Simple Summary
Glioblastoma is a fatal brain tumor treated with surgery and chemoradiotherapy. In addition to known clinical and tumor related factors, certain demographic factors like sex, insurance status and income level have been recently reported to prognosticate for outcomes in patients receiving standard long-course chemoradiotherapy. However, it is not known whether these demographic factors prognosticate in patients who receive short-course chemoradiotherapy, an abbreviated treatment alternative reserved for elderly or low functional status patients. Identifying prognostic factors is important for refining personalized management approaches for this critically ill population. In this study, we investigate the role of demographic factors in patients receiving short-course chemoradiotherapy and find that female sex predicts for shorter time to tumor recurrence and death. These findings are first-in-kind and are contrary to what has been reported in patients who receive long-course chemoradiotherapy, highlighting the need to further investigate the impact of sex on response to different treatment regimens in glioblastoma.
Abstract
Background/Objective: Elucidation of prognostic factors is key to personalizing management approach for patients with glioblastoma (GBM). In patients who are treated with conventionally fractionated radiotherapy (cvRT), sex and other demographic variables (e.g., income level) were recently found to predict for treatment outcomes. However, it is unknown whether these factors predict for outcomes in elderly or poor performance status patients who receive hypofractionated RT (hyRT). In this study, we assess the association of clinical and non-clinical factors to outcomes in GBM patients treated with hyRT concurrent with temozolomide (TMZ). Methods: The records of 61 adult patients with newly diagnosed GBM consecutively treated at our institution with post-operative hyRT (4005 cGy in 15 daily fractions) and TMZ were retrospectively analyzed. Established clinical variables as well as key demographic variables were compared using chi-squared tests. Kaplan–Meier analyses were used to compare overall survival (OS) and progression-free survival (PFS) between clinical and demographic subgroups. Multivariate modeling was performed using Cox proportional hazards regression. Results: Female and male patients composed 44.3% and 55.7% of the study population, respectively, and did not differ significantly in their clinical or tumor characteristics. Most patients were 65 years or older (85.2%), and over half resided in middle/high-income regions (55.7%) and were privately insured (55.7%). On an univariate analysis, female sex was associated with shorter OS (median 10.0 months vs. 13.3 months in males, p = 0.0224) and PFS (median 3.00 months vs. 4.60 months in males, p = 0.0134). Female sex remained significantly associated with inferior outcomes on multivariate analysis. Income level, type of insurance and marital status were not significantly associated with treatment outcomes. Conclusion: Our study is the first to report sex differences in GBM outcomes following hyRT-TMZ. Contrary to responses following cvRT-TMZ, females appear to have inferior outcomes after hyRT-TMZ versus males. Further investigation is warranted to define the optimal treatment approach for sex subgroups in GBM.
1. Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults, occurring at an annual age-adjusted incidence rate of 3.19/100,000, and bearing a dismal prognosis of 12 to 15 months from diagnosis [1]. Population studies have shown that survival in GBM patients declines with increasing age and lower performance status, with an approximate median survival of six to nine months [2,3]. Management of GBM in this poor prognosis cohort is difficult given the higher incidence of comorbidities and increased risk of side-effects [4]. However, prior phase 3 studies demonstrate survival gains with the use of hypofractionated radiotherapy (hyRT) and concurrent temozolomide (TMZ) in this older, frail population [5,6,7].
While age and performance status, in addition to extent of resection (EOR) and methylguanine-DNA methyltransferase (MGMT) promoter methylation status, are well-established prognostic factors, sex and other demographic factors have been recently shown to predict for outcomes in GBM [8,9,10,11,12]. In a recent National Cancer Database (NCDB) analysis, investigators identified male sex, government insurance status and low-income as factors associated with diminished overall survival (OS) in GBM [11]. Moreover, the NRG Radiation Therapy Oncology Group (RTOG), in their 2021 GBM nomogram, identified sex as a significant prognostic factor for OS [13]. These results suggest that certain GBM demographics may be particularly vulnerable to worsened outcomes. However, these prior studies focused exclusively on patients treated with conventionally fractionated RT (cvRT) and TMZ [14,15]. As such, the prognostic impact of sex and other demographic factors remains unknown in GBM patients treated with hyRT-TMZ.
Delineation of potential prognostic factors, clinical and demographic alike, is important in personalizing the management approach for GBM patients. This is particularly critical for patients receiving hyRT, in whom non-clinical prognostic factors are less understood and whose survival is shorter than their counterparts receiving cvRT. In the present study, we conducted a retrospective analysis to determine if sex and demographic factors affect disease outcomes in a cohort of GBM patients treated with hyRT and TMZ. We find that female sex is associated with worse OS and progression-free survival (PFS), contrary to what is observed in patients treated with cvRT-TMZ [16,17].
