Serum Albumin Level as a Predictor of Failure to Rescue in Patients Undergoing Surgery for Spinal Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
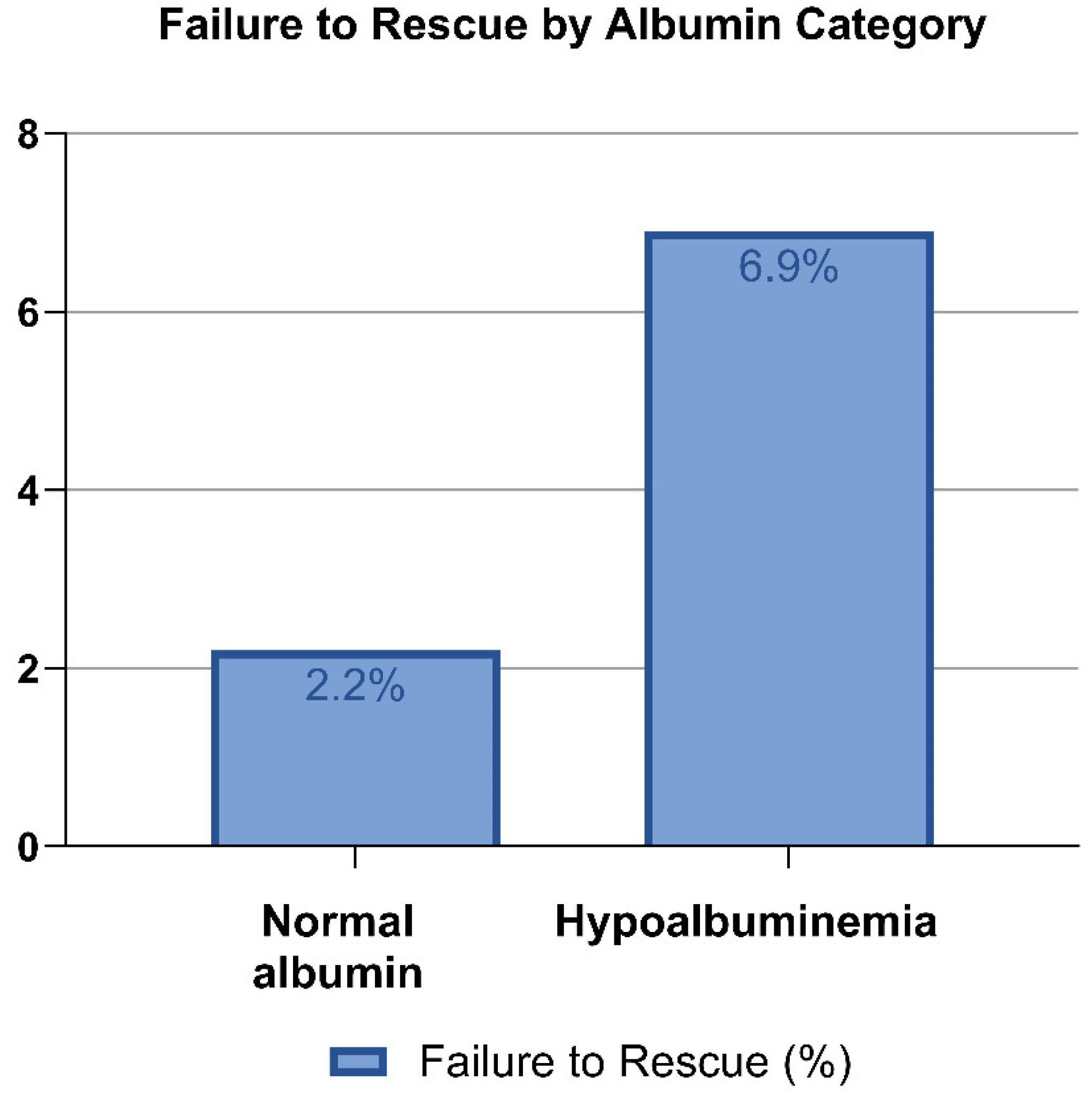
3. Results
3.1. Albumin Level as Continuous Variable
3.2. Albumin Level as Categorical Variable
3.3. Selection Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS-NSQIP | American College of Surgeons National Surgical Quality Improvement Program |
| FTR | Failure to Rescue |
| BMI | Body Mass Index |
| ASA | American Society of Anesthesiologists |
| mFI-5 | modified Frailty Index-5 |
References
- Schoenfeld, A.J.; Le, H.V.; Marjoua, Y.; Leonard, D.A.; Belmont, P.J.; Bono, C.M.; Harris, M.B. Assessing the Utility of a Clinical Prediction Score Regarding 30-Day Morbidity and Mortality Following Metastatic Spinal Surgery: The New England Spinal Metastasis Score (NESMS). Spine J. 2016, 16, 482–490. [Google Scholar] [CrossRef]
- Ando, K.; Kobayashi, K.; Machino, M.; Ota, K.; Morozumi, M.; Tanaka, S.; Imai, R.; Nishida, Y.; Ishiguro, N.; Imagama, S. Fusion Surgery with Instrumentation Following Carbon Ion Radiotherapy for Primary Lumbar Tumors: A Case Series. J. Clin. Neurosci. 2019, 62, 264–268. [Google Scholar] [CrossRef]
- Tarawneh, A.M.; Pasku, D.; Quraishi, N.A. Surgical Complications and Re-Operation Rates in Spinal Metastases Surgery: A Systematic Review. Eur. Spine J. 2021, 30, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Boaro, A.; Wells, M.; Chi, J.; Lu, Y.; Smith, T.R.; Groff, M.W.; Zaidi, H. A National Surgical Quality Improvement Program Analysis of Postoperative Major and Minor Complications in Patients with Spinal Metastatic Disease. World Neurosurg. 2020, 140, e203–e211. [Google Scholar] [CrossRef] [PubMed]
- Paulino Pereira, N.R.; Ogink, P.T.; Groot, O.Q.; Ferrone, M.L.; Hornicek, F.J.; van Dijk, C.N.; Bramer, J.a.M.; Schwab, J.H. Complications and Reoperations after Surgery for 647 Patients with Spine Metastatic Disease. Spine J. 2019, 19, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Rumalla, K.C.; Covell, M.M.; Skandalakis, G.P.; Rumalla, K.; Kassicieh, A.J.; Roy, J.M.; Kazim, S.F.; Segura, A.; Bowers, C.A. The Frailty-Driven Predictive Model for Failure to Rescue among Patients Who Experienced a Major Complication Following Cervical Decompression and Fusion: An ACS-NSQIP Analysis of 3632 Cases (2011–2020). Spine J. 2024, 24, 582–589. [Google Scholar] [CrossRef]
