer. Histopathology 2022, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Green, A.R. Molecular Classification of Breast Cancer: What the Pathologist Needs to Know. Pathology 2017, 49, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Molecular Classification of Breast Cancer: Relevance and Challenges. Arch. Pathol. Lab. Med. 2023, 147, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, J.; Xu, Q.; Gao, Z.; Yang, M.; Wu, X.; Li, X. HER2/PI3K/AKT Pathway in HER2-Positive Breast Cancer: A Review. Medicine 2024, 103, e38508. [Google Scholar]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER 2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Kang, Y.J.; Oh, S.J.; Bae, S.Y.; Kim, E.K.; Lee, Y.J.; Park, E.H.; Jeong, J.; Park, H.K.; Suh, Y.J.; Kim, Y.S. Predictive Biological Factors for Late Survival in Patients with HER2-Positive Breast Cancer. Sci. Rep. 2023, 13, 11008. [Google Scholar] [CrossRef]
- Galogre, M.; Rodin, D.; Pyatnitskiy, M.; Mackelprang, M.; Koman, I. A Review of HER2 Overexpression and Somatic Mutations in Cancers. Crit. Rev. Oncol. Hematol. 2023, 186, 103997. [Google Scholar] [CrossRef]
- Schettini, F.; Prat, A. Dissecting the Biological Heterogeneity of HER2-Positive Breast Cancer. Breast 2021, 59, 339–350. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; Mcshane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Li, Y.; Tsang, J.Y.; Tam, F.; Loong, T.; Tse, G.M. Comprehensive Characterization of HER2-Low Breast Cancers: Implications in Prognosis and Treatment. eBioMedicine 2023, 91, 104571. [Google Scholar] [CrossRef]
- Zhang, H.; Katerji, H.; Turner, B.M.; Hicks, D.G. HER2-Low Breast Cancers: New Opportunities and Challenges. Am. J. Clin. Pathol. 2022, 157, 328–336. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.B. HER2-Low Breast Cancer: Now and in the Future. Cancer Res. Treat. 2024, 56, 700–720. [Google Scholar] [CrossRef]
- Corti, C.; Giugliano, F.; Nicolò, E.; Tarantino, P.; Criscitiello, C.; Curigliano, G. HER2-Low Breast Cancer: A New Subtype? Curr. Treat. Options Oncol. 2023, 24, 468–478. [Google Scholar] [CrossRef]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. Her2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, L. Challenges in HER2-Low Breast Cancer Identification, Detection, and Treatment. Transl. Breast Cancer Res. 2024, 5, 3. [Google Scholar] [CrossRef]
- Tarantino, P.; Viale, G.; Press, M.F.; Hu, X.; Penault-Llorca, F.; Bardia, A.; Batistatou, A.; Burstein, H.J.; Carey, L.A.; Cortes, J.; et al. ESMO Expert Consensus Statements (ECS) on the Definition, Diagnosis, and Management of HER2-Low Breast Cancer. Ann. Oncol. 2023, 34, 645–659. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse Effects of Tyrosine Kinase Inhibitors in Cancer Therapy: Pathophysiology, Mechanisms and Clinical Management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; Madden, S.F.; Gaynor, N.; AlSultan, D.; Le Gal, M.; Eustace, A.J.; Gately, K.A.; Hughes, C.; Davies, A.M.; Mahgoub, T.; et al. Effects of HER Family-Targeting Tyrosine Kinase Inhibitors on Antibody-Dependent Cell-Mediated Cytotoxicity in HER2-Expressing Breast Cancer. Clin. Cancer Res. 2021, 27, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhong, R.; Ma, F. HER2-Low Breast Cancer: Novel Detections and Treatment Advances. Crit. Rev. Oncol. Hematol. 2023, 181, 103883. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Wong, J.S.; Cheah, Y.K. Potential MiRNAs for MiRNA-Based Therapeutics in Breast Cancer. Noncoding RNA 2020, 6, 29. [Google Scholar] [CrossRef]
- Syeda, Z.A.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Devaney, A.; McNeill, R.E.; Davoren, P.A.; Lemetre, C.; Benes, V.; Schmidt, S.; Blake, J.; Ball, G.; et al. MicroRNA Signatures Predict Oestrogen Receptor, Progesterone Receptor and HER2/Neureceptor Status in Breast Cancer. Breast Cancer Res. 2009, 11, R27. [Google Scholar] [CrossRef]
- Nair, M.G.; Mavatkar, A.D.; Naidu, C.M.; VP, S.; CE, A.; Rajarajan, S.; Sahoo, S.; Mohan, G.; Jaikumar, V.S.; Ramesh, R.S.; et al. Elucidating the Role of MicroRNA-18a in Propelling a Hybrid Epithelial–Mesenchymal Phenotype and Driving Malignant Progression in ER-Negative Breast Cancer. Cells 2024, 13, 821. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. MiRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Scognamiglio, I.; Di Martino, M.T.; Campani, V.; Virgilio, A.; Galeone, A.; Gullà, A.; Gallo Cantafio, M.E.; Misso, G.; Tagliaferri, P.; Tassone, P.; et al. Transferrin-Conjugated SNALPs Encapsulating 2’-O-Methylated MiR-34a for the Treatment of Multiple Myeloma. Biomed. Res. Int. 2014, 2014, 217365. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, W.H.; Chen, B.Y.; Nie, J.; Su, Z.R.; Zheng, J.N.; Gong, S.T.; Chen, J.N.; Jiang, D.; Li, Y. MiR-29b Suppresses Proliferation and Induces Apoptosis of Hepatocellular Carcinoma Ascites H22 Cells via Regulating TGF-Β1 and P53 Signaling Pathway. Int. J. Mol. Med. 2021, 48, 157. [Google Scholar] [CrossRef]
- Normann, L.S.; Haugen, M.H.; Aure, M.R.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MiR-101-5p Acts as a Tumor Suppressor in HER2-Positive Breast Cancer Cells and Improves Targeted Therapy. Breast Cancer 2022, 14, 25–39. [Google Scholar] [CrossRef]
- Bozgeyik, E.; Kocahan, S.; Temiz, E.; Bagis, H. MiR-19a and MiR-421 Target PCA3 Long Non-Coding RNA and Restore PRUNE2 Tumor Suppressor Activity in Prostate Cancer. Mol. Biol. Rep. 2021, 49, 6803–6815. [Google Scholar] [CrossRef] [PubMed]
- Trang, P.; Medina, P.P.; Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Homer, R.; Brown, D.; Bader, A.G.; Weidhaas, J.B.; et al. Regression of Murine Lung Tumors by the Let-7 MicroRNA. Oncogene 2010, 29, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. npj Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The Frequency of Low HER2 Expression in Breast Cancer and a Comparison of Prognosis between Patients with HER2-Low and HER2-Negative Breast Cancer by HR Status. Breast Cancer 2022, 29, 234–241. [Google Scholar] [CrossRef]
- Viale, G.; Basik, M.; Niikura, N.; Tokunaga, E.; Brucker, S.; Penault-Llorca, F.; Hayashi, N.; Sohn, J.; Teixeira de Sousa, R.; Brufsky, A.M.; et al. Retrospective Study to Estimate the Prevalence and Describe the Clinicopathological Characteristics, Treatments Received, and Outcomes of HER2-Low Breast Cancer. ESMO Open 2023, 8, 101615. [Google Scholar] [CrossRef] [PubMed]
- Rey-Vargas, L.; Bejarano-Rivera, L.M.; Ballen, D.F.; Serrano-Gómez, S.J. Characterization of HER2-Low Breast Tumors among a Cohort of Colombian Women. Cancers 2024, 16, 3141. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.A.; Habibian, L.; Martin, C.; Semaan, K.; Khaddage, A.; El Kassis, N.; Kesserouani, C.; Kourie, H.R.; Atallah, D. Landscape of HER2-Low Breast Cancer: Insights from a Six-Year Study on Prevalence and Clinicopathological Characteristics. Ann. Diagn. Pathol. 2024, 72, 152326. [Google Scholar] [CrossRef] [PubMed]
- Baez-Navarro, X.; van Bockstal, M.R.; Andrinopoulou, E.R.; van Deurzen, C.H.M. HER2-Low Breast Cancer: Incidence, Clinicopathologic Features, and Survival Outcomes from Real-World Data of a Large Nationwide Cohort. Mod. Pathol. 2023, 36, 100087. Corrigendum in Mod. Pathol. 2023, 36, 100356. https://doi.org/10.1016/j.modpat.2023.100356. [CrossRef]
