Efficacy and Safety of Isatuximab Combination Therapy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Efficacy Outcomes
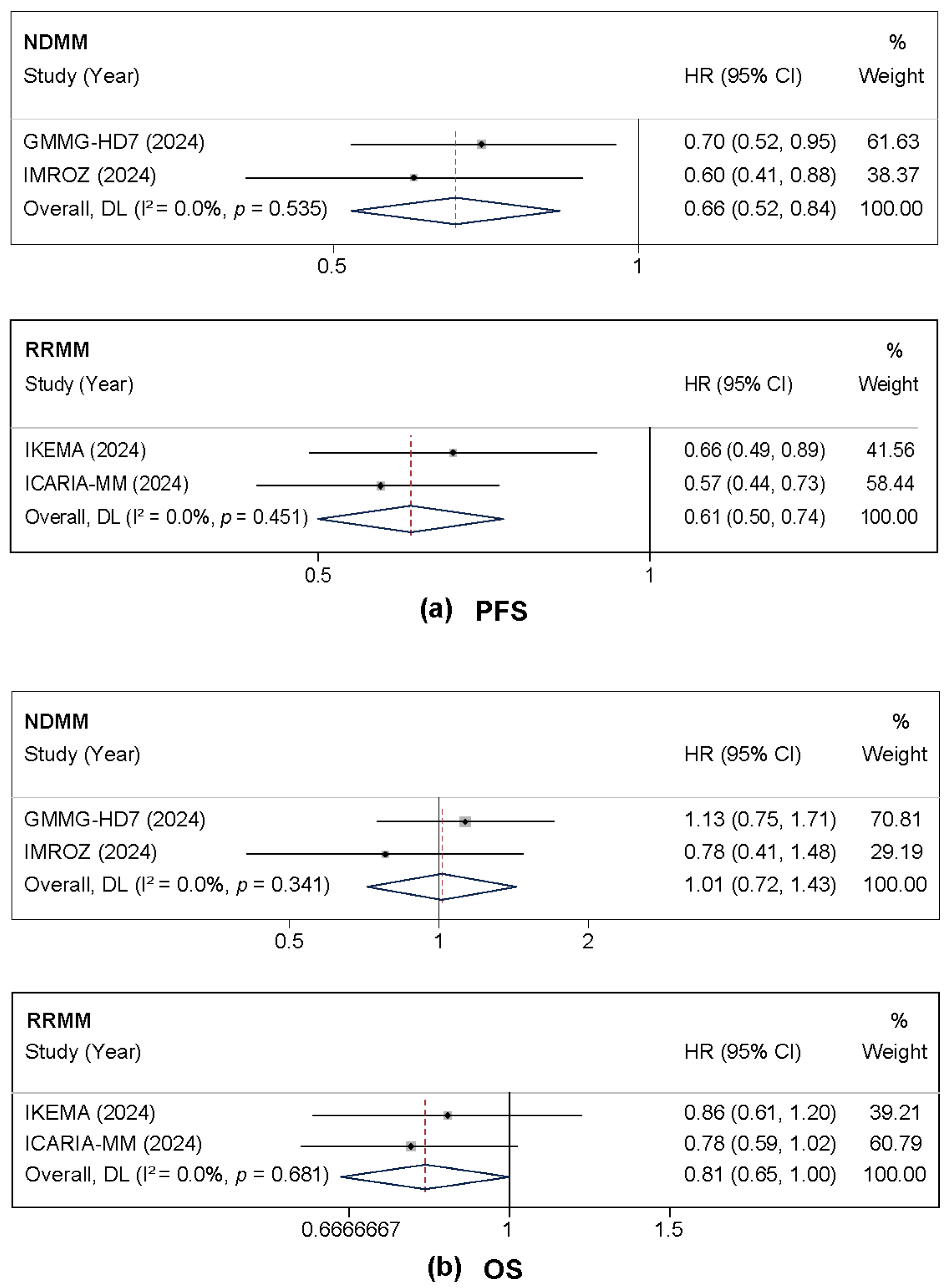
3.2.1. PFS and OS
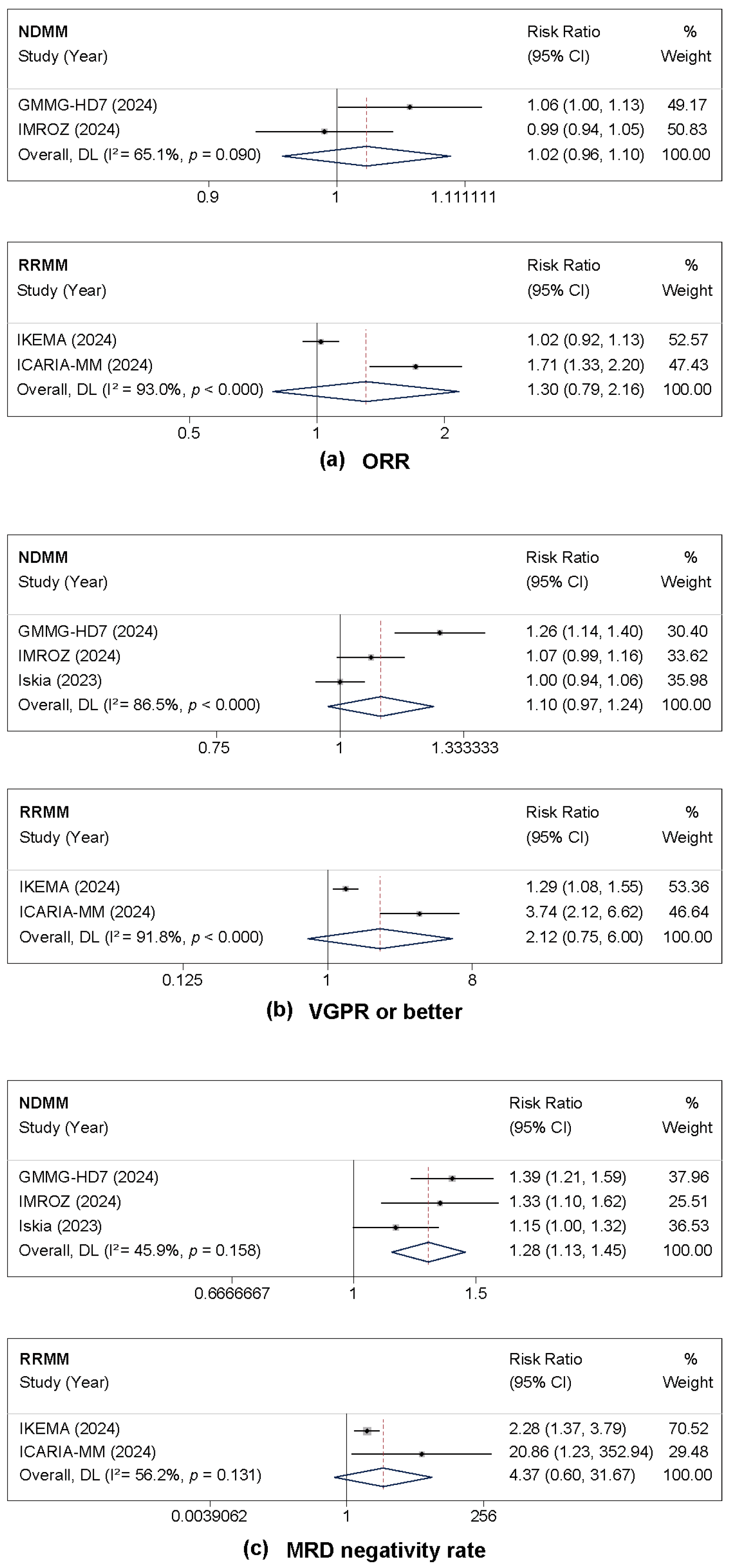
3.2.2. Treatment Response and MRD Negativity
3.3. Safety Outcomes
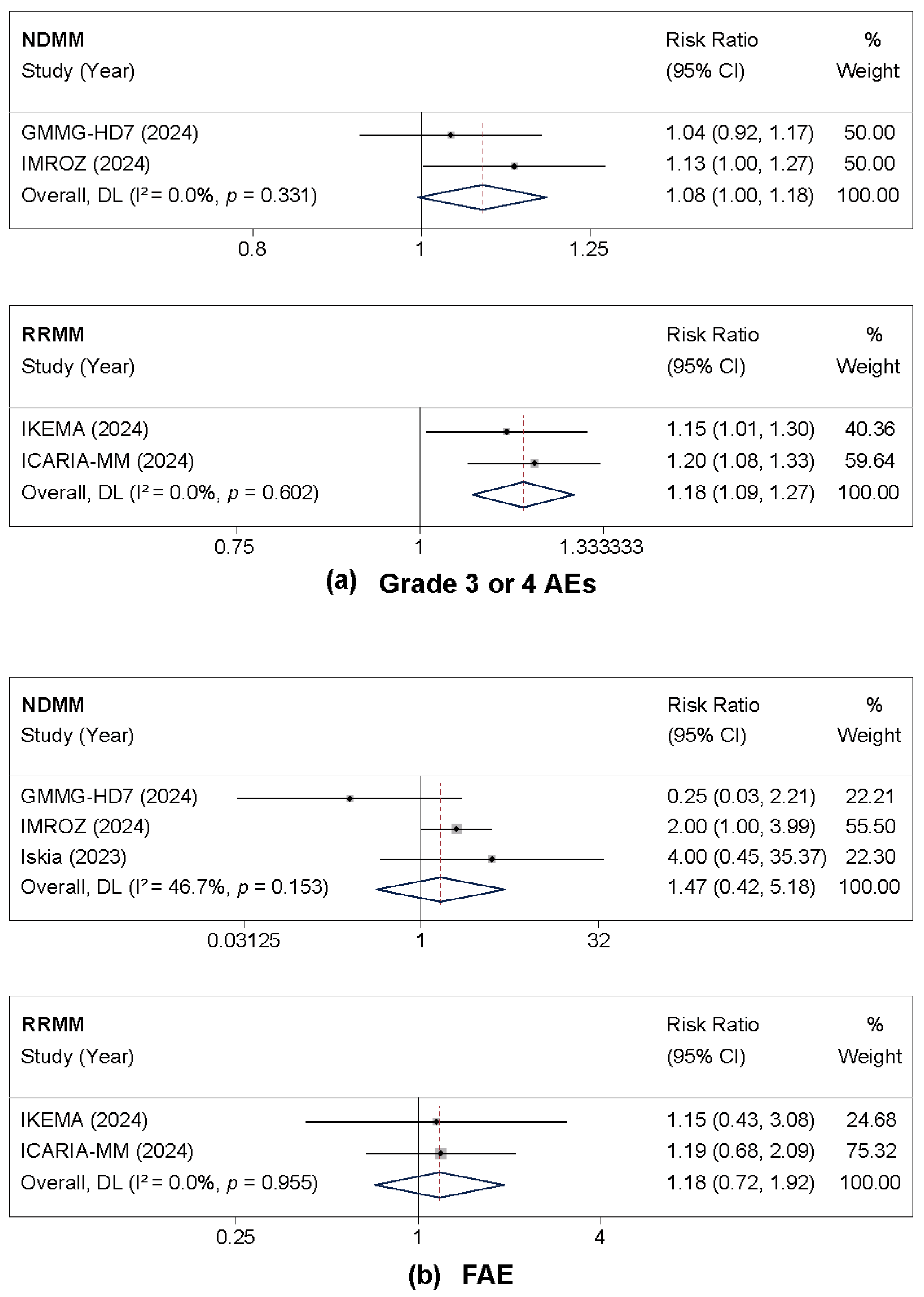
3.3.1. Grade 3 or 4 Adverse Events and Fatal Adverse Events
3.3.2. Hematologic Adverse Events
3.3.3. Non-Hematologic Adverse Events
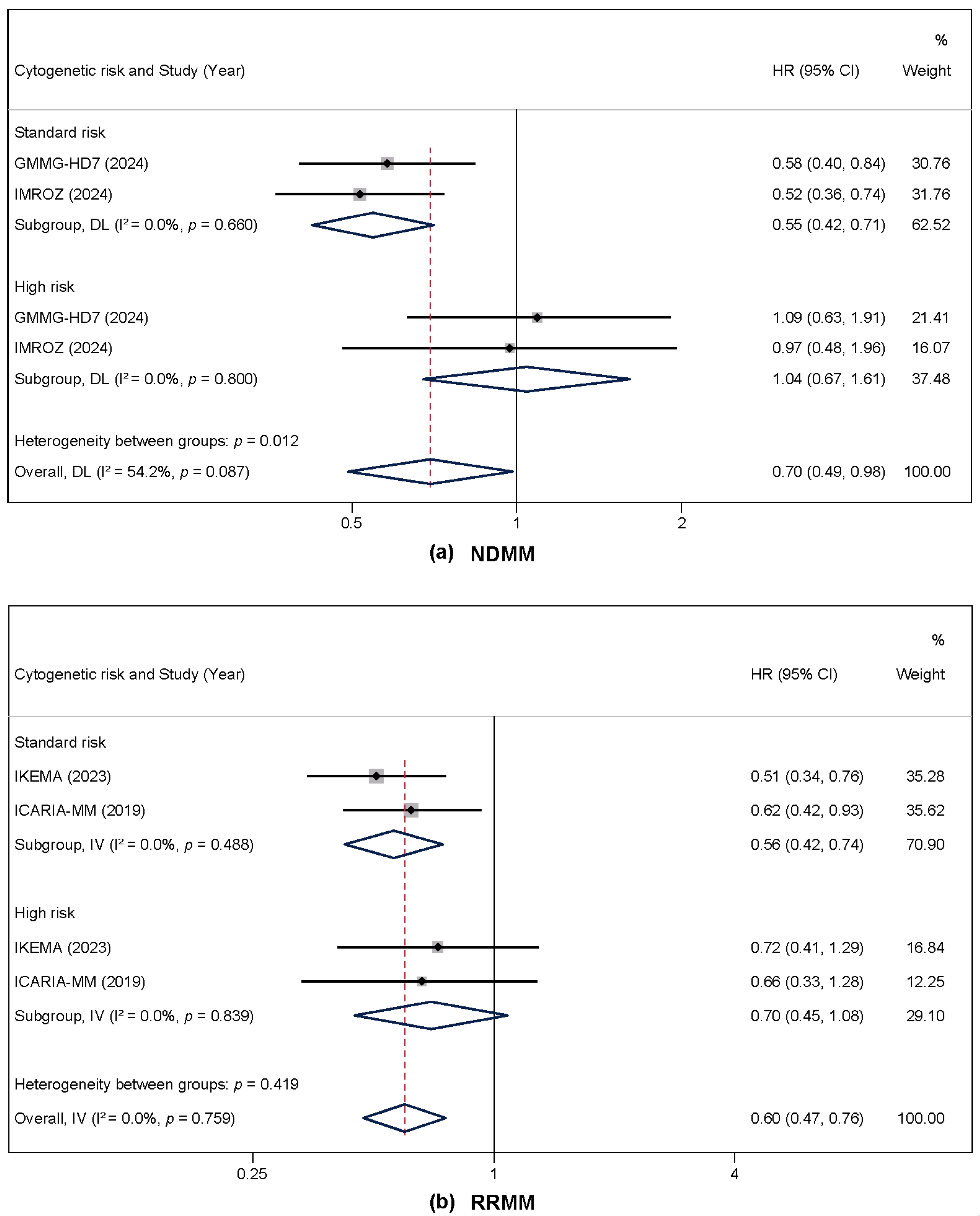
3.4. Subgroup Analysis by Cytogenetic Risk and Disease Status
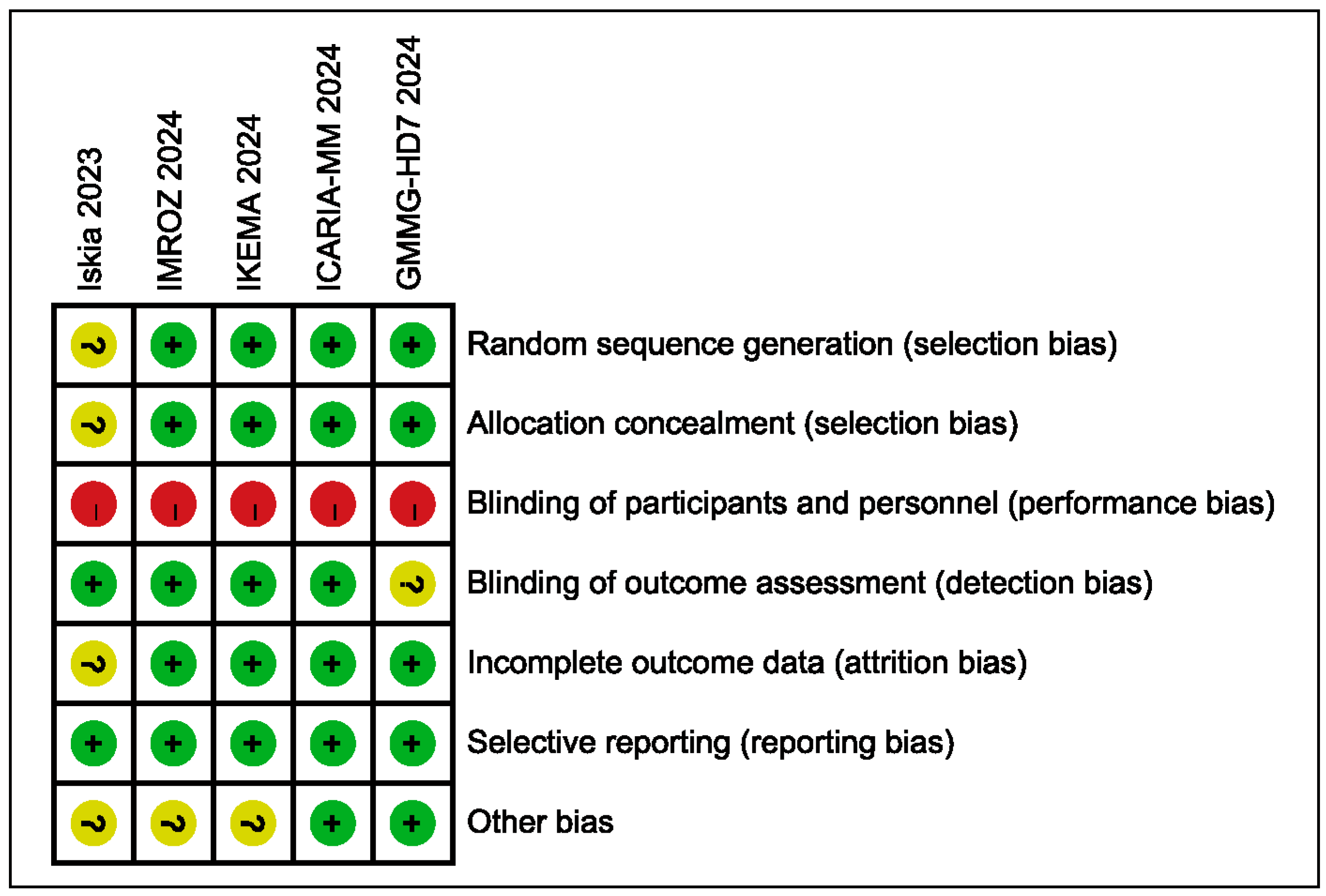
3.5. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef]
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple myeloma. Nat. Rev. Dis. Primers. 2024, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Pawlyn, C. IMiD resistance in multiple myeloma: Current understanding of the underpinning biology and clinical impact. Blood 2023, 142, 131–140. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Beksac, M.; Pour, L.; Delimpasi, S.; Vorobyev, V.; Quach, H.; Spicka, I.; Radocha, J.; Robak, P.; Kim, K.; et al. Belantamab Mafodotin, Pomalidomide, and Dexamethasone in Multiple Myeloma. N. Engl. J. Med. 2024, 391, 408–421. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, D.; Li, H.; Niu, T.; Tong, A. Multiple myeloma: Signaling pathways and targeted therapy. Mol. Biomed. 2024, 5, 32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lan, H.; Wu, J.; Xiao, Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front. Immunol. 2023, 14, 1101495. [Google Scholar] [CrossRef]
