High-Intensity Focused Ultrasound in Dermatology: A Review with Emphasis on Skin Cancer Management and Prevention
Simple Summary
Abstract
1. Introduction
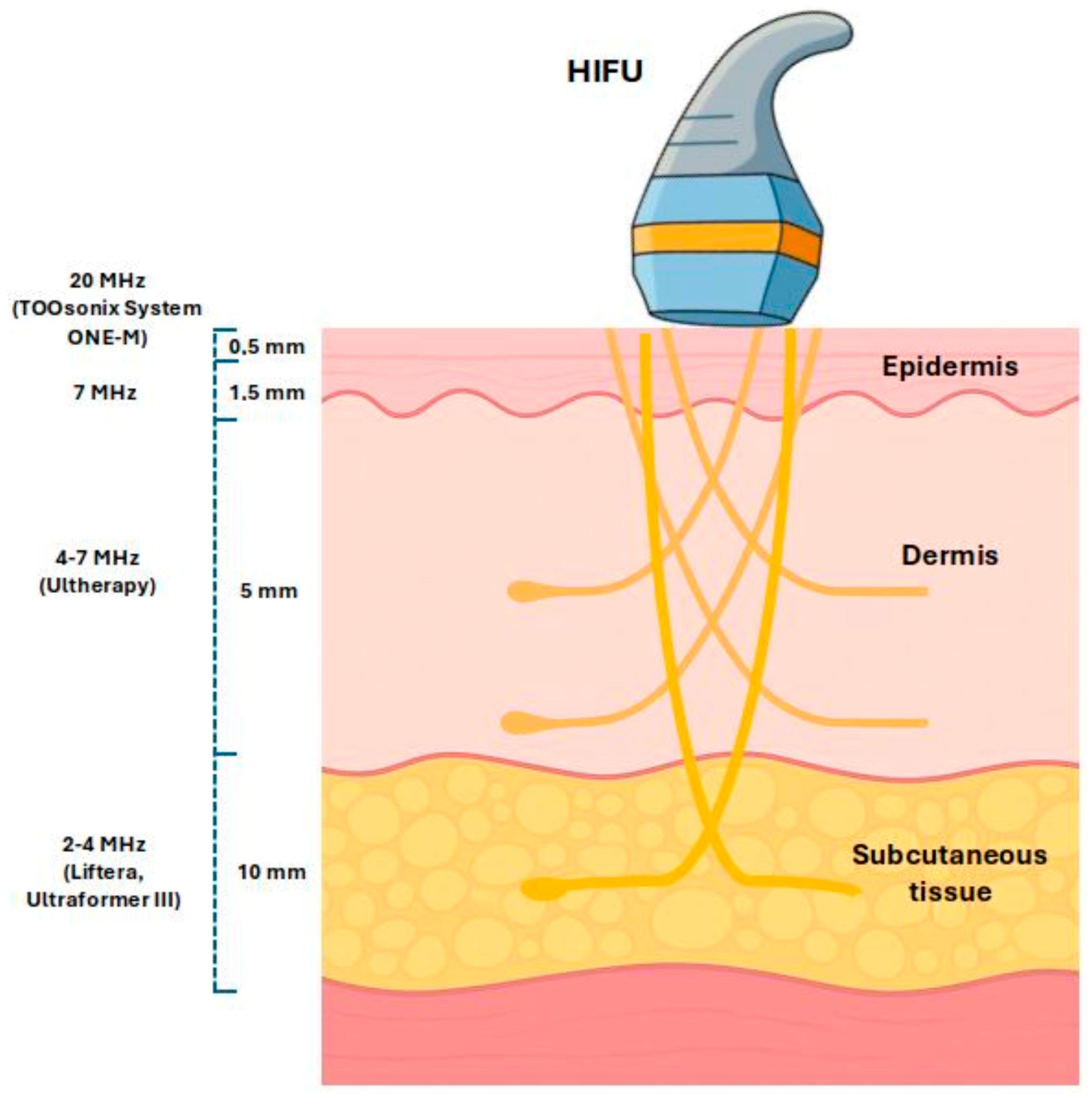
2. Mechanism of HIFU in Dermatology
3. Current Dermatological Uses of HIFU (Cosmetic and Benign Applications)
3.1. Aesthetic Skin Tightening and Rejuvenation
3.2. Benign Cutaneous Lesions
3.2.1. Hypertrophic Scars and Keloids
3.2.2. Seborrheic Keratosis (SK)
3.2.3. Sebaceous Hyperplasia (SH)
3.2.4. Cutaneous Neurofibroma
3.2.5. Benign Vascular Tumors (Cherry Angioma, Congenital Hemangioma)
3.2.6. Vulvar Lichen Sclerosus
3.2.7. Granuloma Annulare and Other Inflammatory Dermatoses
3.2.8. Viral Warts
3.2.9. Healing Process After High-Intensity Focused Ultrasound Treatment of Benign Skin Lesions
4. HIFU for Premalignant Lesions (Actinic Keratosis and Field Cancerization)
5. HIFU for Non-Melanoma Skin Cancers
5.1. Basal Cell Carcinoma (BCC)
5.2. Squamous Cell Carcinoma (SCC)
6. Discussion
7. Conclusions
7.1. Future Directions and Perspectives for Skin Cancer Management and Prevention
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Actinic Keratosis |
| BCC | Basal Cell Carcinoma |
| HIFU | High-Intensity Focused Ultrasound |
| EDC | Electrodesiccation and Curettage |
| PDT | Photodynamic Therapy |
| 5-FU | 5-Fluorouracil |
| NCT | National Clinical Trial (identifier number) |
| SK | Seborrheic Keratosis |
| GA | Granuloma Annulare |
| NMSC | Non-Melanoma Skin Cancer |
References
- Pan, Y.; Tang, B.; Guo, Y.; Cai, Y.; Li, Y.-Y. Global burden of non-melanoma skin cancers among older adults: A comprehensive analysis using machine learning approaches. Sci. Rep. 2025, 15, 15266. [Google Scholar] [CrossRef]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; DiMaio, D.; et al. Basal cell skin cancer, version 2.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1181–1203. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; Breems, N.Y.D.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef]
