Desmoid Tumors—Experience from a Referral Center, Part 1: Multidisciplinary Review and Practical Recommendations
Simple Summary
Abstract
1. Introduction
2. Methodology
3. Diagnostic Approach to DT
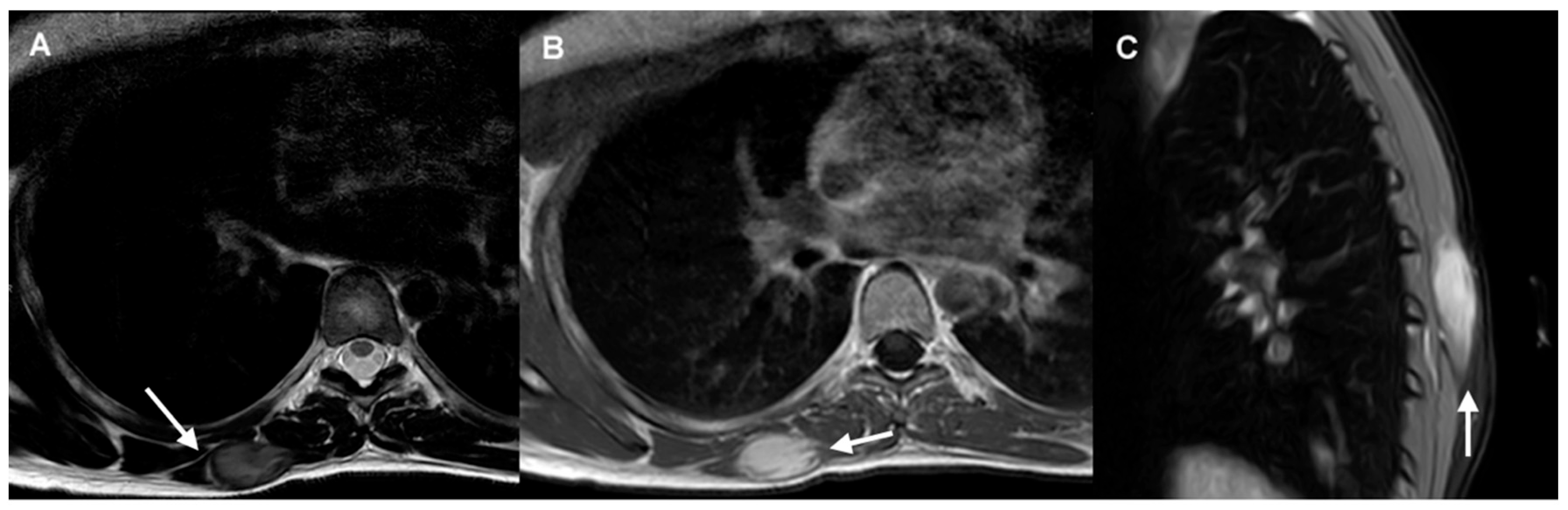
3.1. Imaging Diagnosis
- US is recommended as the first-line imaging modality for the initial evaluation of palpable lesions and for guiding core needle biopsy in DTs (IV, B).
- CT and MRI are the imaging modalities of choice for treatment planning, image-guided procedures, and follow-up in patients with DTs (IV, B).
- MRI is considered the optimal imaging modality for evaluating extra-abdominal DTs (IV, B).
- CT is preferred in the follow-up of intra-abdominal DTs, particularly for evaluating the extent of disease and identifying potential complications (IV, B).
3.2. Biopsy
- Recommendation: Core needle biopsy is the standard method for the diagnosis of DTs (IV, A)
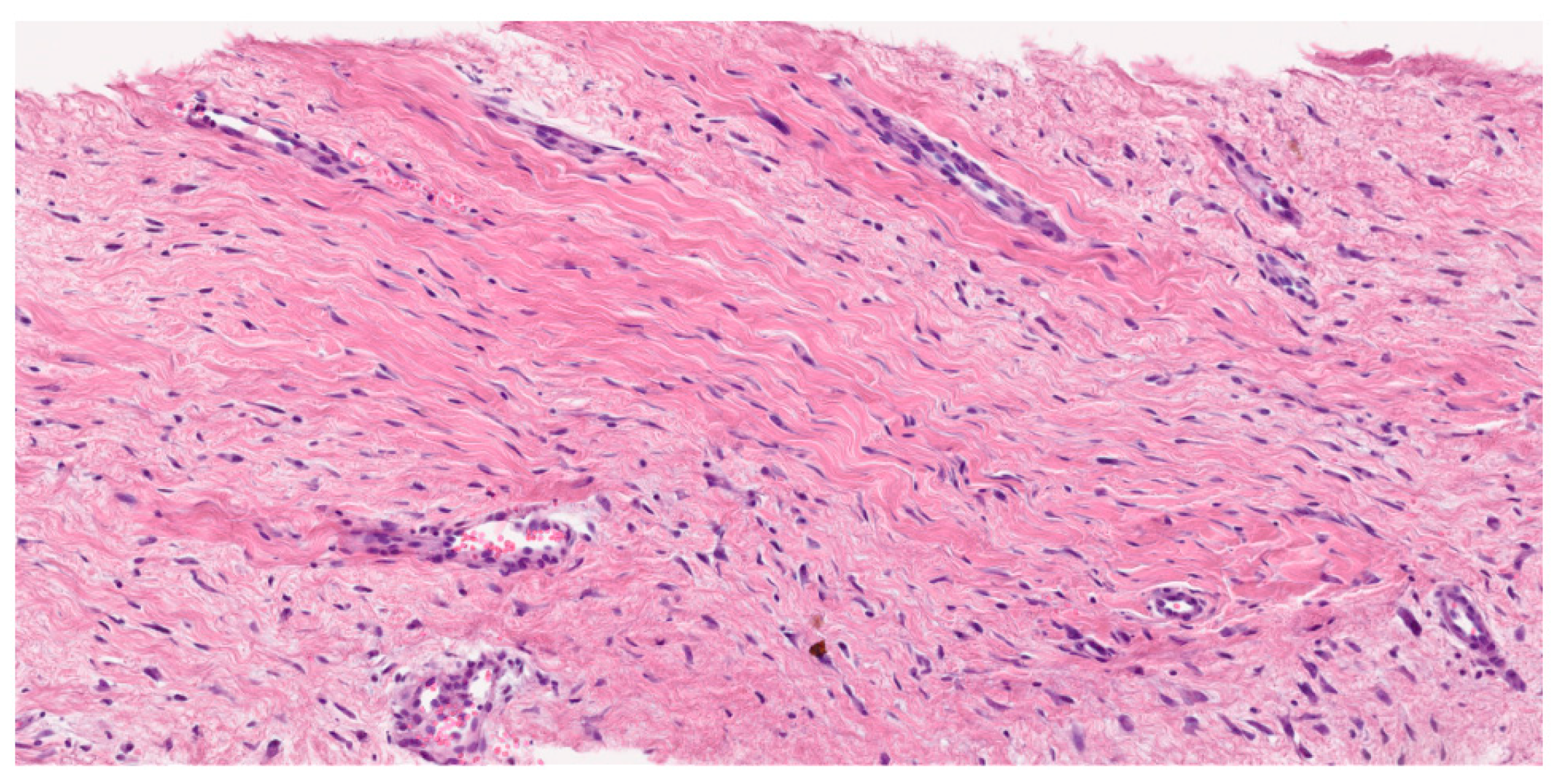
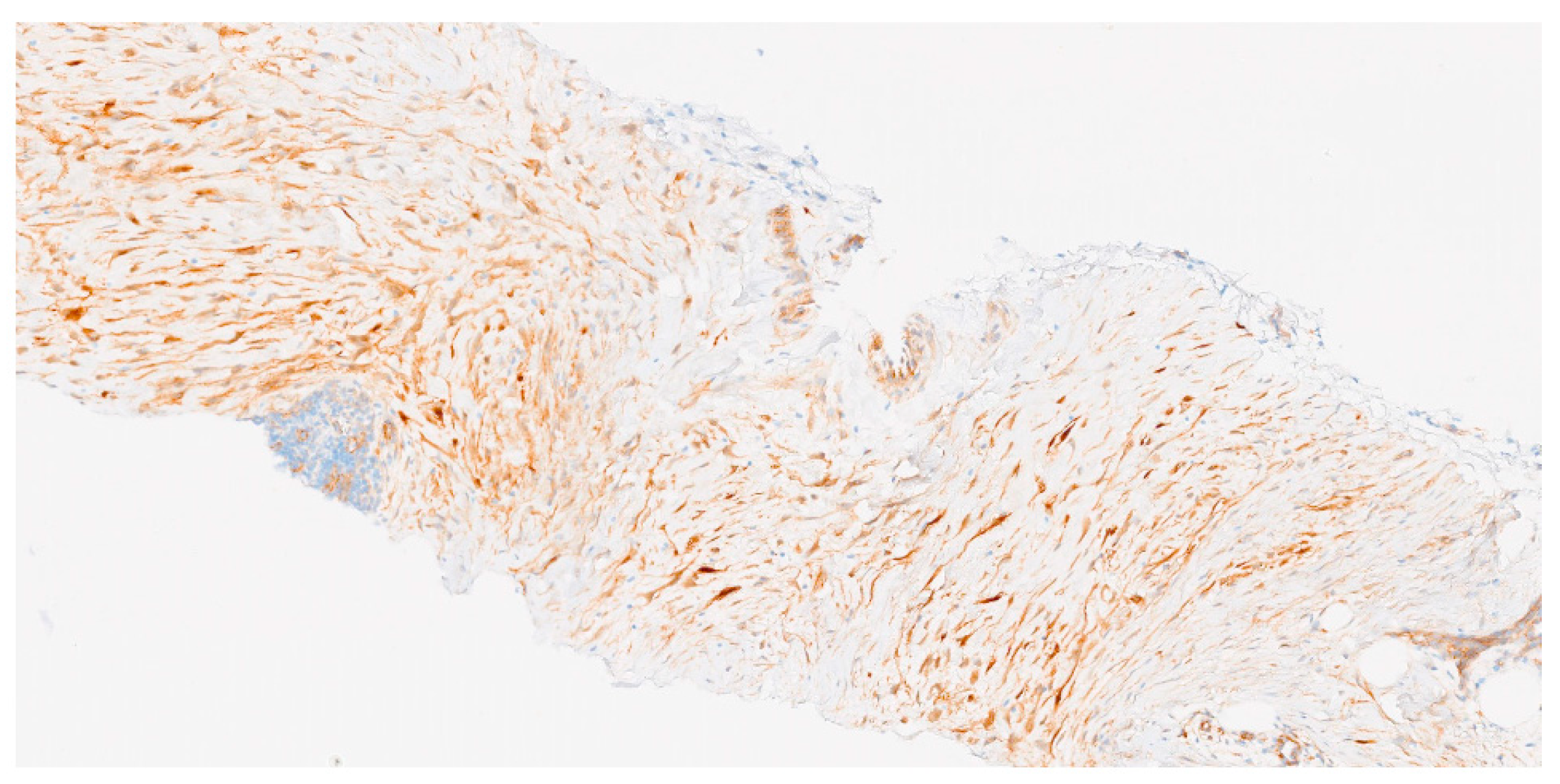
3.3. Histological Diagnosis
3.4. Indication for Molecular Study
- Diagnostic confirmation. Although most desmoid tumors are diagnosed based on histological and clinical features, molecular analysis can aid in confirming the differential diagnosis in complex or doubtful cases when beta-catenin immunostaining is equivocal [2];
- Exclusion of syndromic disease. In patients with a personal or family history of FAP, the analysis of the APC gene is critical. Notably, 85–90% of desmoid tumors associated with FAP harbor APC mutations;
- Assessment of CTNNB1 mutation status and recurrence risk. Certain CTNNB1 mutations, particularly p.Ser45Phe, have been associated with a higher risk of local recurrence. Determining the mutation status may therefore provide valuable prognostic information and guide clinical follow-up and treatment planning;
- Identification of potential therapeutic targets. In selected cases, molecular profiling may help identify therapeutically actionable targets, such as alterations in the Wnt/β-catenin pathway, which may be amenable to targeted therapies, especially in sporadic DTs with CTNNB1 mutations;
- Detection of additional relevant genetic alterations. Beyond CTNNB1 and APC, other genetic changes—including chromosome 22 rearrangements and mutations in genes related to cell proliferation and tumor invasion—may be identified. These findings can contribute to a deeper understanding of DT biology and the mechanisms underlying tumor development.
