Comparative Outcomes of Brachyury Vaccine vs. Imatinib in Advanced Chordoma: A Mayo Clinic Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
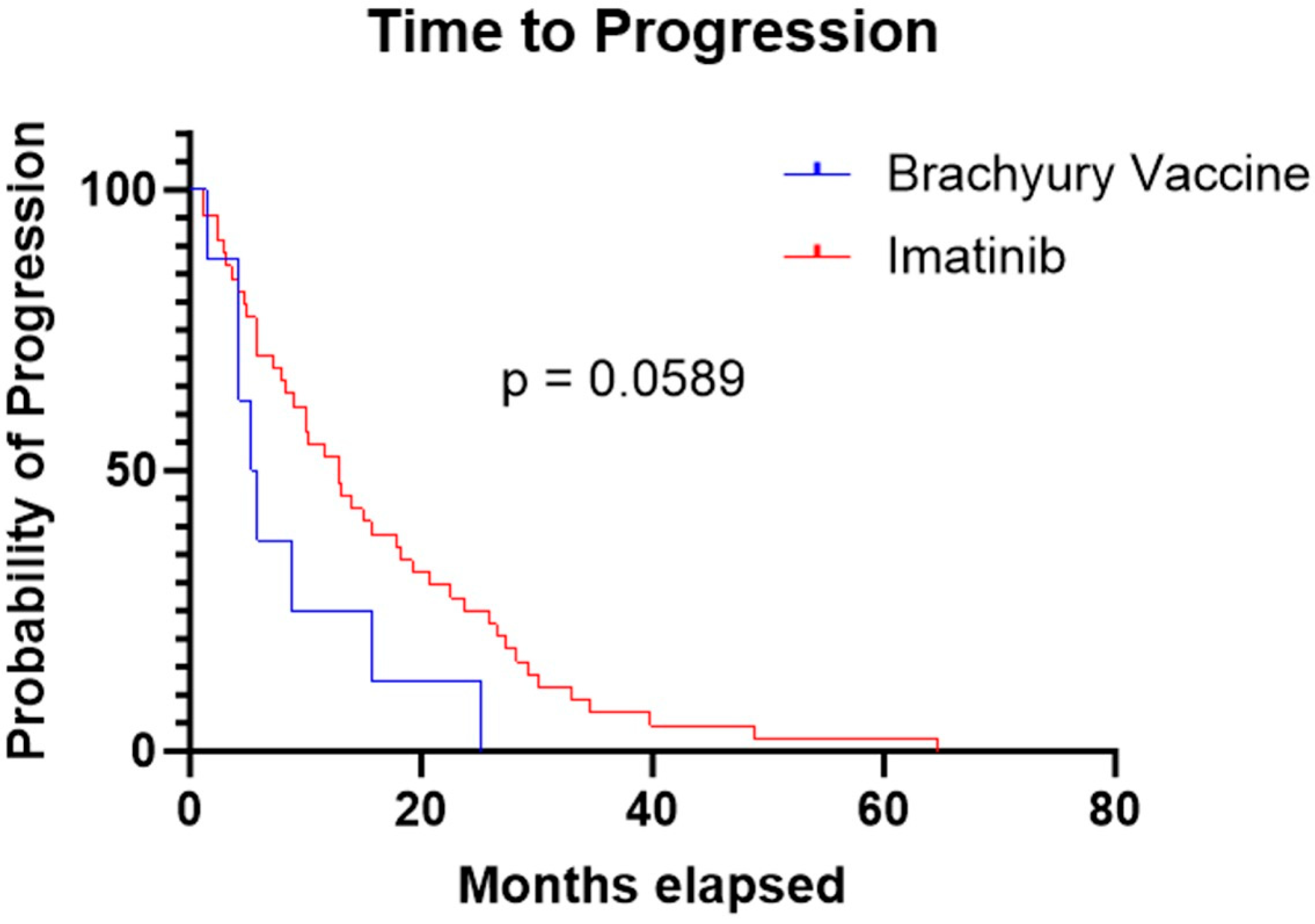
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECOG | Eastern Cooperative Oncology Group |
| TTP | Time to progression |
| OS | Overall survival |
| SOC | Standard of care |
| TKI | Tyrosine kinase inhibitor |
| MVA-BN | modified vaccinia Ankara-Bavarian Nordic |
| FPV | fowlpox Virus |
| RT | Radiation therapy |
| PFS | Progression free survival |
| BMI | Body mass index |
| PDGFB | platelet-derived growth factor β |
References
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Ghaith, A.K.; Akinduro, O.O.; Alexander, A.Y.; Goyal, A.; Bon-Nieves, A.; de Macedo Filho, L.; Otamendi-Lopez, A.; Nathani, K.R.; Abode-Iyamah, K.; Jentoft, M.E.; et al. Immunohistochemical markers predicting long-term recurrence following clival and spinal chordoma resection: A multicenter study. Neurosurg. Focus 2023, 54, E15. [Google Scholar] [CrossRef]
- McMaster, M.L.; Goldstein, A.M.; Bromley, C.M.; Ishibe, N.; Parry, D.M. Chordoma: Incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 2001, 12, 1–11. [Google Scholar] [CrossRef]
- Perez-Vega, C.; Akinduro, O.O.; Ruiz-Garcia, H.J.; Ghaith, A.K.A.; Almeida, J.P.; Jentoft, M.E.; Mahajan, A.; Janus, J.R.; Bendok, B.R.; Choby, G.W.; et al. Extent of Surgical Resection as a Predictor of Tumor Progression in Skull Base Chordomas: A Multicenter Volumetric Analysis. World Neurosurg. 2024, 181, e620–e627. [Google Scholar] [CrossRef]
- Casali, P.G.; Stacchiotti, S.; Sangalli, C.; Olmi, P.; Gronchi, A. Chordoma. Curr. Opin. Oncol. 2007, 19, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Akinduro, O.O.; Garcia, D.P.; Domingo, R.A.; Vivas-Buitrago, T.; Sousa-Pinto, B.; Bydon, M.; Clarke, M.J.; Gokaslan, Z.L.; Kalani, M.A.; Abode-Iyamah, K.; et al. Cervical chordomas: Multicenter case series and meta-analysis. J. Neurooncol. 2021, 153, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.A.; Aston, W.J.; Briggs, T.W.; Cannon, S.R.; Saifuddin, A. Sacral chordoma: Can local recurrence after sacrectomy be predicted? Clin. Orthop. Relat. Res. 2008, 466, 2217–2223. [Google Scholar] [CrossRef]