- Calik, J.; Sauer, N.; Woźniak, B.; Wojnar, A.; Pietkiewicz, P.; Dzięgiel, P. Pilot Study on High-Intensity Focused Ultrasound (HIFU) for Basal Cell Carcinoma: Effectiveness and Safety. J. Clin. Med. 2024, 13, 3277. [Google Scholar] [CrossRef]
- Guinot, J.L.; Rembielak, A.; Perez-Calatayud, J.; Rodríguez-Villalba, S.; Skowronek, J.; Tagliaferri, L.; Guix, B.; Gonzalez-Perez, V.; Valentini, V.; Kovacs, G. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018, 126, 377–385. [Google Scholar] [CrossRef]
- Poltorak, M.; Banatkiewicz, P.; Poltorak, L.; Sobolewski, P.; Zimon, D.; Szwast, M.; Walecka, I. Brachytherapy and 3D printing for skin cancer: A review paper. J. Contemp. Brachytherapy 2024, 16, 156–169. [Google Scholar] [CrossRef]
- Poltorak, M.; Banatkiewicz, P.; Poltorak, L.; Sobolewski, P.; Zimon, D.; Szwast, M.; Walecka, I. Individualized 3D printing for skin cancer brachytherapy: Development, implementation, clinical applications, and treatment assessment. J. Contemp. Brachytherapy 2024, 16, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Półtorak, M.; Sobolewski, P.; Walecka, I. Quantitative Dosimetric Analysis with Independent Software Solutions and Comprehensive Treatment Plan Parameter Evaluation in skin brachytherapy. Pol. J. Med. Phys. Eng. 2024, 30, 169–176. [Google Scholar] [CrossRef]
- Krajewska-Węglewicz, L.; Sobolewski, P.; Walecka, I. Topical 5% Imiquimod for the Treatment of Superficial and Nodular Periocular Basal Cell Carcinoma: A Systematic Review of Clinical Outcomes, Safety, and Treatment Strategies. Cancers 2025, 17, 2111. [Google Scholar] [CrossRef]
- Focused Ultrasound Foundation. State of the Field Report 2024. Available online: https://www.fusfoundation.org/the-foundation/foundation-reports/state-of-the-field-report-2024/ (accessed on 6 July 2025).