- Bisht, K.; Fukao, T.; Chiron, M.; Richardson, P.; Atanackovic, D.; Chini, E.; Chng, W.J.; Van De Velde, H.; Malavasi, F. Immunomodulatory properties of CD38 antibodies and their effect on anticancer efficacy in multiple myeloma. Cancer Med. 2023, 12, 20332–20352. [Google Scholar] [CrossRef]
- Filho, J.T.D.S.; Cantadori, L.O.; Crusoe, E.d.Q.; Hungria, V.; Maiolino, A. Daratumumab-based quadruplet versus triplet induction regimens in transplant-eligible newly diagnosed multiple myeloma: A systematic review and meta-analysis. Blood Cancer J. 2025, 15, 37. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef]
- Martin, T.G.; Corzo, K.; Chiron, M.; van de Velde, H.; Abbadessa, G.; Campana, F.; Solanki, M.; Meng, R.; Lee, H.; Wiederschain, D.; et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells. 2019, 8, 1522. [Google Scholar] [CrossRef]
- Moreno, L.; Perez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3176–3187. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Bringhen, S.; Anttila, P.; Capra, M.; Cavo, M.; Cole, C.; Gasparetto, C.; Hungria, V.; Jenner, M.; Vorobyev, V.; et al. Isatuximab as monotherapy and combined with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood 2021, 137, 1154–1165. [Google Scholar] [CrossRef]
- Frampton, J.E. Isatuximab: A Review of Its Use in Multiple Myeloma. Target. Oncol. 2021, 16, 675–686. [Google Scholar] [CrossRef]
