Simple Summary
SLAMF7 is highly expressed on myeloma cells at all disease stages, with a limited expression on normal tissues, and, potentially, with few side effects. It works by activating immune cells to kill myeloma cells and could represents a platform for next-generation therapies, such as CAR-T cells, bispecific antibodies, and antibody–drug conjugates. SLAMF7’s combination of specificity, stability, and clinical validation makes it an excellent target for current and future multiple myeloma therapies.
Abstract
Signaling Lymphocyte Activation Molecule Family member 7 (SLAMF7) represents a potential target for CAR T-cell therapy in the treatment of multiple myeloma (MM), and it is a promising alternative to classic BCMA-CAR therapy. The receptor is expressed on immune cells, particularly natural killer cells and T cells, that can trigger both activating and inhibitory signals. It is highly expressed in MM cells at all disease stages, playing a crucial role in cell adhesion and communication between immune cells, and being involved in the development and progression of the disease. The target has a proven clinical success with Elotuzumab (anti-SLAMF7 antibody); it works by activating immune cells to kill myeloma cells, and limited expression on normal tissues, with, potentially, few side effects. SLAMF7’s combination of specificity, stability, and clinical validation makes it an excellent target for current and future MM therapies. However, ‘fratricide death’, a phenomenon where the engineered CAR-T cells attack and kill each other, is a critical issue that requires safe engineering solutions. In this work, we will provide an overview on the field with a specific focus on SLAMF7 as an emerging CAR-T cell target in MM.
Keywords:
multiple myeloma; CAR-T cell therapy; SLAMF7; new CAR-T targets; BCMA; elotuzumab; dual CAR-T 1. Introduction
Multiple myeloma (MM) constitutes 1–2% of all cancers and 10–15% of haematological malignancies [1], with an estimated incidence of 4.5–6.0 cases per 100,000 persons per year, in individuals with a median age of 65–70 years [2]. MM has experienced a significant enhancement in survival outcomes over the past two decades, which is indicative of the advancements in medical research and treatment options [3]. Cellular immunotherapies, in particular anti-BCMA autologous chimeric antigen receptor (CAR) T-cell therapy, have played a pivotal role in this progress [4]. The anti-BCMA CAR-T idecabtagenevicleucel (Ide-cel) and ciltacabtageneautoleucel (Cilta-cel), represent a significant development in the field of cancer therapy, as they are the first to be approved for use in patients with relapsed (R) or refractory (R) MM [5,6,7,8]. Despite the initial promise of successful outcomes, most patients ultimately experience disease relapse or progression, with a 5-year overall survival (OS) rate of approximately 60%. Only 10–15% of patients achieve the expected survival rate of the general population [9,10]. Consequently, in the current state of knowledge, MM can still be regarded as an incurable disease. CAR-T cell therapy for MM has several limitations. One is the risk of relapse due to antigen loss or tumour heterogeneity, whereby cancer cells no longer express the target antigen or exhibit significant variation; patients may also experience severe side effects; and the manufacturing process is complex and costly, and further research is needed to improve durability and broaden applicability.
2. SLAMF7 Target
Signaling Lymphocytic Activation Molecule Family Member 7 (SLAMF7) is a cell surface protein expressed on natural killer (NK) cells and in over 95% of malignant plasma cells (mPCs) [11,12]. Its role in PC survival renders it an attractive target for therapeutic intervention. The SLAM family of receptors, which are expressed on hematopoietic cells, plays a crucial role in regulating the immune system [13,14]. The SLAM family comprises six members: CD229, SLAM, NTB-A, 2B4, CD84, and CS1. SLAMF7, also known as CS1/CRACC/CD319, has been shown to promote the proliferation and growth of MM cells due to its high expression in these cells [15]. Furthermore, the expression of this protein is also observed in B cells, T cells, dendritic cells, NK cells, and monocytes. SLAMF7 has been implicated in various biological processes, including cell viability, humoral immunity, autoimmunity, cell adhesion, and lymphocyte development. SLAMF7 plays a crucial role in the pathogenesis of MM, and it possesses unique characteristics that make it a promising target for immunotherapeutic interventions [16]. Novel therapeutic interventions that target SLAMF7 include elotuzumab (ELO), a humanized IgG1 kappa monoclonal antibody [17], and CAR-T [18,19]. ELO, a humanized IgG1 kappa monoclonal antibody, activates NK cells directly and through antibody-dependent cellular cytotoxicity [12,17]. ELO enhances SLAMF7-SLAMF7 interactions between NK cells and myeloma cells, sending costimulatory signals that enhance killing. While ELO has minimal clinical efficacy as a single agent, it is effective in combination with lenalidomide and dexamethasone [20]. CAR T cells require lower antigen density for activation than antibodies do [21]. This is a critical difference: antigen downregulation below the CAR activation threshold leaves the T cell silent, rendering CAR T cell therapy ineffective. The binding affinity of the antigen recognition domain substantially affects the sensitivity of CAR T cells to antigens. The threshold for CAR T cell activation is inversely correlated with scFv affinity [22]. Even though SLAMF7 CAR T cells use the same targeting domain as ELO, the CAR format allows recognition at lower antigen densities. When CAR T cells meet their targeted antigen on a cell’s surface, the cells bind to it, become activated, and proceed to proliferate and become cytotoxic. They kill tumor cells directly through multiple mechanisms, including cytotoxicity and cytokine secretion. Each CAR T cell can also proliferate extensively after encountering antigen, creating a self-amplifying effect. These mechanistic differences mean that CAR T cells are potentially more potent, but they also carry risks of more severe side effects, such as cytokine release syndrome (CRS). CAR T-cells can eliminate tumor cells that might resist antibody therapy due to their lower antigen density threshold and direct cytotoxicity. However, these same properties increase the risk of on-target/off-tumor toxicity against normal SLAMF7+ cells.
The present article will evaluate the potential of SLAMF7 as a CAR-T target in MM, emphasizing the potential role in the advancement of treatment options and instilling a sense of hope and optimism in the audience.
3. The Challenges and Limitations of CAR-T Therapy in Multiple Myeloma
CAR-T in MM is a revolutionary therapy, albeit not yet definitive. Indeed, while offering a valuable therapeutic option for heavily pretreated patients, its durability is still evolving, thus not curative for most patients. The treatment has been shown to have a high initial response rate (RR) (e.g., >70% ORR in Ide-cel and Cilta-cel trials), although many patients relapse within 12–18 months [4,5,6,7,8]. However, the ongoing research and development in the field of CAR-T therapy provide reassurance about the continuous efforts to improve MM treatment.