- Ellens, N.P.K.; Partanen, A. Preclinical MRI-guided focused ultrasound: A review of systems and current practices. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 291–305. [Google Scholar] [CrossRef]
- Guillaumier, S.; Peters, M.; Arya, M.; Afzal, N.; Charman, S.; Dudderidge, T.; Hosking-Jervis, F.; Hindley, R.G.; Lewi, H.; McCartan, N.; et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant non-metastatic prostate cancer. Eur. Urol. 2018, 74, 422–429. [Google Scholar] [CrossRef]
- Barile, A.; Arrigoni, F.; Zugaro, L.; Zappia, M.; Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Brunese, L.; Gangi, A.; Masciocchi, C. Minimally invasive treatments of painful bone lesions: State of the art. Med. Oncol. 2017, 34, 53. [Google Scholar] [CrossRef]
- Lamsam, L.; Johnson, E.; Connolly, I.D.; Wintermark, M.; Hayden Gephart, M. A review of potential applications of MR-guided focused ultrasound for targeting brain tumor therapy. Neurosurg. Focus. 2018, 44, E10. [Google Scholar] [CrossRef]
- Peek, M.C.L.; Wu, F. High-intensity focused ultrasound in the treatment of breast tumours. Ecancermedicalscience 2018, 12, 794. [Google Scholar] [CrossRef]
- Lang, B.H.; Wu, A.L.H. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules—A systematic review. J. Ther. Ultrasound 2017, 5, 11. [Google Scholar] [CrossRef]
- Bove, T.; Zawada, T.; Serup, J.; Jessen, A.; Poli, M. High-frequency (20-MHz) high-intensity focused ultrasound (HIFU) system for dermal intervention: Preclinical evaluation in skin equivalents. Ski. Res. Technol. 2019, 25, 217–228. [Google Scholar] [CrossRef]
- Soegaard, S.; Aarup, V.; Serup, J.; Bove, T.; Zawada, T.; Jessen, A.; Poli, M. High frequency (20 MHz) High Intensity Focused Ultrasound (HIFU) system for dermal intervention: A 12 week local tolerance study in minipigs. Ski. Res. Technol. 2020, 26, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Halabchi, F.; Mazaheri, R.; Abolhasani, M.; Tabesh, M. Review of the mechanisms and effects of noninvasive body contouring devices on cellulite and subcutaneous fat. Int. J. Endocrinol. Metab. 2016, 14, e36727. [Google Scholar] [CrossRef] [PubMed]
- Day, D. Microfocused ultrasound for facial rejuvenation: Current perspectives. Res. Rep. Focus. Ultrasound 2014, 2, 13–17. [Google Scholar] [CrossRef]
- White, M.W.; Makin, I.R.S.; Slayton, M.H.; Barthe, P.G.; Gliklich, R. Selective transcutaneous delivery of energy to porcine soft tissues using Intense Ultrasound (IUS). Lasers Surg. Med. 2008, 40, 67–75. [Google Scholar] [CrossRef]
- White, W.M.; Makin, I.R.S.; Barthe, P.G.; Slayton, M.H.; Gliklich, R.E. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy. Arch. Facial Plast. Surg. 2007, 9, 22–29. [Google Scholar] [CrossRef]
- Hsu, S.-R.; Wu, C.-H.; Chen, S.-J. A review of high-intensity focused ultrasound combined with immunotherapy. J. Radiol. Sci. 2024, 49, 111–116. [Google Scholar] [CrossRef]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- Serup, J.; Bove, T.; Zawada, T.; Jessen, A.; Poli, M. High-frequency (20 MHz) high-intensity focused ultrasound: New treatment of actinic keratosis, basal cell carcinoma, and Kaposi sarcoma. An open-label exploratory study. Ski. Res. Technol. 2020, 26, 824–831. [Google Scholar] [CrossRef]
- Schuetzenberger, K.; Pfister, M.; Messner, A.; Froehlich, V.; Garhoefer, G.; Hohenadl, C.; Schmetterer, L.; Werkmeister, R.M. Comparison of optical coherence tomography and high frequency ultrasound imaging in mice for the assessment of skin morphology and intradermal volumes. Sci. Rep. 2019, 9, 13643. [Google Scholar] [CrossRef]
- Contini, M.; Hollander, M.H.J.; Vissink, A.; Schepers, R.H.; Jansma, J.; Schortinghuis, J. A systematic review of the efficacy of microfocused ultrasound for facial skin tightening. Int. J. Environ. Res. Public Health 2023, 20, 1522. [Google Scholar] [CrossRef]
- Ling, J.; Zhao, H. A systematic review and meta-analysis of the clinical efficacy and patients’ satisfaction of micro-focused ultrasound (MFU) treatment for facial rejuvenation and tightening. Aesth. Plast. Surg. 2023, 47, 1806–1823. [Google Scholar] [CrossRef]