2. Methods
2.1. Study Cohort
Between 2013 and 2019, 61 adult patients (age ≥ 18 years) with newly diagnosed, pathology-proven GBM completed curative intent hyRT-TMZ at our institution. Patients were consecutively treated by a multidisciplinary team of neurosurgeons, radiation oncologists and neuro-oncologists. Patients were excluded if they had received previous brain irradiation, did not complete the full course of radiotherapy, were treated with a non-hyRT schedule and/or did not receive concurrent TMZ. Patients were followed up on until time of death, which occurred in 59 of 61 patients.
2.2. Treatment
All 61 patients underwent surgery in the form of craniotomy and maximal feasible resection. Extent of resection (EOR) was determined by post-operative brain magnetic resonance imaging (MRI). All patients completed adjuvant of external-beam radiotherapy to the post-operative bed and T1- and fluid attenuated inversion recovery (FLAIR)-enhancing regions on post-operative MRI, to a total dose of 4005 cGy in 15 daily fractions as part of the curative intent hypofractionated protocol at our institution. TMZ was administered concurrently with hyRT in all patients.
2.3. Statistical Analyses
Overall survival (OS) time was measured from the date of surgery to the date of death or last follow-up for living patients. Progression-free survival (PFS) time was calculated from the end of radiotherapy to the date of recurrence, which was determined by a follow-up MRI and/or surgical pathology. The following variables were assessed: age, sex, Karnofsky Performance Scale (KPS) score, race/ethnicity, insurance type, marital status, income based on zip code as reported by the U.S. Census Bureau [18], EOR, MGMT promoter methylation status and Charlson Comorbidity Index score. For zip code income, Zip Code Tabulation Areas data were obtained from the U.S. Census Bureau and cross-referenced with the poverty threshold for our region in a given year; zip codes with a median income below this threshold were categorized as ‘low’ and those above the threshold as ‘middle/high.’ Comparisons between variables were performed using the Chi-squared test. The Kaplan–Meier method was used to calculate the probability of tumor control and survival; 95% confidence intervals were estimated according to Altman’s method [19]. OS and PFS curves were compared in a univariate analysis using the log-rank test. Hazard ratios (HR) with confidence intervals (CI) were calculated using the method described by Klein and Moeschberger [20]. For continuous variables such as age, univariate Cox proportional hazards regression was used to determine association with OS and PFS [21]. Variables that were found to be significant (p-value < 0.05) were considered in the multivariate analysis. Multivariate analysis was performed by Cox proportional hazards regression [21].
3. Results
3.1. Composite Demographics
A total of 61 patients who met inclusion and exclusion criteria were identified for our study. Baseline patient demographics and treatment factors are shown in Table 1. Thirty-four patients were male and twenty-seven were female. The median age of the entire cohort was 71.0 years (interquartile range (IQR) 9.5) and 70.5 years (IQR 10) for males, and 72.0 years (IQR 11) for females. The majority of patients (85.2%) were 65 years or older and considered elderly as per historical GBM trials [7,22]. Approximately 28% of patients had suboptimal KPS under 70. Over a quarter (26.2%) of patients had a Charlson comorbidity score above six, which generally reflects two comorbidities or an advanced comorbidity besides solid tumor in a person 71 years of age (median age of our cohort) or older. This comorbidity score was also the median for the entire cohort, irrespective of sex. Gross total resection (GTR) was achieved in 26 patients, and subtotal resection (STR) was performed on the remaining 35 patients. Over two-thirds (68.9%) of GBM tumors lacked methylation of the MGMT promoter. Approximately 44% of patients were government-insured and resided in a low-income zip code. Above one-third of patients (34.4%) were unmarried, widowed or without a domestic partner.

Table 1.
Composite demographics of study cohort. KPS = Karnofsky Performance Status. MGMT = methylguanine-DNA methyltransferase.
