High Rate of Cytokine Release Syndrome-Related Coagulopathy with Low Incidence of Bleeding and Thrombosis in Patients Treated with B-Cell Maturation Antigen (BCMA)-Targeted Chimeric Antigen Receptor T-Cells (CAR-T)
Simple Summary
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Evaluation of Laboratory and Clinical Parameters
2.3. Thromboprophylaxis and Blood Product Support
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. CAR-T-Associated Toxicities
3.3. The Association Between CRS and Coagulopathy
3.4. Thrombosis and Bleeding Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Mikkilineni, L.; Kochenderfer, J.N. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 2017, 130, 2594–2602. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Grant, S.J.; Wildes, T.M. Chimeric Antigen Receptor T-Cell and Bispecific Antibody Therapy in Multiple Myeloma: Moving Into the Future. J. Clin. Oncol. 2023, 41, 4416–4429. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiu, L.; Usmani, S.; Joo, C.W.; Costa, L.; Derman, B.; Du, J.; Einsele, H.; de Larrea, C.F.; Hajek, R.; et al. Consensus guidelines and recommendations for the management and response assessment of chimeric antigen receptor T-cell therapy in clinical practice for relapsed and refractory multiple myeloma: A report from the International Myeloma Working Group Immunotherapy Committee. Lancet Oncol. 2024, 25, e374–e387. [Google Scholar] [CrossRef]
- Kfir-Erenfeld, S.; Asherie, N.; Lebel, E.; Vainstein, V.; Assayag, M.; Sharon, T.D.; Grisariu, S.; Avni, B.; Elias, S.; Alexander-Shani, R.; et al. Clinical evaluation and determinants of response to HBI0101 (BCMA CART) therapy in relapsed/refractory multiple myeloma. Blood Adv. 2024, 8, 4077–4088. [Google Scholar] [CrossRef]
- Lebel, E.; Asherie, N.; Kfir-Erenfeld, S.; Elias, S.; Grisariu, S.; Avni, B.; Assayag, M.; Dubnikov-Sharon, T.; Alexander-Shani, R.; Bessig, N.; et al. Efficacy of HBI0101, an Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) for Relapsed/Refractory Multiple Myeloma. Blood 2024, 144, 1030. [Google Scholar] [CrossRef]
- Lebel, E.; Asherie, N.; Kfir-Erenfeld, S.; Grisariu, S.; Avni, B.; Elias, S.; Assayag, M.; Dubnikov-Sharon, T.; Pick, M.; Alexander-Shani, R.; et al. Efficacy and Safety of Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell for the Treatment of Relapsed and Refractory AL Amyloidosis. J. Clin. Oncol. 2025, 43, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Feldman, S.A.; Wilson, W.H.; Spaner, D.E.; Maric, I.; Stetler-Stevenson, M.