- Dicker, V.; Parra, L.A.; Amado, A.M.; Acevedo, A.; Castelanich, D.; Velasquez, L.; Parra, A.M. High-Intensity Focused Ultrasound Devices for Skin Tightening: A Comparative Literature Review of Device Specifications. Dermatol. Rev. 2025, 6, e70030. [Google Scholar] [CrossRef]
- Anastasova, V.N.; Georgiev, A.A.; Zanzov, E.I.; Velkova, K.G.; Krasteva, E.S. High-Intensity Focused Ultrasound Thermotherapy for Scar Treatment. Ann. Burn. Fire Disasters 2023, 36, 63–67. [Google Scholar]
- Calik, J.; Migdal, M.; Zawada, T.; Bove, T. Treatment of seborrheic keratosis by high frequency focused ultrasound—An early experience with 11 consecutive cases. Clin. Cosmet. Investig. Dermatol. 2022, 15, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Farci, F.; Rapini, R.P. Sebaceous Hyperplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Woźniak, B.; Sauer, N.; Pogorzelska-Antkowiak, A.; Dzięgiel, P.; Calik, J. Treatment of sebaceous hyperplasia by high-frequency focused ultrasound (HIFU): A comprehensive exploration with clinical insights. J. Clin. Med. 2025, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, M.; Bouroubi, A.; Beauchamp, R.; Bocquet, A.; Grégoire, J.-M.; Rauly-Lestienne, I.; Blanco, I.; Wolkenstein, P.; Schmitt, A.-M. Cutaneous neurofibromas: Patients’ medical burden, current management and therapeutic expectations: Results from an online European patient community survey. Orphanet J. Rare Dis. 2019, 14, 286. [Google Scholar] [CrossRef]
- Peltonen, S.; Serup, J.; Tang, M.; Gillstedt, M.; Kantere, D.; Neittaanmäki, N.; Holmström, P.; Blakeley, J.O.; Rosner, K.; Roberts, J.; et al. High-intensity focused ultrasound: Safety and efficacy of a novel treatment modality for neurofibromatosis type 1 cutaneous neurofibroma. JEADV Clin. Pract. 2024, 3, 1049–1060. [Google Scholar] [CrossRef]
- Woźniak, B.; Bove, T.; Zawada, T.; Calik, J. Treatment of cutaneous neurofibromas in patients with neurofibromatosis type 1. Case Rep. Dermatol. 2023, 15, 194–201. [Google Scholar] [CrossRef]
- Qadeer, H.A.; Singal, A.; Patel, B.C. Cherry Hemangioma; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Boull, C.; Maguiness, S.M. Congenital hemangiomas. Semin. Cutan. Med. Surg. 2016, 35, 124–127. [Google Scholar] [CrossRef]
- Aversa, A.J.; Miller, O.F. Cryo-curettage of cherry angiomas. J. Dermatol. Surg. Oncol. 1983, 9, 930–931. [Google Scholar] [CrossRef]
- Patel, B.C. The krypton yellow-green laser for the treatment of facial vascular and pigmented lesions. Semin. Ophthalmol. 1998, 13, 158–170. [Google Scholar] [CrossRef]
- Calik, J.; Zawada, T.; Bove, T. Treatment of superficial benign vascular tumors by high intensity focused ultrasound: Observations in two illustrative cases. J. Cosmet. Dermatol. 2022, 21, 3371–3379. [Google Scholar] [CrossRef]
- Regauer, S.; Liegl, B.; Reich, O. Early vulvar lichen sclerosus: A histopathological challenge. Histopathology 2005, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, M.C.; Visser, P.J.; Overbeek, L.I.; van Beurden, M.; Berkhof, J. Lichen sclerosus: Incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Meng, Y.; Feng, S.; Liu, M.; Xiao, L.; Zhang, X.; Zheng, J.; Chang, S.; Xu, R.X. Therapeutic assessment of high-intensity focused ultrasound for vulvar lichen sclerosus by active dynamic thermal imaging and hyperspectral imaging—A preliminary study. Front. Phys. 2020, 8, 91. [Google Scholar] [CrossRef]
- Jia, R.; Wu, C.; Tang, X.; He, M.; Liu, X.; Su, C.; Li, C. Comparison of the efficacy of focused ultrasound at different focal depths in treating vulvar lichen sclerosus. Int. J. Hyperth. 2023, 40, 2172220. [Google Scholar] [CrossRef]
- Ruan, L.; Xie, Z.; Wang, H.; Jiang, J.; Shi, H.; Xu, J. High-intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva. Int. J. Gynaecol. Obstet. 2010, 109, 167–170. [Google Scholar] [CrossRef]
- Wu, C.; Zou, M.; Xiong, Y.; Wang, L.; Chen, H.; Fan, Y.; Li, C. Short- and long-term efficacy of focused ultrasound therapy for non-neoplastic epithelial disorders of the vulva. BJOG 2017, 124 (Suppl. S3), 87–92. [Google Scholar] [CrossRef]