- Gomes Do Nascimento, R.; Otoni, K.M. Histological and Molecular Classification of Breast Cancer: What Do We Know? Mastology 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Tarantino, P.; Gupta, H.; Hughes, M.E.; Files, J.; Strauss, S.; Kirkner, G.; Feeney, A.M.; Li, Y.; Garrido-Castro, A.C.; Barroso-Sousa, R.; et al. Comprehensive Genomic Characterization of HER2-Low and HER2-0 Breast Cancer. Nat. Commun. 2023, 14, 7496. [Google Scholar] [CrossRef]
- Bansal, R.; Adeyelu, T.; Elliott, A.; Walker, P.; Bustos, M.A.; Rodriguez, E.; Accordino, M.K.; Meisel, J.; Gatti-Mays, M.E.; Hsu, E.; et al. Genomic and Transcriptomic Landscape of HER2-Low Breast Cancer. Breast Cancer Res. Treat. 2025, 209, 323–330. [Google Scholar] [CrossRef]
- Jin, J.; Li, B.; Cao, J.; Li, T.; Zhang, J.; Cao, J.; Zhao, M.; Wang, L.; Wang, B.; Tao, Z.; et al. Analysis of Clinical Features, Genomic Landscapes and Survival Outcomes in HER2-Low Breast Cancer. J. Transl. Med. 2023, 21, 360. [Google Scholar] [CrossRef]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.H.; Lu, Y.S.; Ono, M.; Ueno, T.; et al. HER2 Expression, Copy Number Variation and Survival Outcomes in HER2-Low Non-Metastatic Breast Cancer: An International Multicentre Cohort Study and TCGA-METABRIC Analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef]
- Merlin, J.L.; Husson, M.; Sahki, N.; Gilson, P.; Massard, V.; Harlé, A.; Leroux, A. Integrated Molecular Characterization of HER2-Low Breast Cancer Using Next Generation Sequencing (NGS). Biomedicines 2023, 11, 3164. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and Mechanisms of MicroRNA-Mediated Gene Regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The Widespread Regulation of MicroRNA Biogenesis, Function and Decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Varghese, L.N.; Schwenke, D.O.; Katare, R. Role of Noncoding RNAs in Cardiac Ageing. Front. Cardiovasc. Med. 2023, 10, 1142575. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, Characterization, and Functions of Mirtrons. Wiley Interdiscip. Rev. RNA 2022, 13, 1680. [Google Scholar] [CrossRef]
- Herrera-Carrillo, E.; Berkhout, B. Dicer-Independent Processing of Small RNA Duplexes: Mechanistic Insights and Applications. Nucleic Acids Res. 2017, 45, 10369–10379. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules 2020, 10, 1285. [Google Scholar] [CrossRef]
- Biasini, A.; Abdulkarim, B.; de Pretis, S.; Tan, J.Y.; Arora, R.; Wischnewski, H.; Dreos, R.; Pelizzola, M.; Ciaudo, C.; Marques, A.C. Translation Is Required for MiRNA-dependent Decay of Endogenous Transcripts. EMBO J. 2021, 40, e104569. [Google Scholar] [CrossRef]
- da Sacco, L.; Masotti, A. Recent Insights and Novel Bioinformatics Tools to Understand the Role of MicroRNAs Binding to 5′ Untranslated Region. Int. J. Mol. Sci. 2012, 14, 480–495. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target MRNAs Are Repressed as Efficiently by MicroRNA-Binding Sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Strayer, E.C.; Krishna, S.; Lee, H.; Vejnar, C.; Neuenkirchen, N.; Gupta, A.; Beaudoin, J.D.; Giraldez, A.J. NaP-TRAP Reveals the Regulatory Grammar in 5′UTR-Mediated Translation Regulation during Zebrafish Development. Nat. Commun. 2024, 15, 10898. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yu, J.; Huang, S.; Zhu, H.; Huang, Z. Transcriptional Regulation of Gene Expression by MicroRNAs as Endogenous Decoys of Transcription Factors. Cell Physiol. Biochem. 2014, 33, 1698–1714. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Kumar, A. Paradigm Shift: MicroRNAs Interact with Target Gene Promoters to Cause Transcriptional Gene Activation or Silencing. Exp. Cell Res. 2025, 444, 114372. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, B.; Zhan, X.; Silver, H.; Li, J. Microrna 195-5p Targets Foxo3 Promoter Region to Regulate Its Expression in Granulosa Cells. Int. J. Mol. Sci. 2021, 22, 6721. [Google Scholar] [CrossRef]
- Jie, M.; Feng, T.; Huang, W.; Zhang, M.; Feng, Y.; Jiang, H.; Wen, Z. Subcellular Localization of MiRNAs and Implications in Cellular Homeostasis. Genes 2021, 12, 856. [Google Scholar] [CrossRef]
- Wu, T.; Song, H.; Xie, D.; Hua, K.; Hu, J.; Deng, Y.; Ji, C.; Fang, L. Mir-30b-5p Promotes Proliferation, Migration, and Invasion of Breast Cancer Cells via Targeting ASPP2. Biomed. Res. Int. 2020, 2020, 7907269. [Google Scholar] [CrossRef]
- Ma, T.; Yang, L.; Zhang, J. MiRNA 542 3p Downregulation Promotes Trastuzumab Resistance in Breast Cancer Cells via AKT Activation. Oncol. Rep. 2015, 33, 1215–1220. [Google Scholar] [CrossRef]
- de Mattos-Arruda, L.; Bottai, G.; Nuciforo, P.G.; di Tommaso, L.; Giovannetti, E.; Peg, V.; Losurdo, A.; Pérez-Garcia, J.; Masci, G.; Corsi, F.; et al. MicroRNA-21 Links Epithelial-to-Mesenchymal Transition and Inflammatory Signals to Confer Resistance to Neoadjuvant Trastuzumab and Chemotherapy in HER2-Positive Breast Cancer Patients. Oncotarget 2015, 6, 37269–37280. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Guijarro, L.G.; Casanova, C.; Coca, S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; Asúnsolo, Á. The Regulatory Role of Mitochondrial MicroRNAs (MitomiRs) in Breast Cancer: Translational Implications Present and Future. Cancers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Erturk, E.; Enes Onur, O.; Akgun, O.; Tuna, G.; Yildiz, Y.; Ari, F. Mitochondrial MiRNAs (MitomiRs): Their Potential Roles in Breast and Other Cancers. Mitochondrion 2022, 66, 74–81. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular MiRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating MicroRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ni, J.; Beretov, J.; Wasinger, V.C.; Graham, P.; Li, Y. Recent Advances of Small Extracellular Vesicle Biomarkers in Breast Cancer Diagnosis and Prognosis. Mol. Cancer 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Rouas, R.; Lagneaux, L.; Krayem, M.; Duvillier, H.; Berehab, M.; Lewalle, P. Acute Myeloid Leukemia-Derived Exosomes Deliver MiR-24-3p to Hinder the T-Cell Immune Response through DENN/MADD Targeting in the NF-ΚB Signaling Pathways. Cell Commun. Signal 2023, 21, 253. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-Derived Exosomal MiR-934 Induces Macrophage M2 Polarization to Promote Liver Metastasis of Colorectal Cancer. J. Hematol. Oncol. 2020, 13, 156. Correction in J. Hematol. Oncol. 2021, 14, 33. https://doi.org/10.1186/s13045-021-01042-0. [CrossRef]
- Dayakar, A.; Shanmukha, K.D.; Kalangi, S.K. Spectrum of MicroRNAs and Their Target Genes in Cancer: Intervention in Diagnosis and Therapy. Mol. Biol. Rep. 2022, 49, 6827–6846. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in Cancer: Biomarkers, Functions and Therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ciccarone, F.; Ciriolo, M.R. Editorial: Hallmark of Cancer: Sustained Proliferative Signalling. Front. Oncol. 2023, 13, 1328827. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, W.; Hong, J.Y.; Kang, D.; Park, S.; Suh, J.; You, D.; Park, Y.Y.; Suh, N.; Hwang, J.J.; et al. MiR-96-5p Targets PTEN to Mediate Sunitinib Resistance in Clear Cell Renal Cell Carcinoma. Sci. Rep. 2022, 12, 3537. [Google Scholar] [CrossRef]