- Gleeson, E.M.; Clarke, J.R.; Morano, W.F.; Shaikh, M.F.; Bowne, W.B.; Pitt, H.A. Patient-Specific Predictors of Failure to Rescue after Pancreaticoduodenectomy. HPB 2019, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Portuondo, J.I.; Shah, S.R.; Singh, H.; Massarweh, N.N. Failure to Rescue as a Surgical Quality Indicator: Current Concepts and Future Directions for Improving Surgical Outcomes. Anesthesiology 2019, 131, 426–437. [Google Scholar] [CrossRef]
- Lafonte, M.; Cai, J.; Lissauer, M.E. Failure to Rescue in the Surgical Patient: A Review. Curr. Opin. Crit. Care 2019, 25, 706–711. [Google Scholar] [CrossRef]
- Roy, J.M.; Segura, A.C.; Rumalla, K.; Skandalakis, G.P.; Covell, M.M.; Bowers, C.A. A Predictive Model of Failure to Rescue After Thoracolumbar Fusion. Neurospine 2023, 20, 1337–1345. [Google Scholar] [CrossRef]
- Kang, T.; Park, S.Y.; Lee, J.S.; Lee, S.H.; Park, J.H.; Suh, S.W. Predicting Postoperative Complications in Patients Undergoing Lumbar Spinal Fusion by Using the Modified Five-Item Frailty Index and Nutritional Status. Bone Jt. J. 2020, 102, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.K.; Cheung, Z.B.; Vig, K.S.; Phan, K.; Lima, M.C.; Kim, J.S.; Di Capua, J.; Kaji, D.A.; Arvind, V.; Cho, S.K. Hypoalbuminemia as an Independent Risk Factor for Perioperative Complications Following Surgical Decompression of Spinal Metastases. Glob. Spine J. 2019, 9, 321–330. [Google Scholar] [CrossRef]
- He, Z.; Zhou, K.; Tang, K.; Quan, Z.; Liu, S.; Su, B. Perioperative Hypoalbuminemia Is a Risk Factor for Wound Complications Following Posterior Lumbar Interbody Fusion. J. Orthop. Surg. Res. 2020, 15, 538. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhang, B.-F.; Zhang, Y.-M. The Association between Prealbumin Concentration at Admission and Mortality in Elderly Patients with Hip Fractures: A Cohort Study. Arch. Osteoporos. 2024, 19, 27. [Google Scholar] [CrossRef]
- Gelfand, Y.; De la Garza Ramos, R.; Nakhla, J.P.; Echt, M.; Yanamadala, V.; Yassari, R. Predictive Value of Hypoalbuminemia and Severe Hypoalbuminemia in Oncologic Spine Surgery. Clin. Neurol. Neurosurg. 2021, 210, 107009. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.J.; Park, B.J.; Perez, E.A.; Ternes, S.; Gold, C.; Carnahan, R.; Yamaguchi, S.; Kawasaki, H. Predictors of Survival After Emergent Surgical Decompression for Acutely Presenting Spinal Metastasis. World Neurosurg. 2023, 179, e39–e45. [Google Scholar] [CrossRef]