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 2012, 119, 2709–2720. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, H.; Wang, J.; Li, F. New insights into CAR T-cell hematological toxicities: Manifestations, mechanisms, and effective management strategies. Exp. Hematol. Oncol. 2024, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, K.; Cheng, H.; Cao, J.; Shi, M.; Qiao, J.; Yan, Z.; Jing, G.; Pan, B.; Sang, W.; et al. Coagulation Disorders after Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients with Relapsed and Refractory Hematologic Malignancies. Biol. Blood Marrow Transplant. 2020, 26, 865–875. [Google Scholar] [CrossRef]
- Hashmi, H.; Mirza, A.-S.; Darwin, A.; Logothetis, C.; Garcia, F.; Kommalapati, A.; Mhaskar, R.S.; Bachmeier, C.; Chavez, J.C.; Shah, B.; et al. Venous thromboembolism associated with CD19-directed CAR T-cell therapy in large B-cell lymphoma. Blood Adv. 2020, 4, 4086–4090. [Google Scholar] [CrossRef]
- Blair, P.; Rex, S.; Vitseva, O.; Beaulieu, L.; Tanriverdi, K.; Chakrabarti, S.; Hayashi, C.; Genco, C.A.; Iafrati, M.; Freedman, J.E. Stimulation of Toll-Like Receptor 2 in Human Platelets Induces a Thromboinflammatory Response Through Activation of Phosphoinositide 3-Kinase. Circ. Res. 2009, 104, 346–354. [Google Scholar] [CrossRef]
- Wang, J.; Doran, J. The Many Faces of Cytokine Release Syndrome-Related Coagulopathy. Clin. Hematol. Int. 2021, 3, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Frenzel, L.; Decaux, O.; Macro, M.; Belhadj-Merzoug, K.; Manier, S.; Touzeau, C.; Leleu, X.; Frère, C.; Lecompte, T.; Perrot, A.; et al. Venous thromboembolism prophylaxis and multiple myeloma patients in real-life: Results of a large survey and clinical guidance recommendations from the IFM group. Thromb. Res. 2023, 233, 153–164. [Google Scholar] [CrossRef]
- Frenzel, L. Thromboprophylaxis in multiple myeloma. Res. Pract. Thromb. Haemost. 2025, 9, 102685. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Maier, C.L.; Helms, J.; Umemura, Y.; Moore, H.; Othman, M.; Thachil, J.; Connors, J.M.; Levi, M.; et al. Updated definition and scoring of disseminated intravascular coagulation in 2025: Communication from the ISTH SSC Subcommittee on Disseminated Intravascular Coagulation. J. Thromb. Haemost. 2025, 23, 2356–2362. [Google Scholar] [CrossRef]