- Pathological diagnosis should be made by a sarcoma expert pathologist according to the most current WHO classification of soft tissue and bone tumors (IV, A).
- Molecular testing is recommended to confirm the diagnosis in histologically or immunohistochemically equivocal cases and to exclude syndromic conditions (FAP) in patients with CTNNB1 wild-type DTs (IV, B).
- Genotyping for specific CTNNB1 mutations (S45F) may be considered to estimate the risk of local recurrence and guide surveillance intensity (IV, C).
- Molecular profiling may be useful in selected cases to identify potential therapeutic targets (V, C).
4. Management of DT
4.1. Surgical Approach
4.1.1. Intra-Abdominal DT
- Surgery is indicated in cases of severe intra-abdominal complications in sporadic DTs (IV, B).
- In FAP-associated DTs, surgery should be avoided whenever possible and only considered in life-threatening situations. Surgical intervention may be warranted in cases of tumor progression if AS fails (IV, B).
4.1.2. Abdominal Wall DT
- In sporadic DTs of the abdominal wall requiring active treatment, surgery should be considered a first-line option, alongside systemic therapies and local ablative approaches (IV, B).
4.1.3. Extra-Abdominal DT
- Surgery is indicated in cases of localized and easily resectable tumors with symptomatic disease (pain or functional impairment), especially when previous non-surgical approaches have failed (IV, B).
- Function-preserving surgery is highly recommended, prioritizing quality of life over obtaining wide resection margins (V, B).
4.2. Local Ablative Techniques
- Percutaneous cryoablation can be considered a reasonable local treatment option for small or medium-sized progressive or symptomatic extra-abdominal DTs (II, B).
4.3. Radiation Therapy
- RT should be reserved for progressive, symptomatic, or inoperable tumors when systemic therapies are contraindicated or ineffective, weighing potential long-term toxicities, particularly in patients under 30 years (IV, B).
- RT may be considered as an option in unresectable DMs or after incomplete (R1/R2) resections, particularly in recurrent cases, given its potential to improve local control (III, C).
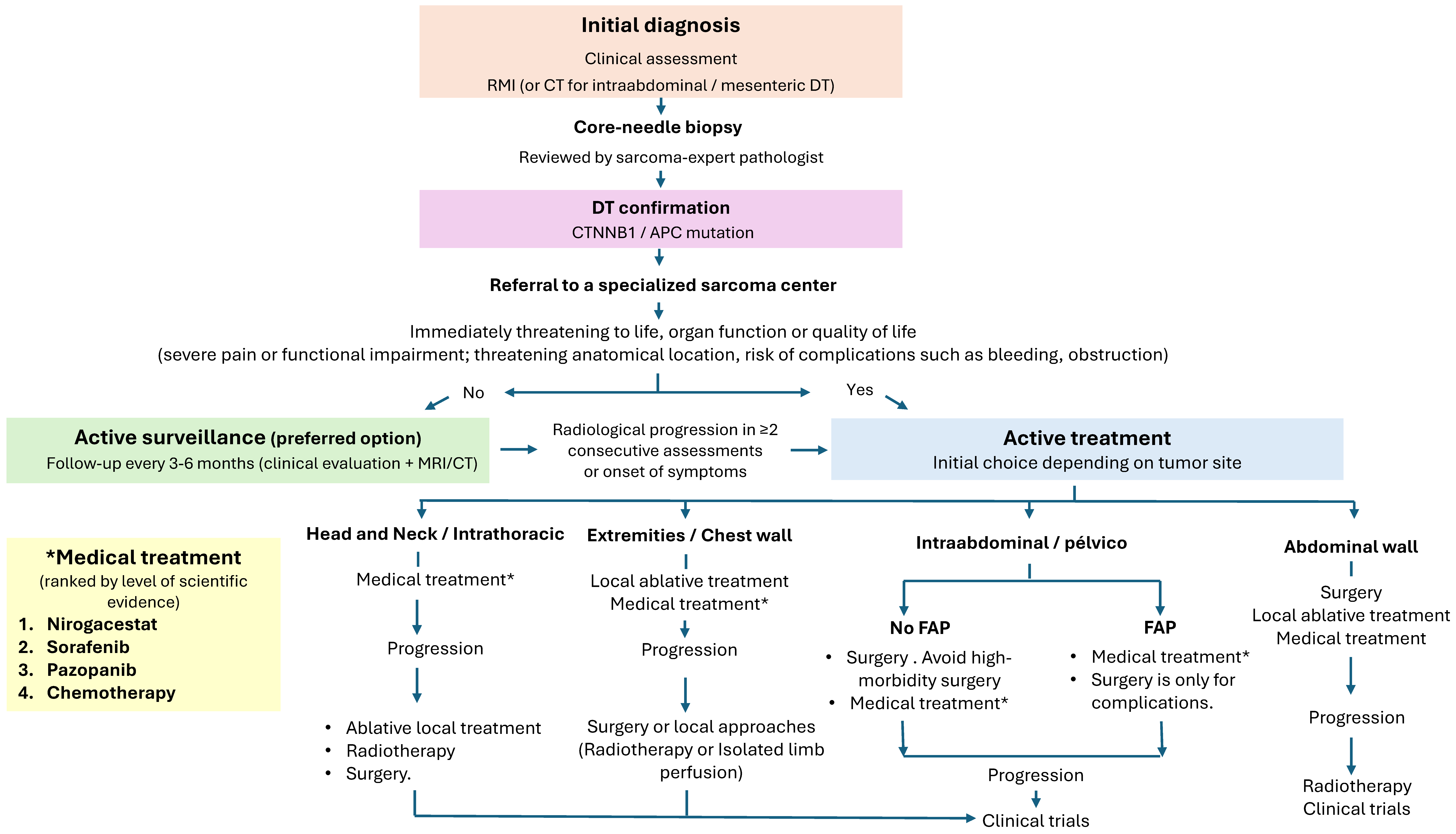
4.4. Active Surveillance
- AS is recommended as the initial approach for asymptomatic or stable patients, given the potential for spontaneous stabilization or regression (III, A).
