1. Introduction
Colorectal cancer (CRC) continues to represent a significant global health challenge. In 2020, more than 1.9 million new cases were diagnosed worldwide, leading to over 930,000 deaths and establishing CRC as the second most common cause of cancer-related mortality [
1]. Alarmingly, projections for 2040 anticipate a 63% increase in annual incidence, culminating in approximately 3.2 million new cases, and a 73% increase in mortality, with an estimated 1.6 million deaths per year [
1]. At initial diagnosis, between 15% and 30% of patients already present with metastatic disease, and approximately 20% of those treated for localized tumors subsequently develop distant metastases [
2]. Survival outcomes vary substantially according to disease stage, ranging from approximately 90% in localized CRC to less than 10% in cases of metastatic dissemination [
3]. However, significant heterogeneity in clinical outcomes persists even among patients classified within the same stage, underscoring the urgent need for additional prognostic markers to refine risk stratification and optimize therapeutic strategies [
4]. In recent years, therapeutic paradigms in CRC have evolved considerably, notably with the introduction of neoadjuvant treatment protocols, which have contributed to improved oncological outcomes [
5,
6,
7]. Nonetheless, these advances concurrently raise critical questions regarding the optimal identification of patients most likely to benefit from specific therapeutic interventions.
Fluorodeoxyglucose positron emission tomography combined with computed tomography (18F-FDG PET/CT) has become a cornerstone modality in oncologic imaging. According to the 2023 guidelines of the European Society for Medical Oncology (ESMO), FDG PET/CT is recommended for staging when elevated tumor markers are present without an identifiable metastatic site and may assist in evaluating the resectability of suspected metastases [
8]. Despite its superior sensitivity compared to conventional computed tomography (CT) for detecting lymph node and distant metastases, FDG PET/CT is not currently endorsed for routine initial staging of CRC [