- Huang, G.; Zhong, X.; Yao, L.; Ma, Q.; Liao, H.; Xu, L.; Zou, J.; Sun, R.; Wang, D.; Guo, X. MicroRNA 449a Inhibits Cell Proliferation and Migration by Regulating Mutant P53 in MDA MB 468 Cells. Exp. Ther. Med. 2021, 22, 1020. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Kulbay, M.; Paimboeuf, A.; Ozdemir, D.; Bernier, J. Review of Cancer Cell Resistance Mechanisms to Apoptosis and Actual Targeted Therapies. J. Cell Biochem. 2022, 123, 1736–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Tumor Viruses and Replicative Immortality—Avoiding the Telomere Hurdle. Semin. Cancer Biol. 2014, 26, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M. Editorial: Hallmark of Cancer: Replicative Immortality. Front. Oncol. 2023, 13, 1204094. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Vimalraj, S. A Concise Review of VEGF, PDGF, FGF, Notch, Angiopoietin, and HGF Signalling in Tumor Angiogenesis with a Focus on Alternative Approaches and Future Directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF Signaling: Role in Angiogenesis and Beyond. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef] [PubMed]
- Karsten, N.; Kolben, T.; Mahner, S.; Beyer, S.; Meister, S.; Kuhn, C.; Schmoeckel, E.; Wuerstlein, R.; Harbeck, N.; Ditsch, N.; et al. The Role of E-Cadherin Expression in Primary Site of Breast Cancer. Arch. Gynecol. Obstet. 2022, 305, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, F.; Cai, Q.; Deng, L.; Ouyang, Q.; Zhang, X.H.F.; Zheng, J. Invasion and Metastasis in Cancer: Molecular Insights and Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 57. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, T.; Huang, C.; Xu, Z.; Wang, L.; Jiang, E.; Wang, H.; Chen, Y.; Liu, K.; Shao, Z.; et al. Melanoma Cell-Secreted Exosomal MiR-155-5p Induce Proangiogenic Switch of Cancer-Associated Fibroblasts via SOCS1/JAK2/STAT3 Signaling Pathway. J. Exp. Clin. Cancer Res. 2018, 37, 242. [Google Scholar] [CrossRef]
- Han, Q.; Tan, S.; Gong, L.; Li, G.; Wu, Q.; Chen, L.; Du, S.; Li, W.; Liu, X.; Cai, J.; et al. Omental Cancer-Associated Fibroblast-Derived Exosomes with Low MicroRNA-29c-3p Promote Ovarian Cancer Peritoneal Metastasis. Cancer Sci. 2023, 114, 1929–1942. [Google Scholar] [CrossRef]
- Li, Z.; Sun, C.; Qin, Z. Metabolic Reprogramming of Cancer-Associated Fibroblasts and Its Effect on Cancer Cell Reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef]
- Xuekai, L.; Yan, S.; Jian, C.; Yifei, S.; Xinyue, W.; Wenyuan, Z.; Shuwen, H.; Xi, Y. Advances in Reprogramming of Energy Metabolism in Tumor T Cells. Front. Immunol. 2024, 15, 1347181. [Google Scholar] [CrossRef]
- Bi, Q.; Wu, J.Y.; Qiu, X.M.; Zhang, J.D.; Sun, Z.J.; Wang, W. Tumor-Associated Inflammation: The Tumor-Promoting Immunity in the Early Stages of Tumorigenesis. J. Immunol. Res. 2022, 2022, 3128933. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Immune Evasion in Cancer: Mechanisms and Cutting-Edge Therapeutic Approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A Novel MiR-155/MiR-143 Cascade Controls Glycolysis by Regulating Hexokinase 2 in Breast Cancer Cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y. Pancreatic Cancer Cell-Derived MicroRNA-155-5p-Containing Extracellular Vesicles Promote Immune Evasion by Triggering EHF-Dependent Activation of Akt/NF-ΚB Signaling Pathway. Int. Immunopharmacol. 2021, 100, 107990. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Soltani, B.M.; Sadeghizadeh, M. MicroRNA-203a Inhibits Breast Cancer Progression through the PI3K/Akt and Wnt Pathways. Sci. Rep. 2024, 14, 4715. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ren, Q.; Wang, J.; He, Y.; Deng, H.; Wang, X.; Liu, C. MiR-199a-3p Promotes Gastric Cancer Progression by Promoting Its Stemness Potential via DDR2 Mediation. Cell Signal 2023, 106, 110636. [Google Scholar] [CrossRef]
- Shi, H.; Pan, B.; Liang, J.; Cai, B.; Wu, G.; Bian, Y.; Shan, G.; Ren, S.; Huang, Y.; Guo, W. MiR-30c-5p Inhibits Esophageal Squamous Cell Carcinoma Progression by Repressing the PI3K/AKT Signaling Pathway. Thorac. Cancer 2024, 15, 2206–2216. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Zhou, Z.S.; Wang, G.Z.; Tu, J.Y.; Cheng, H.B.; Ma, S.Z.; Ke, C.; Wang, Y.; Jian, Q.P.; Shu, Y.H.; et al. MiR-122-5p Regulates the Mevalonate Pathway by Targeting P53 in Non-Small Cell Lung Cancer. Cell Death Dis. 2023, 14, 234. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Tian, Y. MiR-19-3p Targets PTEN to Regulate Cervical Cancer Cell Proliferation, Invasion, and Autophagy. Genet. Res. 2023, 2023, 4784500. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, J.; Wang, J.; Pan, Y.; Fu, J.; Bai, Y.; Zhang, J.; Shao, C. Extracellular MiR-1246 Promotes Lung Cancer Cell Proliferation and Enhances Radioresistance by Directly Targeting DR5. Oncotarget 2016, 7, 32707–32722. [Google Scholar] [CrossRef]
- Chen, J.; Cai, S.; Gu, T.; Song, F.; Xue, Y.; Sun, D. Mir-140-3p Impedes Gastric Cancer Progression and Metastasis by Regulating Bcl2/Becn1-Mediated Autophagy. Onco Targets Ther. 2021, 14, 2879–2892. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, C.; Wang, Y.; Fan, B.; Song, W.; Shen, R. MiR-363-3p Induces Tamoxifen Resistance in Breast Cancer Cells through PTEN Modulation. Sci. Rep. 2024, 14, 32135. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.J.; Tao, H.; Jin, W. Sen. MicroRNA-21 Controls HTERT via PTEN in Human Colorectal Cancer Cell Proliferation. J. Physiol. Biochem. 2015, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Naohiro, S.; Nakayama, Y.; Osaki, M.; Okada, F.; Oshimura, M.; Kugoh, H. MiR-19b Regulates HTERT MRNA Expression through Targeting PITX1 MRNA in Melanoma Cells. Sci. Rep. 2015, 5, 8201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zhang, S.; Huang, Q.; Liu, S.; Qiu, L.; Wei, M.; Deng, X.; Meng, W.; Chen, H.N.; et al. MicroRNA-6084 Orchestrates Angiogenesis and Liver Metastasis in Colorectal Cancer via Extracellular Vesicles. JCI Insight 2025, 10, e189503. [Google Scholar] [CrossRef]
- Chang, R.M.; Fu, Y.; Zeng, J.; Zhu, X.Y.; Gao, Y. Cancer-Derived Exosomal MiR-197-3p Confers Angiogenesis via Targeting TIMP2/3 in Lung Adenocarcinoma Metastasis. Cell Death Dis. 2022, 13, 1032. [Google Scholar] [CrossRef]
- Fan, B.; Niu, Y.; Ren, Z.; Wei, S.; Ma, Y.; Su, J.; Zhang, A. Long Noncoding RNA MMP2-AS1 Contributes to Progression of Renal Cell Carcinoma by Modulating MiR-34c-5p/MMP2 Axis. J. Oncol. 2022, 2022, 7346460. [Google Scholar] [CrossRef]
- Leoni, I.; Galvani, G.; Monti, E.; Vianello, C.; Valenti, F.; Pincigher, L.; Grolla, A.A.; Moro, M.; Coada, C.A.; Perrone, A.; et al. MiR-22/GLUT1 Axis Induces Metabolic Reprogramming and Sorafenib Resistance in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 3808. [Google Scholar] [CrossRef]
- Xu, D.; Liu, B.; Wang, L. MiR-365-3p Inhibits Lung Cancer Proliferation and Migration via CPT1A-Mediated Fatty Acid Oxidation. Sci. Rep. 2025, 15, 7076. [Google Scholar] [CrossRef]
- Wei, J.; Wang, F.; Kong, L.Y.; Xu, S.; Doucette, T.; Ferguson, S.D.; Yang, Y.; McEnery, K.; Jethwa, K.; Gjyshi, O.; et al. MiR-124 Inhibits STAT3 Signaling to Enhance T Cell-Mediated Immune Clearance of Glioma. Cancer Res. 2013, 73, 3913–3926. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic Reticulum Stress-induced Exosomal MiR-27a-3p Promotes Immune Escape in Breast Cancer via Regulating PD-L1 Expression in Macrophages. J. Cell Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef]
