Identification of Phenotypically Distinct Cancer Stem Cell Subpopulations in Oral Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material
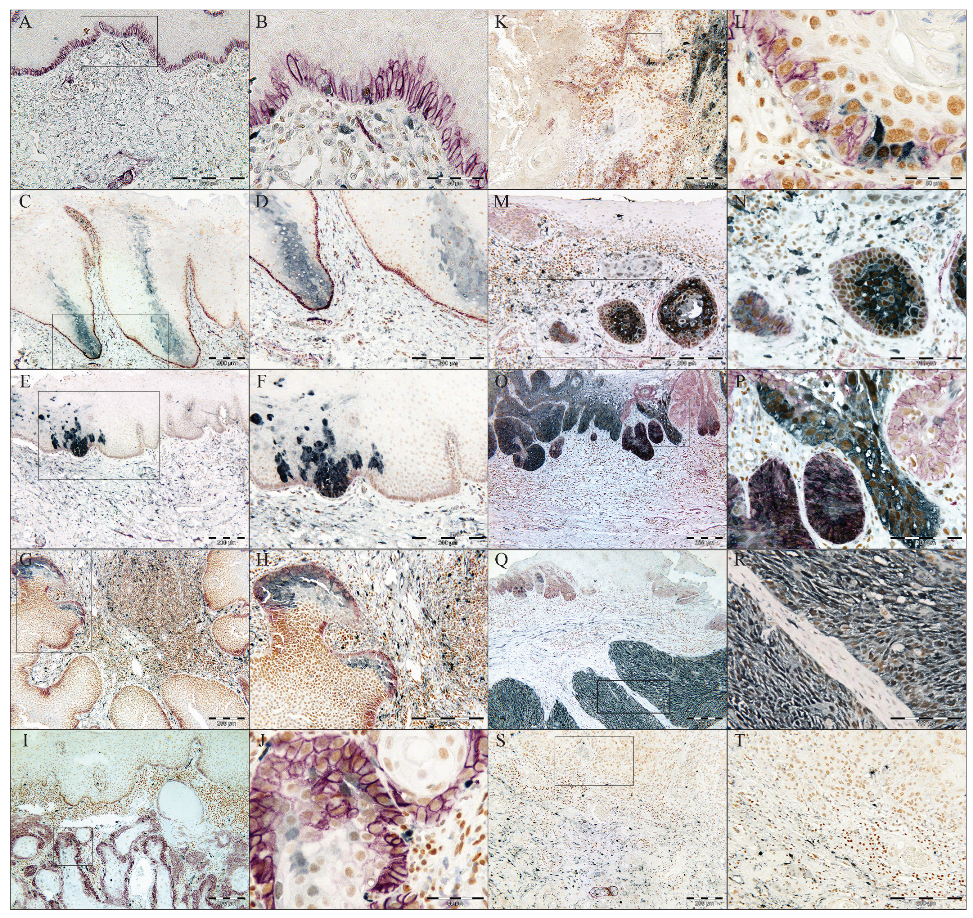
2.2. Triple Immunohistochemistry (IHC)
2.3. Evaluation of the Staining
2.4. Laser Microdissection of FFPE OSCCs
2.5. Data Collection and Entry
2.6. Cell Lines and Culture
2.7. Fluorescence-Activated Cell Sorting (FACS)
2.8. RNA Extraction, cDNA Synthesis and Quantitative RT-PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics of the Study Cohort
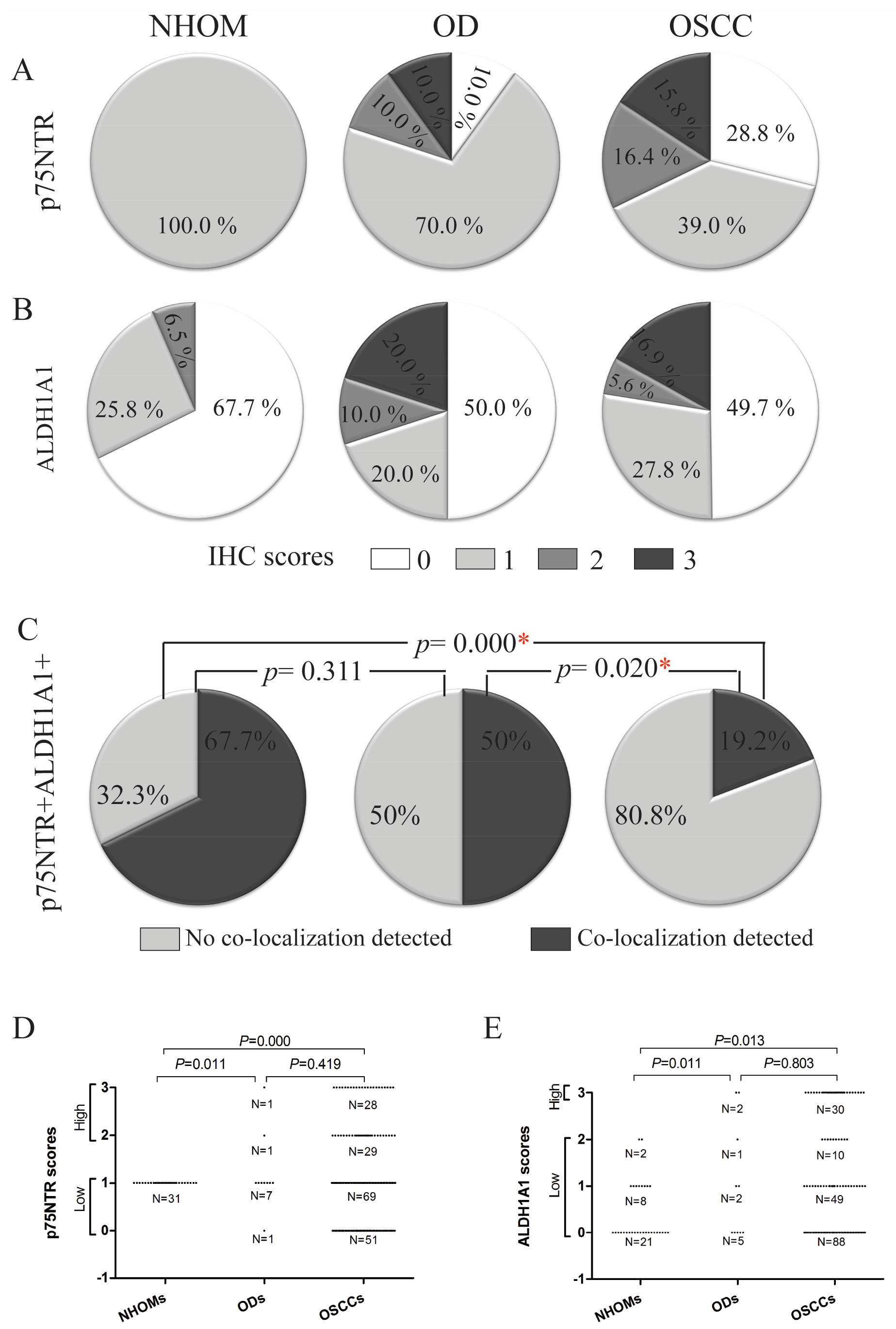
3.2. Increased Frequency of p75NTR+ and ALDH1A1+ Cells in OD and OSCC Compared to NHOM
3.3. Identification of a Pattern Resembling a Clone-Like Distribution of ALDH1+ Cells
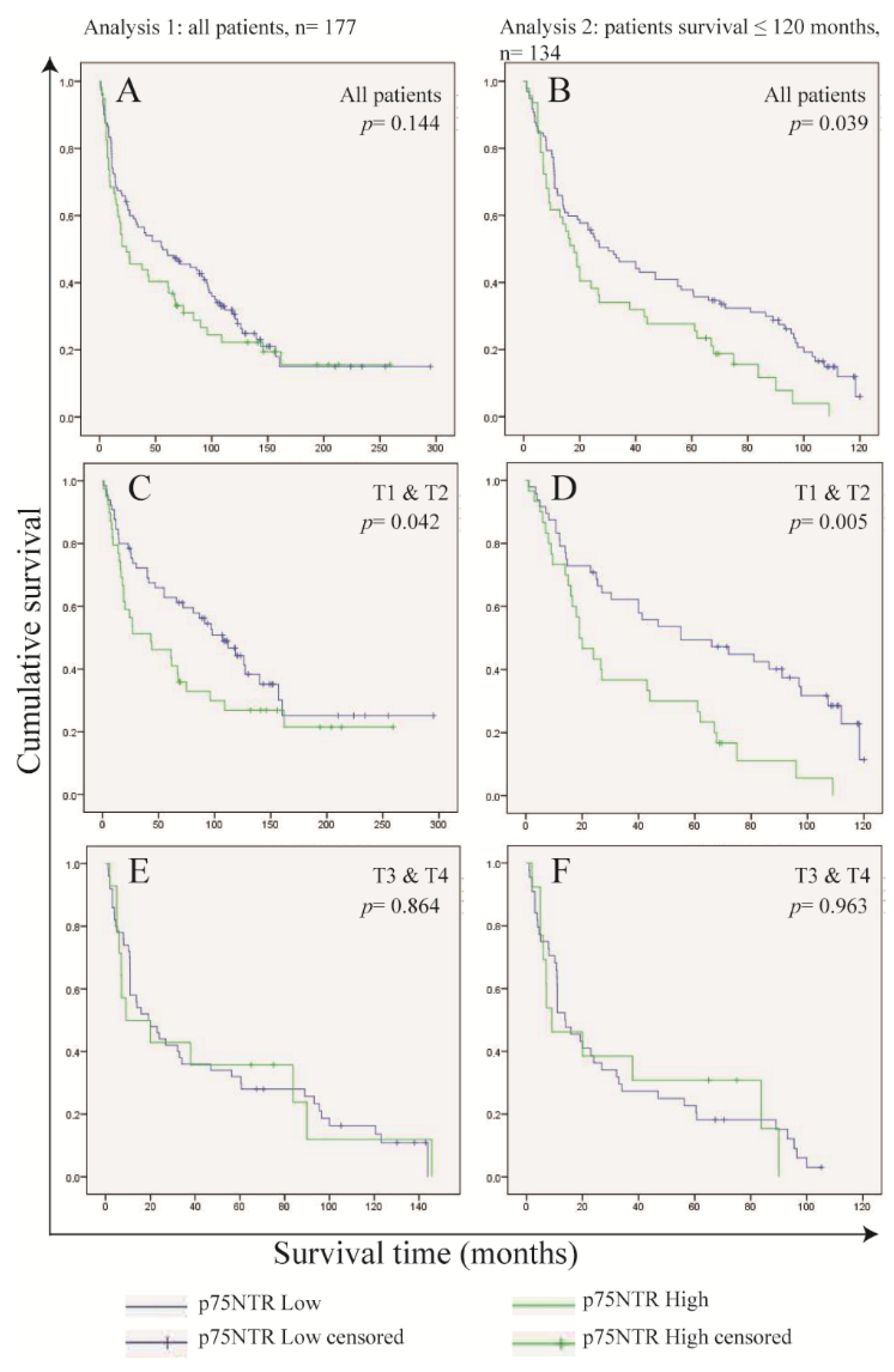
3.4. Frequency of p75NTR+ Cells in OSCC Cells Predicts Survival in Patients with Small Tumor Size (T1 and T2)
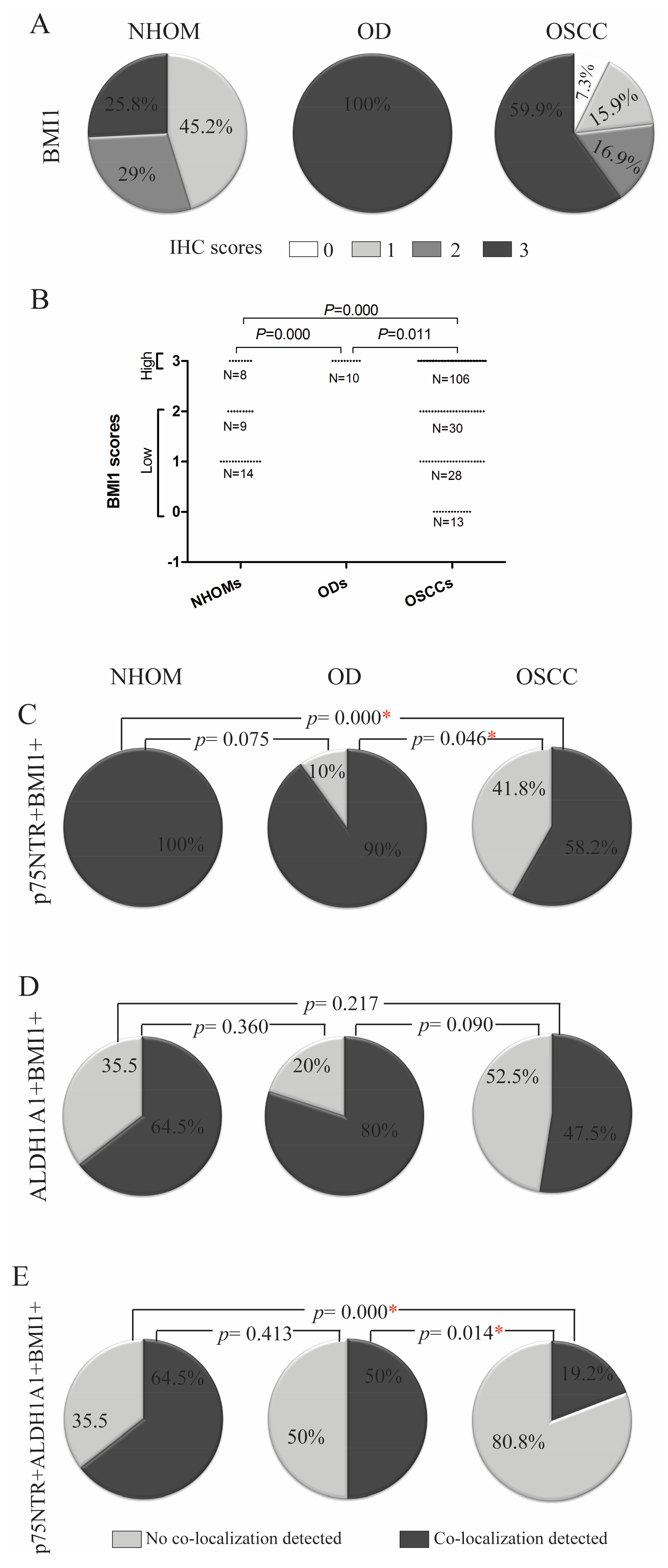
3.5. Highest Expression of Self-Renewing Marker BMI1 Was Detected in OD
3.6. A Greater Number of p75NTR+ Cells Co-Expressed BMI1 Compared to ALDH1A1+ Cells
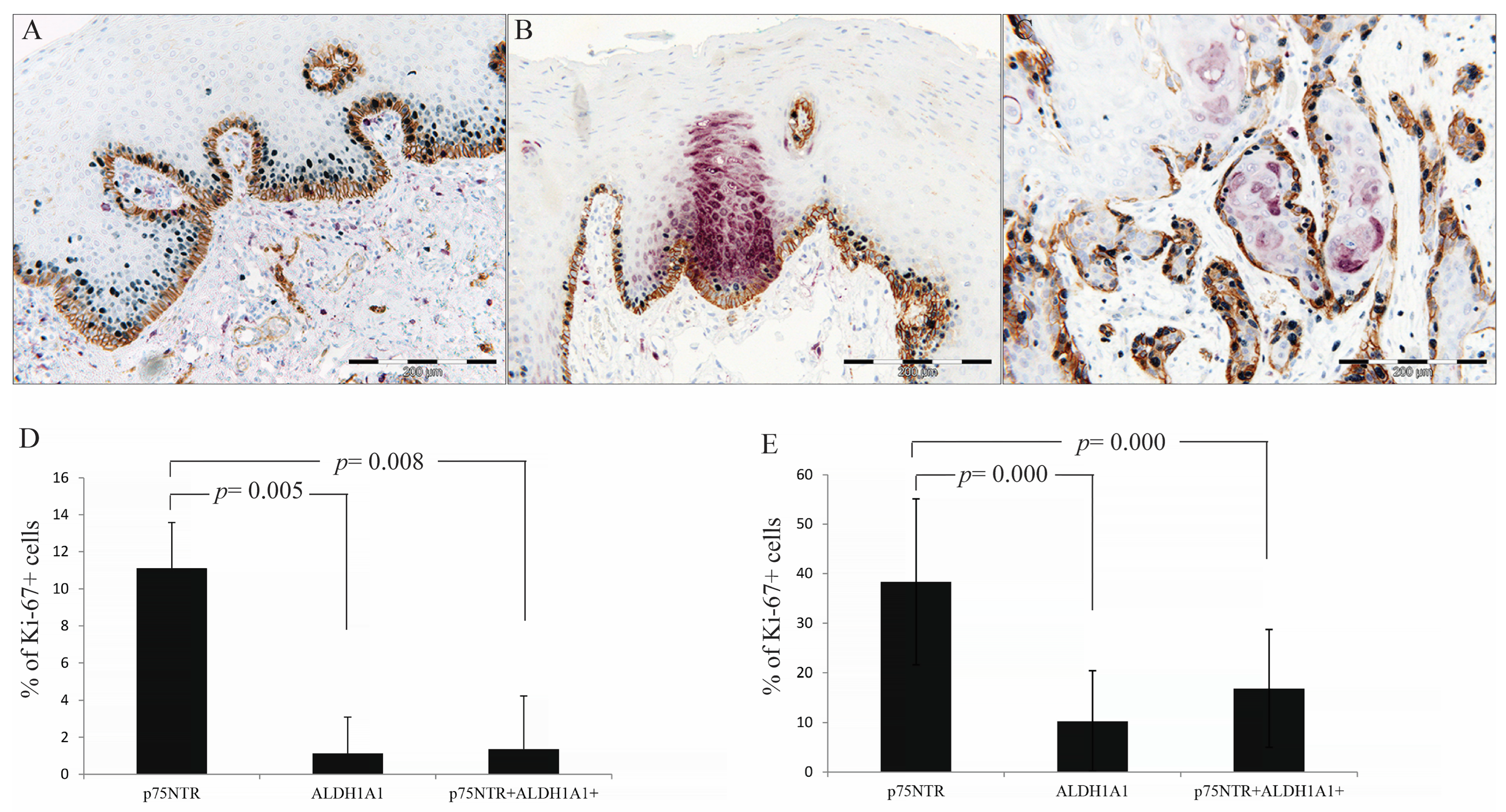
3.7. p75NTR+ Cells Displayed Higher Proliferative Activity than ALDH1A1 in NHOM and OSCC
3.8. Comparable Expression of p75NTR, ALDH1A1, and BMI1 Between Tumor Center, Invading Front, and Lymph Node Metastasis
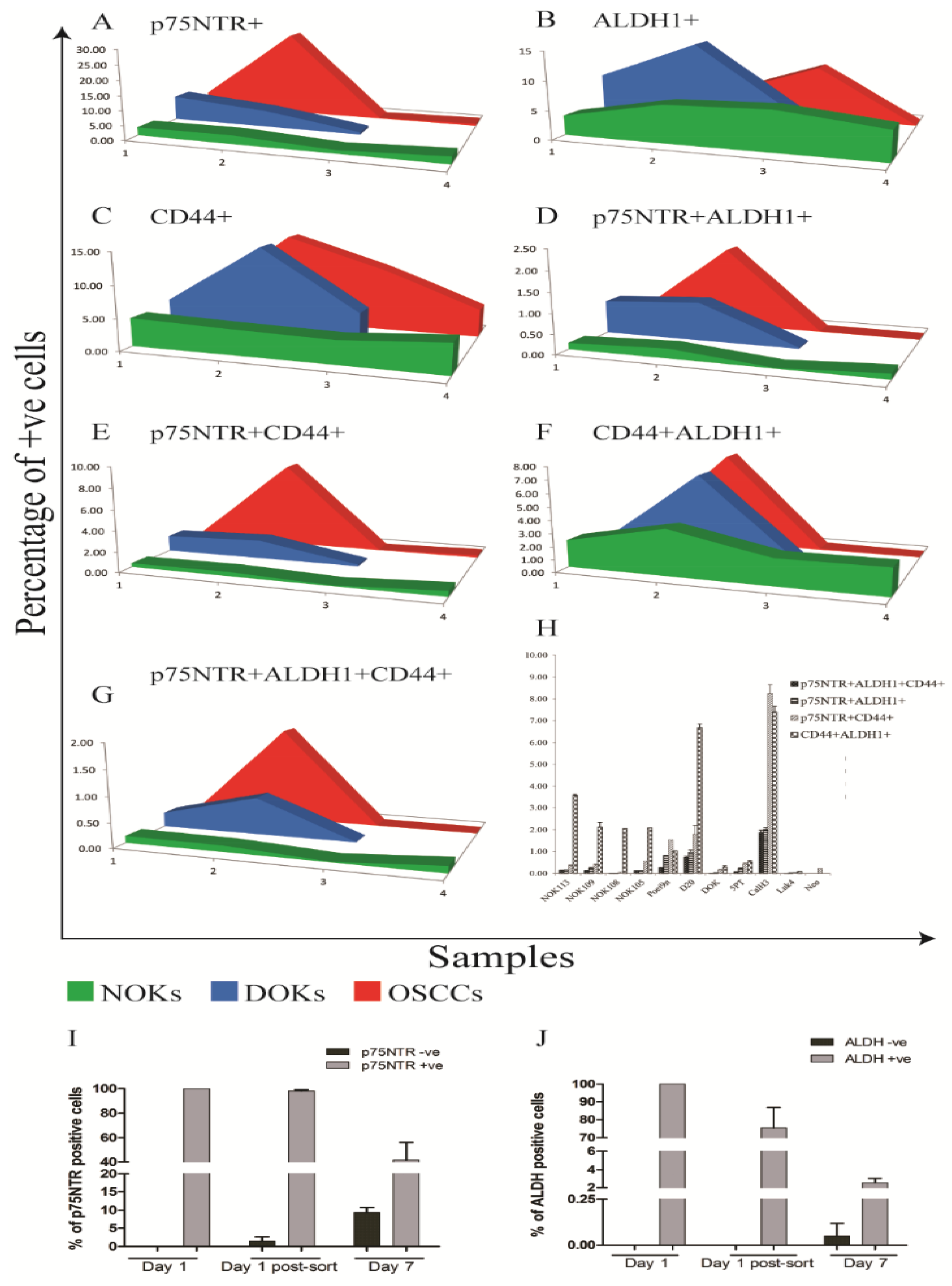
3.9. Increased Heterogeneity of CSC Marker Expression with Cancer Progression In Vitro
3.10. Distinct Kinetics of ALDH1 and p75NTR Expression in OSCC-Derived Cells Propagated In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef]
- You, L.; Guo, X.; Huang, Y. Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med. J. 2018, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.-Q.; Chen, D.-B.; Wang, B.-Q.; Zhang, L.-S. Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in human breast cancer cell lines. Oncol. Lett. 2012, 4, 1264–1268. [Google Scholar] [CrossRef]