- Park, H.; Choi, Y.; Lee, S.; Lee, S.H.; Kim, E.S.; Jang, S.W.; Park, J.H.; Cho, Y.; Jang, G.; Ha, Y.; et al. The Clinical Outcomes of Cervical Spine Chordoma: A Nationwide Multicenter Retrospective Study. Neurospine 2024, 21, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Gokaslan, Z.L.; Zadnik, P.L.; Sciubba, D.M.; Germscheid, N.; Goodwin, C.R.; Wolinsky, J.P.; Bettegowda, C.; Groves, M.L.; Luzzati, A.; Rhines, L.D.; et al. Mobile spine chordoma: Results of 166 patients from the AOSpine Knowledge Forum Tumor database. J. Neurosurg. Spine 2016, 24, 644–651. [Google Scholar] [CrossRef]
- Kerekes, D.; Goodwin, C.R.; Ahmed, A.K.; Verlaan, J.J.; Bettegowda, C.; Abu-Bonsrah, N.; Sciubba, D.M. Local and Distant Recurrence in Resected Sacral Chordomas: A Systematic Review and Pooled Cohort Analysis. Glob. Spine J. 2019, 9, 191–201. [Google Scholar] [CrossRef]
- Ghaith, A.K.; Nguyen, R.; El-Hajj, V.G.; Montaser, A.; De Biase, G.; Ravindran, K.; Perez-Vega, C.; Lee, S.J.; Dominari, A.; Battistin, U.; et al. Proton versus photon adjuvant radiotherapy: A multicenter comparative evaluation of recurrence following spinal chordoma resection. Neurosurg. Focus 2024, 56, E9. [Google Scholar] [CrossRef]
- Dewaele, B.; Maggiani, F.; Floris, G.; Ampe, M.; Vanspauwen, V.; Wozniak, A.; Debiec-Rychter, M.; Sciot, R. Frequent activation of EGFR in advanced chordomas. Clin. Sarcoma Res. 2011, 1, 4. [Google Scholar] [CrossRef]
- Tamborini, E.; Virdis, E.; Negri, T.; Orsenigo, M.; Brich, S.; Conca, E.; Gronchi, A.; Stacchiotti, S.; Manenti, G.; Casali, P.G.; et al. Analysis of receptor tyrosine kinases (RTKs) and downstream pathways in chordomas. Neuro Oncol. 2010, 12, 776–789. [Google Scholar] [CrossRef]