3.2. Univariate Analysis
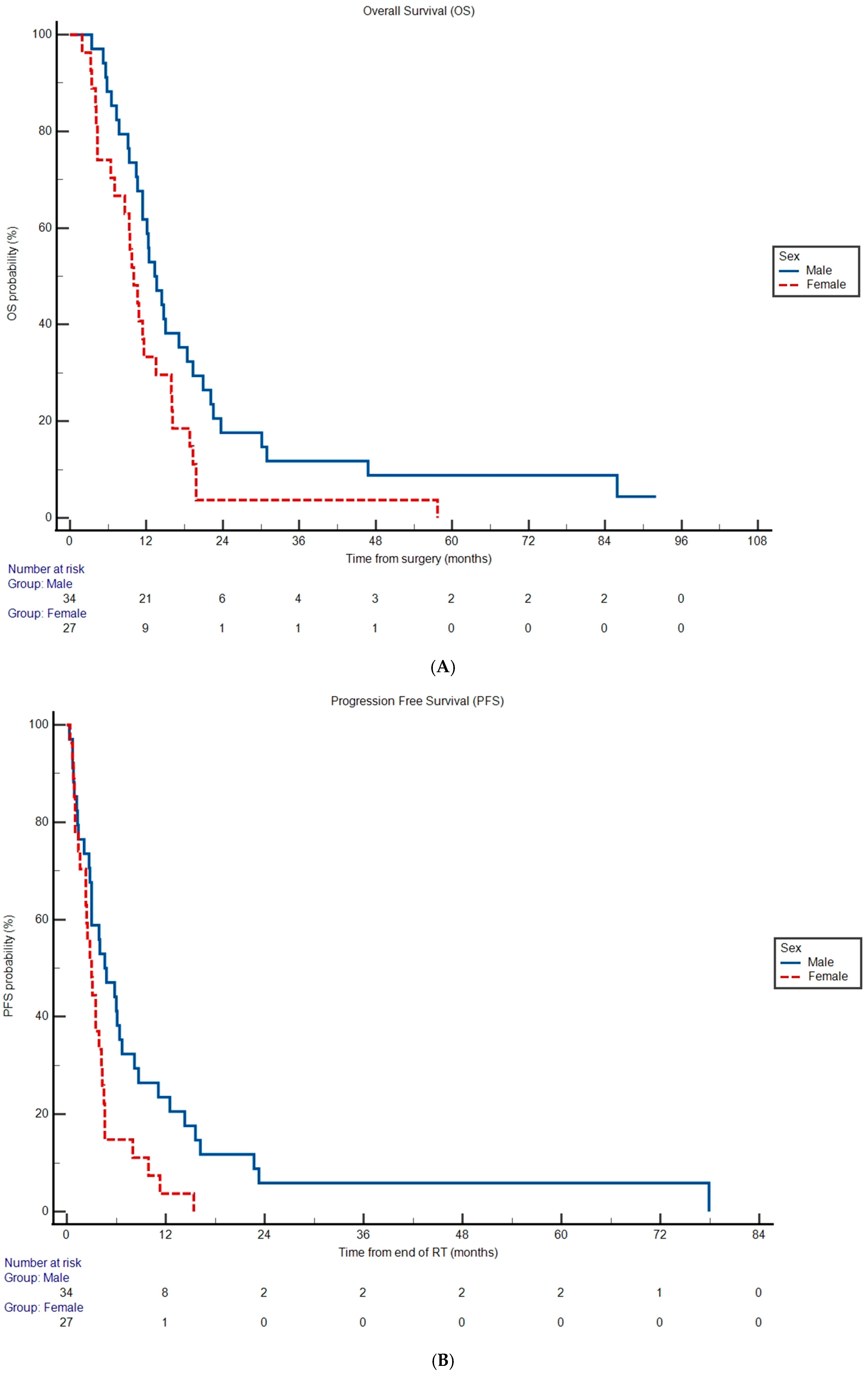
Median OS was 11.6 months (95% CI 10.0 to 14.4) and median PFS was 3.50 months (95% CI 2.80 to 77.9) for the entire cohort. Lack of MGMT promoter methylation was associated with increased hazard rate of death (OS HR 2.11, 95% CI 1.24 to 3.59, p = 0.00570) and disease progression (PFS HR 2.50, 95% CI 1.45 to 4.32, p = 0.00100) compared to patients with MGMT promoter methylation (Table 2, Supplementary Figure S1A,B). Median OS was shorter in females (Figure 1A) at 10.0 months versus 13.3 months in males (HR 1.92, 95% CI 1.10 to 3.37, p = 0.0224; Table 2). Female patients also experienced an increased hazard rate of disease progression (HR 2.07, 95% CI 1.16 to 3.69, p = 0.0134; Table 2) compared to male patients with a median PFS of 3.00 versus 4.60 months, respectively (Figure 1B). The remainder of the covariates analyzed were not significantly associated with OS and PFS (p > 0.05; Table 2).