- Basati, G.; Mohammadpour, H.; Emami Razavi, A. Association of high expression levels of SOX2, NANOG, and OCT4 in gastric cancer tumor tissues with progression and poor prognosis. J. Gastrointest. Cancer 2020, 51, 41–47. [Google Scholar] [CrossRef]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Coleman, M.P.; Gatta, G.; Verdecchia, A.; Esteve, J.; Sant, M.; Storm, H.; Allemani, C.; Ciccolallo, L.; Santaquilani, M.; Berrino, F. EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2003, 14 (Suppl. 5), v128–v149. [Google Scholar]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, Y.W.; Hsu, H.S.; Tseng, L.M.; Huang, P.I.; Lu, K.H.; Chen, D.T.; Tai, L.K.; Yung, M.C.; Chang, S.C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef]
- Clay, M.R.; Tabor, M.; Owen, J.H.; Carey, T.E.; Bradford, C.R.; Wolf, G.T.; Wicha, M.S.; Prince, M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010, 32, 1195–1201. [Google Scholar] [CrossRef]
- Okamoto, A.; Chikamatsu, K.; Sakakura, K.; Hatsushika, K.; Takahashi, G.; Masuyama, K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009, 45, 633–639. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef]
- Nakamura, T.; Endo, K.; Kinoshita, S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells 2007, 25, 628–638. [Google Scholar] [CrossRef]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; van den Broek, M.; et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.D.; Yuan, Y.; Liu, X.H.; Gong, D.J.; Bai, C.G.; Wang, F.; Luo, J.H.; Xu, Z.Y. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 2009, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Kawano, S.; Matsubara, R.; Goto, Y.; Hirano, M.; Jinno, T.; Toyoshima, T.; Kitamura, R.; Oobu, K.; Nakamura, S. Immunohistochemical location of the p75 neurotrophin receptor (p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int. J. Clin. Oncol. 2011, 18, 154–163. [Google Scholar] [CrossRef]