Commercial CAR-T therapies in MM target BCMA [23,24]. Despite the dramatic responses produced by CAR-T cell therapy, most patients will eventually progress. The reasons for this are multifactorial. The hypothesis that tumour cells may downregulate or even lose BCMA has been proposed as a mechanism leading to relapse due to immune evasion [25,26,27]. Several other factors must be considered, including the exhaustion of CAR-T cells, the presence of an immunosuppressive tumor microenvironment, and the persistence of myeloma stem-like cells [28]. CAR-T cell exhaustion, characterized by poor persistence and dysfunction due to persistent antigen stimulation and an immunosuppressive tumour environment, further complicates following treatments [29]. Prior antimyeloma therapy has been identified as a host-related factor influencing CAR-T response, resulting in impaired T cell function and a microenvironmental deficit [30]. Tumor-intrinsic resistance mechanisms have been shown to include complex genomic alterations and immunomodulatory gene mutations [31]. Systemic inflammation prior to CAR-T administration has been identified as a potent biomarker of response, reflecting a pro-inflammatory tumour microenvironment characterized by regulatory T-cell populations and myeloid-derived suppressor cells [32,33].
Putative targeting molecules identification other than BCMA is a key area of clinical study and development [34,35,36]. MM cells express a variety of surface antigens (Figure 1 and Table 1). Expression patterns of these and other antigens can be used for diagnosis, monitoring, and have prognostic value The main targets under study are summarized in Table 2, with their biological characteristics and the pros and cons of their use [37,38,39,40]. While most targets are still in the preclinical stage, only a small number are currently being explored in clinical trials. GPRC5D has been identified as a promising therapeutic target due to its high expression levels in myeloma cells, in contrast to its minimal expression in normal tissues, with an expression restricted primarily to hair follicles, hard keratinizing tissue (hair shaft, nail, tongue center), and a subset of cells in skin [41]. In a recently published meta-analysis, 130 MM patients treated with GPRC5D-targeted CAR T-cell therapy demonstrated an ORR of 87%, with 74% of these patients having received prior BCMA-targeted therapy. The proportion of patients achieving a partial response (PR) was 25%, while 33% achieved a very good partial response (VGPR), and 48% achieved a complete/stringent complete response (CR/sCR). Furthermore, 65% of patients achieved minimal residual disease (MRD) negativity [42]. These results suggest that GPRC5D is an attractive target in the treatment of R/R MM and heavily pretreated patients. GPRC5D is independent of BCMA expression patterns on myeloma cells, and it appears to be the ideal candidate in the relay of myeloma treatment at relapse after anti-BCMA therapies. SLAMF7 is a valid target but is less clinically advanced, with ongoing trials still evaluating its full potential. While individual studies show promising results for each, such as a high ORR for SLAMF7-CAR-T and high CR rates for both BCMA and GPRC5D-CAR-T, a direct head-to-head comparison to determine which is superior is currently not available.
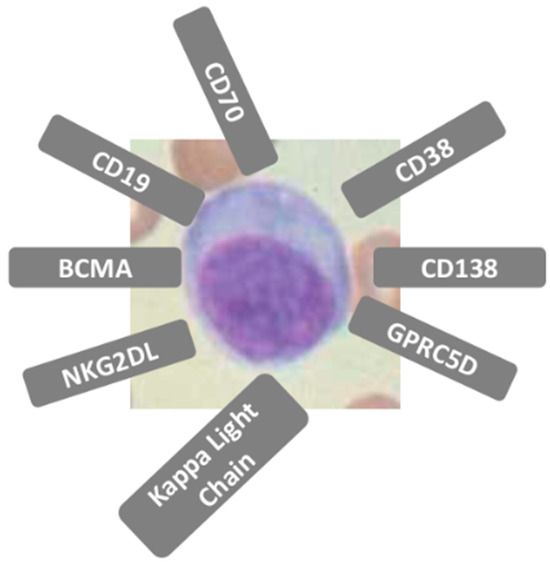
Figure 1.
Multiple myeloma cells express a variety of surface antigens. Expression patterns of these and other antigens can be used for diagnosis, monitoring, and have prognostic value.

Table 1.
SLAMF7 as a CAR-T Target in multiple myeloma.

Table 2.
Targeting molecules other than BCMA in Multiple Myeloma.
Another strategy, which is still under investigation, involves dual-targeting of CARs [43]. Many ongoing investigations are to evaluate dual-targeting strategies with various combinations based on BCMA, CD19, CD138, and SLAMF7, together or in sequence, and some already showed improved clinical activity in early phase clinical studies [44]. Regarding treatment approaches to augment anti-MM activity of a single targeted immunotherapeutic agent or to overcome an immunosuppressive BM microenvironment, a combination of different anti-MM agents with distinct mechanisms of action remains very attractive strategies. The primary objective of this approach is twofold: to enhance tumour coverage and to minimize antigen loss.
CAR-T cell exhaustion prevention is explored through the optimization of CAR-T cell structure, the utilization of early memory T cells, and the inhibition of intracellular exhaustion-related signals through genetic modifications or inhibitors [45]. The enhancement of tumor activity of CAR-T cells and central memory CAR-T cells can be achieved through genetic modifications, such as the incorporation of immune-stimulatory receptors and the deletion of genes associated with CAR-T cell anergy. Research has demonstrated that armored CAR-T cells, which secrete cytokines or express pro-inflammatory ligands, have the capacity to reshape the tumor microenvironment [46]. Overcoming immunosuppressive cells in the bone marrow is crucial in the management of MM. These cells, including osteoclasts, myeloid-derived suppressor cells, tumor-associated macrophages, regulatory T cells, regulatory B cells, tumor-associated neutrophils, and bone marrow stromal cells, engage in signal integration with MM cells, facilitating their survival and proliferation. In addition, these cells have been shown to inhibit the activity of effector T cells, thereby enabling MM cells to evade immune surveillance.
Another significant issue is toxicity, and CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) are prevalent [47]. The incidence of CRS ranges from 80% to 90% among patients, predominantly manifesting as mild symptoms, though in some instances, it can be severe; ICANS is less prevalent but can be life-threatening [48]. Prolonged cytopenias, infections, and hypogammaglobulinemia represent additional risks [49].