- Rajan, A.M.; Kumar, S. New investigational drugs with single-agent activity in multiple myeloma. Blood Cancer J. 2016, 6, e451. [Google Scholar] [CrossRef]
- Richardson, P.G.; Perrot, A.; Miguel, J.S.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; Huang, S.-Y.; et al. Isatuximab-pomalidomide-dexamethasone versus pomalidomide-dexamethasone in patients with relapsed and refractory multiple myeloma: Final overall survival analysis. Haematologica. 2024, 109, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Spicka, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet. 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Yong, K.; Martin, T.; Dimopoulos, M.-A.; Mikhael, J.; Capra, M.; Facon, T.; Hajek, R.; Spicka, I.; Baker, R.; Kim, K.; et al. Isatuximab plus carfilzomib-dexamethasone versus carfilzomib-dexamethasone in patients with relapsed multiple myeloma (IKEMA): Overall survival analysis of a phase 3, randomised, controlled trial. Lancet Haematol. 2024, 11, e741–e750. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Mai, E.K.; Bertsch, U.; Fenk, R.; Nievergall, E.; Tichy, D.; Besemer, B.; Durig, J.; Schroers, R.; von Metzler, I.; et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): Part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol. 2022, 9, e810–e821. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.K.; Bertsch, U.; Pozek, E.; Fenk, R.; Besemer, B.; Hanoun, C.; Schroers, R.; von Metzler, I.; Haenel, M.; Mann, C.; et al. Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial. J. Clin. Oncol. 2025, 43, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.-A.; Leleu, X.P.; Beksac, M.; Pour, L.; Hajek, R.; Liu, Z.; Minarik, J.; Moreau, P.; Romejko-Jarosinska, J.; et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 391, 1597–1609. [Google Scholar] [CrossRef]
- Osama, M.; Khan, M.H.; Khan, S.; Hussain, A.; Tahir, A.; Ullah, M.; Afridi, A.; Ullah, U.; Rehman, W.U. Efficacy and safety of anti-CD38 monoclonal antibodies-based therapy versus standard therapy in newly diagnosed multiple myeloma patients: A systematic review and meta-analysis. Ther. Adv. Hematol. 2025, 16, 20406207251314289. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Nasser, M. Cochrane Handbook for Systematic Reviews of Interventions. Am. J. Public Health 2020, 110, 753–754. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011, 343, d4002. [Google Scholar] [CrossRef]
- Gay, F.; Roeloffzen, W.; Dimopoulos, M.A.; Rosiñol, L.; van der Klift, M.; Mina, R.; Oriol Rocafiguera, A.; Katodritou, E.; Wu, K.L.; Rodriguez Otero, P.; et al. Results of the Phase III Randomized Iskia Trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone vs. Carfilzomib-Lenalidomide-Dexamethasone As Pre-Transplant Induction and Post-Transplant Consolidation in Newly Diagnosed Multiple Myeloma Patients. Blood 2023, 142, 4. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Faini, A.C.; Morandi, F.; Ortolan, E.; Storti, P.; Giuliani, N.; Richardson, P.G.; Malavasi, F. Monoclonal anti-CD38 therapy in human myeloma: Retrospects and prospects. Front. Immunol. 2025, 16, 1519300. [Google Scholar] [CrossRef]