- Soland, T.M.; Brusevold, I.J.; Koppang, H.S.; Schenck, K.; Bryne, M. Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology 2008, 53, 62–72. [Google Scholar] [CrossRef]
- Osman, T.A.; Parajuli, H.; Sapkota, D.; Ahmed, I.A.; Johannessen, A.; Costea, D.E. The low-affinity nerve growth factor receptor p75NTR identifies a transient stem cell-like state in oral squamous cell carcinoma cells. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2015, 44, 410–419. [Google Scholar] [CrossRef]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Huang, E.H.; Hynes, M.J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J.Z.; Wicha, M.S.; Boman, B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009, 69, 3382–3389. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Douville, J.; Beaulieu, R.; Balicki, D. ALDH1 as a Functional Marker of Cancer Stem and Progenitor Cells. Stem Cells Dev. 2008, 18, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, M.; Sadeghi, M.; Ghazi, N.; Saghravanian, N.; Dehghani, M.; Aminian, A. Prognostic value of CD44 expression in oral squamous cell carcinoma: A meta-analysis. Ann. Diagn. Pathol. 2023, 67, 152213. [Google Scholar] [CrossRef]
- Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Milosevic Markovic, M.; Riberti, N.; Jelovac, D.; Milasin, J. MicroRNA-21 as a Regulator of Cancer Stem Cell Properties in Oral Cancer. Cells 2025, 14, 91. [Google Scholar] [CrossRef]