Manufacturing time and production failure represent additional factors that contribute to this phenomenon [50]. Autologous (auto) CAR-T production requires 3–5 weeks (“vein-to-vein” time), and patients with aggressive disease may experience disease progression during this period. The risk of manufacturing failure is high if the T cells collection is impaired due to excessive pretreatment [51]. Based on these considerations, CAR-T therapy is currently available only in specialized centers that require highly trained staff, intensive monitoring, and inpatient capacity for toxicity management. Access is especially limited in low- and middle-income countries [52].
4. SLAMF7-CAR-T Construct
A SLAMF7-CAR-T construct has been developed in Germany, and the preclinical results were published in 2017 [53]. The construct is composed of a lentiviral vector that contains a targeting domain derived from the monoclonal antibody targeting SLAM7, huLuc63, fused to an IgG4-Fc Hinge-CH2-CH3 spacer with a 4/2NQ modification to prevent binding of Fc-receptors, a CD28/CD3zeta signaling module in cis with a T2A element, and a truncated epidermal growth factor receptor (EGFRt). This construct was selected for its ability to reduce FcγR binding and prevent off-target activation. SLAMF7 CAR T-cells were successfully prepared using CD8+ and CD4+ T-cells from MM patients and healthy donors. In vitro, the efficacy of the drug was demonstrated using SLAMF7 myeloma cell lines, with 80% and 90% lysis observed after 4 h and 20 h, respectively [53]. Furthermore, the drug promoted productive proliferation, and no differences were observed between newly diagnosed and RR MM.
CAR-T cells have been shown to selectively eliminate SLAMF7+high lymphocytes, including NK cells, CD4+ and CD8+ T cells, and B cells, while preserving the function of other lymphocytes. The internalization of the SLAMF7 protein has been demonstrated to reduce expression, thereby enabling CD8+ and CD4+ cells to acquire a low SLAMF7 phenotype [14]. A CD8+ fratricide-resistant SLAMF7 CAR-T was developed, thereby ensuring the preservation of functional CD8+ T cell cultures. Fratricide death occurs because SLAMF7 is expressed on the CAR-T cells themselves, leading them to attack each other during manufacturing and after infusion. Given that SLAMF7 is expressed in NK cells, this therapy can result in cell death and deficiencies, potentially leading to severe infections. A suicide gene within the SLAMF7 CAR-T encodes a dimerization domain containing a caspase-9 domain, which is efficacious in the high-tumor-burden myeloma model, despite the fratricide of CD8+CS1-expressing CAR-T cells [13]. This approach facilitates the elimination of SLAMF7 CAR-T cells prior to NK cell depletion, thereby substantially enhancing the development of antitumor activity [53].
5. SLAMF7-CAR-T Clinical Trials
Several researchers have developed anti-SLAMF7 CAR T cells and tested them in preclinical models [14,22,53]. Gogishvili et al. developed an anti-SLAMF7 CAR T cell construct using the target-binding domain of ELO paired with a CD28 co-stimulatory domain [53]. O’Neal et al. [14] generated anti-SLAMF7 CAR T cells with a different target-binding epitope (the distal V2 domain) and a third generation CD28 and 4-1BB combination co-stimulatory domain. These cells effectively killed malignant plasma cells [22]. These CAR T cells were predominantly CD4+, suggesting that CD8+ CAR T cells were destroyed during ex vivo production. Consequently, they used CRISPR/Cas9 technology to delete SLAMF7 and create anti-SLAMF7 CAR T cells that were resistant to fratricide. Despite yielding a more balanced CD4:CD8 T cell profile, SLAMF7-deficient CAR T cells were not significantly more effective in murine xenograft models. Overall, while the consequences of fratricide for anti-SLAMF7 CAR T cells and other immune cells require further investigation, these studies suggest that anti-SLAMF7 CAR T cells could be a promising therapeutic strategy for treating myeloma.
5.1. Autologous Approaches
As previously indicated, SLAMF7 is expressed on NK cells, and a subset of CD8+ T cells. The depletion of these leukocyte subpopulations, induced by drugs targeting the antigen, has been demonstrated to result in an increased risk of infection [14]. Incorporate a suicide gene to enhance safety, particularly with respect to the risk of infectious complications. In the aforementioned “A Phase I Clinical Trial of T-cells Expressing an Anti-SLAMF7 CAR for Treating Multiple Myeloma,” the authors have engineered a novel anti-SLAMF7 CAR capable of specifically recognizing and eradicating SLAMF7-expressing tumors in murine models. The protocol involves the genetic modification of autologous T cells with genes that encode an inducible caspase 9 (IC9) cell suicide system, in conjunction with the anti-SLAMF7 CAR-T cell therapy [54]. The administration of the dimerizer drug Rimiducid is crucial for activating the IC9 suicide gene, thereby leading to the eradication of CAR T cells. In this protocol, the researchers employed the suicide gene system to eliminate CAR-T cells in the event of severe toxicities [55]. One potential drawback is the premature elimination of CAR-T cells [56]. If the dimerizer drug is administered too early or by accident, the therapeutic CAR-T cells may be eliminated before they can fight the tumor, which would compromise the treatment’s effectiveness. Once activated, the suicide mechanism cannot be reversed, necessitating additional CAR-T infusions if the tumor persists. Activation eliminates the potential for durable CAR-T cell persistence, which is crucial for preventing relapse.
CARAMBA-1 is a pioneering clinical trial of adoptive immunotherapy with auto SLAMF7 CAR-T cells in patients with advanced MM who have exhausted conventional therapies [57]. The CARAMBA-1 clinical trial is an open-label, non-randomized, multicenter study that combined a phase I dose-escalation with a phase II dose-expansion part to assess the feasibility, safety, and antimyeloma activity of SLAMF7 CAR-T cells [58]. The trial distinguishing characteristic is its provision of clinical evidence substantiating the notion of virus-free CAR gene transfer through the implementation of advanced “Sleeping Beauty” transposon technology [59]. The transposition of Sleeping Beauty in CAR-T engineering is a compelling prospect, given the high rate of stable CAR gene transfer facilitated by optimized hyperactive SB100X transposase and transposon combinations, encoded by mRNA and minicircle DNA, respectively, as preferred vector embodiments [60]. This approach has the potential to facilitate and expedite vector procurement, CAR-T manufacturing, and distribution, and promises to provide a safe, effective, and economically sustainable treatment. Its main advantages over viral systems like lentiviruses are its non-viral nature, potentially lower manufacturing cost and faster production timeline. CARAMBA cell manufacturing takes 14 days in total. Starting from an unmobilized leukapheresis targeting ≥5 × 109 total white blood cells, the leukocytes are split so that both portions will contain approximately similar total numbers of CD4+ and CD8+ T cells. The CARAMBA is the first clinical trial with virus-free CAR-T cells in Europe, and the first clinical trial that uses advanced sleeping beauty technology worldwide.
