Factors Associated with Bone Union Failure After Frozen Autograft Reconstruction in Lower Limb Osteosarcoma
Simple Summary
Abstract
1. Introduction
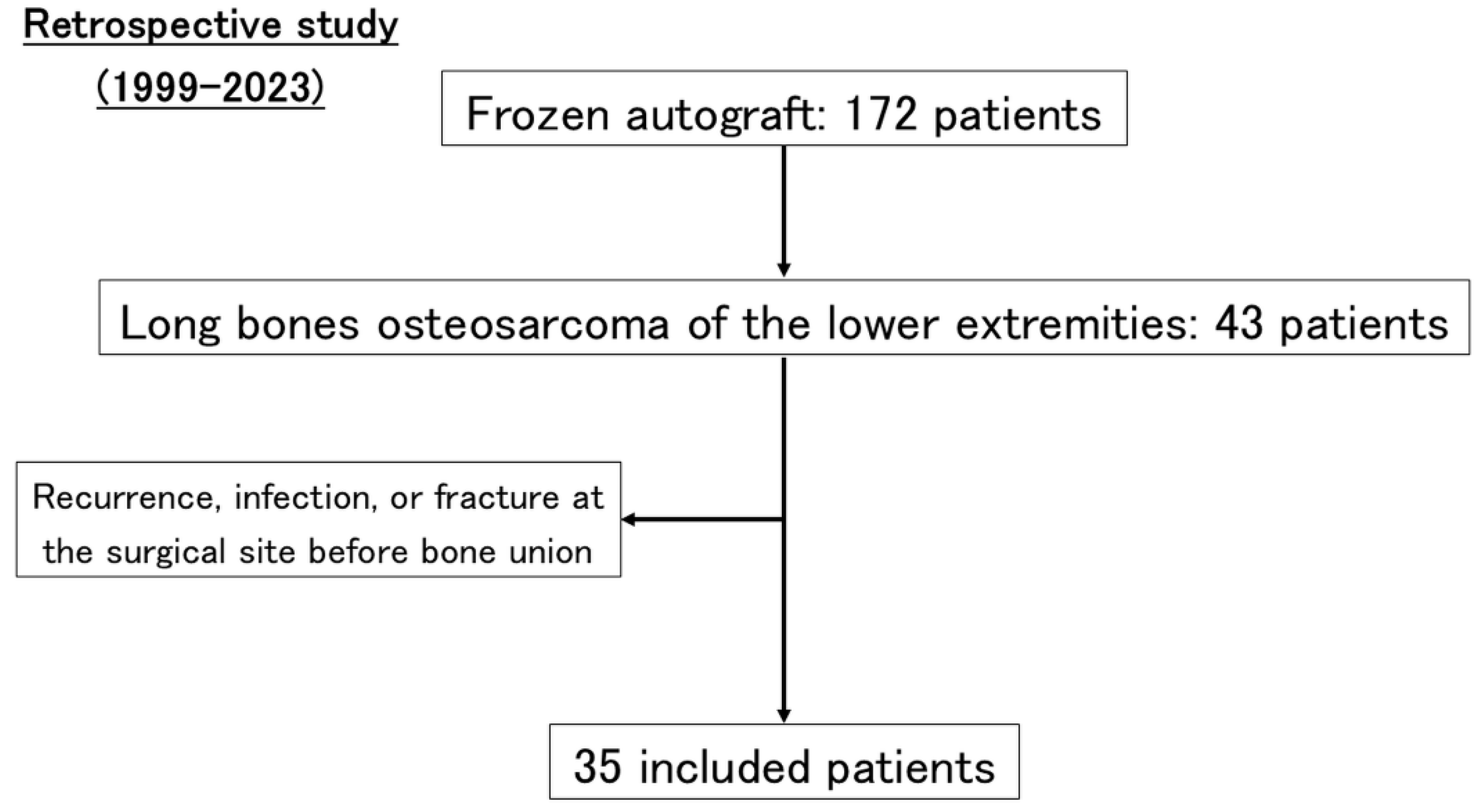
2. Materials and Methods
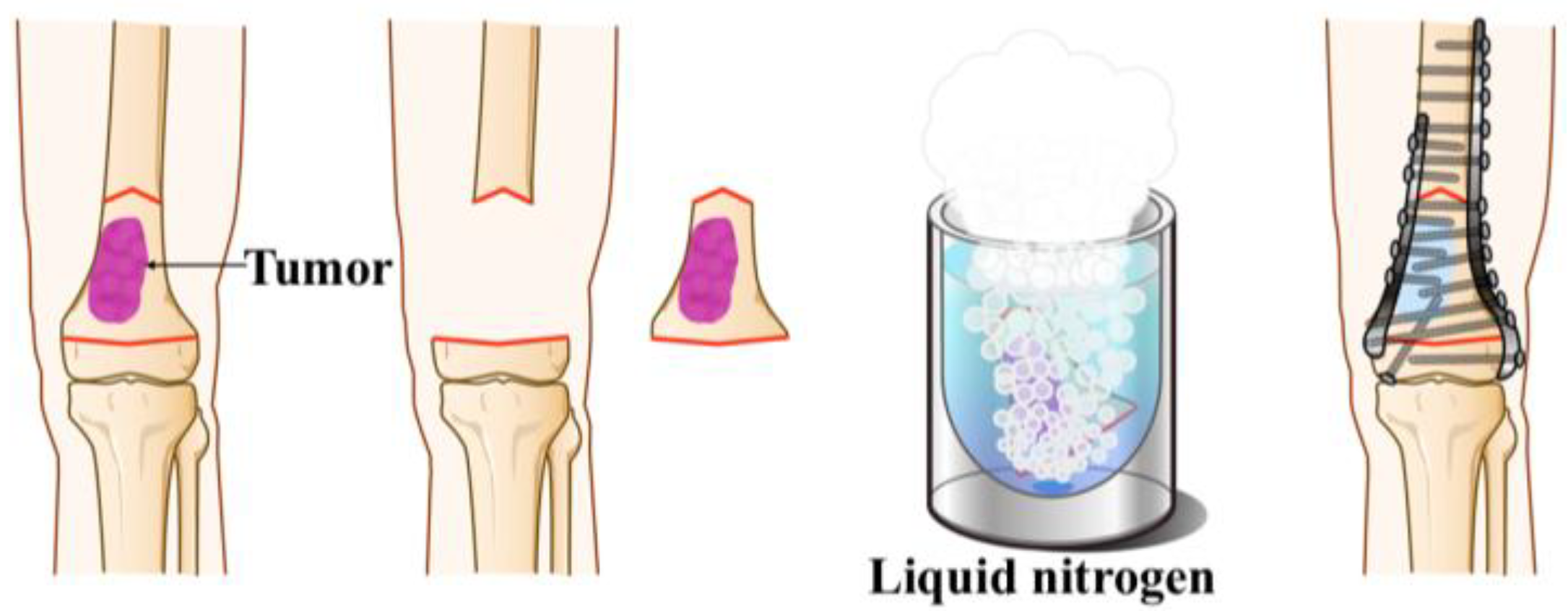
2.1. Surgical Procedure
2.1.1. Free Freezing Technique [18]
2.1.2. Pedicle Freezing Technique [27]
3. Results
3.1. Patients’ Characteristics
3.2. Nonunion Analysis
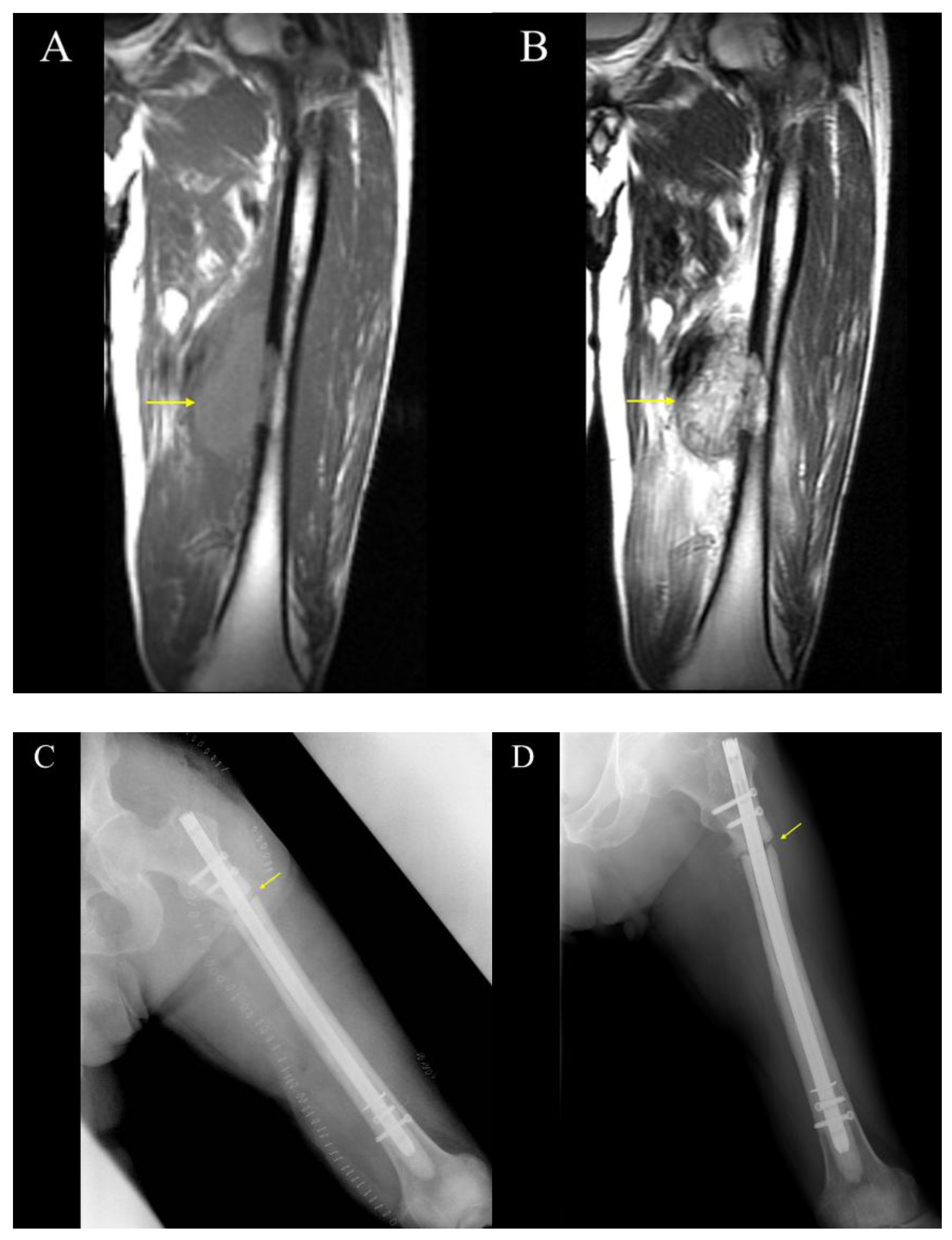
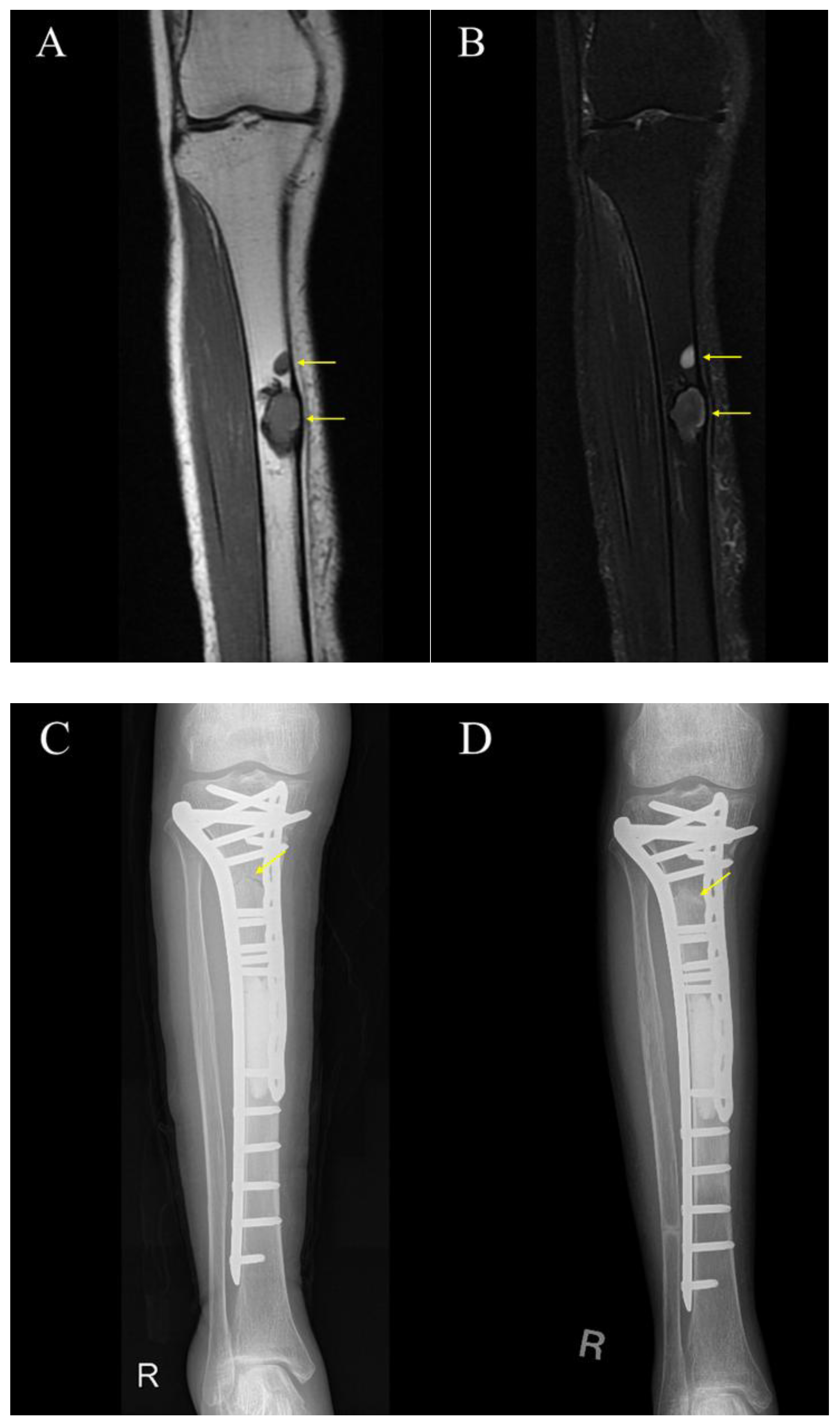
3.3. Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| STIR | Short Tau Inversion Recovery |
| CT | Computed Tomography |
| PMMA | Polymethyl Methacrylate |
| IFN-γ | Interferon-gamma |
| IL-12 | Interleukin-12 |
| HMGB1 | High-Mobility Group Box 1 |
| cGAS | Cyclic GMP–AMP Synthase |
| STING | Stimulator of Interferon Genes |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-Derived Suppressor Cells |
| PD-1 | Programmed Cell Death Protein 1 |
References
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of osteosarcoma and current management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Anninga, J.K.; Gelderblom, H.; Fiocco, M.; Kroep, J.R.; Taminiau, A.H.M.; Hogendoorn, P.C.W.; Egeler, R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur. J. Cancer 2011, 47, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Aljubran, A.H.; Griffin, A.; Pintilie, M.; Blackstein, M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann. Oncol. 2009, 20, 1136–1141. [Google Scholar] [CrossRef]
- Goorin, A.M.; Delorey, M.J.; Lack, E.E.; Gelber, R.D.; Price, K.; Cassady, J.R.; Levey, R.; Tapper, D.; Jaffe, N.; Link, M. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: Analysis of 32 patients. J. Clin. Oncol. 1984, 2, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Seibel, N.L.; Altekruse, S.F.; Ries, L.A.G.; Melbert, D.L.; O’Leary, M.; Smith, F.O.; Reaman, G.H. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J. Clin. Oncol. 2010, 28, 2625–2634. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.; Grimer, R. Surgical modalities in the treatment of bone sarcoma in children. Cancer Treat. Rev. 2010, 36, 342–347. [Google Scholar] [CrossRef]
- Benevenia, J.; Kirchner, R.; Patterson, F.; Beebe, K.; Wirtz, D.C.; Rivero, S.; Palma, M.; Friedrich, M.J. Outcomes of a modular intercalary endoprosthesis as treatment for segmental defects of the femur, tibia, and humerus. Clin. Orthop. Relat. Res. 2016, 474, 539–548. [Google Scholar] [CrossRef]