- Bialkower, M.; Garnier, G. Fibrinogen Diagnostics in Major Hemorrhage. Crit. Rev. Anal. Chem. 2020, 52, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Fries, D.; Martini, W.Z. Role of fibrinogen in trauma-induced coagulopathy. Br. J. Anaesth. 2010, 105, 116–121. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.R.; E Knight, T.; O McNerney, K.; Leick, M.B.; Jain, T.; Ahmed, S.; Frigault, M.J.; A Hill, J.; Jain, M.D.; Johnson, W.T.; et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant. Cell. Ther. 2023, 29, 438.e1–438.e16. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Dong, R.; Wang, Y.; Lin, Y.; Sun, X.; Xing, C.; Zhang, Y.; Wang, H.; Dai, L.; Yu, K.; Jiang, S. The correlation factors and prognostic significance of coagulation disorders after chimeric antigen receptor T cell therapy in hematological malignancies: A cohort study. Ann. Transl. Med. 2022, 10, 975. [Google Scholar] [CrossRef]
- Yamasaki-Morita, M.; Arai, Y.; Ishihara, T.; Onishi, T.; Shimo, H.; Nakanishi, K.; Nishiyama, Y.; Jo, T.; Hiramatsu, H.; Mitsuyoshi, T.; et al. Relative hypercoagulation induced by suppressed fibrinolysis after tisagenlecleucel infusion in malignant lymphoma. Blood Adv. 2022, 6, 4216–4223. [Google Scholar] [CrossRef]
- Johnsrud, A.J.; Craig, J.; Baird, J.H.; Spiegel, J.Y.; Muffly, L.; Zehnder, J.L.; Tamaresis, J.S.; Negrin, R.S.; Johnston, L.; Arai, S.; et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv. 2021, 5, 4465–4475. [Google Scholar] [CrossRef]
- Schorr, C.; Forindez, J.; Espinoza-Gutarra, M.; Mehta, R.; Grover, N.; Perna, F. Thrombotic Events Are Unusual Toxicities of Chimeric Antigen Receptor T-Cell Therapies. Int. J. Mol. Sci. 2023, 24, 8349. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Yu, Q.; Teng, X.; Guo, X.; Wei, G.; Xu, H.; Cui, J.; Chang, A.H.; Hu, Y.; Huang, H. CRS-related coagulopathy in BCMA targeted CAR-T therapy: A retrospective analysis in a phase I/II clinical trial. Bone Marrow Transplant. 2021, 56, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total n = 108 |
|---|---|
| Age in years-mean (range) | 64 (35–84) |
| Females, n (%) | 56 (52%) |
| Diagnosis: MM/AL | 95/13 |
| Comorbidities, n (%) | |
| DM2 | 16 (15%) |
| Hypertension | 31 (29%) |
| Dyslipidemia | 27 (25%) |
| Obesity | 2 (2%) |
| Smoking | 25 (23%) |
| ≥1 cardiovascular risk factors | 46 (43%) |
| IHD (n = 16) or stroke (n = 1) | 17 (16%) |
| History of VTE | 16 (15%) |
| CKD | 2 (2%) |
| Pulmonary disease | 6 (6%) |
| ECOG-PS, n (%) | |
| 0 | 11 (10%) |
| 1 | 46 (43%) |
| 2 | 48 (44%) |
| 3 | 2 (2%) |
| 4 | 1 (1%) |
| Disease-related features | |
| Previous lines of Tx, median (range) | 4 (3–14) |