4.5. Systemic Therapy
- Systemic treatment is indicated for symptomatic patients, rapid tumor progression, anatomical risk, or refractory or recurrent disease (III, A).
- Systemic Treatments for DTs:
- ○
- Sorafenib is strongly recommended, supported by a randomized phase III trial (I, A);
- ○
- Nirogacestat is strongly recommended following the results of the phase III DeFi trial (I, A);
- ○
- Pazopanib may be considered in refractory or progressive cases (II, B);
- ○
- Imatinib is also conditionally recommended in refractory disease. Its efficacy is variable, and evidence is primarily from small, non-randomized series (III, B);
- ○
- Hormonal therapies, such as tamoxifen, are not recommended due to very-low-quality evidence and the lack of a proven benefit in modern clinical studies (IV, D);
- ○
- Doxorubicin, either conventional or liposomal, with or without dacarbazine, is conditionally recommended for patients requiring rapid tumor control or with refractory/aggressive disease (III, B);
- ○
- Methotrexate combined with vinblastine or vinorelbine is recommended as a first-line systemic therapy in pediatric and young adult patients (II, B).
- It is recommended to continue systemic therapies for at least 6 to 12 months before evaluating their effectiveness (IV, B).
- Inclusion in clinical trials for advanced disease patients is highly recommended (V, A).
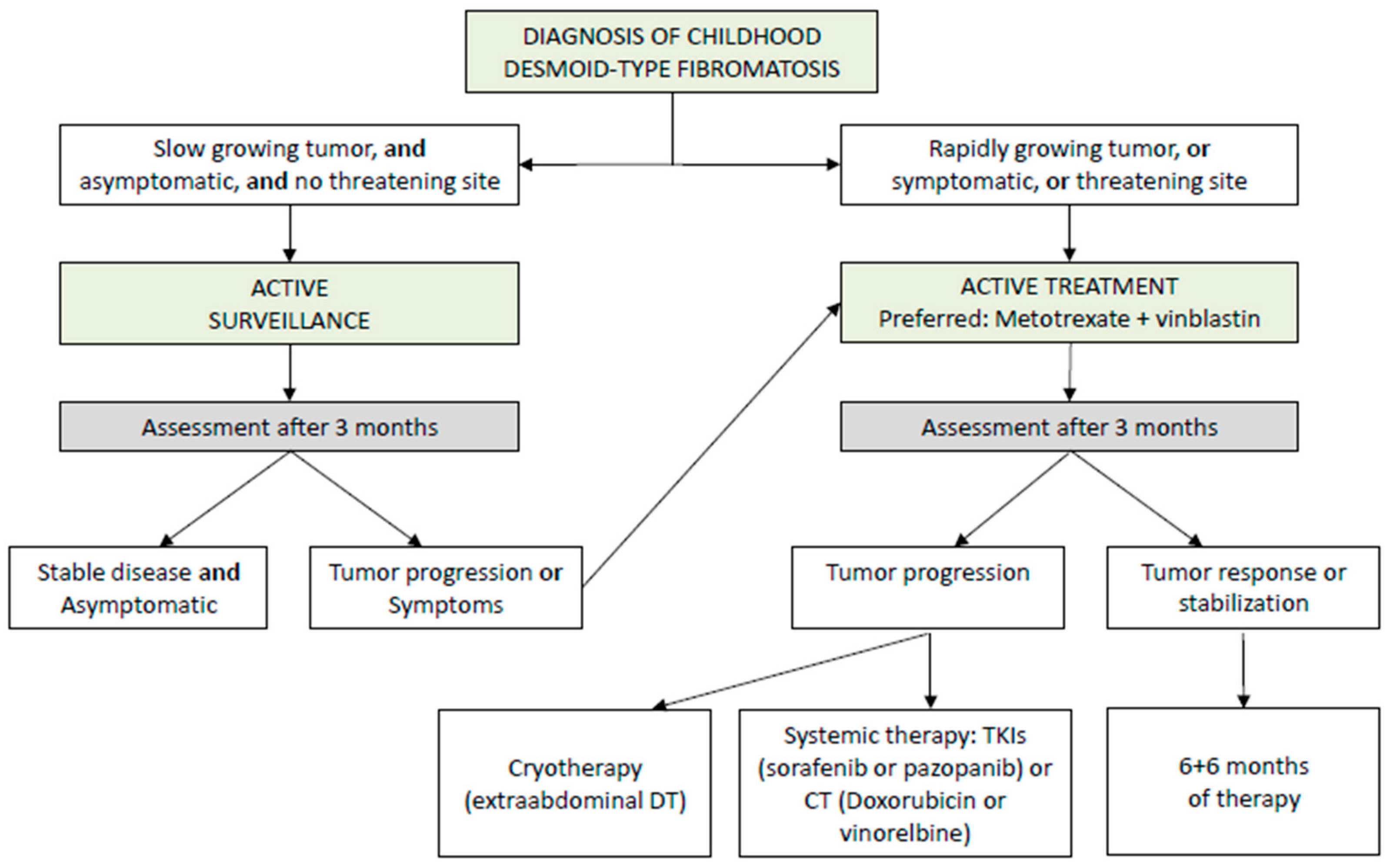
5. Childhood Desmoid Tumors
- Initial management should employ AS for tumors in non-critical sites and without significant symptoms (V, B).
- Active treatment should be considered in cases of clear progression, increasing pain, worsening symptoms, or tumors in high-risk locations (V, B).