- Zou, C.; Xu, Q.; Mao, F.; Li, D.; Bian, C.; Liu, L.Z.; Jiang, Y.; Chen, X.; Qi, Y.; Zhang, X.; et al. MiR-145 Inhibits Tumor Angiogenesis and Growth by N-RAS and VEGF. Cell Cycle 2012, 11, 2137–2145. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a Limits Tumorigenic Inflammation in Colorectal Cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J.W.; Calses, P.; Kemp, C.J.; Taniguchi, T. MiR-96 Downregulates REV1 and RAD51 to Promote Cellular Sensitivity to Cisplatin and PARP Inhibition. Cancer Res. 2012, 72, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ng, W.L.; Zhang, X.; Wang, P.; Zhang, Z.; Mo, Y.Y.; Mao, H.; Hao, C.; Olson, J.J.; Curran, W.J.; et al. Targeting DNA-PKcs and ATM with MiR-101 Sensitizes Tumors to Radiation. PLoS ONE 2010, 5, e11397. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, M.; Okayama, H.; Ishigame, T.; Min, A.K.T.; Saito, K.; Ujiie, D.; Murakami, Y.; Kikuchi, T.; Nakayama, Y.; Noda, M.; et al. MiRNA-148a-3p Regulates Immunosuppression in DNA Mismatch Repair-Deficient Colorectal Cancer by Targeting PD-L1. Mol. Cancer Res. 2019, 17, 1403–1413. [Google Scholar] [CrossRef]
- Singh, V.; Sen, A.; Saini, S.; Dwivedi, S.; Agrawal, R.; Bansal, A.; Shekhar, S. MicroRNA Significance in Cancer: An Updated Review on Diagnostic, Prognostic, and Therapeutic Perspectives. Ejifcc 2024, 35, 265–284. [Google Scholar]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Liu, M.; Mo, F.; Song, X.; He, Y.; Yuan, Y.; Yan, J.; Yang, Y.; Huang, J.; Zhang, S. Exosomal Hsa-MiR-21-5p Is a Biomarker for Breast Cancer Diagnosis. PeerJ 2021, 9, e12147. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.J.; Zhang, L.; Xu, Q.F.; Zhao, Y.M.; Shi, X.Y.; Xu, A.G. Serum MiR-21 Level: A Potential Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Med. 2015, 8, 14759–14763. [Google Scholar]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum MiR-21 as a Diagnostic and Prognostic Biomarker in Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef]
- Kao, H.W.; Pan, C.Y.; Lai, C.H.; Wu, C.W.; Fang, W.L.; Huang, K.H.; Lin, W.C. Urine MiR-21-5p as a Potential Non-Invasive Biomarker for Gastric Cancer. Oncotarget 2017, 8, 56389–56397. [Google Scholar] [CrossRef]
- Stechele, M.; Link, H.; Hirner-Eppeneder, H.; Alunni-Fabbroni, M.; Wildgruber, M.; Salvermoser, L.; Corradini, S.; Schinner, R.; Ben Khaled, N.; Rössler, D.; et al. Circulating MiR-21 as a Prognostic Biomarker in HCC Treated by CT-Guided High-Dose Rate Brachytherapy. Radiat. Oncol. 2023, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Hanif, M.; Ahmed, A.; Jamal, Q.; Mushtaq, S.; Khan, A.; Saqib, M. Circulating MiR-21 as a Prognostic and Predictive Biomarker in Oral Squamous Cell Carcinoma. Pak. J. Med. Sci. 2019, 35, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Ishinaga, H.; Okugawa, Y.; Hou, B.; He, F.; Yin, C.; Murata, M.; Toiyama, Y.; Takeuchi, K. The Role of MiR-21 as a Predictive Biomarker and a Potential Target to Improve the Effects of Chemoradiotherapy against Head and Neck Squamous Cell Carcinoma. J. Radiat. Res. 2023, 64, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lu, Z.; Meng, F.; Wan, Y.; Zhang, L.; Xu, Q.; Wang, Z. Circulating MiR-141 as a Potential Biomarker for Diagnosis, Prognosis and Therapeutic Targets in Gallbladder Cancer. Sci. Rep. 2022, 12, 10072. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal MiRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Eilertsen, M.; Andersen, S.; Al-Saad, S.; Richardsen, E.; Stenvold, H.; Hald, S.M.; Al-Shibli, K.; Donnem, T.; Busund, L.T.; Bremnes, R.M. Positive Prognostic Impact of MiR-210 in Non-Small Cell Lung Cancer. Lung Cancer 2014, 83, 272–278. [Google Scholar] [CrossRef]
- Parrella, P.; Barbano, R.; Pasculli, B.; Fontana, A.; Copetti, M.; Valori, V.M.; Poeta, M.L.; Perrone, G.; Righi, D.; Castelvetere, M.; et al. Evaluation of MicroRNA-10b Prognostic Significance in a Prospective Cohort of Breast Cancer Patients. Mol. Cancer 2014, 13, 142. [Google Scholar] [CrossRef]
- Xin, S.Y.; Feng, X.S.; Zhou, L.Q.; Sun, J.J.; Gao, X.L.; Yao, G.L. Reduced Expression of Circulating MicroRNA-218 in Gastric Cancer and Correlation with Tumor Invasion and Prognosis. World J. Gastroenterol. 2014, 20, 6906–6911. [Google Scholar] [CrossRef]
- Wang, X.X.; Ge, S.J.; Wang, X.L.; Jiang, L.X.; Sheng, M.F.; Ma, J.J. MiR-218 Tissue Expression Level Is Associated with Aggressive Progression of Gastric Cancer. Genet. Mol. Res. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Feng, X.; Yang, P.; Yang, J.; An, P.; Wang, H.; Ye, S.; Yu, C.; He, Y.; et al. Low Expression of MicroRNA-126 Is Associated with Poor Prognosis in Colorectal Cancer. Genes Chromosomes Cancer 2014, 53, 358–365. [Google Scholar] [CrossRef]
- Thugu, T.R.; Jain, M.; Madhavaram, N.J.; Singh, N.; Dash, S.; Alhomaid, A.; Kanagala, S.G.; Byreddi, L.Y.; Jain, A.; Desai, R. CLO25-090: MiR-31 as a Predictive Biomarker of Cetuximab Efficacy in Metastatic Colorectal Cancer Patients. J. Natl. Compr. Canc Netw. 2025, 23, CLO25-090. [Google Scholar] [CrossRef]
- Caramés, C.; Cristobal, I.; Moreno, V.; Marín, J.P.; González-Alonso, P.; Torrejón, B.; Minguez, P.; Leon, A.; Martín, J.I.; Hernández, R.; et al. MicroRNA-31 Emerges as a Predictive Biomarker of Pathological Response and Outcome in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2016, 17, 878. [Google Scholar] [CrossRef]
- Wang, H.; Tan, G.; Dong, L.; Cheng, L.; Li, K.; Wang, Z.; Luo, H. Circulating MiR-125b as a Marker Predicting Chemoresistance in Breast Cancer. PLoS ONE 2012, 7, e34210. [Google Scholar] [CrossRef]
- Patellongi, I.; Amiruddin, A.; Massi, M.N.; Islam, A.A.; Pratama, M.Y.; Sutandyo, N.; Latar, N.H.M.; Faruk, M. Circulating MiR-221/222 Expression as MicroRNA Biomarker Predicting Tamoxifen Treatment Outcome: A Case–Control Study. Ann. Med. Surg. 2023, 85, 3806–3815. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-Tumor Heterogeneity from a Cancer Stem Cell Perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef]
- Alimena, S.; Stephenson, B.J.K.; Webber, J.W.; Wollborn, L.; Sussman, C.B.; Packard, D.G.; Williams, M.; Comrie, C.E.; Wang, J.Y.; Markert, T.; et al. Differences in Serum MiRNA Profiles by Race, Ethnicity, & Socioeconomic Status: Implications for Developing an Equitable Ovarian Cancer Screening Test. Cancer Prev. Res. 2024, 17, 177–185. [Google Scholar]
- Chen, B.; Xia, Z.; Deng, Y.N.; Yang, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Emerging MicroRNA Biomarkers for Colorectal Cancer Diagnosis and Prognosis. Open Biol. 2019, 9, 180212. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The Technology and Biology of Single-Cell RNA Sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor Heterogeneity: Preclinical Models, Emerging Technologies, and Future Applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef] [PubMed]