- Jaksic Karisik, M.; Lazarevic, M.; Mitic, D.; Nikolic, N.; Milosevic Markovic, M.; Jelovac, D.; Milasin, J. Osteogenic and Adipogenic Differentiation Potential of Oral Cancer Stem Cells May Offer New Treatment Modalities. Int. J. Mol. Sci. 2023, 24, 4704. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, T.; Takamaru, N.; Ohe, G.; Akita, K.; Nakagawa, T.; Miyamoto, Y. Expression of CD44, CD44v9, ABCG2, CD24, Bmi-1 and ALDH1 in stage I and II oral squamous cell carcinoma and their association with clinicopathological factors. Oncol. Lett. 2018, 16, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Caspa Gokulan, R.; Devaraj, H. Stem Cell Markers CXCR-4 and CD133 Predict Aggressive Phenotype and Their Double Positivity Indicates Poor Prognosis of Oral Squamous Cell Carcinoma. Cancers 2021, 13, 5895. [Google Scholar] [CrossRef]
- Baillie, R.; Tan, S.T.; Itinteang, T. Cancer Stem Cells in Oral Cavity Squamous Cell Carcinoma: A Review. Front. Oncol. 2017, 7, 112. [Google Scholar] [CrossRef]
- Ravindran, G.; Devaraj, H. Aberrant expression of CD133 and musashi-1 in preneoplastic and neoplastic human oral squamous epithelium and their correlation with clinicopathological factors. Head Neck 2012, 34, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Osman, T.A.; Oijordsbakken, G.; Costea, D.E.; Johannessen, A.C. Successful triple immunoenzymatic method employing primary antibodies from same species and same immunoglobulin subclass. Eur. J. Histochem. EJH 2013, 57, e22. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Qian, D.; Kiel, M.; Becker, M.W.; Pihalja, M.; Weissman, I.L.; Morrison, S.J.; Clarke, M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003, 423, 302–305. [Google Scholar] [CrossRef]
- Gerdes, J. An immunohistological method for estimating cell growth fractions in rapid histopathological diagnosis during surgery. Int. J. Cancer J. Int. Du Cancer 1985, 35, 169–171. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Gammon, L.; Biddle, A.; Heywood, H.K.; Johannessen, A.C.; Mackenzie, I.C. Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PLoS ONE 2013, 8, e62493. [Google Scholar] [CrossRef]