- Druker, B.J.; Sawyers, C.L.; Kantarjian, H.; Resta, D.J.; Reese, S.F.; Ford, J.M.; Capdeville, R.; Talpaz, M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001, 344, 1038–1042. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Longhi, A.; Ferraresi, V.; Grignani, G.; Comandone, A.; Stupp, R.; Bertuzzi, A.; Tamborini, E.; Pilotti, S.; Messina, A.; et al. Phase II study of imatinib in advanced chordoma. J. Clin. Oncol. 2012, 30, 914–920. [Google Scholar] [CrossRef]
- Yang, X.R.; Ng, D.; Alcorta, D.A.; Liebsch, N.J.; Sheridan, E.; Li, S.; Goldstein, A.M.; Parry, D.M.; Kelley, M.J. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat. Genet. 2009, 41, 1176–1178. [Google Scholar] [CrossRef]
- Vujovic, S.; Henderson, S.; Presneau, N.; Odell, E.; Jacques, T.S.; Tirabosco, R.; Boshoff, C.; Flanagan, A.M. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J. Pathol. 2006, 209, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.R.; Guiliano, D.; Presneau, N.; McLean, S.; Frow, R.; Vujovic, S.; Anderson, J.; Sebire, N.; Whelan, J.; Athanasou, N.; et al. A molecular map of mesenchymal tumors. Genome Biol. 2005, 6, R76. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.M.; Conley, A.P.; Attia, S.; Van Tine, B.A.; Seetharam, M.; Chen, Y.L.; Gafoor, Z.; Heery, C.; Pico-Navarro, C.; Adams, T. A phase 2 study of a brachyury-targeting vaccine in combination with radiation therapy for the treatment of advanced chordoma. Cancer 2024, 130, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Trendowski, M.R.; El Charif, O.; Dinh, P.C., Jr.; Travis, L.B.; Dolan, M.E. Genetic and Modifiable Risk Factors Contributing to Cisplatin-induced Toxicities. Clin. Cancer Res. 2019, 25, 1147–1155. [Google Scholar] [CrossRef]
- Joensuu, H.; Dimitrijevic, S. Tyrosine kinase inhibitor imatinib (STI571) as an anticancer agent for solid tumours. Ann. Med. 2001, 33, 451–455. [Google Scholar] [CrossRef]
- Tamborini, E.; Miselli, F.; Negri, T.; Lagonigro, M.S.; Staurengo, S.; Dagrada, G.P.; Stacchiotti, S.; Pastore, E.; Gronchi, A.; Perrone, F.; et al. Molecular and biochemical analyses of platelet-derived growth factor receptor (PDGFR) B, PDGFRA, and KIT receptors in chordomas. Clin. Cancer Res. 2006, 12, 6920–6928. [Google Scholar] [CrossRef]