- Shi, H.H.; Zhu, L.; Yang, Y.; Jin, H.M.; Li, R.Z.; Liang, Z.Q.; Lang, J.H. Multicenter randomized clinical study of high-intensity focused ultrasound and cortical hormone in the treatment of non-neoplastic epithelial disorders of vulva. Zhonghua Yi Xue Za Zhi 2013, 93, 3291–3293. (In Chinese) [Google Scholar]
- Goldstein, A.T.; Creasey, A.; Pfau, R.; Phillips, D.; Burrows, L.J. A double-blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2011, 64, e99–e104. [Google Scholar] [CrossRef]
- He, S.; Jiang, J. High-intensity focused ultrasound therapy for pediatric and adolescent vulvar lichen sclerosus. Int. J. Hyperth. 2022, 39, 579–583. [Google Scholar] [CrossRef]
- Li, R.; Jiang, J. The efficacy and safety of secondary focused ultrasound therapy for recurrence of non-neoplastic epithelial disorders of the vulva. Int. J. Hyperth. 2022, 39, 1310–1314. [Google Scholar] [CrossRef]
- Krapf, J.M.; Mitchell, L.; Holton, M.A.; Goldstein, A.T. Vulvar lichen sclerosus: Current perspectives. Int. J. Womens Health 2020, 12, 11–20. [Google Scholar] [CrossRef]
- Corazza, M.; Schettini, N.; Zedde, P.; Borghi, A. Vulvar lichen sclerosus from pathophysiology to therapeutic approaches: Evidence and prospects. Biomedicines 2021, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Salgado, H.C.; Drumond, D.G.; Pannain, G.D.; de Melo E Costa, L.G.; Sampaio, F.S.; Leite, I.C.G. Randomized clinical trial with fractional CO2 laser and Clobetasol in the treatment of vulvar lichen sclerosus: A clinic study of feasibility. BMC Res. Notes 2023, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mishra, N.; Ghatage, P. Treatment options in vulvar lichen sclerosus: A scoping review. Cureus 2021, 13, e13527. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, S.J.; Harper, C.D.; Schmieder, G.J. Granuloma Annulare; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Calik, J.; Zawada, T.; Sauer, N.; Bove, T. High Intensity Focused Ultrasound (20 MHz) and Cryotherapy as Therapeutic Options for Granuloma Annulare and Other Inflammatory Skin Conditions. Dermatol. Ther. 2024, 14, 1189–1210. [Google Scholar] [CrossRef]
- Li, M.; Tao, M.; Zhang, Y.; Pan, R.; Gu, D.; Xu, Y. Effect of High-Intensity Macro-Focused Ultrasound on a Case of Morbihan Disease. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1949–1954. [Google Scholar] [CrossRef]
- Bove, T.; Zawada, T.; Jessen, A.; Poli, M.; Serup, J. Removal of Common Warts by High-Intensity Focused Ultrasound: An Introductory Observation. Case Rep. Dermatol. 2021, 13, 340–346. [Google Scholar] [CrossRef]
- Calik, J.; Zawada, T.; Bove, T. Treatment of Condylomata Acuminata Using a New Non-Vapor-Generating Focused Ultrasound Method following Imiquimod 5% Cream. Case Rep. Dermatol. 2022, 14, 275–282. [Google Scholar] [CrossRef]
- Calik, J.; Zawada, T.; Bove, T.; Dzięgiel, P.; Pogorzelska-Antkowiak, A.; Mackiewicz, J.; Woźniak, B.; Sauer, N. Healing Process after High-Intensity Focused Ultrasound Treatment of Benign Skin Lesions: Dermoscopic Analysis and Treatment Guidelines. J. Clin. Med. 2024, 13, 931. [Google Scholar] [CrossRef]
- Aggarwal, I.; Puyana, C.; Chandan, N.; Jetter, N.; Tsoukas, M. Field Cancerization Therapies for the Management of Actinic Keratosis: An Updated Review. Am. J. Clin. Dermatol. 2024, 25, 391–405. [Google Scholar] [CrossRef]
- Balcere, A.; Konrāde-Jilmaza, L.; Pauliņa, L.A.; Čēma, I.; Krūmiņa, A. Clinical Characteristics of Actinic Keratosis Associated with the Risk of Progression to Invasive Squamous Cell Carcinoma: A Systematic Review. J. Clin. Med. 2022, 11, 5899. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Zwingers, T.; Willers, C. Recurrence Rates and Patient Assessed Outcomes of 0.5% 5-fluorouracil in Combination with Salicylic Acid Treating Actinic Keratoses. Eur. J. Dermatol. 2012, 22, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 2. Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Quaedvlieg, P.J.; Tirsi, E.; Thissen, M.R.; Krekels, G.A. Actinic Keratosis: How to Differentiate the Good from the Bad Ones? Eur. J. Dermatol. 2006, 16, 335–339. [Google Scholar]