- Panagopoulos, G.N.; Mavrogenis, A.F.; Mauffrey, C.; Lesenský, J.; Angelini, A.; Megaloikonomos, P.D.; Igoumenou, V.G.; Papanastassiou, J.; Savvidou, O.; Ruggieri, P.; et al. Intercalary reconstructions after bone tumor resections: A review of treatments. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Biau, D.J.; Larousserie, F.; Thévenin, F.; Piperno-Neumann, S.; Anract, P. Results of 32 allograft-prosthesis composite reconstructions of the proximal femur. Clin. Orthop. Relat. Res. 2010, 468, 834–845. [Google Scholar] [CrossRef]
- Funovics, P.T.; Bucher, F.; Toma, C.D.; Kotz, R.I.; Dominkus, M. Treatment and Outcome of parosteal Osteosarcoma: Biological versus endoprosthetic Reconstruction. J. Surg. Oncol. 2011, 103, 782–789. [Google Scholar] [CrossRef]
- Grimer, R.J.; Aydin, B.K.; Wafa, H.; Carter, S.R.; Jeys, L.; Abudu, A.; Parry, M. Very long-term outcomes after endoprosthetic replacement for malignant tumours of bone. Bone Jt. J. 2016, 98, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Andreou, D.; Tunn, P.-U.; Leithner, A. Advances in tumour endoprostheses: A systematic review. EFORT Open Rev. 2019, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, D.-G.; Cho, W.H.; Song, W.S.; Kim, B.S. Are pasteurized autografts durable for reconstructions after bone tumor resections? Clin. Orthop. Relat. Res. 2018, 476, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Outani, H.; Takenaka, S.; Hamada, K.; Imura, Y.; Kakunaga, S.; Tamiya, H.; Wakamatsu, T.; Naka, N.; Ueda, T.; Araki, N. A long-term follow-up study of extracorporeal irradiated autografts in limb salvage surgery for malignant bone and soft tissue tumors: A minimum follow-up of 10 years after surgery. J. Surg. Oncol. 2020, 121, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Wan, S.L.; Sakayama, K.; Yamamoto, N.; Nishida, H.; Tomita, K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J. Bone Jt. Surg. Br. 2005, 87, 218–225. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, W.M.; Huang, C.K. Reconstruction after intercalary resection of malignant bone tumours: Comparison between segmental allograft and extracorporeally-irradiated autograft. J. Bone Jt. Surg. Br. 2005, 87, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Manabe, J.; Ahmed, A.R.; Kawaguchi, N.; Matsumoto, S.; Kuroda, H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin. Orthop. Relat. Res. 2004, 419, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; Wu, P.-K.; Chen, C.-F.; Chung, L.-H.; Liu, C.-L.; Chen, T.-H. High-grade osteosarcoma treated with hemicortical resection and biological reconstruction. J. Surg. Oncol. 2012, 105, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Bangcoy, M.L.S.; Taniguchi, Y.; Morinaga, S.; Asano, Y.; Tsuchiya, H. Graft survivals after reconstruction using tumor-bearing frozen bone in the extremities. Cancers 2023, 15, 3926. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, S.; Yang, Y.; Qu, G.; Liu, G.; Liu, X.; Ma, Y.; Wang, X.; Yao, W. Frozen inactivated autograft replantation for bone and soft tissue sarcomas. Front. Oncol. 2024, 14, 1278237. [Google Scholar] [CrossRef] [PubMed]
- Muteti, E.N.; Rotich, G.K.; Opakas, J.E.; de Souza, A.M.G. Limb salvage surgery in osteosarcoma of the femur using liquid-nitrogen-frozen free femur autograft in a developing country. J. Orthop. Case Rep. 2022, 12, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.; Feng, R.; Li, D. Intercalary frozen autografts for reconstruction of bone defects following meta-/diaphyseal tumor resection at the extremities. BMC Musculoskelet. Disord. 2022, 23, 890. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Z.; Shafiq, M.B.; Ali, S.; Rafi, I. Complications and outcome of bone sarcoma patients with limb salvage using liquid nitrogen-treated bone for reconstruction. J. Cancer Allied Spec. 2024, 10, 543. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Nishida, H.; Srisawat, P.; Shirai, T.; Hayashi, K.; Takeuchi, A.; Yamamoto, N.; Tomita, K. Pedicle frozen autograft reconstruction in malignant bone tumors. J. Orthop. Sci. 2010, 15, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yamamoto, N.; Nojima, T.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Tsuchiya, H. The process of bone regeneration from devitalization to revitalization after pedicle freezing with immunohistochemical and histological examination in rabbits. Cryobiology 2020, 92, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Higuchi, T.; Abe, K.; Taniguchi, Y.; Yonezawa, H.; et al. A viability analysis of tumor-bearing frozen autograft for the reconstruction after resection of malignant bone tumors using 99m Tc-MDP scintigraphy. Clin. Nucl. Med. 2023, 48, 25–34. [Google Scholar] [CrossRef]