- Luksanapruksa, P.; Buchowski, J.M.; Hotchkiss, W.; Tongsai, S.; Wilartratsami, S.; Chotivichit, A. Prognostic Factors in Patients with Spinal Metastasis: A Systematic Review and Meta-Analysis. Spine J. 2017, 17, 689–708. [Google Scholar] [CrossRef]
- Leeds, I.L.; Kachalia, A.; Haut, E.R. Rescuing Failure to Rescue-Patient Safety Indicator 04 on the Brink of Obsolescence. JAMA Surg. 2021, 156, 115–116. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Camino-Willhuber, G.; Tani, S.; Schonnagel, L.; Caffard, T.; Haffer, H.; Chiapparelli, E.; Sarin, M.; Shue, J.; Soffin, E.M.; Zelenty, W.D.; et al. Association of Frailty and Preoperative Hypoalbuminemia with the Risk of Complications, Readmission, and Mortality After Spine Surgery. World Neurosurg. 2023, 174, e152–e158. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, J.; Seneviratne, N.; Bangash, A.H.; Goodwin, C.R.; Weber, M.H.; Charest-Morin, R.; Shin, J.H.; Versteeg, A.L.; Fourman, M.S.; Murthy, S.G.; et al. The Utilization of Hypoalbuminemia as a Prognostic Metric in Patients with Spinal Metastases: A Scoping Review. Brain Spine 2025, 5, 104223. [Google Scholar] [CrossRef]
- De la Garza Ramos, R.; Charest-Morin, R.; Goodwin, C.R.; Zuckerman, S.L.; Laufer, I.; Dea, N.; Sahgal, A.; Rhines, L.D.; Gokaslan, Z.L.; Bettegowda, C.; et al. Malnutrition in Spine Oncology: Where Are We and What Are We Measuring? Glob. Spine J. 2025, 15, 29S–46S. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Zarei, B.; Elyasi, S. Albumin: A Comprehensive Review and Practical Guideline for Clinical Use. Eur. J. Clin. Pharmacol. 2024, 80, 1151–1169. [Google Scholar] [CrossRef]
- Mazzaferro, E.M.; Edwards, T. Update on Albumin Therapy in Critical Illness. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 1289–1305. [Google Scholar] [CrossRef]
- Soeters, P.; Bozzetti, F.; Cynober, L.; Forbes, A.; Shenkin, A.; Sobotka, L. Defining Malnutrition: A Plea to Rethink. Clin. Nutr. 2017, 36, 896–901. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.M.; Rumalla, K.; Skandalakis, G.P.; Kazim, S.F.; Schmidt, M.H.; Bowers, C.A. Failure to Rescue as a Patient Safety Indicator for Neurosurgical Patients: Are We There yet? A Systematic Review. Neurosurg. Rev. 2023, 46, 227. [Google Scholar] [CrossRef]
- Christina, N.M.; Tjahyanto, T.; Lie, J.G.; Santoso, T.A.; Albertus, H.; Octavianus, D.; Putri, D.A.U.I.; Andrew, J.; Jatinugroho, Y.D.; Shiady, C.; et al. Hypoalbuminemia and Colorectal Cancer Patients: Any Correlation?: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e32938. [Google Scholar] [CrossRef]