Table 2.
Univariate analysis assessing association of covariates with overall survival (OS) and progression-free survival (PFS). Hazard ratio = HR. CI = confidence interval. KPS = Karnofsky Performance Status. MGMT = methylguanine-DNA methyltransferase.
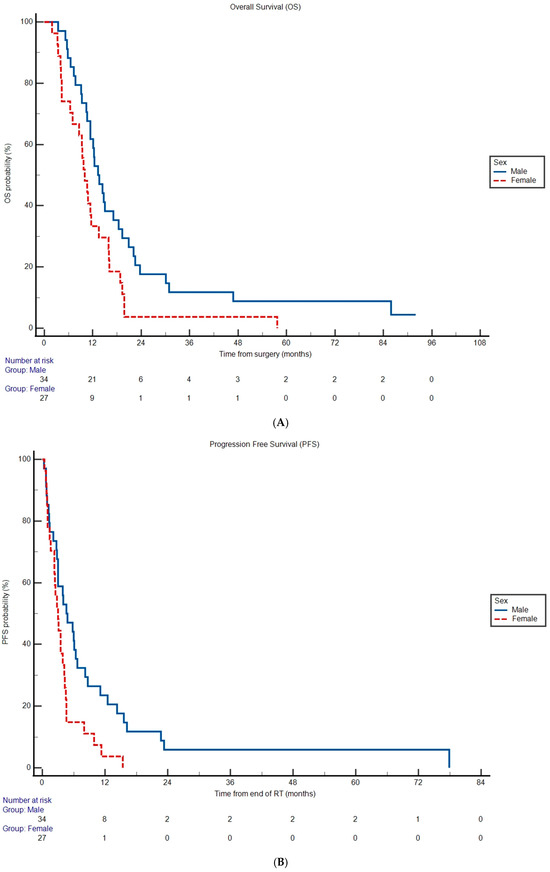
Figure 1.
Overall survival (A) and progression-free survival (B) probabilities according to sex subgroup. Differences in Kaplan–Meier curves were statistically significant in both cases (p < 0.05). Tables under the graphs represent the remaining number of subgroup patients at risk for experiencing an event of interest at given timepoint.
3.3. Multivariate Cox Proportional Hazard Analysis
In multivariable Cox regression analysis (Supplementary Table S1), MGMT promoter methylation status remained significantly associated with OS (HR 2.33, 95% CI 1.33 to 4.17, p = 0.00340) and PFS (HR 2.56, 95% CI 1.43 to 4.55, p = 0.00180). Similarly, sex remained significantly associated with OS (HR 2.02, 95% CI 1.18 to 3.45, p = 0.0108) and PFS (HR 1.91, 95% CI 1.11 to 3.30, p = 0.0191).
3.4. Chi-Squared Analysis for Covariate Association with Sex
Tumor variables such as MGMT methylation status, EOR and tumor multifocality did not differ significant between males and females (p > 0.05; Table 3). Similarly, clinical variables such as comorbidity score and functional status did not significantly differ between sex subgroups (p > 0.05; Table 3). Sex and marital status had a significant association, with more men being married and more women being single, divorced or widowed in our cohort (p = 0.000100; Table 3). The remainder of demographic variables, including income level and insurance status, did not differ between males and females (p > 0.05; Table 3).

Table 3.
Assessment of differences in clinical, tumor and demographic factors between males and females by Chi-square analysis. pX2 = p value for chi-square analysis. KPS = Karnofsky Performance Status. MGMT = methylguanine-DNA methyltransferase.
4. Discussion
Emerging studies are reporting on the prognostic value of demographic factors like sex, income level and insurance status in GBM [10,11,12,16,17,23]. These studies converge with the accumulating neuro-oncology literature that demonstrates demographic-based differences in outcomes across a variety of brain tumors [24,25]. With regard to GBM specifically, the majority of these studies have been performed using the NCDB, which lacks outcomes data beyond OS and omits important RT-related details. However, an understanding of the impact of demographic factors on outcomes besides OS is important, as early-phase trials often rely on endpoints such as PFS to advance promising investigational therapies [26]. Equally important is accounting for specific RT details such as dose and technique, as the availability and completion of different radiation regimens (cvRT vs. hyRT) may be impacted differently by certain demographic variables (e.g., insurance status, geographic location). In this study, we evaluated the association of demographic factors with OS and PFS in a cohort of newly diagnosed GBM patients treated with hyRT-TMZ, an abbreviated chemoradiation schedule that has not been studied in the context of demographic prognostic variables.