8]. FDG PET/CT enables comprehensive quantitative assessment of tumor metabolism through parameters such as standardized uptake value (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG). A growing body of evidence suggests that both MTV and TLG are independent prognostic biomarkers in CRC, providing critical information complementary to traditional clinical and pathological factors [
9,
10,
11,
12,
13]. In a recent investigation conducted by our group, we demonstrated that FDG PET/CT exhibits superior diagnostic accuracy compared with conventional imaging modalities for initial staging of colorectal cancer, leading to substantial alterations in therapeutic management strategies in a considerable proportion of cases [
14]. These findings highlight the potential for FDG PET/CT to play an increasingly central role in the initial assessment of CRC.
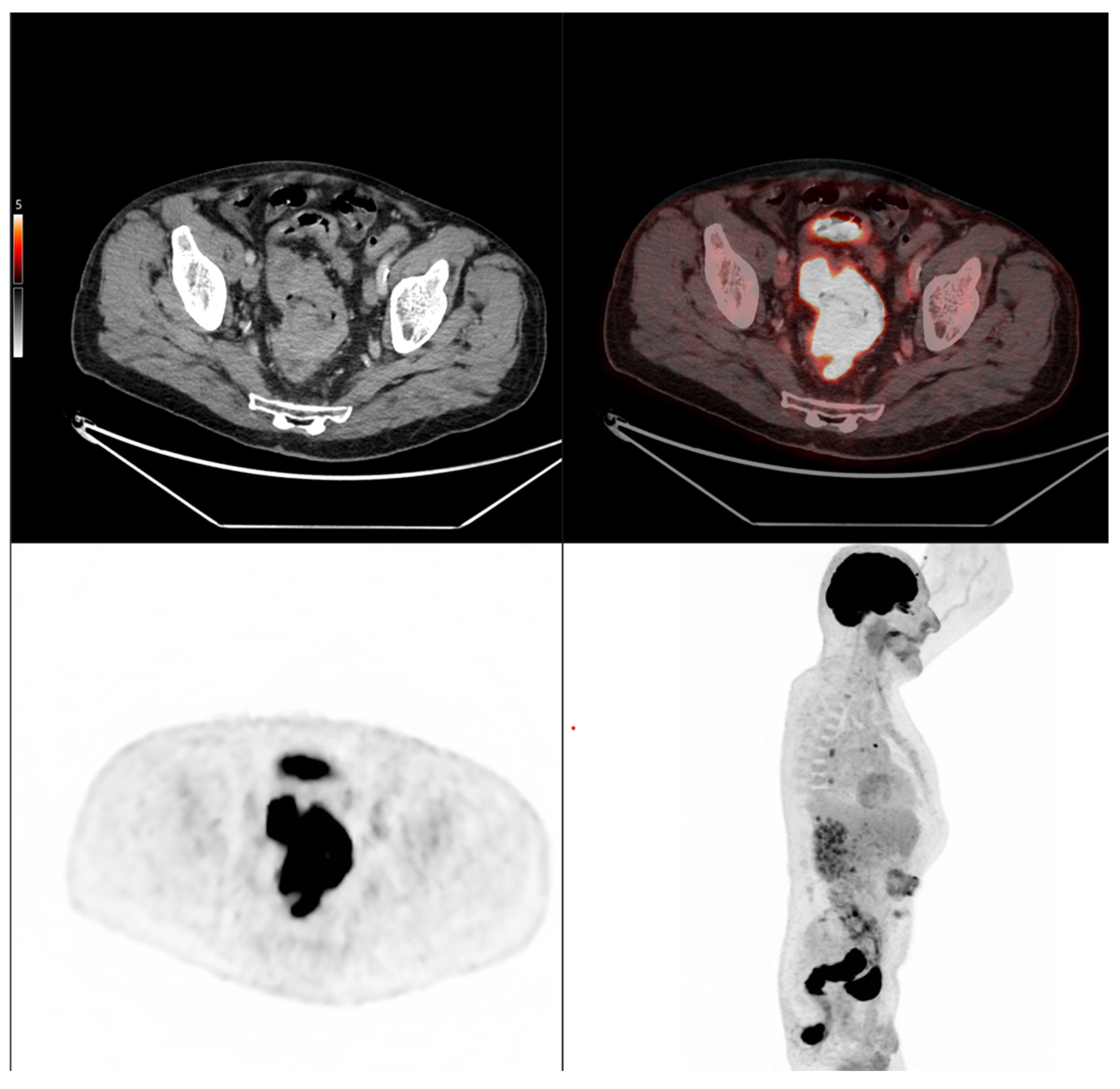
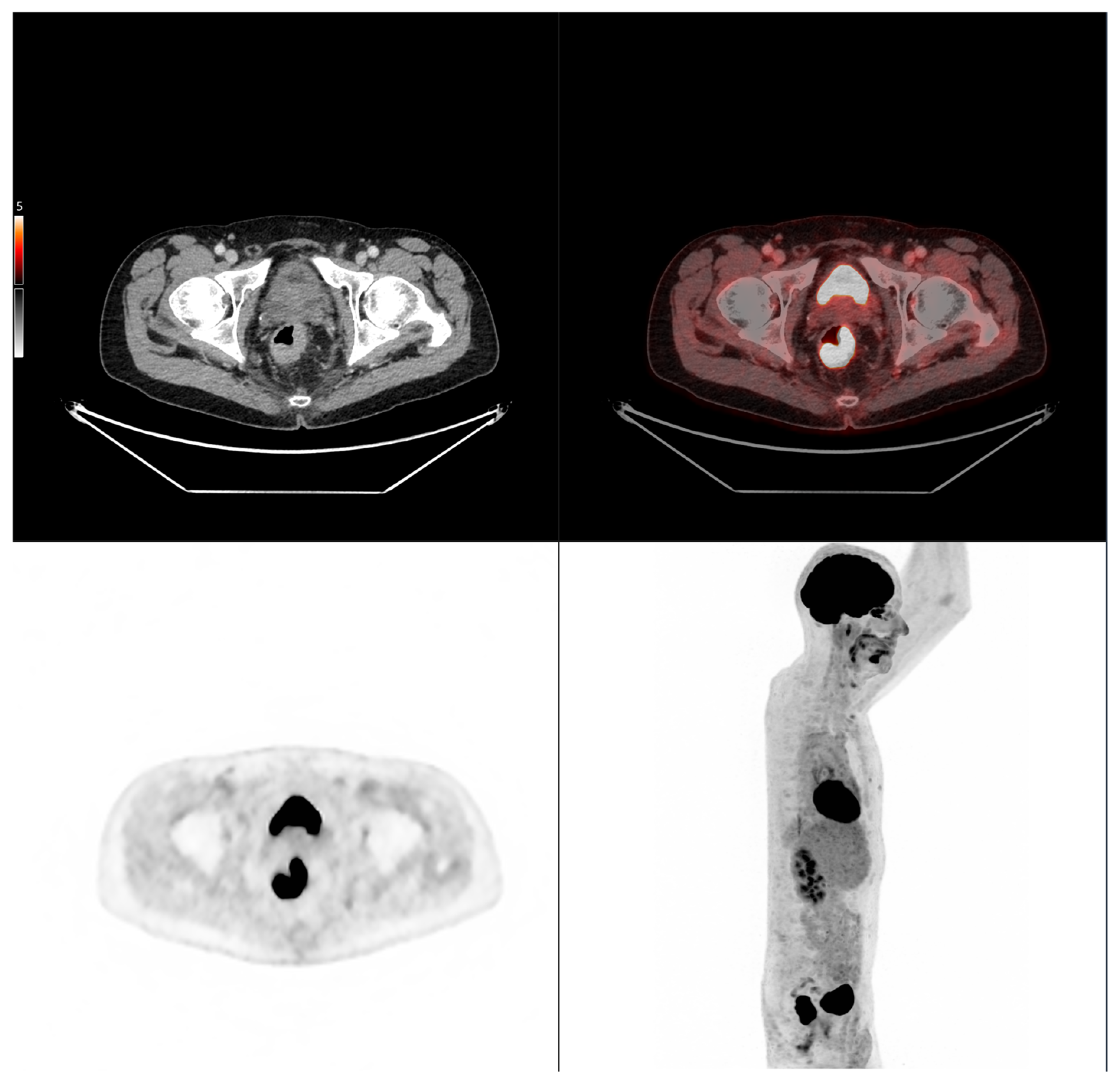
Accordingly, the present study aimed to evaluate whether morpho-metabolic features derived from baseline FDG PET/CT segmentation of the primary tumor could serve as prognostic biomarkers associated with subsequent tumor evolution in colorectal