- Gammon, L.; Biddle, A.; Fazil, B.; Harper, L.; Mackenzie, I.C. Stem cell characteristics of cell sub-populations in cell lines derived from head and neck cancers of Fanconi anemia patients. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2011, 40, 143–152. [Google Scholar] [CrossRef]
- Dalley, A.J.; Abdulmajeed, A.A.; Upton, Z.; Farah, C.S. Organotypic culture of normal, dysplastic and squamous cell carcinoma-derived oral cell lines reveals loss of spatial regulation of CD44 and p75(NTR) in malignancy. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2012, 42, 37–46. [Google Scholar] [CrossRef]
- Muntoni, A.; Fleming, J.; Gordon, K.E.; Hunter, K.; McGregor, F.; Parkinson, E.K.; Harrison, P.R. Senescing oral dysplasias are not immortalized by ectopic expression of hTERT alone without other molecular changes, such as loss of INK4A and/or retinoic acid receptor-beta: But p53 mutations are not necessarily required. Oncogene 2003, 22, 7804–7808. [Google Scholar] [CrossRef]
- Costea, D.E.; Dimba, A.O.; Loro, L.L.; Vintermyr, O.K.; Johannessen, A.C. The phenotype of in vitro reconstituted normal human oral epithelium is essentially determined by culture medium. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2005, 34, 247–252. [Google Scholar] [CrossRef]
- Sapkota, D.; Bruland, O.; Costea, D.E.; Haugen, H.; Vasstrand, E.N.; Ibrahim, S.O. S100A14 regulates the invasive potential of oral squamous cell carcinoma derived cell-lines in vitro by modulating expression of matrix metalloproteinases, MMP1 and MMP9. Eur. J. Cancer 2011, 47, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Schubert, M.; Pietsch, L.; Taubert, I.; Wuchter, P.; Eckstein, V.; Bruckner, T.; Zoeller, M.; Ho, A.D. Aldehyde dehydrogenase activity among primary leukemia cells is associated with stem cell features and correlates with adverse clinical outcomes. Exp. Hematol. 