- Kandolf, L.; Peris, K.; Malvehy, J.; Mosterd, K.; Heppt, M.V.; Fargnoli, M.C.; Berking, C.; Arenberger, P.; Bylaite-Bučinskiene, M.; del Marmol, V.; et al. European Consensus-based Interdisciplinary Guideline for Diagnosis, Treatment and Prevention of Actinic Keratoses, Epithelial UV-induced Dysplasia and Field Cancerization on Behalf of European Associations. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1024–1047. [Google Scholar] [CrossRef]
- Ahmady, S.; Jansen, M.H.E.; Nelemans, P.J.; Kessels, J.P.H.M.; Arits, A.H.M.M.; de Rooij, M.J.M.; Essers, B.A.B.; Quaedvlieg, P.J.F.; Kelleners-Smeets, N.W.J.; Mosterd, K. Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis: Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 634–640. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Cazzaniga, S.; Bossart, S.; Feldmeyer, L.; Pelloni, L.; van Rhyn, M.; Angermeier, S.; Adatto, M.; Hunger, R.E.; Heidemeyer, K. Efficacy Assessment of the High-Frequency High-Intensity Focused Ultrasound as a New Treatment for Actinic Keratosis. Dermatology 2022, 238, 662–667. [Google Scholar] [CrossRef]
- Zawada, T.; Bove, T. Strongly Focused HIFU Transducers with Simultaneous Optical Observation for Treatment of Skin at 20 MHz. Ultrasound Med. Biol. 2022, 48, 1309–1327. [Google Scholar] [CrossRef]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Farberg, A.S.; Marson, J.W.; Soleymani, T. Advances in Photodynamic Therapy for the Treatment of Actinic Keratosis and Nonmelanoma Skin Cancer: A Narrative Review. Dermatol. Ther. 2023, 13, 689–716. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.; Shah, M.; Pon, K.; Alavi, A. Laser Resurfacing Monotherapy for the Treatment of Actinic Keratosis. J. Cutan. Med. Surg. 2021, 25, 634–642. [Google Scholar] [CrossRef]
- Jiang, A.J.; Soon, S.L.; Rullan, P.; Brody, H.J.; Monheit, G.D.; Lee, K.C. Chemical Peels as Field Therapy for Actinic Keratoses: A Systematic Review. Dermatol. Surg. 2021, 47, 1343–1346. [Google Scholar] [CrossRef]
- Korecka, K.; Slian, A.; Czajkowska, J.; Dańczak-Pazdrowska, A.; Polańska, A. The Application of High-Frequency Ultrasonography in Post-Therapeutic Assessment of Actinic Keratosis After Photodynamic Therapy. Cancers 2024, 16, 3778. [Google Scholar] [CrossRef]
- Hobayan, C.G.P.; Gray, A.N.; Waters, M.F.; Mager, L.A.; Kobayashi, S.; Essien, E.W.; Ulman, C.A.; Kaffenberger, B.H. Diagnostic Accuracy of High-Frequency Ultrasound for Cutaneous Neoplasms: A Narrative Review of the Literature. Arch. Dermatol. Res. 2024, 316, 419. [Google Scholar] [CrossRef]
- Mazur, E.; Kwiatkowska, D.; Reich, A. Reflectance Confocal Microscopy and Dermoscopy of Facial Pigmented and Non-Pigmented Actinic Keratosis Features before and after Photodynamic Therapy Treatment. Cancers 2023, 15, 5598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceryn, J.; Lesiak, A.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Noweta, M.; Stasikowska-Kanicka, O.; Ciążyński, K.; Zalaudek, I.; Narbutt, J. Targeting Biomarkers of Proliferation and Inflammation (Ki67, p53, and COX-2) in Actinic Keratoses with Photodynamic Therapy. Biomedicines 2025, 13, 1487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317, Erratum in J. Am. Acad. Dermatol. 2021, 85, 535. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; Del Marmol, V.; Dummer, R.; et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef] [PubMed]
- Paoli, J.; Dahlén Gyllencreutz, J.; Fougelberg, J.; Johansson Backman, E.; Modin, M.; Polesie, S.; Zaar, O. Nonsurgical options for the treatment of basal cell carcinoma. Dermatol. Pract. Concept. 2019, 9, 75–81. [Google Scholar] [CrossRef]
- Serup, J.; Zawada, T.; Bove, T. Recalcitrant Basal Cell Carcinoma after Grenz Ray Therapy: Introduction of High-Intensity Focused Ultrasound for Minimally Invasive Management. Case Rep. Dermatol. 2024, 16, 164–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calik, J.; Oślizło, M.; Słocka-Romaniuk, B.; Elsaftawy, A.; Sauer, N. Case report: Sequential treatment strategy for advanced basal cell carcinoma in Gorlin-Goltz syndrome: Integration of vismodegib, radiotherapy, surgery, and high-intensity focused ultrasound. Front. Oncol. 2024, 14, 1428702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://clinicaltrials.gov/study/NCT05698706 (accessed on 31 July 2025).