In our cohort, females had significantly lower survival and shorter time to recurrence following hyRT-TMZ when compared to males. This difference in outcomes occurred despite comparable clinical (e.g., EOR, KPS) and pathological (e.g., MGMT promoter methylation) characteristics. Although sex-based differences in GBM outcomes have been previously reported in the literature, these reports have generally put forth the opposite association: namely, that female patients respond better to standard-of-care therapy than their male counterparts [16,17,27]. Indeed, findings like these are reflected in the 2021 NRG RTOG GBM nomogram and have culminated in the recent validation of a sex-specific version based on RTOG trials 0525 and 0825 [14,15]. However, it is important to note that these RTOG trials exclusively consisted of patients treated with cvRT-TMZ, and there have been no published reports to-date that focus on patients treated with hyRT-TMZ, a shorter-lived cohort in whom clinical discussions and decisions stand to benefit greatly from improved personalized prognostication.
Sexual dimorphism in GBM incidence, biology and outcomes has previously been described in the literature and is speculated to arise from hormonal, metabolic, genetic and immune differences between males and females [27,28]. Preclinical studies, for instance, demonstrate that estrogen increases the apoptosis of GBM cells and suppresses the oncogenic mitogen-activated protein kinase pathway in a sex-dependent manner [29]. Other immune studies in preclinical GBM models show a monocytic predominance in myeloid cells in male mice versus granulocytes in female mice, as well as differing cytokine profiles [30,31]. These studies in particular generally demonstrate a more pro-inflammatory tumor milieu in female versus male subjects. However, how biological factors such as estrogen, immune subsets or cytokines are modulated by cvRT versus hyRT, and to what extent this may impact GBM growth and recurrence, remains to be studied. At best, these preclinical studies provide a conjectural basis for differences in de novo GBM tumorigenesis between males and females, but do not offer or investigate a sex-dependent link between chemoradiation treatment and outcomes. Additional studies are therefore critically needed to elucidate how different chemoradiation regimens may benefit distinct sex subgroups, and how these may be applied to personalized treatment approaches in the clinic. Translational studies should consider capturing data in a sex-stratified manner from easily accessible tests (e.g., cytokine assay from peripheral blood, radiomic features from MRI) during the course of treatment to correlate with chemoradiotherapy outcomes prospectively.
Studying sex as a predictive variable for treatment response is highly relevant in GBM as new chemoradiation schedules are increasingly being tested in the clinic. Two U.S. institutions recently reported their experiences with dose-escalated hyRT, which delivers a biologically effective dose (BED) closer to cvRT and potentially confers survival gains over classical hyRT [32,33]. Sex-specific considerations in BED selection are particularly important in light of recent reports of female GBM patients harboring higher tumor volume and areas of necrosis on MRI compared to males [34]. If this is the case, it is possible that higher tumor and/or necrotic volume may necessitate a higher BED than that conferred by classical hyRT. While, in our study we did not observe sex differences in multifocality or subtotal resection rates—surrogates of disease burden—it is important to note that we did not directly quantitate tumor volume or necrosis. Future radiological and histological studies should, therefore, consider investigating the correlation of these metrics with sex and the possible impact on response to radiotherapy regimens with different BED levels. Potential immunophenotyping studies could also be performed in a BED-specific manner on tumor specimens collected post-radiotherapy at recurrence.
In our study, we did not find that insurance status or income based on zip code associated with treatment outcomes. This stands in contrast to prior studies demonstrating a correlation between government insurance/uninsured status as well as low-income to inferior outcomes in GBM [10,12,23]. It is possible that in our cohort of GBM patients receiving hyRT the disease was so aggressive that the impact of insurance status and income did not significantly influence treatment outcomes. Of note, we did observe that a higher proportion of females in our study were unmarried or without a domestic partner compared to males. Previous studies using the Surveillance, Epidemiology, and End Results (SEER) database demonstrate a protective effect of marriage in GBM outcomes, particularly in elderly males, and is congruent with observations in other types of cancer [10,35,36,37]. However, due to the sample size of our cohort we could not determine with high statistical confidence the degree to which this covariate influenced treatment response within sex subgroups. As mentioned, independently, marital status did not predict for OS or PFS. Additional studies are warranted to investigate the potential impact of marriage, companionship and family support in GBM patients undergoing hyRT.