- Facon, T.; Moreau, P.; Weisel, K.; Goldschmidt, H.; Usmani, S.Z.; Chari, A.; Plesner, T.; Orlowski, R.Z.; Bahlis, N.; Basu, S.; et al. Daratumumab/lenalidomide/dexamethasone in transplant-ineligible newly diagnosed myeloma: MAIA long-term outcomes. Leukemia 2025, 39, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Suzuki, K.; Plesner, T.; et al. Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Nawaz, M.A.; Zuo, Y.; Zeng, P. Next-generation immunotherapy in relapsed/refractory multiple myeloma: Strategies to achieve sustained MRD negativity. Crit. Rev. Oncol. Hematol. 2025, 214, 104913. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; Garcia-Sanchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.J.; Vidriales, M.B.; García-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Richardson, P.G.; O’Donnell, E.K.; O’Gorman, P.; Leypoldt, L.B.; Laubach, J.; Gay, F.; Leleu, X.; Facon, T.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab for the treatment of multiple myeloma: Current clinical advances and future directions. Expert Opin. Investig. Drugs 2025, 34, 571–589. [Google Scholar] [CrossRef]
- Lin, Z.; Dong, R.; Zhang, W.; Liu, R.; Fu, B.; He, A. Efficacy and safety of anti-CD38 monoclonal antibodies in patients with newly diagnosed multiple myeloma: An updated systematic review and meta-analysis based on randomized controlled trials. Leuk. Lymphoma 2025, 66, 1839–1849. [Google Scholar] [CrossRef]