cancer patients treated with standard therapy. Rather than assessing treatment efficacy, this study sought to identify imaging features potentially reflective of the tumor’s intrinsic biological behavior.
4. Discussion
Colorectal cancer (CRC) remains one of the most pressing global oncologic challenges, with a steadily increasing incidence and mortality burden. While current staging systems are fundamental for clinical management, they fall short in accounting for the substantial heterogeneity in outcomes among patients within the same stage [
4]. Accordingly, the present study focused on evaluating whether baseline FDG PET/CT features of the primary tumor could serve as prognostic biomarkers in colorectal cancer patients treated with standard therapy. The aim was to explore imaging characteristics potentially reflective of intrinsic tumor behavior, rather than to assess treatment efficacy.
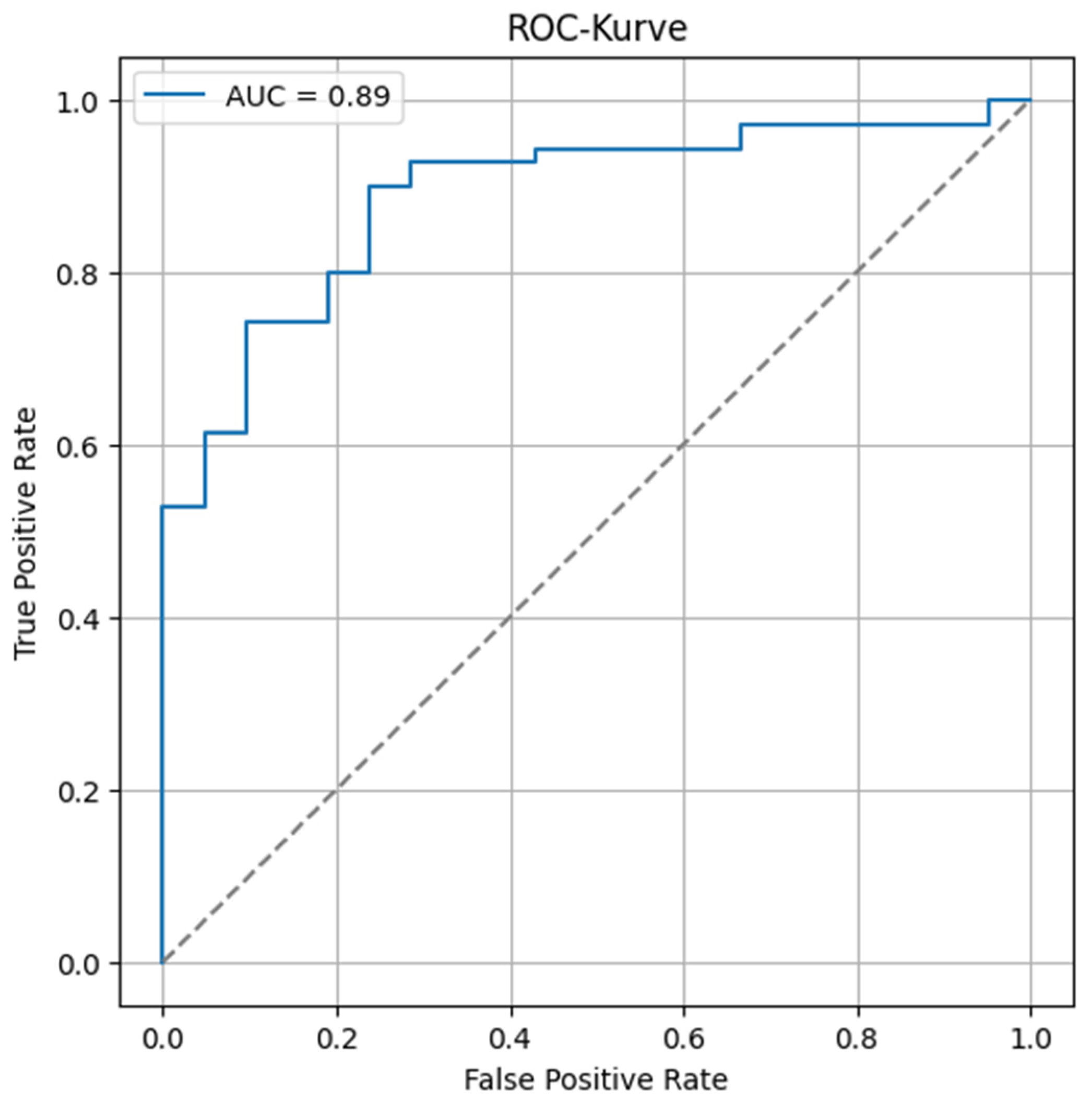
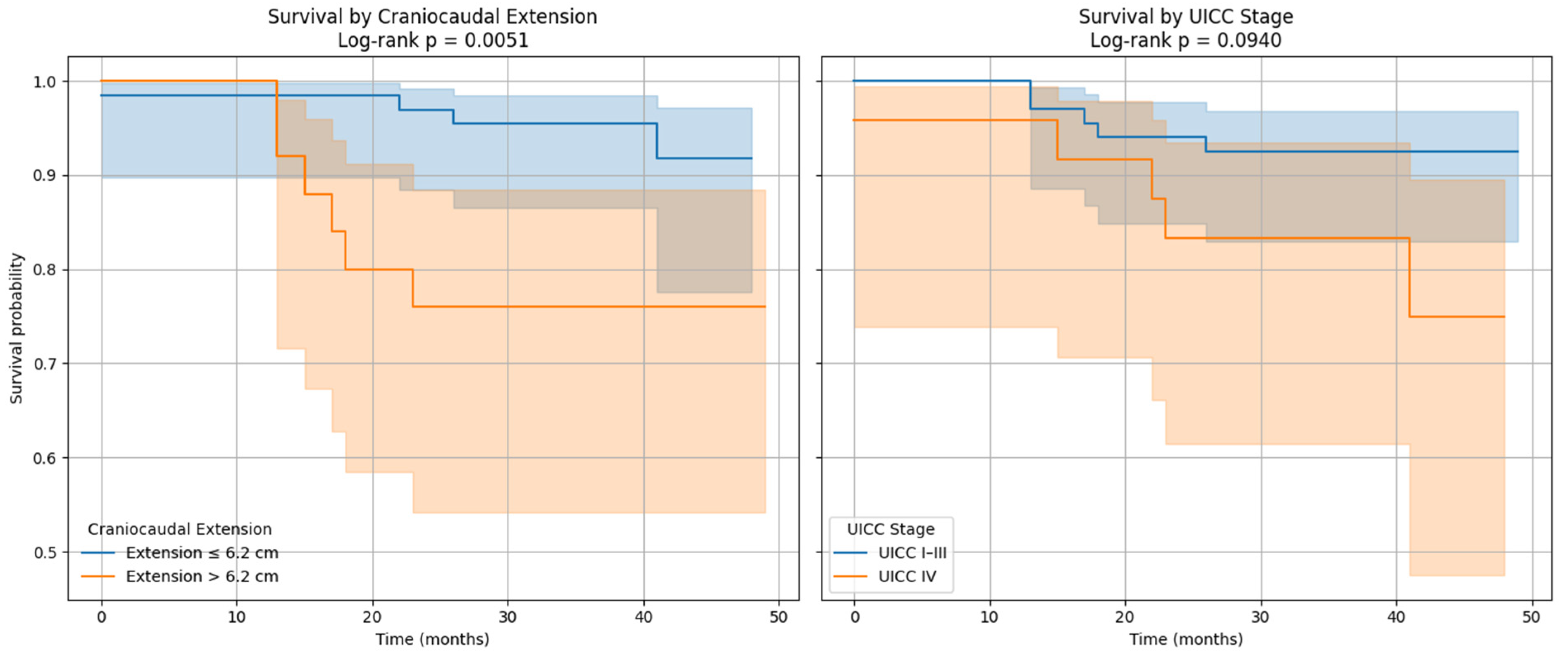
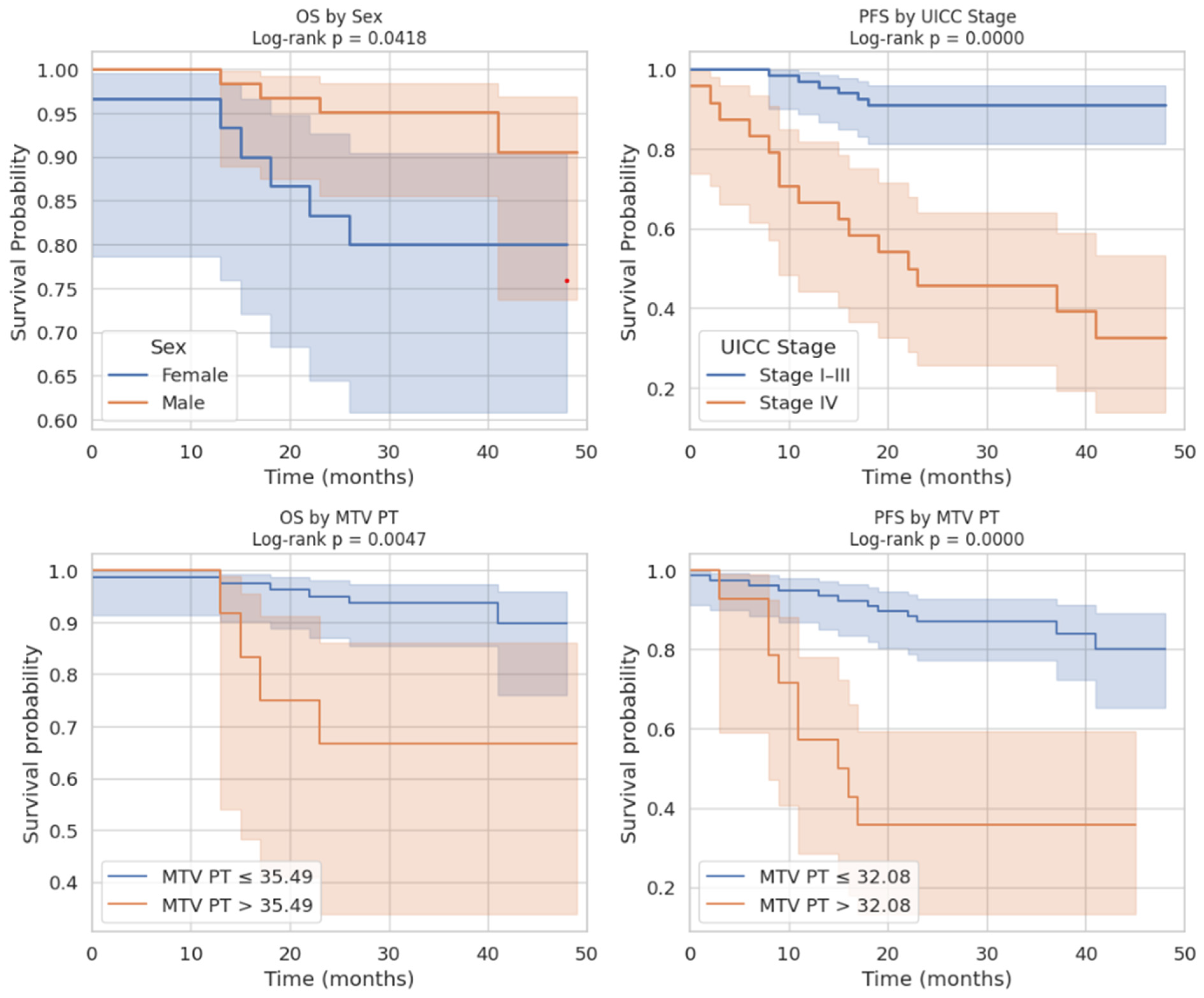
We demonstrated that cranio-caudal extension was the strongest prognostic biomarker of short-term treatment response, as defined by clinical benefit (CB), with a high discriminative performance (AUC of 0.89). This finding highlights the potential of morphological tumor burden, easily measured from standard PET/CT acquisitions, to guide early clinical decisions. In contrast, MTV was not associated with CB, but showed a significant correlation with progression-free survival (PFS), confirming its role as a prognostic biomarker of systemic tumor aggressiveness. This dual prognostic behavior is biologically coherent. Cranio-caudal extension likely reflects local invasiveness and resectability, two factors closely tied to immediate treatment outcomes [
13]. Conversely, MTV captures the global glycolytic activity of the tumor, which may indicate biological aggressiveness and a higher likelihood of dissemination. The stronger association of MTV with PFS than with overall survival (OS) aligns with prior studies reporting that metabolic burden predicts early relapse but may not fully translate into reduced OS due to salvage therapies and treatment heterogeneity [
9,
10,
11,
12,
13,
15,
16,
17].