- Pandiani, C.; Strub, T.; Nottet, N.; Cheli, Y.; Gambi, G.; Bille, K.; Husser, C.; Dalmasso, M.; Béranger, G.; Lassalle, S.; et al. Single-Cell RNA Sequencing Reveals Intratumoral Heterogeneity in Primary Uveal Melanomas and Identifies HES6 as a Driver of the Metastatic Disease. Cell Death Differ. 2021, 28, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ma, N.; Li, X.; Gou, Y.; Duan, Y.; Liu, B.; Xia, J.; Zhao, X.; Wang, X.; Li, Q.; et al. Advances in Single-Cell RNA Sequencing and Its Applications in Cancer Research. J. Hematol. Oncol. 2023, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, I.; Zaza, N.; Das, S.; Li, B.D. Precision Medicine and Targeted Therapies in Breast Cancer. Surg. Oncol. Clin. N. Am. 2020, 29, 51–62. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Guidi, L.; Pellizzari, G.; Tarantino, P.; Valenza, C.; Curigliano, G. Resistance to Antibody-Drug Conjugates Targeting HER2 in Breast Cancer: Molecular Landscape and Future Challenges. Cancers 2023, 15, 1130. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Fardi, E.; Ghaderi, H.; Palizdar, S.; Khorram, R.; Vafadar, R.; Ghanaatian, M.; Rezaei-Tazangi, F.; Baziyar, P.; Ahmadi, A.; et al. Receptor Tyrosine Kinase Inhibitors in Cancer. Cell. Mol. Life Sci. 2023, 80, 104. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, H.; Solanki, R.; Rawat, L.; Tabasum, S.; Tanwar, P.; Pal, S.; Sabarwal, A. Recent Developments in Receptor Tyrosine Kinase Inhibitors: A Promising Mainstay in Targeted Cancer Therapy. Med. Drug Discov. 2024, 23, 100195. [Google Scholar] [CrossRef]
- Iancu, G.; Serban, D.; Badiu, C.D.; Tanasescu, C.; Tudosie, M.S.; Tudor, C.; Costea, D.O.; Zgura, A.; Iancu, R.; Vasile, D. Tyrosine Kinase Inhibitors in Breast Cancer. Exp. Ther. Med. 2021, 23, 114. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Rajendran, R.L.; Miruka, C.O.; Sivamani, P.; Maran, B.A.V.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.C. A Review on Tyrosine Kinase Inhibitors for Targeted Breast Cancer Therapy. Pathol. Res. Pract. 2024, 263, 155607. [Google Scholar] [CrossRef]
- Roskoski, R. Classification of Small Molecule Protein Kinase Inhibitors Based upon the Structures of Their Drug-Enzyme Complexes. Pharmacol. Res. 2016, 103, 26–48. [Google Scholar] [CrossRef]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. In Small Molecules in Oncology; Recent Results in Cancer Research, Volume 211; Springer: Cham, Switzerland, 2018; pp. 19–44. [Google Scholar]
- Eli, L.D.; Kavuri, S.M. Mechanisms of Neratinib Resistance in HER2-Mutant Metastatic Breast Cancer. Cancer Drug Resist. 2022, 5, 873–881. [Google Scholar] [CrossRef]
- Lee, A. Tucatinib: First Approval. Drugs 2020, 80, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.M.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus Capecitabine versus Lapatinib plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer (PHOEBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xia, Y.; Zhu, Y.; Yang, Y.; Lin, Q.; Liu, Q.; Yang, W.; Ling, L.; Zhong, J.; Duan, Z.; et al. Preclinical Study and Phase 2 Trial of Neoadjuvant Pyrotinib Combined with Chemotherapy in Luminal/HER2-Low Breast Cancer: PILHLE-001 Study. Cell Rep. Med. 2024, 5, 101807. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Harbeck, N.; Beckmann, M.W.; Rody, A.; Schneeweiss, A.; Müller, V.; Fehm, T.; Marschner, N.; Gluz, O.; Schrader, I.; Heinrich, G.; et al. HER2 Dimerization Inhibitor Pertuzumab—Mode of Action and Clinical Data in Breast Cancer. Breast Care 2013, 8, 49–55. [Google Scholar] [CrossRef]
- Behl, A.; Wani, Z.A.; Das, N.N.; Parmar, V.S.; Len, C.; Malhotra, S.; Chhillar, A.K. Monoclonal Antibodies in Breast Cancer: A Critical Appraisal. Crit. Rev. Oncol. Hematol. 2023, 183, 103915. [Google Scholar] [CrossRef]
- Vu, T.; Claret, F.X. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef]
- Vo, T.H.; EL-Sherbieny Abdelaal, E.; Jordan, E.; O’Donovan, O.; McNeela, E.A.; Mehta, J.P.; Rani, S. MiRNAs as Biomarkers of Therapeutic Response to HER2-Targeted Treatment in Breast Cancer: A Systematic Review. Biochem. Biophys. Rep. 2023, 37, 101588. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-Targeted Therapies in Cancer: A Systematic Review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef]
- Pérez-Garcia, J.; Muñoz-Couselo, E.; Cortés, J.; Scaltriti, M. Therapeutic Antibodies in Breast Cancer. Semin. Oncol. 2014, 41, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Jiao, D.C.; Qiao, J.H.; Wang, C.Z.; Sun, X.F.; Lu, Z.D.; Li, L.F.; Zhang, C.J.; Yan, M.; Wei, Y.; et al. De-Escalated Neoadjuvant Weekly Nab-Paclitaxel with Trastuzumab and Pertuzumab versus Docetaxel, Carboplatin, Trastuzumab, and Pertuzumab in Patients with HER2-Positive Early Breast Cancer (HELEN-006): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2025, 26, 27–36. [Google Scholar] [PubMed]
- Peng, D.M.; Li, J.; Qiu, J.X.; Zhao, L. Neoadjuvant Pertuzumab plus Trastuzumab in Combination with Chemotherapy for Human Epidermal Growth Factor Receptor 2 Positive Breast Cancer: A Real-World Retrospective Single-Institutional Study in China. World J. Surg. Oncol. 2024, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Falcón González, A.; Cruz Jurado, J.; Llabrés Valenti, E.; Urbano Cubero, R.; Álamo de la Gala, M.C.; Martínez Guisado, M.A.; Álvarez Ambite, R.; Rodríguez González, C.J.; Amérigo Góngora, M.; Rodríguez Pérez, L.; et al. Real-World Experience with Pertuzumab and Trastuzumab Combined with Chemotherapy in Neoadjuvant Treatment for Patients with Early-Stage HER2-Positive Breast Cancer: The NEOPERSUR Study. Clin. Transl. Oncol. 2024, 26, 2217–2226. [Google Scholar] [CrossRef]
- Arshad, M.; Azad, A.; Chan, P.Y.K.; Vigneswara, V.; Feldinger, K.; Nafi, S.N.M.; Laporte-Maguire, E.; De Santo, C.; Zuo, J.; Shaaban, A.M.; et al. Neratinib Could Be Effective as Monotherapy or in Combination with Trastuzumab in HER2-Low Breast Cancer Cells and Organoid Models. Br. J. Cancer 2024, 130, 1990–2002. [Google Scholar] [CrossRef]
- Mark, C.; Lee, J.S.; Cui, X.; Yuan, Y. Antibody–Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int. J. Mol. Sci. 2023, 24, 13726. [Google Scholar] [CrossRef]
- Fan, P.; Xu, K. Antibody-Drug Conjugates in Breast Cancer: Marching from HER2-Overexpression into HER2-Low. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188849. [Google Scholar] [CrossRef]
- Martín, M.; Pandiella, A.; Vargas-Castrillón, E.; Díaz-Rodríguez, E.; Iglesias-Hernangómez, T.; Martínez Cano, C.; Fernández-Cuesta, I.; Winkow, E.; Perelló, M.F. Trastuzumab Deruxtecan in Breast Cancer. Crit. Rev. Oncol. Hematol. 2024, 198, 104355. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab Emtansine: Mechanisms of Action and Drug Resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Eng. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results from DESTINY-Breast03, a Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer: Long-Term Survival Analysis of the DESTINY-Breast03 Trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective Estrogen Receptor Modulators (SERMs) and Selective Estrogen Receptor Degraders (SERDs) in Cancer Treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Wibowo, E.; Pollock, P.A.; Hollis, N.; Wassersug, R.J. Tamoxifen in Men: A Review of Adverse Events. Andrology 2016, 4, 776–788. [Google Scholar] [CrossRef]
- Shen, J.; He, Y.; Li, S.; Chen, H. Crosstalk of Methylation and Tamoxifen in Breast Cancer. Mol. Med. Rep. 2024, 30, 180. [Google Scholar] [CrossRef] [PubMed]
- Generali, D.; Berardi, R.; Caruso, M.; Cazzaniga, M.; Garrone, O.; Minchella, I.; Paris, I.; Pinto, C.; De Placido, S. Aromatase Inhibitors: The Journey from the State of the Art to Clinical Open Questions. Front. Oncol. 2023, 13, 1249160. [Google Scholar] [CrossRef] [PubMed]