- Wells, C.I.; Varghese, C.; Boyle, L.J.; McGuinness, M.J.; Keane, C.; O’Grady, G.; Gurney, J.; Koea, J.; Harmston, C.; Bissett, I.P. “Failure to Rescue” Following Colorectal Cancer Resection: Variation and Improvements in a National Study of Postoperative Mortality. Ann. Surg. 2023, 278, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Farjah, F.; Backhus, L.; Cheng, A.; Englum, B.; Kim, S.; Saha-Chaudhuri, P.; Wood, D.E.; Mulligan, M.S.; Varghese, T.K. Failure to Rescue and Pulmonary Resection for Lung Cancer. J. Thorac. Cardiovasc. Surg. 2015, 149, 1365–1371; discussion 1371–1373.e3. [Google Scholar] [CrossRef]
- Parkin, C.J.; Moritz, P.; Kirkland, O.; Glover, A. What Is the Accuracy of the ACS-NSQIP Surgical Risk Calculator in Emergency Abdominal Surgery? A Meta-Analysis. J. Surg. Res. 2021, 268, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.; Arora, S.; King, D.; Bouras, G.; Almoudaris, A.M.; Davis, R.; Darzi, A. A Systematic Review to Identify the Factors That Affect Failure to Rescue and Escalation of Care in Surgery. Surgery 2015, 157, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Williams, S.V.; Krakauer, H.; Schwartz, J.S. Hospital and Patient Characteristics Associated with Death after Surgery. A Study of Adverse Occurrence and Failure to Rescue. Med. Care 1992, 30, 615–629. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value, % |
|---|---|
| Age (mean, SD) | 62, 12 |
| Male sex (%) | 1044 (59.6%) |
| Dependent functional status (%) | 158 (9.06%) |
| BMI (mean kg/m2, SD) | 27.3, 6.2 |
| ASA class (median, IQR) | 3, 3–3 |
| Modified Frailty Index 5 (continuous) | |
| 0 | 806 (46.1%) |
| 1 | 616 (35.2%) |
| 2 | 266 (15.1%) |
| 3 | 61 (3.4%) |
| Chronic steroid use (%) | 358 (20.4%) |
| Mean preoperative albumin level (g/dL, SD) | 3.63, 0.642 |
| Normal albumin (%) | 1050 (60%) |
| Hypoalbuminemia (%) | 699 (40%) |
| Elective case | 1041 (59.5%) |
| Emergent case | 708 (40.5%) |
| Parameter | Value, % |
|---|---|
| Operative time (median hours, IQR) | 3.1 (2.1–4.6) |
| Perioperative transfusion (%) | 297 (16.98%) |
| Corpectomy (%) | 395 (22.55%) |
| Fusion procedure (%) | 990 (56.5%) |
| Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (continuous) | 1.01 | 0.99–1.03 | 0.264 |
| Male sex | 1.53 | 0.91–2.55 | 0.103 |
| Dependent functional status | 2.62 | 1.68–4.08 | <0.001 * |
| BMI (continuous) | 0.97 | 0.93–1.01 | 0.198 |
| ASA class | 2.13 | 1.43–3.18 | <0.001 * |