- Akhavan-Sigari, R.; Gaab, M.R.; Rohde, V.; Abili, M.; Ostertag, H. Expression of PDGFR-alpha, EGFR and c-MET in spinal chordoma: A series of 52 patients. Anticancer Res. 2014, 34, 623–630. [Google Scholar]
- Hindi, N.; Casali, P.G.; Morosi, C.; Messina, A.; Palassini, E.; Pilotti, S.; Tamborini, E.; Radaelli, S.; Gronchi, A.; Stacchiotti, S. Imatinib in advanced chordoma: A retrospective case series analysis. Eur. J. Cancer 2015, 51, 2609–2614. [Google Scholar] [CrossRef]
- Battistin, U.; Nguyen, R.; Ghaith, A.K.; El-Hajj, V.G.; Soltan, F.; Ghaith, S.; Weinberg, J.H.; Elmi-Terander, A.; Grossbach, A.J.; Akinduro, O.O. The impact of socioeconomic determinants on the access to care and survival in patients with spinal chordomas—A national cancer database analysis. J. Neurooncol. 2024, 169, 359–368. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Suarez-Meade, P.; Garcia, D.; Brown, D.A.; Sarabia-Estrada, R.; Attia, S.; Gokaslan, Z.L.; Quinones-Hinojosa, A. Targeted Therapy for Chordoma: Key Molecular Signaling Pathways and the Role of Multimodal Therapy. Target. Oncol. 2021, 16, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, N.A.; Rekhi, B.; Thorat, K.; Dikshit, R.; Agrawal, M.; Puri, A. Revisiting chordoma with brachyury, a “new age” marker: Analysis of a validation study on 51 cases. Arch. Pathol. Lab. Med. 2010, 134, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Oakley, G.J.; Fuhrer, K.; Seethala, R.R. Brachyury, SOX-9, and podoplanin, new markers in the skull base chordoma vs chondrosarcoma differential: A tissue microarray-based comparative analysis. Mod. Pathol. 2008, 21, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Heery, C.R.; Palena, C.; McMahon, S.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Dirmeier, U.; Cordes, L.; Marte, J.; Dahut, W.; et al. Phase I Study of a Poxviral TRICOM-Based Vaccine Directed Against the Transcription Factor Brachyury. Clin. Cancer Res. 2017, 23, 6833–6845. [Google Scholar] [CrossRef]