- Chapman, M.W. The effect of reamed and nonreamed intramedullary nailing on fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S230–S238. [Google Scholar] [CrossRef]
- Indrekvam, K.; Lekven, J.; Engesaeter, L.B.; Langeland, N. Effects of intramedullary reaming and nailing on blood flow in rat femora. Acta Orthop. Scand. 1992, 63, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Agarwal, A.K.; Gupta, N.; Vijay, V. Plate augmentation with retention of intramedullary nail is effective for resistant femoral shaft non-union. J. Orthop. 2016, 13, 242–245. [Google Scholar] [CrossRef]
- Said, G.Z.; Said, H.G.; El-Sharkawi, M.M. Failed intramedullary nailing of femur: Open reduction and plate augmentation with the nail in situ. Int. Orthop. 2011, 35, 1089–1092. [Google Scholar] [CrossRef][Green Version]
- Perisano, C.; Cianni, L.; Polichetti, C.; Cannella, A.; Mosca, M.; Caravelli, S.; Maccauro, G.; Greco, T. Plate augmentation in aseptic femoral shaft nonunion after intramedullary nailing: A literature review. Bioengineering 2022, 9, 560. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xing, Z.; Ji, T.; Guo, W. Factors influencing nonunion and fracture following biological intercalary reconstruction for lower-extremity bone tumors: A systematic review and pooled analysis. Orthop. Surg. 2022, 14, 3261–3267. [Google Scholar] [CrossRef]
- Takeuchi, A.; Tsuchiya, H.; Setsu, N.; Gokita, T.; Tome, Y.; Asano, N.; Minami, Y.; Kawashima, H.; Fukushima, S.; Takenaka, S.; et al. What are the complications, function, and survival of tumor-devitalized autografts used in patients with limb-sparing surgery for bone and soft tissue tumors? A Japanese musculoskeletal oncology group multi-institutional study. Clin. Orthop. Relat. Res. 2023, 481, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kafchinski, L.A.; Gundle, K.R.; Saidi, K.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Intercalary allograft augmented with intramedullary cement and plate fixation is a reliable solution after resection of a diaphyseal tumour. Bone Jt. J. 2017, 99, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wagner, E.R.; Stans, A.A.; Shin, A.Y.; Bishop, A.T.; Sim, F.H.; Moran, S.L. What is the outcome of allograft and intramedullary free fibula (Capanna technique) in pediatric and adolescent patients with bone tumors? Clin. Orthop. Relat. Res. 2016, 474, 660–668. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yamamoto, N.; Hayashi, K.; Matsubara, H.; Miwa, S.; Igarashi, K.; Tsuchiya, H. Joint-preservation surgery for pediatric osteosarcoma of the knee joint. Cancer Metastasis Rev. 2019, 38, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Xin, Z.; Liu, Y. Abscopal effect: From a rare phenomenon to a new frontier in cancer therapy. Biomark. Res. 2024, 12, 98. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Guo, Z.; Si, T.; Xing, W.; Yu, W.; Wang, Y. Cryoablation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Oncotarget 2019, 10, 4180–4191. [Google Scholar] [CrossRef]
- Kaplan, J.L.; Turker, I.; Chumsri, S.; Gabriel, E. Abscopal effect following cryoablation in a patient with metastatic breast cancer. Front. Biosci. (Schol. Ed.) 2023, 15, 2. [Google Scholar] [CrossRef]
- Benzon, B.; Glavaris, S.A.; Simons, B.W.; Hughes, R.M.; Ghabili, K.; Mullane, P.; Miller, R.; Nugent, K.; Shinder, B.; Tosoian, J.; et al. Combining immune Check Point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 126–136. [Google Scholar] [CrossRef]
- Soule, E.; Bandyk, M.; Matteo, J. Percutaneous ablative cryoimmunotherapy for micrometastaic abscopal effect: No complications. Cryobiology 2018, 82, 22–26. [Google Scholar] [CrossRef]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy plus cryotherapy: Potential augmented abscopal effect for advanced cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef]
- Aarts, B.M.; Klompenhouwer, E.G.; Rice, S.L.; Imani, F.; Baetens, T.; Bex, A.; Horenblas, S.; Kok, M.; Haanen, J.B.A.G.; Beets-Tan, R.G.H.; et al. Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019, 10, 53. [Google Scholar] [CrossRef]
- Niu, L.; Mu, F.; Zhang, C.; Li, Y.; Liu, W.; Jiang, F.; Li, L.; Liu, C.; Zeng, J.; Yao, F.; et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology 2013, 67, 17–22. [Google Scholar] [CrossRef]
- Li, F.; Guo, Z.; Yu, H.; Zhang, X.; Si, T.; Liu, C.; Yang, X.; Qi, L. Anti-tumor immunological response induced by cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell prostate cancer murine model. Neoplasma 2014, 61, 659–671. [Google Scholar] [CrossRef]