5.2. Allogeneic (‘Off-the-Shelf’) Approaches
The first allo-CAR-T anti-MM to be tested within a clinical trial is UCARTCS1, an off-the-shelf SLAMF7-targeting allo-CAR T-cell product derived from healthy donors. The product was manufactured using TALEN gene editing technology to eliminate endogenous expression of T-cell receptor (TCR) and SLAMF7 [61,62]. The product has been shown to inhibit the TRAC gene, thereby preventing GVHD by disrupting TCR assembly. The product also knocks out SLAMF7 to facilitate robust expansion and yield, while avoiding the “fratricide” effect. UCARTCS1A is equipped with an RQR8 safety switch, which functions as a CD20 mimotope. If necessary, this switch enables the use of rituximab, a monoclonal antibody, to target and eliminate the affected cells. It has been demonstrated that UCARTCS1 has significant anti-MM activity, as evidenced by its effects on MM cell lines, primary mPCsandin vivo in a MM mouse model [62]. The activity of UCARTCS1 was found to be independent of the proportion of tumor cells, the SLAMF7 expression level on MM cells, or the frequency of regulatory T-cells in MM bone marrow samples. Furthermore, UCARTCS1 cells exhibited equivalent antitumor activity in samples from both newly diagnosed and heavily pretreated patients, indicating that mechanisms of resistance to prior therapies did not result in diminished susceptibility to UCARTCS1-mediated lysis. The authors evaluated the potential of UCARTCS1 to induce on-target/off-tumor toxicity. According to the data presented, UCARTCS1 exerts its cytotoxic effects on a subset of B cells, NK cells, and T cells that exhibit the highest levels of SLAMF7, while demonstrating minimal impact on cells with low or no SLAMF7 expression. The impact of UCARTCS1 on CD8+ T-cells was more pronounced than on CD4+ T-cells, which is likely related to the lower SLAMF7 expression on CD4+ T-cells. These results are consistent with the published data, which show fratricide killing of CD8+ T-cells during the CAR T-cell manufacturing process when SLAMF7 is not genetically deleted. Indeed, SLAMF7 knockout proved instrumental in preventing CD8+ T-cell fratricide effect during the production process, thereby enhancing the CD4+/CD8+ T-cell ratio in the final CAR T-cell product. The manipulation of the SLAMF7 gene may result in the production of less differentiated cells, as T-cells undergo reduced antigenic stimulation during the manufacturing process. In conclusion, the authors demonstrated that gene editing did not impact the cytotoxic capacity of SLAMF7-targeting CAR T cells.
Preliminary data from the phase 1 MELANI-01 trial (NCT04142619) presented at the 2021 American Society of Gene and Cell Therapy Annual Meeting [63] indicate that UCARTCS1A demonstrated early antitumor activity in patients with RR MM after failure of CAR T-cell therapy and/or transplant. The expansion and persistence of UCARTCS1A have been observed to correlate with clinically meaningful antimyeloma activity and serum cytokine changes in patients who have undergone extensive pretreatment. The allo-CAR T-cell product consistently remained present in patients, regardless of donor and batch. These preliminary data validated SLAMF7 as a target for allo-CAR T cells. A notable advantage of employing an allo-CAR T-cell over an auto approach is the accessibility of a ready-to-use product. The process of rapid manufacturing has been demonstrated to reduce expenses and yield more than 100 doses from a single batch of donor cells. T cells collected from healthy donors are likely to be more potent because these individuals have not been exposed to high doses of steroids, chemotherapy, or undergone an autologous transplant. However, the Phase I MELANI-01 trial was subject to a clinical hold by the FDA, which was initiated in July 2020 following the occurrence of a fatal cardiac event in a patient, and no results are published. The hold was lifted in November 2020 following the implementation of safety protocol adjustments to the study by Cellectis. Notwithstanding this course of action, the sponsor elected to bring the study to a close, a decision that was made based on its own internal considerations. It is noteworthy that this decision was not influenced by any concerns about safety [64].
Allogeneic CAR-T therapy has several challenges, including issues with managing toxicity. The risk of graft-versus-host disease (GvHD) requires additional genetic modifications, such as TRAC knockout, which can complicate manufacturing and introduce new failure modes [65]. Maintaining cell quality while manufacturing at scale is technically challenging. Immunogenicity concerns may necessitate immunosuppression protocols that could compromise efficacy. Engineering cells to evade immune rejection while preventing GvHD is a delicate process that may compromise CAR-T persistence, a key driver of durable responses.
5.3. Dual-Targeting Strategies
Moares et al. developed the CARtein platform, a CAR system that utilizes split inteins to precisely link CAR modules, allowing for the creation of structurally seamless, dual-targeting CARs against BCMA and SLAMF7 [43]. This innovative protein splicing approach enhances the modular design of CARs, which was further refined using advanced protein structure prediction software. The result is a CARtein construct that exhibits robust and precise T-cell activation against cancer cells expressing these antigens. The CARtein platform offers a versatile and potent strategy to overcome limitations in current CAR T-cell therapy, with the potential to improve the safety and effectiveness of treatments for MM. A phase 1/2 trial of a dual-targeted CAR with anti-BCMA and anti-SLAMF7 domains in 16 patients showed an overall response rate of 81% with 38% complete responses, and a 1-year duration of response of 56% [66]. Toxicities included cytokine release syndrome in 38% of patients (grade ≥3 in 6%), no immune effector cell-associated neurotoxicity syndrome, but significant cytopenias in 100% (grade ≥3 in 100%), and infections in 38%. Other groups have developed different CAR constructs co-targeting BCMA and SLAMF7, and one is being clinically tested (NCT0595011) [67,68].