- Jin, M.C.; Connolly, I.D.; Ravi, K.; Tobert, D.G.; MacDonald, S.M.; Shin, J.H. Unraveling molecular advancements in chordoma tumorigenesis and treatment response: A review of scientific discoveries and clinical implications. Neurosurg. Focus 2024, 56, E18. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Q.; Wang, B.; Ji, T.; Guo, W.; Ren, T.; Tang, X. The role of tumor immune microenvironment in chordoma: Promising immunotherapy strategies. Front. Immunol. 2023, 14, 1257254. [Google Scholar] [CrossRef]
- Xia, C.; Huang, W.; Chen, Y.L.; Fu, H.B.; Tang, M.; Zhang, T.L.; Li, J.; Lv, G.H.; Yan, Y.G.; Ouyang, Z.H.; et al. Coexpression of HHLA2 and PD-L1 on Tumor Cells Independently Predicts the Survival of Spinal Chordoma Patients. Front. Immunol. 2021, 12, 797407. [Google Scholar] [CrossRef]
- Bishop, A.J.; Amini, B.; Lin, H.; Raza, S.M.; Patel, S.; Grosshans, D.R.; Ghia, A.; Farooqi, A.; Guadagnolo, B.A.; Mitra, D.; et al. Immune Checkpoint Inhibitors Have Clinical Activity in Patients With Recurrent Chordoma. J. Immunother. 2022, 45, 374–378. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Q.; Lou, J.; Wang, B.; Wang, W.; Niu, J.; Guo, L.; Chen, C.; Yu, Y.; Huang, Y.; et al. Chordoma recruits and polarizes tumor-associated macrophages via secreting CCL5 to promote malignant progression. J. Immunother. Cancer 2023, 11, e006808. [Google Scholar] [CrossRef]
- Eissa, L.; Bastawi, R.A. Skull base “intrinsic” bony mass lesions: Conventional, diffusion and perfusion imaging with a proposed imaging approach. Egypt. J. Radiol. Nucl. Med. 2024, 55, 188. [Google Scholar] [CrossRef]
- Seeling, C.; Lechel, A.; Svinarenko, M.; Moller, P.; Barth, T.F.E.; Mellert, K. Molecular features and vulnerabilities of recurrent chordomas. J. Exp. Clin. Cancer Res. 2021, 40, 244. [Google Scholar] [CrossRef] [PubMed]
- Bangash, A.H.; Ryvlin, J.; Chakravarthy, V.; Akinduro, O.O.; Zadnik Sullivan, P.L.; Niu, T.; Galgano, M.A.; Shin, J.H.; Gokaslan, Z.L.; Fourman, M.S.; et al. Unmasking Racial, Ethnic, and Socioeconomic Disparities in United States Chordoma Clinical Trials: Systematic Review. Cancers 2025, 17, 225. [Google Scholar] [CrossRef] [PubMed]
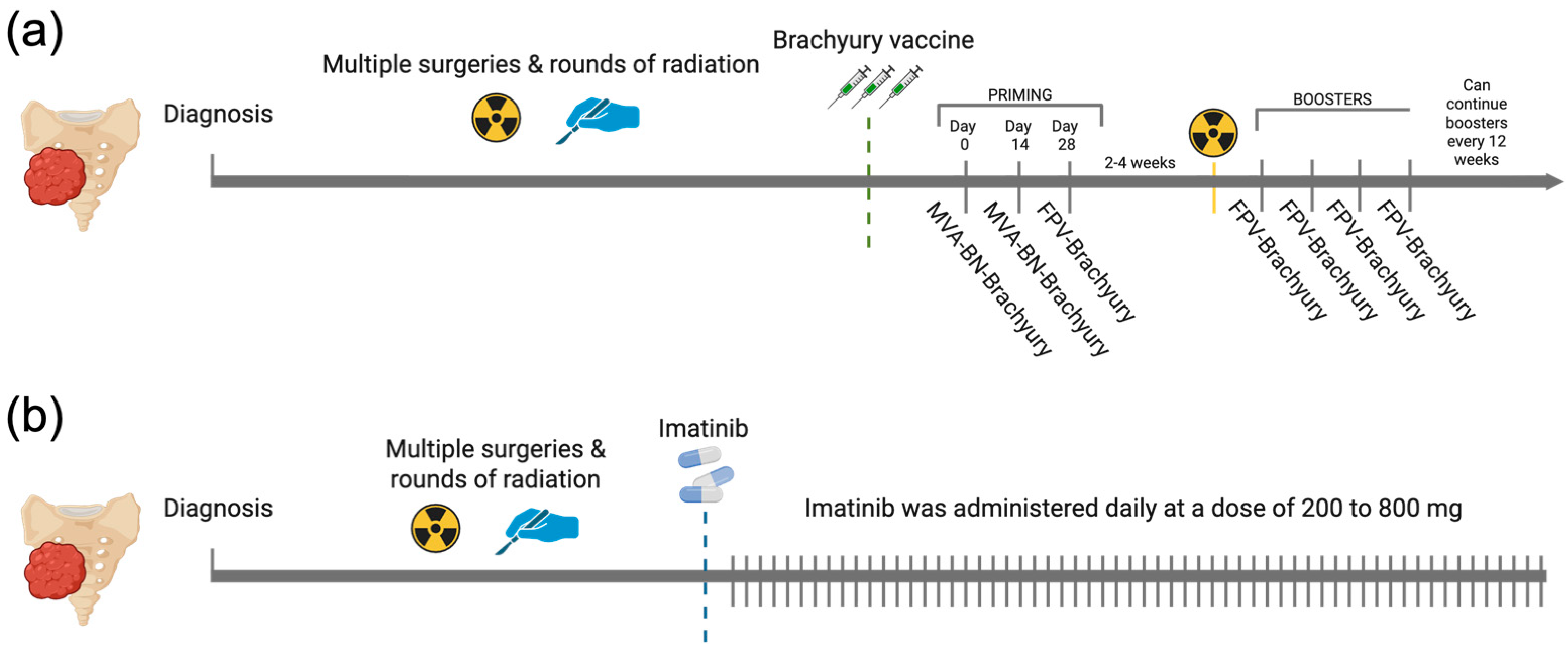

| Variable | Brachyury Vaccine (N = 8) | Imatinib (N = 44) | p Value |