- Karimbanathottathil, M.F.; Yoosuf, B.T.; Mamatha, M.; Bansal, D. Comprehensive safety evaluation of isatuximab in multiple myeloma using disproportionality analysis of FAERS and meta-analysis of randomized controlled trials. Sci. Rep. 2024, 14, 31859. [Google Scholar] [CrossRef]
- Quach, H.; Parmar, G.; Mateos, M.V.; Ailawadhi, S.; Leleu, X. Recent Developments in Convenience of Administration of the Anti-CD38 Antibody Isatuximab: Subcutaneous Delivery and Fast Intravenous Infusion in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2024, 24, 358–363. [Google Scholar] [CrossRef]
- Bonello, F.; Grasso, M.; D’Agostino, M.; Celeghini, I.; Castellino, A.; Boccadoro, M.; Bringhen, S. The Role of Monoclonal Antibodies in the First-Line Treatment of Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma. Pharmaceuticals 2020, 14, 20. [Google Scholar] [CrossRef]
- Chen, W.; You, J.; Lin, L.; Yan, X.; An, G.; Wang, Y.; Tian, W.; Ding, K.; Zhang, X.; Chen, W.; et al. Real-world outcomes of isatuximab with pomalidomide and dexamethasone for relapsed and/or refractory multiple myeloma. Chin. Med. J. (Engl.), 29 May 2025; Accepted Manuscript. [Google Scholar] [CrossRef] [PubMed]

| Study | Year & | Phase | Population | Patients | Median Age (Year) | Intervention | Follow-Up # (Months) | Primary End-Point | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | ||||||