- Kharb, R.; Haider, K.; Neha, K.; Yar, M.S. Aromatase Inhibitors: Role in Postmenopausal Breast Cancer. Arch. Pharm. 2020, 353, e2000081. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.; Ingle, J.N.; Dudenkov, T.M.; Kalari, K.R.; Carlson, E.E.; Na, J.; Buzdar, A.U.; Robson, M.E.; Ellis, M.J.; Goss, P.E.; et al. Pharmacogenomics of Aromatase Inhibitors in Postmenopausal Breast Cancer and Additional Mechanisms of Anastrozole Action. JCI Insight 2020, 5, e137571. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Burrett, J.; Clarke, M.; Davies, C.; Duane, F.; Evans, V.; Gettins, L.; Godwin, J.; Gray, R.; Liu, H.; et al. Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet 2015, 386, 1341–1352. [Google Scholar]
- Ruhstaller, T.; Giobbie-Hurder, A.; Colleoni, M.; Jensen, M.B.; Ejlertsen, B.; De Azambuja, E.; Neven, P.; Láng, I.; Jakobsen, E.H.; Gladieff, L.; et al. Adjuvant Letrozole and Tamoxifen Alone or Sequentially for Postmenopausal Women with Hormone Receptor–Positive Breast Cancer: Long-Term Follow-Up of the BIG 1-98 Trial. J. Clin. Oncol. 2019, 37, 105–114. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.K.; Liu, Z.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; et al. Aromatase Inhibitors versus Tamoxifen in Premenopausal Women with Oestrogen Receptor-Positive Early-Stage Breast Cancer Treated with Ovarian Suppression: A Patient-Level Meta-Analysis of 7030 Women from Four Randomised Trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef]
- Demir Cetinkaya, B.; Biray Avci, C. Molecular Perspective on Targeted Therapy in Breast Cancer: A Review of Current Status. Med. Oncol. 2022, 39, 149. [Google Scholar] [CrossRef]
- Ligasová, A.; Frydrych, I.; Koberna, K. Basic Methods of Cell Cycle Analysis. Int. J. Mol. Sci. 2023, 24, 3674. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef]
- Purohit, L.; Jones, C.; Gonzalez, T.; Castrellon, A.; Hussein, A. The Role of CD4/6 Inhibitors in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 1242. [Google Scholar] [CrossRef]
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/MTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Alves, C.L.; Ditzel, H.J. Drugging the PI3K/AKT/MTOR Pathway in ER+ Breast Cancer. Int. J. Mol. Sci. 2023, 24, 4522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, X.; Ren, Y.; Fan, Z.; Zhang, J.; He, G.; Fu, L. Targeting PI3K Family with Small-Molecule Inhibitors in Cancer Therapy: Current Clinical Status and Future Directions. Mol. Cancer 2024, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K Inhibitors in Advanced Breast Cancer. Ann. Oncol. 2019, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, S.E.; Mayer, I.A. Targeting the PI3K/AKT/MTOR Pathway in Hormone Positive Breast Cancer. Drugs 2020, 80, 1685–1697. [Google Scholar] [CrossRef]
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110. [Google Scholar] [CrossRef]
- Kenna, M.M.; McGarrigle, S.; Pidgeon, G.P. The next Generation of PI3K-Akt-MTOR Pathway Inhibitors in Breast Cancer Cohorts. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 185–197. [Google Scholar]
- Zhang, H.P.; Jiang, R.Y.; Zhu, J.Y.; Sun, K.N.; Huang, Y.; Zhou, H.H.; Zheng, Y.B.; Wang, X.J. PI3K/AKT/MTOR Signaling Pathway: An Important Driver and Therapeutic Target in Triple-Negative Breast Cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Shaw, A.L.; Parson, M.A.H.; Truebestein, L.; Jenkins, M.L.; Leonard, T.A.; Burke, J.E. ATP-Competitive and Allosteric Inhibitors Induce Differential Conformational Changes at the Autoinhibitory Interface of Akt1. Structure 2023, 31, 343–354. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; DeMichele, A.; Clark, A.S.; Zelnak, A.; Yardley, D.A.; Karuturi, M.; Sanft, T.; Blau, S.; Hart, L.; et al. Phase I/II Trial of Triplet Therapy (Exemestane, Ribociclib, and Everolimus) after Progression on a CDK4/6 Inhibitor in HR+/HER2− Advanced Breast Cancer (TRINITI-1). Clin. Cancer Res. 2021, 27, 4177–4185. [Google Scholar] [CrossRef]
- Önder, T.; Ateş, Ö.; Öner, I.; Karaçin, C. Relationship between HER2-Low Status and Efficacy of CDK4/6 Inhibitors in Advanced Breast Cancer: A Real-World Study. Int. J. Clin. Oncol. 2024, 29, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Dai, Y.; Chen, L.; Liang, Y.; He, J.; Chen, S.; Song, X.; Luo, W.; Xu, R.; Chen, Q. Effect of HER2-Low Expression on the Efficacy of CDK4/6 Inhibitors in Breast Cancer: A Meta-Analysis. J. Clin. Oncol. 2024, 42, e13034. [Google Scholar] [CrossRef]
- Zattarin, E.; Presti, D.; Mariani, L.; Sposetti, C.; Leporati, R.; Menichetti, A.; Corti, C.; Benvenuti, C.; Fucà, G.; Lobefaro, R.; et al. Prognostic Significance of HER2-Low Status in HR-Positive/HER2-Negative Advanced Breast Cancer Treated with CDK4/6 Inhibitors. npj Breast Cancer 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sottotetti, F.; Tagliaferri, B.; Rizzo, G.; Palumbo, R.; Chessa, G.; Raso, C.; Perrone, L.; Malovini, A.; Tibollo, V.; Locati, L.D.; et al. Patterns of Treatment and Outcomes of Patients with Metastatic HER2-Low Breast Cancer Treated with CDK4/6 Inhibitors and Hormone Therapy. Drugs Context 2025, 14, 2024-12-1. [Google Scholar] [CrossRef]
- Kim, E.H.; Ryu, Y.; Choi, J.; Park, D.; Lee, J.W.; Chi, S.G.; Kim, S.H.; Yang, Y. Targeting MiR-21 to Overcome P-Glycoprotein Drug Efflux in Doxorubicin-Resistant 4T1 Breast Cancer. Biomater. Res. 2024, 28, 0095. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Sohal, I.S.; Iyer, S.G.; Sudarshan, K.; Orellana, E.A.; Ozcan, K.E.; dos Santos, A.P.; Low, P.S.; Kasinski, A.L. Selective Targeting of Chemically Modified MiR-34a to Prostate Cancer Using a Small Molecule Ligand and an Endosomal Escape Agent. Mol. Ther. Nucleic Acids 2024, 35, 102193. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Lu, M.; Cheng, C.; Feng, Z.; Zhao, R.; Liang, J.; Han, J.; Jiang, J.; Xu-Welliver, M.; et al. Let-7b-3p Inhibits Tumor Growth and Metastasis by Targeting the BRF2-Mediated MAPK/ERK Pathway in Human Lung Adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1841–1856. [Google Scholar] [CrossRef]
- Pan, W.; Tan, Y.; Chen, X.; Zeng, L.; Lv, Y.; Yang, J. MiRNA-204-5p Acts as a Tumor Suppressor in Gastric Cancer by Inhibiting Cell Migration, Invasion, and Glycolysis via the RAB22A/PI3K/AKT Axis. Sci. Rep. 2025, 15, 29536. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, J.; Yu, J.; Liao, J.; Liu, S.; Cai, N.; Liang, H.; Chen, X.; Ding, Z.; Zhang, B. MicroRNA-148a-3p Inhibits Progression of Hepatocelluar Carcimoma by Repressing SMAD2 Expression in an Ago2 Dependent Manner. J. Exp. Clin. Cancer Res. 2020, 39, 150. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Liang, L.; He, X. A Narrative Review of MicroRNA Therapeutics: Understanding the Future of MicroRNA Research. Precis. Cancer Med. 2021, 4, 33. [Google Scholar] [CrossRef]
- Segal, M.; Slack, F.J. Challenges Identifying Efficacious MiRNA Therapeutics for Cancer. Expert. Opin. Drug Discov. 2020, 15, 987–992. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent Progress in MicroRNA Delivery for Cancer Therapy by Non-Viral Synthetic Vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In Vivo Delivery of MiRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Chaudhary, V.; Jangra, S.; Yadav, N.R. Nanotechnology Based Approaches for Detection and Delivery of MicroRNA in Healthcare and Crop Protection. J. Nanobiotechnol. 2018, 16, 40. [Google Scholar] [CrossRef]