| Extramedullary multiple myeloma | 24/95 (25%) |
| High-risk cytogenetics multiple myeloma | 42/95 (44%) |
| Triple-refractory multiple myeloma * | 81/95 (85%) |
| Lymphodepletion regimen, n (%) | |
| Fludarabine/cyclophosphamide | 102 (94%) |
| Bendamustine | 6 (6%) |
| FIB > 200 (mg/dL) | 100 < FIB < 200 (mg/dL) | FIB < 100 (mg/dL) | p Value | |
|---|---|---|---|---|
| Total patients, n = 108 (%) | 29 (27%) | 59 (54%) | 20 (19%) | |
| Female gender (n = 56) | 19 (34%) | 29 (52%) | 8 (14%) | 0.18 |
| Age (avg) | 61.8 | 63.3 | 68.5 | 0.047 |
| Comorbidities | ||||
| ≥1 cardiovascular RF (n = 46) | 10 | 26 | 10 | 0.52 |
| IHD (n = 16) or stroke (n = 1) | 7 | 8 | 2 | 0.68 |
| History of VTE (n = 16) | 2 | 9 | 5 | 0.38 |
| CRS grade | ||||
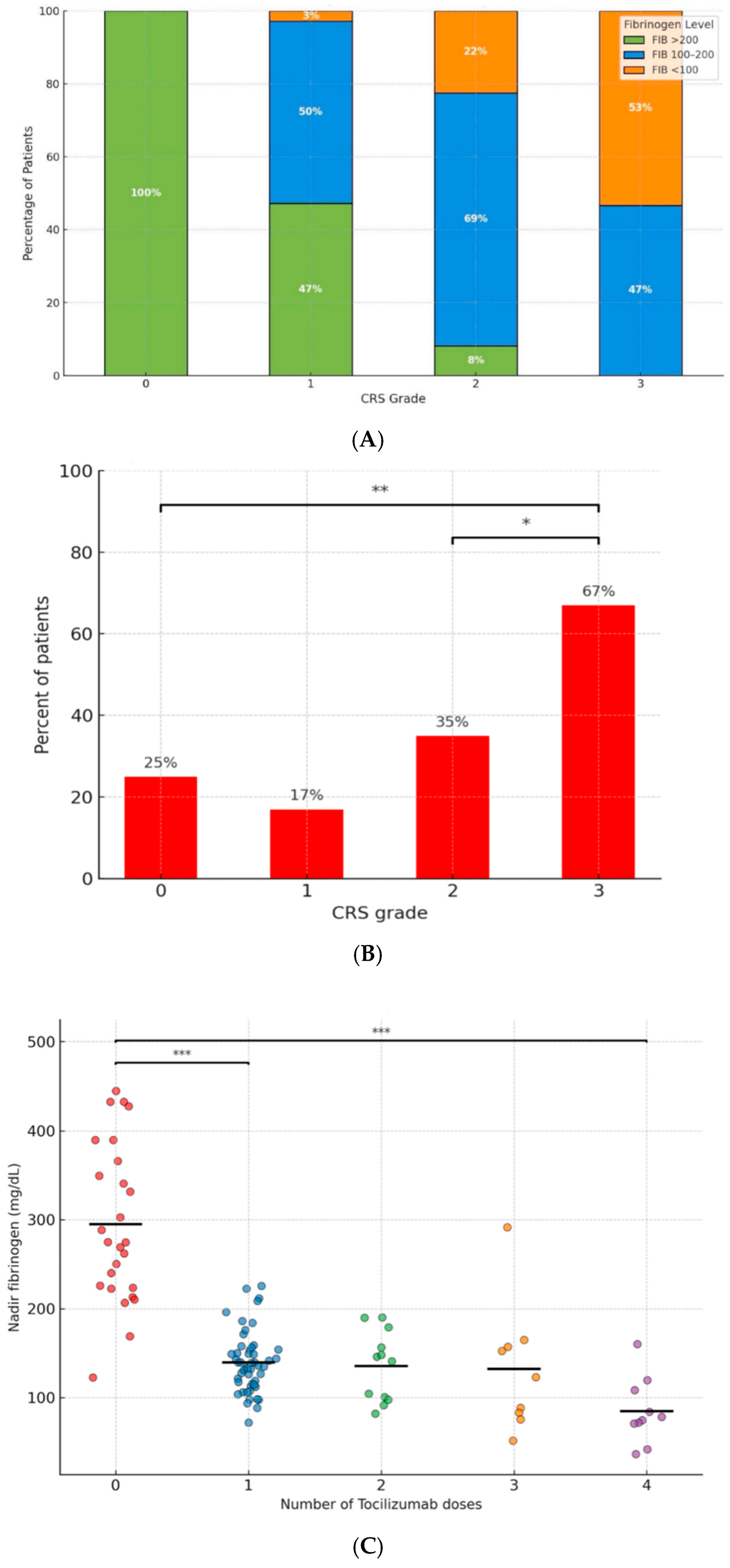
| 0 (n = 8) | 8 | 0 | 0 | <0.001 |
| 1 (n = 36) | 17 | 18 | 1 | <0.001 |
| 2 (n = 49) | 4 | 34 | 11 | <0.001 |
| 3 (n = 15) | 0 | 7 | 8 | <0.001 |
| Tocilizumab doses | ||||
| 0 (n = 26) | 24 | 2 | 0 | <0.001 |
| 1 (n = 51) | 4 | 41 | 6 | <0.001 |
| 2 (n = 12) | 0 | 9 | 3 | <0.001 |
| 3 (n = 9) | 1 | 4 | 4 | <0.001 |
| 4 (n = 10) | 0 | 3 | 7 | <0.001 |
| Laboratory values | ||||
| Peak CRP (mg/dL) | 8.9 | 6.5 | 5.5 | 0.72 |
| Peak ferritin (ng/mL) | 1262 | 3115 | 7893 | 0.001 |
| Peak LDH (U/L) | 396 | 423 | 1082 | 0.006 |
| Nadir neutrophil count (109/L) | 0.4 | 0.4 | 0.2 | 0.033 |
| Nadir platelet count (109/L) | 69 | 70 | 41 | 0.044 |
| Nadir hemoglobin (g/dL) | 8 | 8.2 | 7.9 | |
| Peak PTT (sec) | 35.7 | 34.3 | 36.2 | 0.287 |
| Peak PT/INR (sec) | 1.1 | 1.2 | 1.6 | <0.001 |
| Blood product support (No. of patients receiving ≥ 1 units) | ||||
| PLTs | 11 (38%) | 11 (19%) | 8 (40%) | 0.06 |
| PCs | 16 (55%) | 27 (48%) | 16 (80%) | 0.029 |
| FFP | 1 (3%) | 6 (10%) | 15 (75%) | NA |
| Thrombosis events | 1 | 3 | 1 | NA |
| Minor bleeding events | 1 | 2 | 0 | NA |
| Case | Age and Gender | Cardiovascular Comorbidities | Myeloma Characteristics | Previous Lines of Tx | EMD | % Plasma Cells in BM | Thrombosis or Bleeding Event | Days post-CAR-T Infusion | CRS Grade | Tocilizumab Doses | Fibrinogen at Event (mg/dL) | Fibrinogen 2 Weeks post-CAR-T Infusion | Fibrinogen 4 Weeks post-CAR-T Infusion | Delta Fibrinogen * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 (F) | HTN, DM2, dyslipidemia | FLC lambda, +1q, t(4:14), del17p | 3 | Yes | 50% | NSTEMI | 12 | 2 | 3 | 174 | 167 | 152 | 191 |
| 2 | 61 (F) | CHF | IgG-K, +1q, del13(q) | 4 | No | 40% | DVT | 17 | 1 | 0 | 443 | 443 | 433 | 0 |