- Waitz, R.; Solomon, S.B.; Petre, E.N.; Trumble, A.E.; Fassò, M.; Norton, L.; Allison, J.P. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012, 72, 430–439. [Google Scholar] [CrossRef]
- Joosten, J.J.; Muijen, G.N.; Wobbes, T.; Ruers, T.J. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: An experimental study. Cryobiology 2001, 42, 49–58. [Google Scholar] [CrossRef]
- Sabel, M.S.; Arora, A.; Su, G.; Chang, A.E. Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology 2006, 53, 360–366. [Google Scholar] [CrossRef]
- Sabel, M.S.; Nehs, M.A.; Su, G.; Lowler, K.P.; Ferrara, J.L.M.; Chang, A.E. Immunologic response to cryoablation of breast cancer. Breast Cancer Res. Treat. 2005, 90, 97–104. [Google Scholar] [CrossRef]
- Urano, M.; Tanaka, C.; Sugiyama, Y.; Miya, K.; Saji, S. Antitumor effects of residual tumor after cryoablation: The combined effect of residual tumor and a protein-bound polysaccharide on multiple liver metastases in a murine model. Cryobiology 2003, 46, 238–245. [Google Scholar] [CrossRef]
- Nishida, H.; Shirai, T.; Hayashi, K.; Takeuchi, A.; Tanzawa, Y.; Mizokami, A.; Namiki, M.; Tsuchiya, H. Cryotreatment against metastatic renal cell bone tumour reduced multiple lung metastases. Anticancer Res. 2011, 31, 2927–2930. [Google Scholar]
- Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Shinmura, K.; Yokogawa, N.; Fang, X.; et al. Systemic antitumor immune response following reconstruction using frozen autografts for total en bloc spondylectomy. Spine J. 2014, 14, 1567–1571. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.A.; Ayerza, M.A.; Albergo, J.I.; Farfalli, G.L. Do massive allograft reconstructions for tumors of the femur and tibia survive 10 or more years after implantation? Clin. Orthop. Relat. Res. 2020, 478, 517–524. [Google Scholar] [CrossRef]
- Ikuta, K.; Nishida, Y.; Sugiura, H.; Tsukushi, S.; Yamada, K.; Urakawa, H.; Arai, E.; Hamada, S.; Ishiguro, N. Predictors of complications in heat-treated autograft reconstruction after intercalary resection for malignant musculoskeletal tumors of the extremity. J. Surg. Oncol. 2018, 117, 1469–1478. [Google Scholar] [CrossRef]