While the results of this exploratory study are interesting and can be used to generate hypotheses, they must be considered in light of several limitations. First, the retrospective nature of this study limits our conclusions to associations between variables and clinical outcomes, and causality cannot be established. Secondly, the small sample size of our cohort, while comparable to other U.S. retrospective studies of elderly/frail GBM patients, further limits the ability to detect true differences between subgroups [38,39]. We recognize that large, prospective studies are needed in order to confirm any veritable relationship between sex and survival outcomes. Moreover, our study only included patients who completed hyRT in our analyses and, thus, we cannot account for any disparities that may exist in the referral and/or RT completion phases. While out of the scope of our original objectives, we did confirm that there were no major differences in median time to treatment completion between females (21 days, IQR 1.75) and males (22 days, IQR 1). Lastly, we were unable to ascertain differences in post-RT TMZ adherence, corticosteroid use, surveillance schedule and salvage therapies between patient subgroups due to limitations of available data points. Future studies should prospectively collect and assess the impact of these variables on outcomes.
5. Conclusions
Survival continues to be dismal for GBM patients treated with hypofractionated chemoRT. Additional research is needed to elucidate sex-based differences in outcomes based on the type of chemoradiotherapy schedule administered.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17213486/s1, Figure S1: Kaplan-Meier (A) overall survival (OS) and (B) progression-free survival (PFS) estimates for patient cohort stratified by methylguanine-DNA methyltransferase (MGMT) promoter methylation status. Differences in Kaplan-Meier curves were statistically significant in both cases (p < 0.05); Table S1: Multivariable Cox proportional hazards analysis for overall survival (OS) and progression-free survival (PFS). Hazard ratios (HR) with 95% confidence intervals (CI). MGMT = methylguanine methylation.
Author Contributions
O.P., Conceptualization, methodology, data collection, data analysis, article writing and editing, supervision. M.T.: Data collection, article writing and editing. N.M., article writing and editing. E.J.B., Conceptualization and data analysis. M.B.S., Data curation. J.N.B., Data curation. G.M.M., Data curation. S.K.C., Data curation. T.J.C.W., Conceptualization, methodology, data curation, article editing, resources, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with Columbia University Medical Center Institutional Review Board, initially approved on August 9, 2013 under protocol AAAM2358.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used and analyzed during the current study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
T.J.C.W. reports personal fees and non-financial support from AbbVie, personal fees from Cancer Panels, personal fees from Doximity, personal fees and non-financial support from Elekta, personal fees and non-financial support from Merck, personal fees and non-financial support from Novocure, personal fees and non-financial support from RTOG Foundation, personal fees from Wolters Kluwer, grants and non-financial support from Genentech, grants and non-financial support from Varian, personal fees from Iylon Precision Oncology, outside the submitted work. These entities had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| GBM | glioblastoma |
| hyRT | hypofractionated radiotherapy |
| cvRT | conventionally fractionated radiotherapy |
| cGy | centi-Gray |
| TMZ | temozolomide |
| OS | overall survival |
| PFS | progression-free survival |
| EOR | extent of resection |
| MGMT | methylguanine-DNA methyltransferase |
| KPS | Karnofsky Performance Scale |
| NCDB | National Cancer Database |
| RTOG | Radiation Therapy Oncology Group |
| SEER | Surveillance, Epidemiology, and End Results |
| MRI | Magnetic Resonance Imaging |
| BED | Biologically Effective Dose |