|---|---|---|---|
| Age (IQR) | 68.5 (26) | 62.5 (66) | 0.449 |
| Sex | 0.701 | ||
| Male | 6 (75%) | 30 (68.2%) | |
| Female | 2 (25%) | 14 (31.8%) | |
| Race/Ethnicity | 0.739 | ||
| Hispanic | 0 (0%) | 4 (9.1%) | |
| White | 8 (100%) | 35 (79.5%) | |
| Asian | 0 (0%) | 2 (4.5%) | |
| Middle Eastern | 0 (0%) | 2 (4.5%) | |
| Unknown | 0 (0%) | 1 (2.3%) | |
| Body Mass Index kg/m2 (IQR) | 29.1 (12.6) | 25.8 (25.5) | 0.635 |
| Essential Hypertension | 3 (37.5%) | 23 (52.3%) | 0.442 |
| Coronary Artery Disease | 2 (25%) | 4 (9.1%) | 0.195 |
| Congestive Heart Failure | 0 (0%) | 5 (11.4%) | 0.316 |
| Atrial Fibrillation | 0 (0%) | 5 (11.4%) | 0.316 |
| Stroke | 0 (0%) | 5 (11.4%) | 0.316 |
| Diabetes Mellitus | 1 (12.5%) | 5 (11.4%) | 0.926 |
| Obesity | 4 (50%) | 12 (27.3%) | 0.200 |
| Anticoagulation | 4 (50%) | 20 (45.5%) | 0.813 |
| Location | 0.564 | ||
| Skull Base | 0 (0%) | 9 (20.5%) | |
| Cervical | 2 (25%) | 7 (15.9%) | |
| Thoracic | 0 (0%) | 3 (6.8%) | |
| Lumbar | 1 (12.5%) | 6 (13.6%) | |
| Sacrococcygeal | 5 (62.5%) | 20 (45.5%) | |
| Disease Severity | |||
| Pain | 7 (87.5%) | 33 (75%) | 0.440 |
| Motor Deficit | 3 (37.5%) | 18 (40.9%) | 0.857 |
| Sensory Deficit | 4 (50%) | 17 (38.6%) | 0.547 |
| Urinary Incontinence | 2 (25%) | 12 (27.3%) | 0.894 |
| Bowel Incontinence | 1 (12.5%) | 9 (20.5%) | 0.599 |
| ECOG | 0.796 | ||
| 0 | 2 (25%) | 11 (35.5%) | |
| 1 | 6 (75%) | 18 (58.1%) | |
| 2 | 0 (0%) | 1 (3.2%) | |
| 3 | 0 (0%) | 1 (3.2%) | |
| 4 | 0 (0%) | 0 (0%) | |
| Metastatic disease | 0.042 | ||
| Yes | 8 (100%) | 27 (61.4%) | |
| No | 0 (0%) | 17 (38.6%) | |
| Metastasis location | 0.419 | ||
| Local 1 | 2 (25%) | 13 (48.2%) | |
| Distant 2 | 6 (75%) | 14 (51.8%) |
| Variable | Brachyury Vaccine (N = 8) | Imatinib (N = 44) | p Value |
|---|---|---|---|
| Surgical Resection | 0.999 | ||
| Biopsy only | 1 (12.5%) | 7 (15.9%) | |
| One | 2 (25%) | 22 (50%) | |
| Two | 3 (37.5%) | 10 (22.7%) | |
| Three | 2 (25%) | 5 (11.4%) | |
| Radiation (photons) | 0.249 | ||
| No | 2 (25%) | 24 (54.5%) | |
| One round | 3 (37.5%) | 14 (31.8%) | |