Importantly, our findings take on added significance in the context of the shifting therapeutic paradigm in CRC. The adoption of neoadjuvant treatment protocols, especially for rectal cancer and increasingly for selected colon cancers, reflects a move toward earlier systemic intervention and organ preservation [
14,
15,
16,
17,
18,
19]. In this setting, early imaging biomarkers that predict subsequent tumor evolution under standard of care prior to therapy are critical. Our findings indicate that cranio-caudal extension and MTV, derived from a single baseline FDG PET/CT scan, are associated with tumor evolution and may help stratify patients according to the intrinsic aggressiveness of their disease. These imaging parameters could inform clinical decision-making regarding treatment intensity, timing of surgery, and follow-up strategies. Such prognostic insights are particularly relevant in the context of precision oncology, where integrating imaging and molecular biomarkers can enhance patient-tailored management.
Recent studies have further expanded the prognostic relevance of PET/CT in CRC. Beyond traditional metabolic indices, PET/CT-derived features have been linked to microsatellite instability status, which holds significant implications for prognosis and immunotherapy eligibility [
20]. Additionally, radiomic and texture analyses extracted from FDG PET/CT enhance risk stratification, particularly in stage II disease, and in some studies outperform conventional T-stage in predicting survival [
21,
22,
23]. Quantitative indices such as TLG and measures of intratumor metabolic heterogeneity have also been shown to provide incremental prognostic information for recurrence and disease-free survival [
24,
25,
26]. Recently published investigations have demonstrated that early metabolic changes on PET/CT can predict pathologic response and long-term outcomes following neoadjuvant therapy [
18,
19,
20,
21,
22,
23,
24,
25]. Moreover, inflammatory markers derived from FDG uptake in the bone marrow and spleen have recently been identified as additional prognostic indicators linked to systemic immune and inflammatory responses [
27].