- When treatment is required, a multidisciplinary approach in reference centers is recommended, prioritizing non-mutilating strategies and avoiding upfront aggressive surgery (V, C) Figure 5.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Penel, N.; Coindre, J.M.; Bonvalot, S.; Italiano, A.; Neuville, A.; Le Cesne, A.; Terrier, P.; Ray-Coquard, I.; Ranchere-Vince, D.; Robin, Y.M.; et al. Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France. Eur. J. Cancer 2016, 58, 90–96. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours, 4th ed.; Fletcher, C., Bridge, J.A., Hogendoorn, P.C.W., Mertens, F., Eds.; World Health Organization: Geneva, Switzerland, 2013; Volume 5, p. 468. [Google Scholar]
- Kasper, B.; Ströbel, P.; Hohenberger, P. Desmoid Tumors: Clinical Features and Treatment Options for Advanced Disease. Oncologist 2011, 16, 682–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotiligam, D.; Lazar, A.J.; Pollock, R.E.; Lev, D. Desmoid tumor: A disease opportune for molecular insights. Histol. Histopathol. 2008, 23, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, M.H.M.; Lefevre, J.H.; Bülow, S.M.; Järvinen, H.M.; Bertario, L.M.; Kernéis, S.; Parc, Y.M.; Vasen, H.F.A.M. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: An international cohort study. Dis. Colon Rectum 2011, 54, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Santti, K.; Ihalainen, H.; Rönty, M.; Karlsson, C.; Haglund, C.; Sampo, M.; Tarkkanen, M.; Blomqvist, C. Estrogen receptor beta expression correlates with proliferation in desmoid tumors. J. Surg. Oncol. 2019, 119, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Coppola, S.; Cannell, A.J.; Colombo, C.; Bertagnolli, M.M.; George, S.; Le Cesne, A.; Gladdy, R.A.; Casali, P.G.; Swallow, C.J.; et al. Desmoid-type fibromatosis and pregnancy: A multi-institutional analysis of recurrence and obstetric risk. Ann. Surg. 2014, 259, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Cates, J.M. Pregnancy does not increase the local recurrence rate after surgical resection of desmoid-type fibromatosis. Int. J. Clin. Oncol. 2015, 20, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Quintini, C.; Ward, G.; Shatnawei, A.; Xhaja, X.; Hashimoto, K.; Steiger, E.; Hammel, J.; Uso, T.D.; Burke, C.A.; Church, J.M.M. Mortality of intra-abdominal desmoid tumors in patients with familial adenomatous polyposis: A single center review of 154 patients. Ann. Surg. 2012, 255, 511–516. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Soft Tissue Sarcoma. Version 5.2024. Plymouth Meeting (PA): NCCN. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 10 March 2025).
- Van Houdt, W.J.; Wei, I.H.; Kuk, D.; Qin, L.X.; Jadeja, B.; Villano, A.; Hameed, M.; Singer, S.; Crago, A.M. Yield of Colonoscopy in Identification of Newly Diagnosed Desmoid-Type Fibromatosis with Underlying Familial Adenomatous Polyposis. Ann. Surg. Oncol. 2019, 26, 765–771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dykewicz, C.A. Centers for Disease C, and Prevention. Infectious Diseases Society of A. American Society of B, Marrow T Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2001, 33, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Martinetti, C.; Piscopo, F.; Buccicardi, D.; Schettini, D.; Neumaier, C.E.; Gandolfo, N.; Grazioli, L.; Gastaldo, A. Multimodality imaging features of desmoid tumors: A head-to-toe spectrum. Insights Imaging 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braschi-Amirfarzan, M.; Keraliya, A.R.; Krajewski, K.M.; Tirumani, S.H.; Shinagare, A.B.; Hornick, J.L.; Baldini, E.H.; George, S.; Ramaiya, N.H.; Jagannathan, J.P. Role of Imaging in Management of Desmoid-type Fibromatosis: A Primer for Radiologists. Radiographics 2016, 36, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.C.; Iyer, R.B.; Rashid, A.; Ellis, L.; Whitman, G.J. Desmoid tumor of the small bowel and the mesentery. AJR Am. J. Roentgenol. 2004, 183, 118. [Google Scholar] [CrossRef] [PubMed]
- Subhawong, T.K.; Feister, K.; Sweet, K.; Alperin, N.; Kwon, D.; Rosenberg, A.; Trent, J.; Wilky, B.A. MRI Volumetrics and Image Texture Analysis in Assessing Systemic Treatment Response in Extra-Abdominal Desmoid Fibromatosis. Radiol. Imaging Cancer 2021, 3, e210016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinauer, P.A.; Brixey, C.J.; Moncur, J.T.; Fanburg-Smith, J.C.; Murphey, M.D. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007, 27, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Krajewski, K.M.; Giardino, A.A.; Butrynski, J.E.; Raut, C.P. A to Z of desmoid tumors. AJR Am. J. Roentgenol. 2011, 197, W1008–W1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Oh, S.N.; Choi, M.H.; Rha, S.E.; Jung, S.E.; Byun, J.Y. The imaging features of desmoid tumors: The usefulness of diffusion weighted imaging to differentiate between desmoid and malignant soft tissue tumors. Investig. Magn. Reson. Imaging 2017, 21, 162–170. [Google Scholar] [CrossRef]