| GMMG-HD7 [19,20] | 2024 | III | NDMM | 331 | 329 | 59 (54–64) | 60 (54–65) | Isa-RVd | RVd | 48 | MRD negative rate |
| IMROZ [21] | 2024 | III | NDMM | 265 | 181 | 72 (60–80) | 72 (55–80) | Isa-VRd | VRd | 59.7 | PFS |
| IKEMA [18] | 2024 | III | RRMM | 179 | 123 | 65 (55–77) | 63 (57–70) | Isa-Kd | Kd | 56.6 | PFS |
| ICARIA-MM [16] | 2024 | III | RRMM | 154 | 153 | 68 (60–74) | 66 (59–71) | Isa-Pd | Pd | 52.4 | PFS |
| Iskia * [29] | 2023 | III | NDMM | 151 | 151 | 61 | 60 | Isa-KRd | KRd | 20 | MRD negative rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xu, Z.; Jiang, M.; Chen, Y.; Lan, Y. Efficacy and Safety of Isatuximab Combination Therapy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2025, 17, 3494. https://doi.org/10.3390/cancers17213494
Wang C, Xu Z, Jiang M, Chen Y, Lan Y. Efficacy and Safety of Isatuximab Combination Therapy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. Cancers. 2025; 17(21):3494. https://doi.org/10.3390/cancers17213494
Chicago/Turabian StyleWang, Chi, Zhengyang Xu, Meilin Jiang, Yuzhe Chen, and Yu Lan. 2025. "Efficacy and Safety of Isatuximab Combination Therapy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials" Cancers 17, no. 21: 3494. https://doi.org/10.3390/cancers17213494
APA StyleWang, C., Xu, Z., Jiang, M., Chen, Y., & Lan, Y. (2025). Efficacy and Safety of Isatuximab Combination Therapy in Multiple Myeloma: A Meta-Analysis of Randomized Controlled Trials. Cancers, 17(21), 3494. https://doi.org/10.3390/cancers17213494