| Two rounds | 3 (37.5%) | 4 (9.1%) | |
| Three rounds | 0 | 2 (4.5) | |
| Radiation (protons) | 0.443 | ||
| No | 4 (50%) | 29 (65.9%) | |
| One round | 3 (37.5%) | 15 (34.1%) | |
| Two rounds | 1 (12.5%) | 0 (0%) | |
| Cryoablation | 0.401 | ||
| Yes | 1 (12.5%) | 2 (4.6%) | |
| No | 7 (87.5%) | 42 (95.5%) | |
| Other targeted therapies 1 | 0.130 | ||
| Yes | 3 (37.5%) | 6 (13.6%) | |
| No | 5 (62.5%) | 38 (86.4%) |
| Variable | Brachyury Vaccine (N = 8) | Imatinib (N = 44) | p Value |
|---|---|---|---|
| Adverse Events | 6 (75%) | 14 (31.8%) | 0.021 |
| Chills | 4 | 0 | |
| Fatigue | 3 | 2 | |
| Fever | 1 | 0 | |
| Headache | 1 | 1 | |
| Rash | 1 | 1 | |
| Pruritus | 1 | 1 | |
| Nausea | 0 | 2 | |
| Diarrhea | 0 | 2 | |
| Hepatotoxicity 1 | 0 | 2 | |
| Muscle Cramping | 0 | 2 | |
| Febrile neutropenia 2 | 0 | 1 | |
| Neck fasciculations | 0 | 1 | |
| Anasarca | 0 | 1 | |
| Lower Extremity Edema | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Garcia de Llano, J.P.; Iyer, H.G.; Hoffman, H.C.; Seetharam, M.; Attia, S.; Akinduro, O.O. Comparative Outcomes of Brachyury Vaccine vs. Imatinib in Advanced Chordoma: A Mayo Clinic Experience. Cancers 2025, 17, 3493. https://doi.org/10.3390/cancers17213493
Navarro-Garcia de Llano JP, Iyer HG, Hoffman HC, Seetharam M, Attia S, Akinduro OO. Comparative Outcomes of Brachyury Vaccine vs. Imatinib in Advanced Chordoma: A Mayo Clinic Experience. Cancers. 2025; 17(21):3493. https://doi.org/10.3390/cancers17213493
Chicago/Turabian StyleNavarro-Garcia de Llano, Juan P., Harshvardhan G. Iyer, Harry C. Hoffman, Mahesh Seetharam, Steven Attia, and Oluwaseun O. Akinduro. 2025. "Comparative Outcomes of Brachyury Vaccine vs. Imatinib in Advanced Chordoma: A Mayo Clinic Experience" Cancers 17, no. 21: 3493. https://doi.org/10.3390/cancers17213493
APA StyleNavarro-Garcia de Llano, J. P., Iyer, H. G., Hoffman, H. C., Seetharam, M., Attia, S., & Akinduro, O. O. (2025). Comparative Outcomes of Brachyury Vaccine vs. Imatinib in Advanced Chordoma: A Mayo Clinic Experience. Cancers, 17(21), 3493. https://doi.org/10.3390/cancers17213493