- Wang, W.T.; Chen, Y.Q. Circulating MiRNAs in Cancer: From Detection to Therapy. J. Hematol. Oncol. 2014, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in MiRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cazares, M.B.; Saavedra-Leos, M.Z.; Jordan-Alejandre, E.; Nunez-Olvera, S.I.; Compean-Martinez, I.; Lopez-Camarillo, C. Lipid-Based Nanoparticles for the Therapeutic Delivery of Non-Coding RNAs in Breast Cancer. Oncol. Rep. 2020, 44, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Kong, W.; Rong, X.; Zhong, Z.; Jiang, L.; Chen, S.; Li, C.; Zhang, F.; Jiang, J. Delivery of MiRNAs Using Nanoparticles for the Treatment of Osteosarcoma. Int. J. Nanomed. 2024, 19, 8641–8660. [Google Scholar] [CrossRef]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. MiRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Polyester-Based Nanoparticles for Nucleic Acid Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Liang, Z.; Wang, X.; Gu, J.; Yao, Q.; Chen, C. New Polymer of Lactic-Co-Glycolic Acid-Modified Polyethylenimine for Nucleic Acid Delivery. Nanomedicine 2016, 11, 1971–1991. [Google Scholar] [CrossRef] [PubMed]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small Interfering RNA for Cancer Treatment: Overcoming Hurdles in Delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Siddiqui, B.; Qindeel, M.; Rahdar, A.; Bilal, M.; Behzadmehr, R.; Mirinejad, S.; Pandey, S. Chitosan Nanocarriers for MicroRNA Delivery and Detection: A Preliminary Review with Emphasis on Cancer. Carbohydr. Polym. 2022, 290, 119489. [Google Scholar] [CrossRef]
- Denizli, M.; Aslan, B.; Mangala, L.S.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Sood, A.K. Chitosan Nanoparticles for MiRNA Delivery. Methods Mol. Biol. 2017, 1632, 219–230. [Google Scholar]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic Potential of RNA-Enriched Extracellular Vesicles: The next Generation in RNA Delivery via Biogenic Nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Picon, M.A.; Wang, L.; Da Fonseca Ferreira, A.; Dong, C.; Marzouka, G.R. Extracellular Vesicles as Delivery Systems in Disease Therapy. Int. J. Mol. Sci. 2023, 24, 17134. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Telford, B.J.; Yahyanejad, S.; de Gunst, T.; den Boer, H.C.; Vos, R.M.; Stegink, M.; van den Bosch, M.T.J.; Alemdehy, M.F.; van Pinxteren, L.A.H.; Schaapveld, R.Q.J.; et al. Multi-Modal Effects of 1B3, a Novel Synthetic MiR-193a-3p Mimic, Support Strong Potential for Therapeutic Intervention in Oncology. Oncotarget 2021, 12, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Duurland, C.L.; de Gunst, T.; den Boer, H.C.; van den Bosch, M.T.J.; Telford, B.J.; Vos, R.M.; Xie, X.; Zang, M.; Wang, F.; Shao, Y.; et al. INT-1B3, an LNP Formulated MiR-193a-3p Mimic, Promotes Anti-Tumor Immunity by Enhancing T Cell Mediated Immune Responses via Modulation of the Tumor Microenvironment and Induction of Immunogenic Cell Death. Oncotarget 2024, 15, 470–485. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. First-in-Human Study of INT-1B3 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT04675996 (accessed on 30 October 2025).
- Ouyang, B.; Bi, M.; Jadhao, M.; Bick, G.; Zhang, X. MiR-205 Regulates Tamoxifen Resistance by Targeting Estrogen Receptor Coactivator MED1 in Human Breast Cancer. Cancers 2024, 16, 3992. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yin, W.; Yu, Z.H.; Zhou, Y.J.; Chi, J.R.; Ge, J.; Cao, X.C. MiR-190 Enhances Endocrine Therapy Sensitivity by Regulating SOX9 Expression in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 22. [Google Scholar] [CrossRef]
- Ghanbari, M.; Karari, K.; Altalebi, S.A.R.; Majeed, S.A.; Haghi, M. The Role of MicroRNAs in Breast Cancer: Diagnostic and Therapeutic Implications. Int. J. Biol. Macromol. 2025, 321, 146386. [Google Scholar] [CrossRef]
- Aboutalebi Vand Beilankouhi, E.; Sanaat, Z.; Hosseinpour Feizi, M.A.; Mehdizadeh, A.; Safaralizadeh, R. Investigation of Circulating MiR-182-3p, MiR -382-3p and MiR-93, MiR-142-3p Involved in Tamoxifen Resistance and Sensitivity in Luminal-Subtype Breast Cancer Patients: A Case–Control Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 7505–7516. [Google Scholar] [CrossRef]
- Fu, R.; Tong, J.S. MiR-126 Reduces Trastuzumab Resistance by Targeting PIK3R2 and Regulating AKT/MTOR Pathway in Breast Cancer Cells. J. Cell Mol. Med. 2020, 24, 7600–7608. [Google Scholar] [CrossRef]
- Gong, C.; Yao, Y.; Wang, Y.; Liu, B.; Wu, W.; Chen, J.; Su, F.; Yao, H.; Song, E. Up-Regulation of MiR-21 Mediates Resistance to Trastuzumab Therapy for Breast Cancer. J. Biol. Chem. 2011, 286, 19127–19137. [Google Scholar] [CrossRef]
- Tang, H.; Song, C.; Ye, F.; Gao, G.; Ou, X.; Zhang, L.; Xie, X.; Xie, X. MiR-200c Suppresses Stemness and Increases Cellular Sensitivity to Trastuzumab in HER2+ Breast Cancer. J. Cell Mol. Med. 2019, 23, 8114–8127. [Google Scholar] [CrossRef]
- Fogazzi, V.; Kapahnke, M.; Cataldo, A.; Plantamura, I.; Tagliabue, E.; Di Cosimo, S.; Cosentino, G.; Iorio, M.V. The Role of MicroRNAs in HER2-Positive Breast Cancer: Where We Are and Future Prospective. Cancers 2022, 14, 5326. [Google Scholar] [CrossRef]
- Tormo, E.; Adam-Artigues, A.; Ballester, S.; Pineda, B.; Zazo, S.; González-Alonso, P.; Albanell, J.; Rovira, A.; Rojo, F.; Lluch, A.; et al. The Role of MiR-26a and MiR-30b in HER2+ Breast Cancer Trastuzumab Resistance and Regulation of the CCNE2 Gene. Sci. Rep. 2017, 7, 41309. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Shah, N.; Lee, J.S.; Markoutsa, E.; Jie, C.; Liu, S.; Botbyl, R.; Reisman, D.; Xu, P.; Chen, H. A Novel Double-Negative Feedback Loop between MiR-489 and the HER2-SHP2-MAPK Signaling Axis Regulates Breast Cancer Cell Proliferation and Tumor Growth. Oncotarget 2016, 7, 18295–18308. [Google Scholar] [CrossRef] [PubMed]
- Venturutti, L.; Cordo Russo, R.I.; Rivas, M.A.; Mercogliano, M.F.; Izzo, F.; Oakley, R.H.; Pereyra, M.G.; De Martino, M.; Proietti, C.J.; Yankilevich, P.; et al. MiR-16 Mediates Trastuzumab and Lapatinib Response in ErbB-2-Positive Breast and Gastric Cancer via Its Novel Targets CCNJ and FUBP1. Oncogene 2016, 35, 6189–6202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yuan, Y.; Li, L.; Wang, X.; Quan, Y.; Liu, C.; Yu, M.; Hu, X.; Meng, X.; Zhou, Z.; et al. HER2-Intronic MiR-4728-5p Facilitates HER2 Expression and Accelerates Cell Proliferation and Migration by Targeting EBP1 in Breast Cancer. PLoS ONE 2021, 16, 0245832. [Google Scholar] [CrossRef]
- Newie, I.; Søkilde, R.; Persson, H.; Grabau, D.; Rego, N.; Kvist, A.; Von Stedingk, K.; Axelson, H.; Borg, Å.; Vallon-Christersson, J.; et al. The HER2-Encoded MiR-4728-3p Regulates ESR1 through a Non-Canonical Internal Seed Interaction. PLoS ONE 2014, 9, 97200. [Google Scholar] [CrossRef]
- Mutlu, M.; Saatci, Ö.; Ansari, S.A.; Yurdusev, E.; Shehwana, H.; Konu, Ö.; Raza, U.; Şahin, Ö. MiR-564 Acts as a Dual Inhibitor of PI3K and MAPK Signaling Networks and Inhibits Proliferation and Invasion in Breast Cancer. Sci. Rep. 2016, 6, 32541. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, D.; Wei, X. MiR-22 Suppresses Tumorigenesis and Improves Radiosensitivity of Breast Cancer Cells by Targeting Sirt1. Biol. Res. 2017, 50, 27. [Google Scholar] [CrossRef]
- Normann, L.S.; Aure, M.R.; Leivonen, S.K.; Haugen, M.H.; Hongisto, V.; Kristensen, V.N.; Mælandsmo, G.M.; Sahlberg, K.K. MicroRNA in Combination with HER2-Targeting Drugs Reduces Breast Cancer Cell Viability in Vitro. Sci. Rep. 2021, 11, 10893. [Google Scholar] [CrossRef]
- Rezaei, Z.; Sebzari, A.; Kordi-Tamandani, D.M.; Dastjerdi, K. Involvement of the Dysregulation of MiR-23b-3p, MiR-195-5p, MiR-656-5p, and MiR-340-5p in Trastuzumab Resistance of HER2-Positive Breast Cancer Cells and System Biology Approach to Predict Their Targets Involved in Resistance. DNA Cell Biol. 2019, 38, 184–192. [Google Scholar] [CrossRef]