- Puri, A.; Byregowda, S.; Gulia, A.; Patil, V.; Crasto, S.; Laskar, S. Reconstructing diaphyseal tumors using radiated (50 Gy) autogenous tumor bone graft. J. Surg. Oncol. 2018, 118, 138–143. [Google Scholar] [CrossRef]
- Wu, P.-K.; Chen, C.-F.; Chen, C.-M.; Cheng, Y.-C.; Tsai, S.-W.; Chen, T.-H.; Chen, W.-M. Intraoperative extracorporeal irradiation and frozen treatment on tumor-bearing autografts show equivalent outcomes for biologic reconstruction. Clin. Orthop. Relat. Res. 2018, 476, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Crenn, V.; Quinette, Y.; Bouthors, C.; Missenard, G.; Viard, B.; Anract, P.; Boisgard, S.; Mascard, E.; Gouin, F.; de l’Ouest, S.d. Intercalary allograft reconstruction following femoral tumour resection: Mid- and long-term results and benefits of adding a vascularised fibula autograft. World J. Surg. Oncol. 2022, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Ogink, P.T.; Teunissen, F.R.; Massier, J.R.; Raskin, K.A.; Schwab, J.H.; Lozano-Calderon, S.A. Allograft reconstruction of the humerus: Complications and revision surgery. J. Surg. Oncol. 2019, 119, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, H.; Matsumoto, S.; Shimoji, T.; Tanizawa, T.; Ae, K.; Shinomiya, K.; Okawa, A.; Kawaguchi, N. Long-term results from use of pasteurized bone. J. Orthop. Sci. 2012, 17, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Cho, W.H.; Jeon, D.-G.; Kong, C.-B.; Duo, J.; Lee, S.-Y. A comparison of tumor prosthesis implantation and pasteurized autograft-prosthesis composite for proximal tibial tumor. J. Orthop. Sci. 2012, 17, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, N.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Higuchi, T.; Abe, K.; Taniguchi, Y.; Aiba, H.; Araki, Y.; et al. Clinical course of grafted cartilage in osteoarticular frozen autografts for reconstruction after resection of malignant bone and soft-tissue tumor involving an epiphysis. J. Bone Oncol. 2020, 24, 100310. [Google Scholar] [CrossRef]
- Cecilia Belzarena, A.; Cook, J.L. Does liquid nitrogen recycled autograft for treatment of bone sarcoma impact local recurrence rate? A systematic review. J. Bone Oncol. 2024, 48, 100628. [Google Scholar] [CrossRef]
- Chun Wai, C.; Anderson SM, L.; Raymond CH, Y.; Kenneth WY, H.; Albert YL, L. Outcome of limb salvaging procedure for bone tumor using liquid nitrogen technique: A case series of 21 patients. J. Orthop. Surg. Tech. 2023, 6, 519–523. [Google Scholar] [CrossRef]