An important aspect of our findings lies in the distinct prognostic roles observed for morphological and metabolic features on pre-treatment FDG PET/CT. While various previous studies have focused either on metabolic parameters such as SUV, MTV, or TLG, or on morphological descriptors in isolation, our analysis demonstrates that these two dimensions do not behave uniformly [
9,
10,
11,
18,
19,
28,
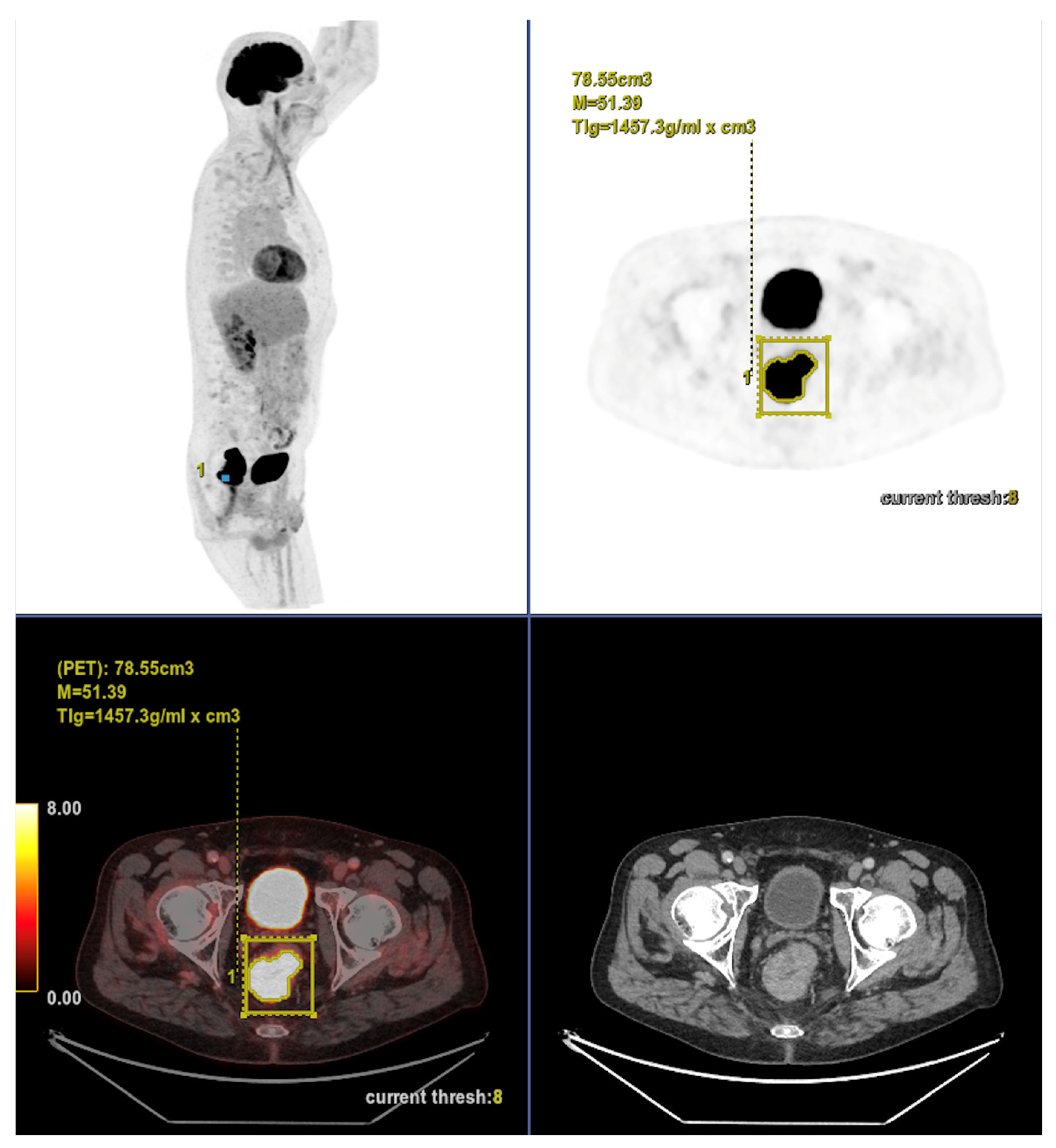
29]. This temporal dissociation is, to our knowledge, novel and highlights the complementary value of morphology and metabolism in understanding tumor behavior. Furthermore, a notable methodological aspect of our study is the decision to segment only the primary tumor. This choice was both pragmatic and justified: fewer than 25% of patients in our cohort had metastatic disease, suggesting that the primary lesion accounts for the bulk of tumor burden in most cases. In fact, primary tumor segmentation is significantly faster, more robust, and clinically implementable than whole-body segmentation, which requires advanced software and trained personnel. While excluding nodal and distant metastases may underestimate the total tumor burden in advanced disease, this limitation is likely to have had a modest impact in our predominantly non-metastatic cohort. Nevertheless, future studies should assess whether incorporating whole-body volumetric data enhances predictive accuracy in more advanced stages.
From a methodological standpoint, our iterative approach to cut-off determination, testing values between the 10th and 90th percentiles, adds robustness by avoiding arbitrary threshold selection and maximizing discrimination between groups.
Finally, we focused on stratification prior to any treatment initiation. This design distinguishes our work from several earlier studies that primarily examined patients who were either refractory to chemotherapy or analyzed outcomes in the post-surgical setting [
10,
11,
29,
30]. By evaluating patients at baseline, we aimed to capture the intrinsic prognostic value of morphological and metabolic tumor features before any potential modification induced by systemic or local therapies.