- Clark, C.M.; Furniss, M.; Mackay-Wiggan, J.M. Basal cell carcinoma: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S.J.; Aslam, N.; Saleem, H.; Rahman, J.; Khan, S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus 2020, 12, e9211. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/study/NCT05133427 (accessed on 31 July 2025).
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melodelima, D.; Prat, F.; Fritsch, J.; Theillere, Y.; Cathignol, D. Treatment of esophageal tumors using high intensity intraluminal ultrasound: First clinical results. J. Transl. Med. 2008, 6, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caudill, J.; Thomas, J.E.; Burkhart, C.G. The risk of metastases from squamous cell carcinoma of the skin. Int. J. Dermatol. 2023, 62, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Sandhu, S.S.; Sivamani, R.K. Clinical utility of daylight photodynamic therapy in the treatment of actinic keratosis-a review of the literature. Clin. Cosmet. Dermatol. 2019, 12, 427–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tchanque-Fossuo, C.N.; Eisen, D.B. A systematic review on the use of cryotherapy versus other treatments for basal cell carcinoma. Dermatol. Online J. 2018, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.K.; Georgouras, K. Complications of cutaneous cryotherapy. Med. J. Aust. 1994, 161, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Zane, C.; Facchinetti, E.; Arisi, M.; Ortel, B.; Calzavara-Pinton, P. Pulsed CO2 Laser Ablation of Superficial Basal Cell of Limbs and Trunk: A Comparative Randomized Clinical Trial With Cryotherapy and Surgical Ablation. Dermatol. Surg. 2017, 43, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hibler, B.P.; Sierra, H.; Cordova, M.; Phillips, W.; Rajadhyaksha, M.; Nehal, K.S.; Rossi, A.M. Carbon dioxide laser ablation of basal cell carcinoma with visual guidance by reflectance confocal microscopy: A proof-of-principle pilot study. Br. J. Dermatol. 2016, 174, 1359–1364. [Google Scholar] [CrossRef]
- Humphreys, T.R.; Malhotra, R.; Scharf, M.J.; Marcus, S.M.; Starkus, L.; Calegari, K. Treatment of superficial basal cell carcinoma and squamous cell carcinoma in situ with a high-energy pulsed carbon dioxide laser. Arch. Dermatol. 1998, 134, 1247–1252. [Google Scholar] [CrossRef]
- Erlendsson, A.M.; Olesen, U.H.; Haedersdal, M.; Rossi, A.M. Ablative fractional laser-assisted treatments for keratinocyte carcinomas and its precursors–Clinical review and future perspectives. Adv. Drug Deliv. Rev. 2020, 153, 185–194. [Google Scholar] [CrossRef]
- Benson, T.A.; Hibler, B.P.; Kotliar, D.; Avram, M. Nonablative fractional laser treatment is associated with a decreased risk of subsequent facial keratinocyte carcinoma development. Dermatol. Surg. 2023, 49, 149–154. [Google Scholar] [CrossRef]
- Borgognoni, L.; Pescitelli, L.; Gerlini, G.; Brandani, P.; Gelli, R.; Giannotti, V.; Bellucci, F.; Sestini, S. Efficacy of Electrochemotherapy in the Treatment of Cutaneous Melanoma Metastases and Rare Non-melanoma Skin Cancer. Anticancer. Res. 2020, 40, 6485–6492. [Google Scholar] [CrossRef]
- Di Monta, G.; Caracò, C.; Benedetto, L.; La Padula, S.; Marone, U.; Tornesello, M.L.; Buonaguro, F.M.; Simeone, E.; Ascierto, P.A.; Mozzillo, N. Electrochemotherapy as “new standard of care” treatment for cutaneous Kaposi’s sarcoma. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014, 40, 61–66. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, X.Y.; Liu, Y.; Sankin, G.N.; Pua, E.C.; Morse, M.A.; Lyerly, H.K.; Clay, T.M.; Zhong, P. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J. Transl. Med. 2007, 5, 34. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, X.Y.; Liu, Y.; Morse, M.A.; Lyerly, H.K.; Clay, T.M.; Zhong, P. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem. Biophys. Res. Commun. 2005, 335, 124–131. [Google Scholar] [CrossRef]
- Joiner, J.B.; Pylayeva-Gupta, Y.; Dayton, P.A. Focused ultrasound for immunomodulation of the tumor microenvironment. J. Immunol. 2020, 205, 2327–2341. [Google Scholar] [CrossRef]