- Kurtz, J.E.; Buy, X.; Deschamps, F.; Sauleau, E.; Bouhamama, A.; Toulmonde, M.; Honoré, C.; Bertucci, F.; Brahmi, M.; Chevreau, C.; et al. CRYODESMO-O1: A prospective, open phase II study of cryoablation in desmoid tumour patients progressing after medical treatment. Eur. J. Cancer 2021, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Woodhead, G.; Hannallah, J.; Young, S. Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foster, C.R.; Strauss, M.; Hornick, J.L.; Habeeb, O. Desmoid Fibromatosis with TP53 Mutation and Striking Nuclear Pleomorphism. Int. J. Surg. Pathol. 2023, 31, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.W.; Fletcher, C.D. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: Analysis of a series and review of the literature. Histopathology 2007, 51, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. ESMO Guidelines Committee, EURACAN and GENTURIS. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Baldini, E.H.; Bonvalot, S.; Callegaro, D.; Cardona, K.; Colombo, C.; Corradini, N.; Crago, A.M.; Tos, A.P.D.; Dileo, P.; et al. Desmoid Tumor Working Group. Current Management of Desmoid Tumors: A Review. JAMA Oncol. 2024, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Schut, A.W.; Timbergen, M.J.M.; van Broekhoven, D.L.M.; van Dalen, T.; van Houdt, W.J.; Bonenkamp, J.J.; Sleijfer, S.; Grunhagen, D.J.; Verhoef, C.A. Nationwide Prospective Clinical Trial on Active Surveillance in Patients With Non-intraabdominal Desmoid-type Fibromatosis: The GRAFITI Trial. Ann. Surg. 2023, 277, 689–696, Erratum in Ann. Surg. 2023, 278, e911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nieuwenhuis, M.H.; Casparie, M.; Mathus-Vliegen, L.M.; Dekkers, O.M.; Hogendoorn, P.C.; Vasen, H.F. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int. J. Cancer 2011, 129, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Crago, A.M.; Chmielecki, J.; Rosenberg, M.; O’Connor, R.; Byrne, C.; Wilder, F.G.; Thorn, K.; Agius, P.; Kuk, D.; Socci, N.D.; et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer 2015, 54, 606–615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janssen, M.L.; van Broekhoven, D.L.; Cates, J.M.; Bramer, W.M.; Nuyttens, J.J.; Gronchi, A.; Salas, S.; Bonvalot, S.; Grünhagen, D.J.; Verhoef, C. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br. J. Surg. 2017, 104, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Canovai, E.; Butler, A.; Clark, S.; Latchford, A.; Sinha, A.; Sharkey, L.; Rutter, C.; Russell, N.; Upponi, S.; Amin, I. Treatment of Complex Desmoid Tumors in Familial Adenomatous Polyposis Syndrome by Intestinal Transplantation. Transplant. Direct. 2024, 10, e1571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilkinson, M.J.; Chan, K.E.; Hayes, A.J.; Strauss, D.C. Surgical outcomes following resection for sporadic abdominal wall fibromatosis. Ann. Surg. Oncol. 2014, 21, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Catania, G.; Ruggeri, L.; Iuppa, G.; Di Stefano, C.; Cardi, F.; Iuppa, A. Abdominal wall reconstruction with intraperitoneal prosthesis in desmoid tumors surgery. Updates Surg. 2012, 64, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.J.; Thomas, J.M. Desmoid tumours of the anterior abdominal wall. Eur. J. Surg. Oncol. 1999, 25, 398–400, Erratum in Eur. J. Surg. Oncol 1999, 25, 558. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Chiappa, A.; Testori, A.; Mazzarol, G.; Biffi, R.; Martella, S.; Pace, U.; Soteldo, J.; Vigna, P.D.; Lembo, R.; et al. Desmoid tumors of the anterior abdominal wall: Results from a monocentric surgical experience and review of the literature. Ann. Surg. Oncol. 2009, 16, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Ternès, N.; Fiore, M.; Bitsakou, G.; Colombo, C.; Honoré, C.; Marrari, A.; Le Cesne, A.; Perrone, F.; Dunant, A.; et al. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann. Surg. Oncol. 2013, 20, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Baumgarten, C.; Bonvalot, S.; Haas, R.; Haller, F.; Hohenberger, P.; Moreau, G.; van der Graaf, W.; Gronchi, A. Management of sporadic desmoid-type fibromatosis: A European consensus approach based on patients’ and professionals’ expertise—a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur. J. Cancer 2015, 51, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.; Gronchi, A. Desmoid tumours (extra-abdominal), a surgeon’s nightmare. Bone Jt. J. 2023, 105-B, 729–734, Erratum in Bone Jt. J. 2023, 105-B, 1030. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.M.; Bell, T.; Tumminello, B.; Khan, S.; Zhou, S.; Oton, A.B. Disease and economic burden of surgery in desmoid tumors: A review. Expert. Rev. Pharmacoecon Outcomes Res. 2023, 23, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hamada, S.; Kawai, A.; Kunisada, T.; Ogose, A.; Matsumoto, Y.; Ae, K.; Toguchida, J.; Ozaki, T.; Hirakawa, A.; et al. Risk factors of local recurrence after surgery in extraabdominal desmoid-type fibromatosis: A multicenter study in Japan. Cancer Sci. 2020, 111, 2935–2942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papalexis, N.; Savarese, L.G.; Peta, G.; Errani, C.; Tuzzato, G.; Spinnato, P.; Ponti, F.; Miceli, M.; Facchini, G. The New Ice Age of Musculoskeletal Intervention: Role of Percutaneous Cryoablation in Bone and Soft Tissue Tumors. Curr. Oncol. 2023, 30, 6744–6770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pal, K.; Awad, A.; Yevich, S.; Kuban, J.D.; Tam, A.L.; Huang, S.Y.; Odisio, B.C.; Gupta, S.; Habibollahi, P.; Bishop, A.J.; et al. Safety and Efficacy of Percutaneous Cryoablation for Recurrent or Metastatic Soft-Tissue Sarcoma in Adult Patients. AJR Am. J. Roentgenol. 2024, 223, e2431490, Erratum in AJR Am. J. Roentgenol. 2024, 223, e2432283. [Google Scholar] [CrossRef] [PubMed]