2009, 37, 1423–1434. [Google Scholar] [CrossRef]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Barker, P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Progress. Neurobiol. 2002, 67, 203–233. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Barker, P.A.; Murphy, R.A. The nerve growth factor receptor: A multicomponent system that mediates the actions of the neurotrophin family of proteins. Mol. Cell. Biochem. 1992, 110, 1–15. [Google Scholar] [CrossRef]
- Tomellini, E.; Lagadec, C.; Polakowska, R.; Le Bourhis, X. Role of p75 neurotrophin receptor in stem cell biology: More than just a marker. Cell. Mol. Life Sci. CMLS 2014, 71, 2467–2481. [Google Scholar] [CrossRef]
- Okumura, T.; Shimada, Y.; Imamura, M.; Yasumoto, S. Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene 2003, 22, 4017–4026. [Google Scholar] [CrossRef]
- Moscatelli, I.; Pierantozzi, E.; Camaioni, A.; Siracusa, G.; Campagnolo, L. p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Exp. Cell Res. 2009, 315, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzidis, E.; Elena-Costea, D.; Parkinson, E.K.; Waseem, A.; Wan, H.; Teh, M.T. Induction of human epithelial stem/progenitor expansion by FOXM1. Cancer Res. 2010, 70, 9515–9526. [Google Scholar] [CrossRef]
- Teh, M.T.; Hutchison, I.L.; Costea, D.E.; Neppelberg, E.; Liavaag, P.G.; Purdie, K.; Harwood, C.; Wan, H.; Odell, E.W.; Hackshaw, A.; et al. Exploiting FOXM1-orchestrated molecular network for early squamous cell carcinoma diagnosis and prognosis. Int. J. Cancer J. Int. Du Cancer 2013, 132, 2095–2106. [Google Scholar] [CrossRef]
- Eneroth, C.M.; Hjertman, L.; Moberger, G. Squamous cell carcinomas of the palate. Acta Oto-Laryngol. 1972, 73, 418–427. [Google Scholar] [CrossRef]