| Modified Frailty Index 5 (continuous) | 1.59 | 1.24–2.05 | <0.001 * |
| Chronic steroid use | 1.55 | 0.91–2.63 | 0.104 |
| Preoperative albumin level (continuous) | 0.34 | 0.24–0.48 | <0.001 * |
| Hypoalbuminemia (<3.5) | 3.88 | 2.36–6.39 | <0.001 * |
| Operative time (continuous) | 0.99 | 0.99–1.00 | 0.417 |
| Perioperative transfusion | 2.02 | 1.13–3.68 | 0.016 * |
| Corpectomy | 0.76 | 0.41–1.40 | 0.385 |
| Fusion procedure | 0.73 | 0.45–1.18 | 0.212 |
| Emergent case | 2.81 | 1.71–4.63 | <0.001 * |
| Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Perioperative transfusion | 1.81 | 0.99–3.30 | 0.050 |
| Modified Frailty Index 5 (continuous) | 1.12 | 0.79–1.59 | 0.521 |
| Dependent functional status | 1.53 | 0.82–2.87 | 0.176 |
| Preoperative albumin level (continuous) | 0.39 | 0.26–0.61 | <0.001 * |
| Emergent case | 2.40 | 1.36–4.33 | <0.003 * |
| Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Perioperative transfusion | 1.82 | 1.00–3.31 | 0.047 * |
| Modified Frailty Index 5 (continuous) | 1.13 | 0.80–1.60 | 0.478 |
| Dependent functional status | 1.63 | 0.88–3.02 | 0.119 |
| Hypoalbuminemia (<3.5) | 2.99 | 1.67–5.33 | <0.001 * |
| Emergent case | 2.52 | 1.41–4.49 | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájera Samaniego, E.; Fluss, R.; Bangash, A.H.; Kirnaz, S.; Murthy, S.; Gelfand, Y.; Yassari, R.; De La Garza Ramos, R. Serum Albumin Level as a Predictor of Failure to Rescue in Patients Undergoing Surgery for Spinal Metastases. Cancers 2025, 17, 3477. https://doi.org/10.3390/cancers17213477
Nájera Samaniego E, Fluss R, Bangash AH, Kirnaz S, Murthy S, Gelfand Y, Yassari R, De La Garza Ramos R. Serum Albumin Level as a Predictor of Failure to Rescue in Patients Undergoing Surgery for Spinal Metastases. Cancers. 2025; 17(21):3477. https://doi.org/10.3390/cancers17213477
Chicago/Turabian StyleNájera Samaniego, Esli, Rose Fluss, Ali Haider Bangash, Sertac Kirnaz, Saikiran Murthy, Yaroslav Gelfand, Reza Yassari, and Rafael De La Garza Ramos. 2025. "Serum Albumin Level as a Predictor of Failure to Rescue in Patients Undergoing Surgery for Spinal Metastases" Cancers 17, no. 21: 3477. https://doi.org/10.3390/cancers17213477
APA StyleNájera Samaniego, E., Fluss, R., Bangash, A. H., Kirnaz, S., Murthy, S., Gelfand, Y., Yassari, R., & De La Garza Ramos, R. (2025). Serum Albumin Level as a Predictor of Failure to Rescue in Patients Undergoing Surgery for Spinal Metastases. Cancers, 17(21), 3477. https://doi.org/10.3390/cancers17213477