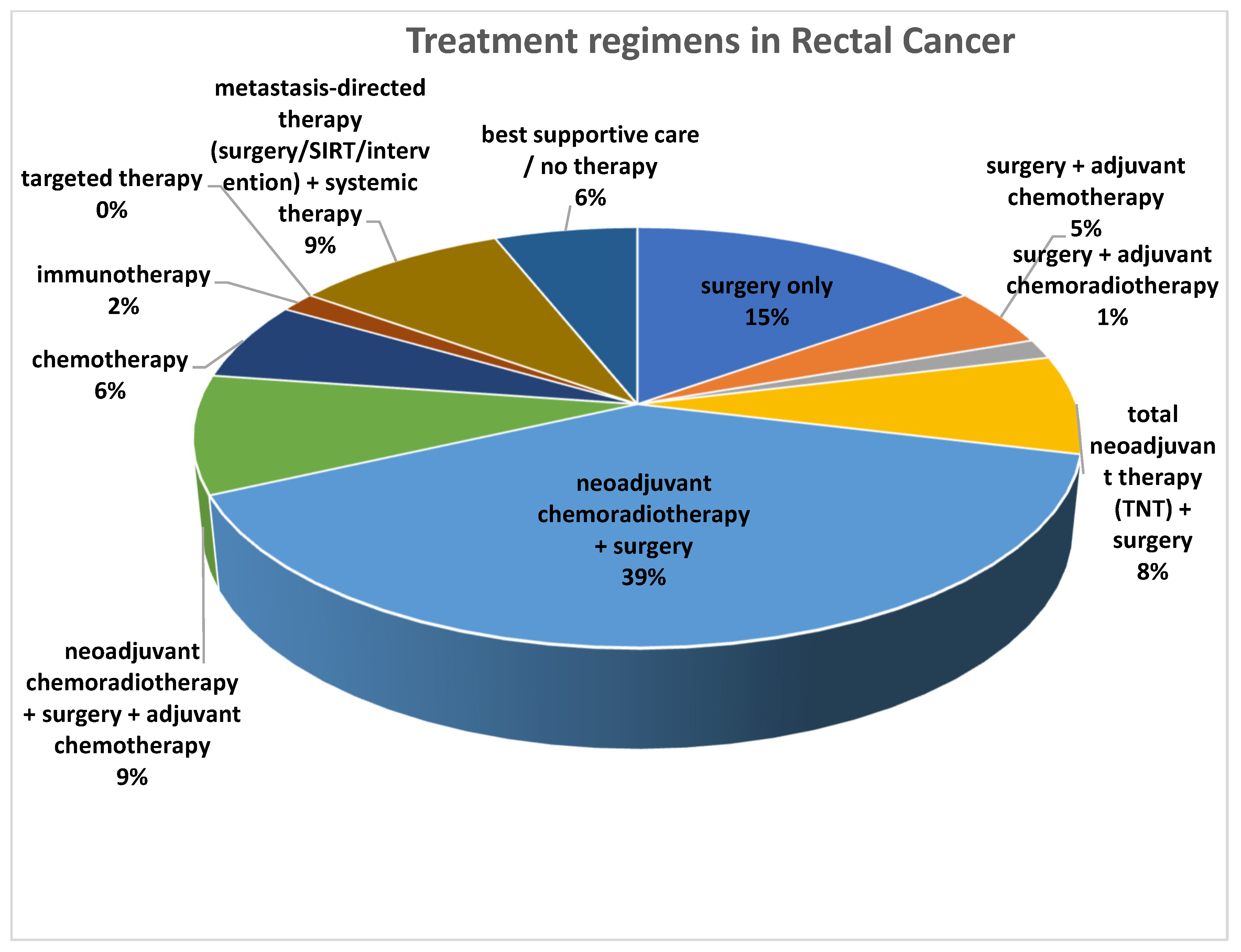
However, several limitations must be acknowledged. First, the retrospective single-center design may limit generalizability. Second, variability in acquisition protocols (including the use of contrast medium), and thresholding methods (41% SUVmax threshold used) may introduce measurement bias. Third, our use of clinical benefit as a binary endpoint, while clinically meaningful, may oversimplify heterogeneous biological responses. Additionally, the cohort included patients who received different treatment regimens, including neoadjuvant, adjuvant, and palliative strategies. Given the limited sample size, subgroup analyses based on treatment modality were not feasible. These issues underscore the need for prospective multicenter studies with standardized imaging and therapeutic protocols to validate the clinical significance and practical application value of the current findings.
Finally, our results contribute to the growing body of research supporting the integration of FDG PET/CT into initial staging and risk stratification in CRC, even when distant metastases are not present. Current ESMO guidelines restrict routine PET/CT use to specific clinical scenarios [
5,
6,
7,
8], but our findings support its broader application as a source of actionable prognostic information, especially when combined with clinical markers.