Researchers developed a dual-CAR T cell (DCAR) therapy by targeting CD38 and SLAMF7 on MM cells. In this therapy, CRISPR/Cas9 was used to remove the CD38 gene from T cells to prevent on-target toxicity [69]. The efficacy of the modified DCAR system has been demonstrated in laboratory and animal studies, with the study showing potent anti-MM activity. In comparison to conventional anti-CD38 CAR T cells, the edited DCAR system offers enhanced safety by reducing adverse reactions to normal hematopoietic cells. The engineered T cells are equipped with two CARs to recognize and bind to two distinct antigens, CD38 and SLAMF7. The CD38 gene is deleted from the T cells using CRISPR/Cas9 technology. This deletion renders the engineered T cells incapable of reacting to CD38 on their own surface or on other immune cells (such as NK cells and erythrocytes) and normal tissues. Consequently, this results in an improvement to the safety profile of the therapy.
6. Expert Opinion
MM is characterised by phenotypic heterogeneity, suggesting that targeting a single antigen with immunotherapeutic approaches may not be the optimal choice. Presently, anti-BCMA CAR-Ts represent the sole target employed in clinical practice. The expression of BCMA on MM cells is subject to variation, as evidenced by the emergence of BCMA-negative relapses and the presence of MM cells expressing low levels of BCMA prior to anti-BCMA therapy [25,70].
SLAMF7 targets have been identified as a promising alternative antigen due to their absence in both epithelial tissues and hematopoietic stem cells Furthermore, over 95% of bone marrow MM cells express SLAMF7, and this expression persists throughout relapse and treatment with standard therapies. SLAMF7 is expressed on a subset of CD8+ T cells, NK cells, thus its targeting can lead to the depletion of these leukocyte subpopulations and potentially increase the risk of infectious sequelae. The preliminary results of the ongoing trials demonstrate that anti-SLAMF7 CAR-Ts exhibit a significantly enhanced potency against MM cells compared to the anti-SLAMF7 antibody elotuzumab (ELO) [53].
Several relevant unanswered questions regarding SLAMF7 CAR T-cell therapy in MM persist, including the long-term efficacy and durability of the therapy, as well as comparative analyses of its efficacy, safety profiles, and patient quality of life with those of other available therapies. Long-term durability can be assessed by extended follow-up in trials and monitoring CAR-T persistence.
To develop effective treatment strategies, it is essential to understand the mechanisms of resistance and relapse. This includes investigating whether mPCs downregulate SLAMF7 expression or develop alternative escape mechanisms, and how the tumour influences these processes. Furthermore, SLAMF7 expression has been observed on specific immune cells, which has the potential to result in on-target, off-tumour toxicity. Consequently, there is a necessity to analyse strategies that can be employed to enhance the safety profile, such as affinity tuning or controlled CAR expression. To enhance safety, particularly in terms of the risk of infection, the anti-SLAMF7 CAR-Ts currently under investigation incorporate a suicide gene. TALEN gene editing is a technology that has the capacity to suppress endogenous TCR and SLAMF7 expression, thereby mitigating the risk of GVHD and fratricide killing [71]. TALENs require a complex and time-consuming process of protein engineering in order to create custom DNA-binding domains for each target sequence. This makes them costly and challenging to produce on a large scale. The process of protein engineering has been made much easier and faster by CRISPR, which has simplified the process from RNA-coding problems [72]. However, achieving high specificity remains challenging, as does the delivery of CRISPR components. Although CRISPR has transformed gene editing by significantly reducing the barriers to entry for many applications, successfully implementing it in a clinical setting requires overcoming the challenges of off-target effects and efficient delivery [73]. TALENs remain a viable option for specific applications where their precision outweighs their complexity. Ongoing research into CRISPR is making it a more powerful and viable tool for manufacturing therapeutic agents, including those for fratricide.
However, the toxicity profile is different from BCMA-CAR-T, and the on-target/off-tumor effects on immune cells are a key differentiator. The fundamental distinction is that SLAMF7-CAR-T causes immunosuppression through direct depletion of effector immune cells (NK, CD8+ T cells), while BCMA-CAR-T causes immunosuppression primarily through B-cell depletion and hypogammaglobulinemia.
Furthermore, it is essential to understand the optimal cellular dose and infusion schedule, as well as the methods for streamlining production to ensure wider availability and cost-effectiveness. It is recommended that future studies investigate the immunosuppressive microenvironment in myeloma and its impact on the persistence of CAR T cells. Furthermore, such studies should identify combinations or modifications that can enhance activity within this environment.
A pivotal consideration is the selection of patients; if there are biomarkers (e.g., soluble SLAMF7, genetic markers) that predict responsiveness, it is also important to ascertain which patient populations (e.g., RR vs. newly diagnosed) wilbenefit for the most.
Another open issue is whether SLAMF7 CAR T can be combined with other immunotherapies, such as checkpoint inhibitors or monoclonal antibodies to improve outcomes, and what preclinical or clinical evidence supports combination approaches.
Early-phase SLAMF7-CAR-T trials would likely target a carefully selected patient population to maximize safety assessment while demonstrating proof-of-concept efficacy. Ideal candidates would be patients who have received three to four prior lines of therapy, including proteasome inhibitors, immunomodulatory drugs and anti-CD38 antibodies, and who have progressive disease despite standard treatments, measurable disease by serum/urine M-protein or serum free light chains, and refractory disease to BCMA-targeted therapy. They would also have prior exposure to BCMA-directed therapies (CAR-T, bispecific antibodies, or ADCs), and disease progression during or after BCMA therapy. Confirmed SLAMF7 expression on myeloma cells would be demonstrated by flow cytometry or immunohistochemistry. This population would enable careful assessment of on-target/off-tumour effects (NK and T cell depletion) while addressing the unmet need of BCMA-refractory disease, where SLAMF7 remains a viable target.