- Auloge, P.; Cazzato, R.L.; Rousseau, C.; Caudrelier, J.; Koch, G.; Rao, P.; Chiang, J.B.; Garnon, J.; Gangi, A. Complications of Percutaneous Bone Tumor Cryoablation: A 10-year Experience. Radiology 2019, 291, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Alaseem, A.; Alsaikhan, N.; AlSudairi, A.M.; Alsehibani, Y.A.; Alhuqbani, M.N.; Aldosari, Z.A.; Aldosari, O.A.; Almuhanna, A.; Alshaygy, I.S. Systematic review of transarterial chemoembolization for desmoid tumors: A promising locoregional treatment for challenging tumor. Discov. Oncol. 2025, 16, 1193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chetan, M.; Gillies, M.; Rehman, S.; McCarthy, C.; Cosker, T.; Wu, F.; Lyon, P.C. High-intensity focused ultrasound treatment of unresectable soft tissue sarcoma and desmoid tumours—A systematic review. Clin. Radiol. 2025, 87, 106977. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Assi, T.; Khoury, R.; Ngo, C.; Faron, M.; Verret, B.; Lévy, A.; Honoré, C.; Hénon, C.; Le Péchoux, C.; et al. Desmoid-type fibromatosis: Current therapeutic strategies and future perspectives. Cancer Treat. Rev. 2024, 123, 102675. [Google Scholar] [CrossRef] [PubMed]
- Matsunobu, T.; Kunisada, T.; Ozaki, T.; Iwamoto, Y.; Yoshida, M.; Nishida, Y. Definitive radiation therapy in patients with unresectable desmoid tumors: A systematic review. Jpn. J. Clin. Oncol. 2020, 50, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Nuyttens, J.J.; Rust, P.F.; Thomas, C.R., Jr.; Turrisi, A.T., 3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000, 88, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Colombo, C.; Le Péchoux, C.; Dei Tos, A.P.; Le Cesne, A.; Marrari, A.; Penel, N.; Grignani, G.; Blay, J.Y.; Casali, P.G.; et al. Sporadic desmoid-type fibromatosis: A stepwise approach to a non-metastasising neoplasm--a position paper from the Italian and the French Sarcoma Group. Ann. Oncol. 2014, 25, 578–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guadagnolo, B.A.; Zagars, G.K.; Ballo, M.T. Long-term outcomes for desmoid tumors treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Keus, R.B.; Nout, R.A.; Blay, J.Y.; de Jong, J.M.; Hennig, I.; Saran, F.; Hartmann, J.T.; Sunyach, M.P.; Gwyther, S.J.; Ouali, M.; et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis--an EORTC STBSG and ROG study (EORTC 62991-22998). Ann Oncol. 2013, 24, 2672–2676. [Google Scholar] [CrossRef] [PubMed]
- Timbergen, M.J.M.; Schut, A.W.; Grünhagen, D.J.; Sleijfer, S.; Verhoef, C. Active surveillance in desmoid-type fibromatosis: A systematic literature review. Eur. J. Cancer 2020, 137, 18–29. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, V.P.; Keohan, M.L.; D’Adamo, D.R.; Antonescu, C.R.; Brennan, M.F.; Singer, S.; Ahn, L.S.; Maki, R.G. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010, 116, 2258–2265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constantinidou, A.; Jones, R.L.; Scurr, M.; Al-Muderis, O.; Judson, I. Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur. J. Cancer 2009, 45, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Azzarelli, A.; Gronchi, A.; Bertulli, R.; Tesoro, J.D.; Baratti, D.; Pennacchioli, E.; Dileo, P.; Rasponi, A.; Ferrari, A.; Pilotti, S.; et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer 2001, 92, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Pulido, M.; Ray-Coquard, I.; Andre, T.; Isambert, N.; Chevreau, C.; Penel, N.; Bompas, E.; Saada, E.; Bertucci, F.; et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol. 2019, 20, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Gruenwald, V.; Reichardt, P.; Bauer, S.; Rauch, G.; Limprecht, R.; Sommer, M.; Dimitrakopoulou-Strauss, A.; Pilz, L.; Haller, F.; et al. Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur. J. Cancer 2017, 76, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Mahoney, M.R.; Van Tine, B.A.; Ravi, V.; Attia, S.; Deshpande, H.A.; Gupta, A.A.; Milhem, M.M.; Conry, R.M.; Movva, S.; et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 2018, 379, 2417–2428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gounder, M.; Ratan, R.; Alcindor, T.; Schöffski, P.; van der Graaf, W.T.; Wilky, B.A.; Riedel, R.F.; Lim, A.; Smith, L.M.; Moody, S.; et al. Nirogacestat, a γ-Secretase Inhibitor for Desmoid Tumors. N. Engl. J. Med. 2023, 388, 898–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braggio, D.A.; Costas Cde Faria, F.; Koller, D.; Jin, F.; Zewdu, A.; Lopez, G.; Batte, K.; Casadei, L.; Welliver, M.; Horrigan, S.K.; et al. Preclinical efficacy of the Wnt/β-catenin pathway inhibitor BC2059 for the treatment of desmoid tumors. PLoS ONE 2022, 17, e0276047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gounder, M.; Jones, R.L.; Chugh, R.; Agulnik, M.; Singh, A.S.; Van Tine, B.A.; Andelkovic, V.; Choy, E.; Lewing, J.H.; Ratan, R.; et al. RINGSIDE phase 2/3 trial of AL102 for treatment of desmoid tumors (DT): Phase 2 results. J. Clin. Oncol. 2023, 41 (Suppl. 16), 11515. [Google Scholar] [CrossRef]
- Orbach, D.; Brennan, B.; Bisogno, G.; Van Noesel, M.; Minard-Colin, V.; Daragjati, J.; Casanova, M.; Corradini, N.; Zanetti, I.; De Salvo, G.L.; et al. The EpSSG NRSTS 2005 treatment protocol for desmoid-type fibromatosis in children: An international prospective case series. Lancet Child Adolesc. Health 2017, 1, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Brennan, B.; Casanova, M.; Corradini, N.; Berlanga, P.; Schoot, R.A.; Ramirez-Villar, G.L.; Safwat, A.; Guillen Burrieza, G.; Dall’Igna, P.; et al. Pediatric Non-Rhabdomyosarcoma Soft Tissue Sarcomas: Standard of Care and Treatment Recommendations from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer Manag. Res. 2022, 14, 2885–2902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constantinidou, A.; Jones, R.L.; Scurr, M.; Judson, I. Advanced aggressive fibromatosis: Effective palliation with chemotherapy. Acta Oncol. 2011, 50, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Orbach, D.; Affinita, M.C.; Chiaravalli, S.; Corradini, N.; Meazza, C.; Bisogno, G.; Casanova, M. Evidence of hydroxyurea activity in children with pretreated desmoid-type fibromatosis: A new option in the armamentarium of systemic therapies. Pediatr. Blood Cancer 2019, 66, e27472. [Google Scholar] [CrossRef] [PubMed]