- Eranki, A.; Srinivasan, P.; Ries, M.; Kim, A.; Lazarski, C.A.; Rossi, C.T.; Khokhlova, T.D.; Wilson, E.; Knoblach, S.M.; Sharma, K.V.; et al. High-intensity focused ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin. Cancer Res. 2020, 26, 1152–1161. [Google Scholar] [CrossRef]
- Vaezy, S.; Shi, X.; Martin, R.W.; Chi, E.; Nelson, P.I.; Bailey, M.R.; Crum, L.A. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med. Biol. 2001, 27, 33–42. [Google Scholar] [CrossRef]
- Lee, S.W.; Goo, B.L. High-intensity focused ultrasound enhances drug penetration into the human skin in the Franz diffusion cell. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1711–1721. [Google Scholar] [CrossRef]
- Han, H.; Lee, H.; Kim, K.; Kim, H. Effect of high intensity focused ultrasound (HIFU) in conjunction with a nanomedicines-microbubble complex for enhanced drug delivery. J. Control Release 2017, 266, 75–86. [Google Scholar] [CrossRef]
- Tu, J.; Yu, A.C.H. Ultrasound-mediated drug delivery: Sonoporation mechanisms, biophysics, and critical factors. BME Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef] [PubMed]



| Study | Patient Sample | Lesion Type | HIFU Parameters | Clinical Outcomes | Cosmetic Results | Adverse Effects | Follow-Up |
|---|---|---|---|---|---|---|---|
| Serup et al., 2020 [29] | 8 patients with 201 AKs; 1 patient with 7 BCCs | AK, BCC, Kaposisarcoma | 20 MHz, 0.6–1.2 J/shot, 150 ms, focal depth 1.3 mm, 1–3 sessions. | 97% complete response in AK. | Good with no scar after treatment. | Transient erythema and superficial crusts (resolving within 2 weeks); median VAS pain score: 4. | 3–6 months |
| Seyed Jafari et al., 2022 [73] | 21 patients with 108 AKs | AK | 20 MHz, 0.9–1.2 J/shot, 150 ms, focal depth 1.3 mm, 1 session. | 98.2% total response (72.2% complete, 26.0% partial). | Good | Short pain during the procedure; only a small proportion of lesions showed hypopigmentation, hyperpigmentation, or erythema. | 3 months |
| Calik et al., 2024 [8] | 8 patients with 15 BCCs | BCC | 20 MHz, 1.3 J/shot, 150 ms, focal depth 0.8–1.8 mm, 1 session. | 100% response; no recurrence detected during the follow-up period. | 75% of patients reported their satisfaction as “very high”. | Transient erythema, crusting, edema, and short-term urticaria; no infections or significant scarring reported. | 6 months |
| Serup et al., 2024 [86] | 1 patient with 8BCCs and 2 AKs | BCC, AK | 20 MHz, 0.9 J/shot, 150 ms, focal depth 1.3 mm, 1 session. | 87.5% clinical response was observed in BCC; 1 lesion recurring after 15 months. | Good | No procedure-related complications occurred. | 6–15 months |
| Calik et al., 2024 [87] | 1 patient with 2 BCCs (associated with Gorlin–Goltz syndrome) | BCC | 20 MHz; 0.7–1.3 J/shot; 150 ms pulse duration; focal depth 1.3 mm; single session; combined with vismodegib. | 100% | Cosmetic outcome reported as “excellent” by the authors. | No acute or delayed adverse effects were recorded. | 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, B.; Sobolewski, P.; Sauer, N.; Koper, M.; Calik, J. High-Intensity Focused Ultrasound in Dermatology: A Review with Emphasis on Skin Cancer Management and Prevention. Cancers 2025, 17, 3518. https://doi.org/10.3390/cancers17213518
Woźniak B, Sobolewski P, Sauer N, Koper M, Calik J. High-Intensity Focused Ultrasound in Dermatology: A Review with Emphasis on Skin Cancer Management and Prevention. Cancers. 2025; 17(21):3518. https://doi.org/10.3390/cancers17213518
Chicago/Turabian StyleWoźniak, Bartosz, Piotr Sobolewski, Natalia Sauer, Mateusz Koper, and Jacek Calik. 2025. "High-Intensity Focused Ultrasound in Dermatology: A Review with Emphasis on Skin Cancer Management and Prevention" Cancers 17, no. 21: 3518. https://doi.org/10.3390/cancers17213518
APA StyleWoźniak, B., Sobolewski, P., Sauer, N., Koper, M., & Calik, J. (2025). High-Intensity Focused Ultrasound in Dermatology: A Review with Emphasis on Skin Cancer Management and Prevention. Cancers, 17(21), 3518. https://doi.org/10.3390/cancers17213518