7. Conclusions
MM is an active area of clinical research, and, in addition to both mild and aggressive chemotherapy regimens, new immunotherapies are being investigated and integrated into the standard of care. The convergence of robust preclinical data, novel manufacturing methods, and initial clinical safety indications establishes SLAMF7 as a promising alternative or supplementary target to BCMA for myeloma CAR-T therapy. SLAMF7 is uniformly expressed on malignant plasma cells in newly diagnosed myeloma and is still present in relapsed myeloma after intensive therapy, making it an ideal target. SLAMF7 expression on myeloma cells is consistently high and is not affected by cytogenetic abnormalities, genomic mutations, or disease stage. Importantly, SLAMF7 is not expressed in any other normal human tissue outside the haematopoietic system, which reduces concerns about off-tumour toxicity. However, success will depend on rigorous clinical validation, toxicity mitigation, and strategic positioning within the evolving immunotherapy landscape.
Author Contributions
Conceptualization, M.M. and C.A.; methodology, G.P. (Gaetana Porto); validation, A.R., M.R.; data curation, E.B., M.B.G., I.D.V.; writing—original draft preparation, M.P., M.M.; writing—review and editing, M.E.A.; D.G., A.A., E.P.; supervision, G.P. (Giorgia Policastro), G.U.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Malard, F.; Neri, P.; Bahlis, N.J.; Terpos, E.; Moukalled, N.; Hungria, V.T.M.; Manier, S.; Mohty, M. Multiple myeloma. Nat. Rev. Dis. Primers 2024, 10, 45. [Google Scholar] [CrossRef]
- Sun, K.; Wu, H.; Zhu, Q.; Gu, K.; Wei, H.; Wang, S.; Li, L.; Wu, C.; Chen, R.; Pang, Y.; et al. Global landscape and trends in lifetime risks of haematologic malignancies in 185 countries: Population-based estimates from GLOBOCAN 2022. EClinicalMedicine 2025, 83, 103193. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Moreau, P.; Mateos, M.V.; Zweegman, S.; Cook, G.; Engelhardt, M.; Delforge, M.; Hajek, R.; et al. EHA-EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2025, 22, 680–700. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Moreau, P.; San-Miguel, J.F.; Mateos, M.V.; Dimopoulos, M.A.; Zweegman, S.; Gay, F.; Engelhardt, M.; Mina, R.; Zamagni, E.; et al. Optimising T-cell immunotherapy in patients with multiple myeloma: Practical considerations from the European Myeloma Network. Lancet Haematol. 2025, 12, e635–e649. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; Bahlis, N.J.; et al. Ide-cel vs standard regimens in triple-class-exposed relapsed and refractory multiple myeloma: Updated KarMMa-3 analyses. Blood 2024, 144, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lin, X.; Xu, Z.; Kanapuru, B.; Theoret, M.R.; Sokolic, R.; Fashoyin-Aje, L.A. FDA Approval Summary: Idecabtagene Vicleucel for the Treatment of Triple-Class-Exposed, Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2025, 31, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Martin, T.G.; Lin, Y.; Cohen, A.D.; Raje, N.; Htut, M.; Deol, A.; Agha, M.; Berdeja, J.G.; Lesokhin, A.M.; et al. Long-Term (≥5-Year) Remission and Survival After Treatment with Ciltacabtagene Autoleucel in CARTITUDE-1 Patients with Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2025, 43, 2766–2771. [Google Scholar] [CrossRef]
- Natrajan, K.; Kaushal, M.; George, B.; Kanapuru, B.; Theoret, M.R. FDA Approval Summary: Ciltacabtagene Autoleucel for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2024, 30, 2865–2871. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Myeloma. NIH. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 8 April 2021).
- Engelhardt, M.; Kortüm, K.M.; Goldschmidt, H.; Merz, M. Functional cure and long-term survival in multiple myeloma: How to challenge the previously impossible. Haematologica 2024, 109, 2420–2435. [Google Scholar] [CrossRef]
- Chu, E.; Wu, J.; Kang, S.S.; Kang, Y. SLAMF7 as a Promising Immunotherapeutic Target in Multiple Myeloma Treatments. Curr. Oncol. 2023, 30, 7891–7903. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.; Bhutani, M.; Voorhees, P.M.; Usmani, S.Z. Clinical potential of SLAMF7 antibodies-focus on elotuzumab in multiple myeloma. Drug Des. Devel. Ther. 2017, 11, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Guo, H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit. Rev. Oncol. Hematol. 2013, 88, 168–177. [Google Scholar] [CrossRef]
- O’Neal, J.; Ritchey, J.K.; Cooper, M.L.; Niswonger, J.; González, S.L.; Street, E.; Rettig, M.P.; Gladney, S.W.; Gehrs, L.; Abboud, R.; et al. CS1 CAR-T targeting the distal domain of CS1 (SLAMF7) shows efficacy in high tumor burden myeloma model despite fratricide of CD8+CS1 expressing CAR-T cells. Leukemia 2022, 36, 1625–1634. [Google Scholar] [CrossRef]
- Luo, G.; Ni, R.; Huang, X.; Li, Y.; Luo, D. The role of signaling lymphocytic activation molecule (SLAM) family receptors in health and disease progression: Focusing on cancer and therapy. Clin. Immunol. 2025, 280, 110574. [Google Scholar] [CrossRef]
- Farhangnia, P.; Ghomi, S.M.; Mollazadehghomi, S.; Nickho, H.; Akbarpour, M.; Delbandi, A.A. SLAM-family receptors come of age as a potential molecular target in cancer immunotherapy. Front. Immunol. 2023, 14, 1174138. [Google Scholar] [CrossRef]
- Raedler, L.A. Empliciti (Elotuzumab): First SLAMF7 Antibody Therapy Approved for the Treatment of Patients with Previously Treated Multiple Myeloma. Am. Health Drug Benefits 2016, 9, 74–77. [Google Scholar] [PubMed] [PubMed Central]