- Chen, L.; Ye, L.; Liang, Y.; Luo, W.; Zuo, Q.; Huang, P.; Hu, Y.; Dai, Y.; Wu, Y.; Guo, Q.; et al. Neratinib Enhances the Efficacy of CDK4/6 Inhibitor plus Endocrine Therapy in HR+/HER2-Low Breast Cancer Cell Line ZR-75-1 via Hsa-MiR-23a-5p. Sci. Rep. 2024, 14, 31062. [Google Scholar] [CrossRef]
| Hallmark | miRNA | Main Target | Effect | Ref. |
|---|---|---|---|---|
| Sustained Proliferative Signaling + Resistance to cell death | miR-203a | PIK3CA; Wnt2b | Upregulated in BC; Inhibits PI3K/Akt and Wnt/β-catenin pathways by reducing PIK3CA and Wnt2b expression to induce cell cycle arrest and decrease proliferation, and promotes Bax expression, increasing the rate of apoptosis | [101] |
| Activating invasion and metastases | miR-199a-3p | DDR2 | Downregulated in GC; Promotes EMT via NFATc1/SOX2/Snai1, autophagy and DNA damage in CSCs, and promotes proliferation, invasion and metastasis by upregulating DDR2 | [102] |
| Sustained proliferative signaling + Activating invasion and metastases | miR-30c-5p | PIK3CA | Downregulated in ESCC; Activates PI3K/Akt pathway by upregulating PIK3CA to increase proliferation, invasion and migration | [103] |
| Sustained proliferative signaling + Evading growth suppressors + Resistance to cell death + Reprogramming of energy metabolism | miR-122-5p | p53 | Upregulated NSCLC; Induces cell proliferation and migration and decreases apoptosis by decreasing p53, and activates MVA pathway | [104] |
| Sustained proliferative signaling + Evading growth suppressors + Resistance to cell death + Activating Invasion and Metastases | miR-19-3p | PTEN | Upregulated in cervical cancer; Increases proliferation and invasion by inhibiting PTEN, which activates PI3K/Akt pathway and reduces autophagy and early apoptosis | [105] |
| Sustained proliferative signaling + Resistance to cell death | miR-1246 (extracellular) | DR5 | Upregulated in lung cancer; Promotes radioresistance and decreases radiosensitivity, contributing to cell proliferation by reducing DR5 expression | [106] |
| Sustained proliferative signaling + Resistance to cell death + Activating invasion and metastases | miR-140-3p | BCl-2 | Downregulated in GC; Enhances proliferation, migration and invasion, while reducing apoptosis by inhibiting BCL2/BECN1-dependent autophagy | [107] |
| Sustained proliferative signaling + Evading Growth Suppressors + Resistance to cell death | miR-363-3p | PTEN | Upregulated in BC; Activates the PI3K/Akt signaling pathway by downregulating PTEN, which increases cell proliferation and tamoxifen resistance | [108] |
| Sustained proliferative signaling + Evading growth suppressors + Enabling replicative immortality | miR-21 | PTEN | Upregulated in CRC; Promotes the activation of ERK1/2 pathway by reducing PTEN to increase hTERT expression and maintain telomere length | [109] |
| Sustained proliferative signaling + Evading growth suppressors + Enabling replicative immortality | miR-19b | PITX1 | Upregulated in melanoma; Reactivates hTERT activity and promotes proliferation by inhibiting PITX1 expression | [110] |
| Activating angiogenesis + Activating invasion and metastasis | miR-6084 | ANGPTL4 | Upregulated in CRC; Under normal conditions, it inactivates the JAK2/STAT3 signaling pathway by downregulating ANGPTL4, which reduces angiogenesis; under hypoxia, it increases HIF1A expression causing SP1 degradation, thereby enhancing angiogenesis and leading to liver metastasis | [111] |
| Sustained proliferative signaling + Activating angiogenesis + Activating invasion and metastasis | miR-197-3p | TIMP2/3 | Upregulated in metastatic LUAD; Promotes proliferation, migration and tube formation by downregulating TIMP2/3, thereby enhancing VEGFR2 expression, ERK signaling, MMP1 and MT1-MMP expressions. | [112] |
| Sustained proliferative signaling + Activating invasion and metastasis | miR-34c-5p | MMP2 | Downregulated in renal cell carcinoma; MMP2-AS1 sponges this miRNA, which prevents MMP2 repression, thereby enhancing proliferation, migration, EMT and invasion | [113] |
| Resistance to cell death + Reprogramming energy metabolism | miR-22-3p | GLUT1 | Downregulated in HCC; Promotes migration and EMT markers’ expression and reduces apoptosis; Increases GLUT1 expression to increase glucose uptake and therapeutic resistance to sorafenib | [114] |
| Sustained proliferative signaling + Activating invasion and metastasis + Reprogramming energy metabolism | miR-365-3p | CPT1A | Downregulated in lung cancer; Promotes FAO pathway activation, thereby increasing ATP production and enhances proliferation and migration by upregulating CPT1A | [115] |
| Evading immune destruction | miR-124 | STAT3 | Downregulated in GBM; When it is upregulated, it inhibits STAT3 that reduces cytokine and chemokine expressions and increases IL-2, TNF-α and IFN-γ expression, thereby reducing the immunosuppression response | [116] |
| Evading immune destruction + Tumor-promoting inflammation | miR-27a-3p | MAGI2 | Upregulated in BC; It reduces MAGI2 expression, which decreases PTEN and activates PI3K/Akt pathway, promoting PD-L1 expression on macrophages and reduces CD8+ T cells | [117] |
| Activating angiogenesis + Activating invasion and metastases | miR-145 | N-RAS | Downregulated in BC; It targets N-RAS/VEGF-A, which increases angiogenesis and promotes cell invasion | [118] |
| Tumor-promoting inflammation | miR-146a | RIPK2 | Downregulated in CRC; It upregulates RIPK2, which stimulates IL-17 production by myeloid cells | [119] |
| Resistance to cell death + Genome instability and mutations | miR-96 | RAD51D; REV1 | Upregulated in BC and OC; It reduces RAD51D to decrease the activity of HR repair and targets REV1 to increase chemosensitivity | [120] |
| Resistance to cell death + Genome instability and mutations | miR-101 | DNA-PKcs; ATM | Upregulated in GBM; It targets DNA-PKcs and ATM to impair the DNA DBS repair mechanism, which increases cells sensitivity to irradiation | [121] |
| Evading immune destruction + Genome instability and mutations | miR-148a-3p | PD-L1 | Upregulated in CRC; It targets PD-L1 to stimulate IFN-γ production to the TME, which is indirectly correlated with MMR deficiency, increasing genome instability | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, E.; Schmitt, F.; Vale, N. Interplay Between MicroRNAs and Breast Cancer Therapies: Personalized Therapeutic Potential for HER2-Low Breast Cancer. Cancers 2025, 17, 3672. https://doi.org/10.3390/cancers17223672
Carvalho E, Schmitt F, Vale N. Interplay Between MicroRNAs and Breast Cancer Therapies: Personalized Therapeutic Potential for HER2-Low Breast Cancer. Cancers. 2025; 17(22):3672. https://doi.org/10.3390/cancers17223672
Chicago/Turabian StyleCarvalho, Eduarda, Fernando Schmitt, and Nuno Vale. 2025. "Interplay Between MicroRNAs and Breast Cancer Therapies: Personalized Therapeutic Potential for HER2-Low Breast Cancer" Cancers 17, no. 22: 3672. https://doi.org/10.3390/cancers17223672
APA StyleCarvalho, E., Schmitt, F., & Vale, N. (2025). Interplay Between MicroRNAs and Breast Cancer Therapies: Personalized Therapeutic Potential for HER2-Low Breast Cancer. Cancers, 17(22), 3672. https://doi.org/10.3390/cancers17223672