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Paszat, L.; Laperriere, N.; Groome, P.; Schulze, K.; Mackillop, W.; Holowaty, E. A population-based study of glioblastoma multiforme. Int. J. Radiat. Oncol. 2001, 51, 100–107. [Google Scholar] [CrossRef]
- Laperriere, N.; Weller, M.; Stupp, R.; Perry, J.R.; Brandes, A.A.; Wick, W.; Bent, M.J.v.D. Optimal management of elderly patients with glioblastoma. Cancer Treat. Rev. 2013, 39, 350–357. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Benevento, F.; Scopece, L.; Mazzocchi, V.; Bacci, A.; Agati, R.; Calbucci, F.; Ermani, M. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma. Cancer 2009, 115, 3512–3518. [Google Scholar] [CrossRef]
- Keime-Guibert, F.; Chinot, O.; Taillandier, L.; Cartalat-Carel, S.; Frenay, M.; Kantor, G.; Guillamo, J.-S.; Jadaud, E.; Colin, P.; Bondiau, P.-Y.; et al. Radiotherapy for Glioblastoma in the Elderly. N. Engl. J. Med. 2007, 356, 1527–1535. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; E Hegi, M.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.; Laperriere, N.; O’cAllaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Cioffi, G.; Chunduru, P.; Molinaro, A.M.; Berger, M.S.; E Sloan, A.; Barnholtz-Sloan, J.S. An independently validated nomogram for isocitrate dehydrogenase-wild-type glioblastoma patient survival. Neuro-Oncol. Adv. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Malay, S.; Somasundaram, E.; Patil, N.; Buerki, R.; Sloan, A.; Barnholtz-Sloan, J.S. Treatment and surgical factors associated with longer-term glioblastoma survival: A National Cancer Database study. Neuro-Oncol. Adv. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Xie, J.; Yang, S.; Liu, X.; Zhao, Y. Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Med. 2018, 7, 3722–3742. [Google Scholar] [CrossRef]
- Yusuf, M.B.; Gaskins, J.; Amsbaugh, M.J.; Woo, S.; Burton, E. Survival impact of prolonged postoperative radiation therapy for patients with glioblastoma treated with combined-modality therapy. Neuro-Oncol. Pract. 2018, 6, 112–123. [Google Scholar] [CrossRef]
- Hsu, E.J.; Thomas, J.; Timmerman, R.D.; Wardak, Z.; Dan, T.D.; Patel, T.R.; Sanford, N.N.; Vo, D.T. Socioeconomic and demographic determinants of radiation treatment and outcomes in glioblastoma patients. Front. Neurol. 2022, 13, 1024138. [Google Scholar] [CrossRef]
- Patil, N.; Somasundaram, E.; Waite, K.A.; Lathia, J.D.; Machtay, M.; Gilbert, M.R.; Connor, J.R.; Rubin, J.B.; Berens, M.E.; Buerki, R.A.; et al. Independently validated sex-specific nomograms for predicting survival in patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. J. Neuro-Oncol. 2021, 155, 363–372. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; E Berens, M.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro-Oncology 2018, 20, 576–577. [Google Scholar] [CrossRef]
- Putz, F.; Knippen, S.; Lahmer, G.; Fietkau, R.; Semrau, S. A Model to Predict the Feasibility of Concurrent Chemoradiotherapy With Temozolomide in Glioblastoma Multiforme Patients Over Age 65. Am. J. Clin. Oncol. 2017, 40, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Income by Zip Code Tabulation Area-Census Bureau Maps. Available online: https://data.census.gov/map?q=Income+by+Zip+code+tabulation+area&layer=VT_2022_860_Z2_PY_D1&loc=43.3751,-113.1138,z2.6270 (accessed on 19 September 2025).
- Clark, T.G.; Bradburn, M.J.; Love, S.B.; Altman, D.G. Survival Analysis Part I: Basic concepts and first analyses. Br. J. Cancer 2003, 89, 232–238. [Google Scholar] [CrossRef]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data, 2nd ed.; Springer: New York, NY, USA, 2003; Volume 9, pp. 302–308. [Google Scholar]
- Bradburn, M.J.; Clark, T.G.; Love, S.B.; Altman, D.G. Survival Analysis Part II: Multivariate data analysis—An introduction to concepts and methods. Br. J. Cancer 2003, 89, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Bauman, G.S.; Gaspar, L.E.; Fisher, B.J.; Halperin, E.C.; Macdonald, D.R.; Cairncross, J.G. A prospective study of short-course radiotherapy in poor prognosis glioblastoma multiforme. Int. J. Radiat. Oncol. 1994, 29, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Young, J.S.; Ore, C.D.; Dayani, F.; Lau, D.; Wadhwa, H.; Rick, J.W.; Nguyen, A.T.; McDermott, M.W.; Berger, M.S.; et al. Insurance type impacts the economic burden and survival of patients with newly diagnosed glioblastoma. J. Neurosurg. 2020, 133, 89–99. [Google Scholar] [CrossRef]