- Bruno, B.; Wäsch, R.; Engelhardt, M.; Gay, F.; Giaccone, L.; D’Agostino, M.; Rodríguez-Lobato, L.G.; Danhof, S.; Gagelmann, N.; Kröger, N.; et al. European Myeloma Network perspective on CAR T-Cell therapies for multiple myeloma. Haematologica 2021, 106, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.; Madduri, D.; Hocking, J. CAR-T cell therapy in Multiple Myeloma: Current status and future challenges. Blood Cancer J. 2024, 14, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lonial, S.; Vij, R.; Harousseau, J.L.; Facon, T.; Moreau, P.; Mazumder, A.; Kaufman, J.L.; Leleu, X.; Tsao, L.C.; Westland, C.; et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J. Clin. Oncol. 2012, 30, 1953–1959. [Google Scholar] [CrossRef]
- Majzner, R.G.; Rietberg, S.P.; Sotillo, E.; Dong, R.; Vachharajani, V.T.; Labanieh, L.; Myklebust, J.H.; Kadapakkam, M.; Weber, E.W.; Tousley, A.M.; et al. Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov. 2020, 10, 702–723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amatya, C.; Pegues, M.A.; Lam, N.; Vanasse, D.; Geldres, C.; Choi, S.; Hewitt, S.M.; Feldman, S.A.; Kochenderfer, J.N. Development of CAR T Cells Expressing a Suicide Gene Plus a Chimeric Antigen Receptor Targeting Signaling Lymphocytic-Activation Molecule F7. Mol. Ther. 2021, 29, 702–717. [Google Scholar] [CrossRef]
- Grywalska, E.; Sosnowska-Pasiarska, B.; Smok-Kalwat, J.; Pasiarski, M.; Niedźwiedzka-Rystwej, P.; Roliński, J. Paving the Way toward Successful Multiple Myeloma Treatment: Chimeric Antigen Receptor T-Cell Therapy. Cells 2020, 9, 983. [Google Scholar] [CrossRef]
- Mishra, A.K.; Gupta, A.; Dagar, G.; Das, D.; Chakraborty, A.; Haque, S.; Prasad, C.P.; Singh, A.; Bhat, A.A.; Macha, M.A.; et al. CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond. Vaccines 2023, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rasche, L.; Kortüm, K.M.; Mersi, J.; Einsele, H. BCMA loss in the epoch of novel immunotherapy for multiple myeloma: From biology to clinical practice. Haematologica 2023, 108, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Maiti, R.; Mohan, P.; Gupta, P. Antigen loss following CAR-T cell therapy: Mechanisms, implications, and potential solutions. Eur. J. Haematol. 2024, 112, 211–222. [Google Scholar] [CrossRef]
- Rejeski, K.; Jain, M.D.; Smith, E.L. Mechanisms of Resistance and Treatment of Relapse after CAR T-cell Therapy for Large B-cell Lymphoma and Multiple Myeloma. Transpl. Cell. Ther. 2023, 29, 418–428. [Google Scholar] [CrossRef]
- Tedder, B.; Bhutani, M. Resistance Mechanisms to BCMA Targeting Bispecific Antibodies and CAR T-Cell Therapies in Multiple Myeloma. Cells 2025, 14, 1077. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, Z.; Zhang, Y. Chimeric Antigen Receptor T Cell Exhaustion during Treatment for Hematological Malignancies. Biomed. Res. Int. 2020, 2020, 8765028. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, W.; Wang, H.; Liu, X.; Fu, R. Challenges and strategies associated with CAR-T cell therapy in blood malignancies. Exp. Hematol. Oncol. 2024, 13, 22. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updates 2021, 59, 100796. [Google Scholar] [CrossRef]
- Valtis, Y.K.; Shouval, R. The fire or the forest: Untangling the role of inflammation and bone marrow health in CAR-T outcomes in acute lymphoblastic leukemia. Transplant. Cell. Ther. 2025, 31, 474–476. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics 2022, 12, 6273–6290. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Vicidomini, R.; Poondla, N.; Tanzadehpanah, H.; Dirbaziyan, A.; Mahaki, H.; Manoochehri, H.; Kalhor, N.; Dama, P. CAR T therapies in multiple myeloma: Unleashing the future. Cancer Gene Ther. 2024, 31, 667–686. [Google Scholar] [CrossRef]
- Cho, S.F.; Xing, L.; Anderson, K.C.; Tai, Y.T. Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers 2021, 13, 6136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vural, E.; Beksaç, M. Current Anti-Myeloma Chimeric Antigen Receptor-T Cells: Novel Targets and Methods. Balkan Med. J. 2025, 42, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, A.; Ormhøj, M.; Choi, B.D.; Taylor, A.O.; Bouffard, A.A.; Scarfò, I.; Larson, R.C.; Frigault, M.J.; Gallagher, K.; Castano, A.P.; et al. Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv. 2019, 3, 3248–3260. [Google Scholar] [CrossRef]
- Larson, R.C.; Kann, M.C.; Graham, C.; Mount, C.W.; Castano, A.P.; Lee, W.H.; Bouffard, A.A.; Takei, H.N.; Almazan, A.J.; Scarfó, I.; et al. Anti-TACI single and dual-targeting CAR T cells overcome BCMA antigen loss in multiple myeloma. Nat. Commun. 2023, 14, 7509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, S.T.; Yazdanifar, M.; Williams, C.; Sanders, A.; Brouwer, C.; Maher, J.; Mukherjee, P. Mucin-1-Targeted Chimeric Antigen Receptor T Cells Are Effective and Safe in Controlling Solid Tumors in Immunocompetent Host. J. Immunother. 2024, 47, 77–88. [Google Scholar] [CrossRef]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ming, X.; Zheng, R.; Zhu, X.; Xiao, Y. Application of GPRC5D Targeting Therapy in Relapsed Refractory Multiple Myeloma. Cancer Med. 2025, 14, e70764. [Google Scholar] [CrossRef]
- Robat-Jazi, B.; Mahalleh, M.; Dashti, M.; Nejati, N.; Ahmadpour, M.; Alinejad, E.; Mohammadi, S.; Lorestani, P.; Hamidieh, A.A.; Habibi, M.A.; et al. A Systematic Review and Meta-analysis on the Safety and Efficacy of CAR T Cell Therapy Targeting GPRC5D in Patients with Multiple Myeloma: A New Insight in Cancer Immunotherapy. Anticancer Agents Med. Chem. 2025, 25, 1017–1028. [Google Scholar] [CrossRef]