| 3 | 69 (M) | DVT, PE | FLC kappa, t(4;11) | 4 | Yes | 40% | DVT + PE | 5 | 3 | 3 | 84 | 83 | 83 | 225 |
| 4 | 58 (M) | HTN, dyslipidemia, smoking | IgA-L, del13(q), del(14) | 2 | Yes | 40% | STEMI | 30 | 1 | 1 | 188 | 214 | 154 | 349 |
| 5 | 63 (F) | HTN, dyslipidemia | IgG-K, +1q, -13 | 4 | Yes | 0.02% | DVT | 10 | 1 | 1 | 171 | 157 | 157 | 287 |
| 6 | 58 (F) | - | FLC-L, IGH rearrangement, t(11q13), +1q | 3 | No | 15% | Hematuria | 13 | 2 | 1 | 154 | 154 | 159 | 218 |
| 7 | 55 (F) | HTN, DM2, CHF | IgG-K | 4 | Yes | 20% | Epistaxis | 3 | 0 | 0 | 428 | 428 | 428 | 0 |
| 8 | 70 (M) | Smoking | IgA-L, + 1q, -P53 (73%), t(4:14) | 4 | Yes | 8% | Cutaneous bleeding | 20 | 2 | 2 | 144 | 142 | 142 | 239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arad, A.; Katz, M.; Lebel, E.; Kalish, Y.; Assayag, M.; Avni, B.; Elias, S.; Grisariu, S.; Shai, E.; Kfir-Erenfeld, S.; et al. High Rate of Cytokine Release Syndrome-Related Coagulopathy with Low Incidence of Bleeding and Thrombosis in Patients Treated with B-Cell Maturation Antigen (BCMA)-Targeted Chimeric Antigen Receptor T-Cells (CAR-T). Cancers 2025, 17, 3551. https://doi.org/10.3390/cancers17213551
Arad A, Katz M, Lebel E, Kalish Y, Assayag M, Avni B, Elias S, Grisariu S, Shai E, Kfir-Erenfeld S, et al. High Rate of Cytokine Release Syndrome-Related Coagulopathy with Low Incidence of Bleeding and Thrombosis in Patients Treated with B-Cell Maturation Antigen (BCMA)-Targeted Chimeric Antigen Receptor T-Cells (CAR-T). Cancers. 2025; 17(21):3551. https://doi.org/10.3390/cancers17213551
Chicago/Turabian StyleArad, Ariela, Maya Katz, Eyal Lebel, Yosef Kalish, Miri Assayag, Batia Avni, Shlomo Elias, Sigal Grisariu, Ela Shai, Shlomit Kfir-Erenfeld, and et al. 2025. "High Rate of Cytokine Release Syndrome-Related Coagulopathy with Low Incidence of Bleeding and Thrombosis in Patients Treated with B-Cell Maturation Antigen (BCMA)-Targeted Chimeric Antigen Receptor T-Cells (CAR-T)" Cancers 17, no. 21: 3551. https://doi.org/10.3390/cancers17213551
APA StyleArad, A., Katz, M., Lebel, E., Kalish, Y., Assayag, M., Avni, B., Elias, S., Grisariu, S., Shai, E., Kfir-Erenfeld, S., Asherie, N., Gatt, M. E., Stepensky, P., & Zimran, E. (2025). High Rate of Cytokine Release Syndrome-Related Coagulopathy with Low Incidence of Bleeding and Thrombosis in Patients Treated with B-Cell Maturation Antigen (BCMA)-Targeted Chimeric Antigen Receptor T-Cells (CAR-T). Cancers, 17(21), 3551. https://doi.org/10.3390/cancers17213551