- Curry, W.T.; Barker, F.G. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J. Neuro-Oncol. 2009, 93, 25–39. [Google Scholar] [CrossRef]
- Mukherjee, D.; Patil, C.G.; Todnem, N.; Ugiliweneza, B.; Nuño, M.; Kinsman, M.; Lad, S.P.; Boakye, M. Racial disparities in medicaid patients after brain tumor surgery. J. Clin. Neurosci. 2013, 20, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Pilz, L.R.; Manegold, C.; Schmid-Bindert, G. Statistical considerations and endpoints for clinical lung cancer studies: Can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer? Transl. Lung Cancer Res. 2012, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef] [PubMed]
- Schiffgens, S.; Wilkens, L.; Brandes, A.A.; Meier, T.; Franceschi, E.; Ermani, M.; Hartmann, C.; Sandalcioglu, I.E.; Dumitru, C.A. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget 2016, 7, 55169–55180. [Google Scholar] [CrossRef]
- Liu, M.; Hurn, P.D.; E Roselli, C.; Alkayed, N.J. Role of P450 Aromatase in Sex-Specific Astrocytic Cell Death. J. Cereb. Blood Flow Metab. 2007, 27, 135–141. [Google Scholar] [CrossRef]
- Bayik, D.; Zhou, Y.; Park, C.; Hong, C.; Vail, D.; Silver, D.J.; Lauko, A.; Roversi, G.; Watson, D.C.; Lo, A.; et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020, 10, 1210–1225. [Google Scholar] [CrossRef]
- Alban, T.J.; Bayik, D.; Otvos, B.; Rabljenovic, A.; Leng, L.; Jia-Shiun, L.; Roversi, G.; Lauko, A.; Momin, A.A.; Mohammadi, A.M.; et al. Glioblastoma Myeloid-Derived Suppressor Cell Subsets Express Differential Macrophage Migration Inhibitory Factor Receptor Profiles That Can Be Targeted to Reduce Immune Suppression. Front. Immunol. 2020, 11, 1191. [Google Scholar] [CrossRef]
- Beckham, T.H.; Rooney, M.K.; McAleer, M.F.; Ghia, A.J.; Tom, M.C.; Perni, S.; McGovern, S.; Grosshans, D.; Chung, C.; Wang, C.; et al. Hypofractionated radiotherapy for glioblastoma: A large institutional retrospective assessment of 2 approaches. Neuro-Oncol. Prac. 2024, 11, 266–274. [Google Scholar] [CrossRef]
- Perlow, H.K.; Yaney, A.; Yang, M.; Klamer, B.; Matsui, J.; Raval, R.R.; Blakaj, D.M.; Arnett, A.; Beyer, S.; Elder, J.B.; et al. Dose-escalated accelerated hypofractionation for elderly or frail patients with a newly diagnosed glioblastoma. J. Neuro-Oncol. 2022, 156, 399–406. [Google Scholar] [CrossRef]
- Bilello, M.; Akbari, H.; Da, X.; Pisapia, J.M.; Mohan, S.; Wolf, R.L.; O’rOurke, D.M.; Martinez-Lage, M.; Davatzikos, C. Population-based MRI atlases of spatial distribution are specific to patient and tumor characteristics in glioblastoma. NeuroImage Clin. 2016, 12, 34–40. [Google Scholar] [CrossRef]
- Chang, S.M.; Barker, F.G. Marital status, treatment, and survival in patients with glioblastoma multiforme. Cancer 2005, 104, 1975–1984. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Yang, K.-B.; Zhang, Y.-Z.; Wu, C.-F.; Wen, D.-W.; Lv, J.-W.; Zhu, G.-L.; Du, X.-J.; Chen, L.; Zhou, G.-Q.; et al. Assessment of Modifiable Factors for the Association of Marital Status With Cancer-Specific Survival. JAMA Netw. Open 2021, 4, e2111813. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Chen, M.-H.; McCarthy, E.P.; Mendu, M.L.; Koo, S.; Wilhite, T.J.; Graham, P.L.; Choueiri, T.K.; Hoffman, K.E.; Martin, N.E.; et al. Marital Status and Survival in Patients With Cancer. J. Clin. Oncol. 2013, 31, 3869–3876. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kirkpatrick, J.P.; McSherry, F.; Herndon, J.E.; Lipp, E.S.; Desjardins, A.; Randazzo, D.M.; Friedman, H.S.; Ashley, D.M.; Peters, K.B.; et al. Adjuvant Radiation in Older Patients With Glioblastoma: A Retrospective Single Institution Analysis. Front. Oncol. 2021, 11, 631618. [Google Scholar] [CrossRef] [PubMed]
- Dohopolski, M.; Schmitt, L.G.; Vis J., de; Mostardeiro, T.R.; Anand, S.; Youssef, M.; Noch, E.; Maher, E.; Sun, M.; Patel, T.; et al. Comparative Outcomes of Standard Radiation Therapy and 5-Fraction Adaptive Stereotactic Radiation Therapy in Newly Diagnosed Glioblastoma: A Propensity Score–Matched Analysis. Adv. Radiat. Oncol. 2025, 10, 101813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).