- Moares, N.; Gonzalez-Garcia, P.; Yi-He, W.; Muñoz-Miranda, J.P.; Gabucio, A.; Luna-Espejo, R.; Ocaña-Cuesta, J.; Fernandez-Cisnal, R.; Fernandez-Ponce, C.M.; Garcia-Cozar, F. Dual targeting of BCMA and SLAMF7 with the CARtein system: Chimeric antigen receptors with intein-mediated splicing elicit specific T cell activation against multiple myeloma. Front. Immunol. 2025, 16, 1613222. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, M.; Ma, L.; Guan, T.; Su, L. From spear to trident: Upgrading arsenal of CAR-T cells in the treatment of multiple myeloma. Heliyon 2024, 10, e29997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghorai, S.K.; Pearson, A.N. Current Strategies to Improve Chimeric Antigen Receptor T (CAR-T) Cell Persistence. Cureus 2024, 16, e65291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.D.; Smith, M.; Shah, N.N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 2023, 141, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.R. Late complications and long-term care of adult CAR T-cell patients. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 109–115. [Google Scholar] [CrossRef]
- Dulobdas, V.; Kirkwood, A.A.; Serpenti, F.; Gautama, B.; Panopoulou, A.; Mathew, A.; Gabriel, S.; Malladi, R.; Pealing, J.; Bonney, D.; et al. Risk factors for CAR T-cell manufacturing failure and patient outcomes in large B-cell lymphoma: A report from the UK National CAR T Panel. Blood Cancer J. 2025, 15, 30. [Google Scholar] [CrossRef]
- Ayala Ceja, M.; Khericha, M.; Harris, C.M.; Puig-Saus, C.; Chen, Y.Y. CAR-T cell manufacturing: Major process parameters and nex Tea Gogishvili, t-generation strategies. J. Exp. Med. 2024, 221, e20230903. [Google Scholar] [CrossRef]
- Gonçalves, E. CAR-T cell therapies: Patient access and affordability solutions. Future Sci. OA 2025, 11, 2483613. [Google Scholar] [CrossRef] [PubMed]
- Gogishvili, T.; Danhof, S.; Prommersberger, S.; Rydzek, J.; Schreder, M.; Brede, C.; Einsele, H.; Hudecek, M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 2017, 130, 2838–2847. [Google Scholar] [CrossRef]
- Diaconu, I.; Ballard, B.; Zhang, M.; Chen, Y.; West, J.; Dotti, G.; Savoldo, B. Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Mol. Ther. 2017, 25, 580–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://clinicaltrials.gov/study/NCT03958656 (accessed on 8 April 2021).
- Kandra, P.; Nandigama, R.; Eul, B.; Huber, M.; Kobold, S.; Seeger, W.; Grimminger, F.; Savai, R. Utility and Drawbacks of Chimeric Antigen Receptor T Cell (CAR-T) Therapy in Lung Cancer. Front. Immunol. 2022, 13, 903562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Study Details | A Phase I/IIa Clinical Trial to Assess Feasibility, Safety and Antitumor Activity of Autologous SLAMF7 CAR-T Cells in Multiple Myeloma. Available online: https://clinicaltrials.gov/study/NCT04499339 (accessed on 8 April 2021).
- Prommersberger, S.; Reiser, M.; Beckmann, J.; Danhof, S.; Amberger, M.; Quade-Lyssy, P.; Einsele, H.; Hudecek, M.; Bonig, H.; Ivics, Z. CARAMBA: A first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 2021, 28, 560–571. [Google Scholar] [CrossRef]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvak, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef]
- Hackett, P.B.; Largaespada, D.A.; Cooper, L.J.N. A transposon and transposase system for human application. Mol. Ther. 2010, 18, 674–683. [Google Scholar] [CrossRef]
- Mathur, R.; Zhang, Z.; He, J.; Galetto, R.; Gouble, A.; Chion-Sotinel, I.; Filipe, S.; Gariboldi, A.; Veeramachaneni, T.; Manasanch, E.E.; et al. Universal SLAMF7-specific CAR T-cells as treatment for multiple myeloma. Blood 2017, 130, 502. [Google Scholar]
- Korst, C.L.B.M.; O’Neill, C.; Bruins, W.S.C.; Cosovic, M.; Twickler, I.; Verkleij, C.P.M.; Le Clerre, D.; Themeli, M.; Chion-Sotinel, I.; Zweegman, S.; et al. Preclinical activity of allogeneic SLAMF7-specific CAR T-cells (UCARTCS1) in multiple myeloma. J. Immunother. Cancer 2024, 12, e008769. [Google Scholar] [CrossRef]
- Allogeneic CAR T-Cell Therapy UCARTCS1A Shows Promise for Multiple Myeloma. Available online: https://www.cgtlive.com/view/allogeneic-car-t-cell-therapy-ucartcs1a-shows-promise-for-multiple-myeloma (accessed on 8 April 2021).
- FDA Lifts Clinical Hold on Study Evaluating UCARTCS1 for Myeloma. ASH Clinical News. American Society of Hematology. Available online: https://ashpublications.org/ashclinicalnews/news/5433/FDA-Lifts-Clinical-Hold-on-Study-Evaluating (accessed on 8 April 2021).
- Lonez, C.; Breman, E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells 2024, 13, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Xu, J.; Luo, W.; Liao, D.; Xie, W.; Wei, Q.; Zhang, Y.; Wang, X.; Wu, Z.; Kang, Y.; et al. Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma. Leukemia 2024, 38, 149–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zah, E.; Nam, E.; Bhuvan, V.; Tran, U.; Ji, B.Y.; Gosliner, S.B.; Wang, X.; Brown, C.E.; Chen, Y.Y. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat. Commun. 2020, 11, 2283. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Pinz, K.G.; Liu, H.; Shuai, X.; Chen, X.; Wang, X.; Brown, C.E.; Chen, Y.Y. A compound chimeric antigen receptor strategy for targeting multiple myeloma. Leukemia 2018, 32, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Geis, M.; Nowotny, B.; Bohn, M.D.; Kouhestani, D.; Einsele, H.; Bumm, T.; Stuhler, G. Combinatorial targeting of multiple myeloma by complementing T cell engaging antibody fragments. Commun. Biol. 2021, 4, 44. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Kolanu, N.D. CRISPR-Cas9 Gene Editing: Curing Genetic Diseases by Inherited Epigenetic Modifications. Glob. Med. Genet. 2024, 11, 113–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, T.; Wang, Y.; Zhang, Y.; Yang, Y.; Cao, J.; Huang, J.; Chen, J.; Chen, H.; Zhang, J.; Wang, L.; et al. Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy. Leukemia 2024, 38, 2517–2543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seijas, A.; Cora, D.; Novo, M.; Al-Soufi, W.; Sánchez, L.; Arana, Á.J. CRISPR/Cas9 Delivery Systems to Enhance Gene Editing Efficiency. Int. J. Mol. Sci. 2025, 26, 4420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).