- Luther, J.A.; Birren, S.J. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. J. Neurosci. 2009, 29, 5411–5424. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Yang, C.; Zhao, H.; Jian, C. Involvement of p75NTR in the effects of Aβ on L-type Ca2+ channel in cultured neuronal networks. Life Sci. 2020, 243, 117293. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Dai, Y.; Qian, C.; Dong, Y.; Chen, Z.; Meng, L.; Jiang, Z.; Huang, T.; Hu, J. Voltage-gated sodium channel Nav1. 5 promotes proliferation, migration and invasion of oral squamous cell carcinoma. Acta Biochim. Et Biophys. Sin. 2019, 51, 562–570. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Zhu, X.; Chen, X.; Wang, H.; Jiang, Y. Preliminary study on the role of voltage-gated sodium channel subtype Nav1. 5 in lymph node metastasis of oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi Chin. J. Stomatol. 2017, 52, 188–193. [Google Scholar]
- Walch, E.T.; Marchetti, D. Role of neurotrophins and neurotrophins receptors in the in vitro invasion and heparanase production of human prostate cancer cells. Clin. Exp. Metastasis 1999, 17, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Zhang, Y.; Ma, Q.; Shimahara, Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol. 2006, 21, 850–858. [Google Scholar] [CrossRef]
- Okumura, T.; Tsunoda, S.; Mori, Y.; Ito, T.; Kikuchi, K.; Wang, T.C.; Yasumoto, S.; Shimada, Y. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 5096–5103. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.; Okumura, T.; Ito, T.; Mori, Y.; Soma, T.; Watanabe, G.; Kaganoi, J.; Itami, A.; Sakai, Y.; Shimada, Y. Significance of nerve growth factor overexpression and its autocrine loop in oesophageal squamous cell carcinoma. Br. J. Cancer 2006, 95, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Y.; Di, B.; Li, J.; Geng, J.; Lu, X.; He, Z. Biological and clinical significance of p75NTR expression in laryngeal squamous epithelia and laryngocarcinoma. Acta Oto-Laryngol. 2012, 132, 314–324. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.M.; Bertucci, F.; Jacquemier, J.; et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef]