| Characteristic | Number | |
| Mean age | 18 (6–62) | |
| Sex | Male | 17 |
| Female | 18 | |
| Histology | Conventional osteosarcoma | 33 |
| (Osteoblastic) | 27 | |
| (Chondroblastic) | 3 | |
| (Fibroblastic) | 3 | |
| Paroosteal osteosarcoma | 1 | |
| Small cell osteosarcoma | 1 | |
| Location | Femur | 19 |
| Tibia | 16 | |
| Fixation method | Plate | 25 |
| Nail | 10 | |
| Freezing method | Pedicle freezing | 13 |
| Free freezing | 22 | |
| Chemotherapy | + | 31 |
| - | 4 | |
| Bone union | + | 28 |
| - | 7 |
| Variables | Bone union | Fisher’s exact test | ||
| + | - | p value | ||
| gender | male | 14 | 3 | 1 |
| female | 14 | 4 | ||
| age | <20 | 24 | 4 | 0.13 |
| ≥20 | 4 | 3 | ||
| Localization | Femur | 17 | 2 | 0.21 |
| Tibia | 11 | 5 | ||
| Reconstruction method | Plate | 23 | 2 | 0.043 |
| Nail | 6 | 4 | ||
| Freezing method | Pedicle | 10 | 3 | 1 |
| Free | 18 | 4 | ||
| Chemotherapy | - | 3 | 1 | 1 |
| + | 25 | 6 |
| Variable | Bone union | Fisher’s exact test | ||
| + | - | p value | ||
| Plate | Single | 6 | 1 | 0.49 |
| Multiple | 17 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morinaga, S.; Hayashi, K.; Miwa, S.; Higuchi, T.; Yonezawa, H.; Asano, Y.; Tsuchiya, H.; Demura, S. Factors Associated with Bone Union Failure After Frozen Autograft Reconstruction in Lower Limb Osteosarcoma. Cancers 2025, 17, 3601. https://doi.org/10.3390/cancers17223601
Morinaga S, Hayashi K, Miwa S, Higuchi T, Yonezawa H, Asano Y, Tsuchiya H, Demura S. Factors Associated with Bone Union Failure After Frozen Autograft Reconstruction in Lower Limb Osteosarcoma. Cancers. 2025; 17(22):3601. https://doi.org/10.3390/cancers17223601
Chicago/Turabian StyleMorinaga, Sei, Katsuhiro Hayashi, Shinji Miwa, Takashi Higuchi, Hirotaka Yonezawa, Yohei Asano, Hiroyuki Tsuchiya, and Satoru Demura. 2025. "Factors Associated with Bone Union Failure After Frozen Autograft Reconstruction in Lower Limb Osteosarcoma" Cancers 17, no. 22: 3601. https://doi.org/10.3390/cancers17223601
APA StyleMorinaga, S., Hayashi, K., Miwa, S., Higuchi, T., Yonezawa, H., Asano, Y., Tsuchiya, H., & Demura, S. (2025). Factors Associated with Bone Union Failure After Frozen Autograft Reconstruction in Lower Limb Osteosarcoma. Cancers, 17(22), 3601. https://doi.org/10.3390/cancers17223601