- Sparber-Sauer, M.; Orbach, D.; Navid, F.; Hettmer, S.; Skapek, S.; Corradini, N.; Casanova, M.; Weiss, A.; Schwab, M.; Ferrari, A. Rationale for the use of tyrosine kinase inhibitors in the treatment of paediatric desmoid-type fibromatosis. Br. J. Cancer 2021, 124, 1637–1646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borovkov, A.; Orbach, D.; Bonneau-Lagacherie, J.; Carausu, L.; Guimard, G.; Haouy, S.; Jannier, S.; Pagnier, A.; Proust, S.; Verite, C.; et al. Desmoid-TypeFibromatosis Tumors in Children After First-Line Failure: Clinical Aspects and Approaches for Subsequent Therapeutic Lines. Pediatr. Blood Cancer 2025, 72, e31866. [Google Scholar] [CrossRef] [PubMed]
- van Maren, S.A.; van Noesel, M.M.; Husson, O.; van der Graaf, W.T.A. Clinical trials in desmoid-type fibromatosis in children and adults: A systematic review. Pediatr. Blood Cancer 2022, 69, e29831. [Google Scholar] [CrossRef] [PubMed]
- Vora, B.M.K.; Munk, P.L.; Somasundaram, N.; Ouellette, H.A.; Mallinson, P.I.; Sheikh, A.; Abdul Kadir, H.; Tan, T.J.; Yan, Y.Y. Cryotherapy in extra-abdominal desmoid tumors: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0261657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Levels of Evidence | |
|---|---|
| I | Evidence from at least one large, randomized, controlled trial of good methodological quality (low potential for bias), or meta-analyses of well-conducted randomized trials without heterogeneity |
| II | Small randomized trials or large randomized trials with a suspicion of bias (lower methodological quality), or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III | Prospective cohort studies |
| IV | Retrospective cohort studies or case–control studies |
| V | Studies without a control group, case reports, and experts’ opinions |
| Grades of recommendation | |
| A | Strong evidence for efficacy with a substantial clinical benefit, strongly recommended |
| B | Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C | Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (adverse events, costs…), optional |
| Drug | Study Type (Key Trial) | N | Dose and Administration | ORR | PFS/Main Outcome | Toxicity |
|---|---|---|---|---|---|---|
| Conventional chemotherapy [52,53,54] |
| 51 (MTS/VBL) 62 (Anthracyclines) 40 |
|
|
|
|
| Sorafenib [57] (TKI) | Phase III randomized, double-blind | 50 sorafenib 37 placebo |
| 33% vs. 20% (placebo) | 2-yr PFS 81% vs. 36%; HR 0.13 (0.05–0.31) | Rash, fatigue, hypertension, diarrhea (mostly G1–2) |
| Pazopanib [55] (TKI) | Phase II randomized non-comparative | 48 pazopanib 24 MTS-VBL | 800 mg orally once daily | 37% PR (PZ) vs. 25% PR (MTX/VBL) | 6-mo non-progression 83.7% (PZ) vs. 45.0% (MTX/VBL) | Pazopanib G3–4 toxicity: Hypertension (21%), diarrhea (15%) |
| Imatinib [56] (TKI) | Phase II multicenter. Retrospective series | 51/40 | 400–800 mg orally once daily | 6–19% (phase II); up to 23% (retrospective) | 1-year PFS: 66–80%; 2-yr PFS 45–55% | Edema, fatigue, nausea, rash, mild cytopenias |
| Nirogacestat [58] (GSI) | Phase III randomized, double-blind. | 70 nirogacestat 72 placebo | 150 mg orally twice daily. | ~41% vs. 8% (placebo) | HR 0.29 (0.15–0.55); Estimated 2-yr PFS 76% vs. 44% Significant improvement in pain and function | Diarrhea, nausea, fatigue, rash, hypophosphatemia; ovarian dysfunction (~75%, reversible in ~74%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, A.A.; Carolina, A.P.; Marta, A.V.; Francisco, A.; Adriana, F.G.; Natalia, G.; Pablo, L.L.; Cristina, M.F.; Lidia, M.S.; Ulrike, N.; et al. Desmoid Tumors—Experience from a Referral Center, Part 1: Multidisciplinary Review and Practical Recommendations. Cancers 2025, 17, 3470. https://doi.org/10.3390/cancers17213470
Rosa AA, Carolina AP, Marta AV, Francisco A, Adriana FG, Natalia G, Pablo LL, Cristina MF, Lidia MS, Ulrike N, et al. Desmoid Tumors—Experience from a Referral Center, Part 1: Multidisciplinary Review and Practical Recommendations. Cancers. 2025; 17(21):3470. https://doi.org/10.3390/cancers17213470
Chicago/Turabian StyleRosa, Alvarez Alvarez, Agra Pujol Carolina, Arregui Valles Marta, Alijo Francisco, Fernández Gonzalo Adriana, Gutiérrez Natalia, Lozano Lominchar Pablo, Mata Fernández Cristina, Mediavilla Santos Lidia, Novo Ulrike, and et al. 2025. "Desmoid Tumors—Experience from a Referral Center, Part 1: Multidisciplinary Review and Practical Recommendations" Cancers 17, no. 21: 3470. https://doi.org/10.3390/cancers17213470
APA StyleRosa, A. A., Carolina, A. P., Marta, A. V., Francisco, A., Adriana, F. G., Natalia, G., Pablo, L. L., Cristina, M. F., Lidia, M. S., Ulrike, N., Marina, S., Guillermo, H. T., Henar, C. G., & Ana, G.-O. d. l. T. (2025). Desmoid Tumors—Experience from a Referral Center, Part 1: Multidisciplinary Review and Practical Recommendations. Cancers, 17(21), 3470. https://doi.org/10.3390/cancers17213470