- Biddle, A.; Liang, X.; Gammon, L.; Fazil, B.; Harper, L.J.; Emich, H.; Costea, D.E.; Mackenzie, I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011, 71, 5317–5326. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, T.A.-H.; Rikardsen, O.; Teh, M.-T.; Sapkota, D.; Liang, K.X.; Neppelberg, E.; Biddle, A.; Mackenzie, I.; Uhlin-Hansen, L.; Johannessen, A.C.; et al. Identification of Phenotypically Distinct Cancer Stem Cell Subpopulations in Oral Squamous Cell Carcinoma. Cancers 2025, 17, 3547. https://doi.org/10.3390/cancers17213547
Osman TA-H, Rikardsen O, Teh M-T, Sapkota D, Liang KX, Neppelberg E, Biddle A, Mackenzie I, Uhlin-Hansen L, Johannessen AC, et al. Identification of Phenotypically Distinct Cancer Stem Cell Subpopulations in Oral Squamous Cell Carcinoma. Cancers. 2025; 17(21):3547. https://doi.org/10.3390/cancers17213547
Chicago/Turabian StyleOsman, Tarig Al-Hadi, Oddveig Rikardsen, Muy-Teck Teh, Dipak Sapkota, Kristina Xiao Liang, Evelyn Neppelberg, Adrian Biddle, Ian Mackenzie, Lars Uhlin-Hansen, Anne Christine Johannessen, and et al. 2025. "Identification of Phenotypically Distinct Cancer Stem Cell Subpopulations in Oral Squamous Cell Carcinoma" Cancers 17, no. 21: 3547. https://doi.org/10.3390/cancers17213547
APA StyleOsman, T. A.-H., Rikardsen, O., Teh, M.-T., Sapkota, D., Liang, K. X., Neppelberg, E., Biddle, A., Mackenzie, I., Uhlin-Hansen, L., Johannessen, A. C., & Costea, D. E. (2025). Identification of Phenotypically Distinct Cancer Stem Cell Subpopulations in Oral Squamous Cell Carcinoma. Cancers, 17(21), 3547. https://doi.org/10.3390/cancers17213547






