BALAD-2 Emerges as the Most Accurate Prognostic Model in Hepatocellular Carcinoma: Results from a Biobank-Based Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection
2.3. Serum Biomarker Measurements and Calculation of Scoring Models
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Baseline Characteristics
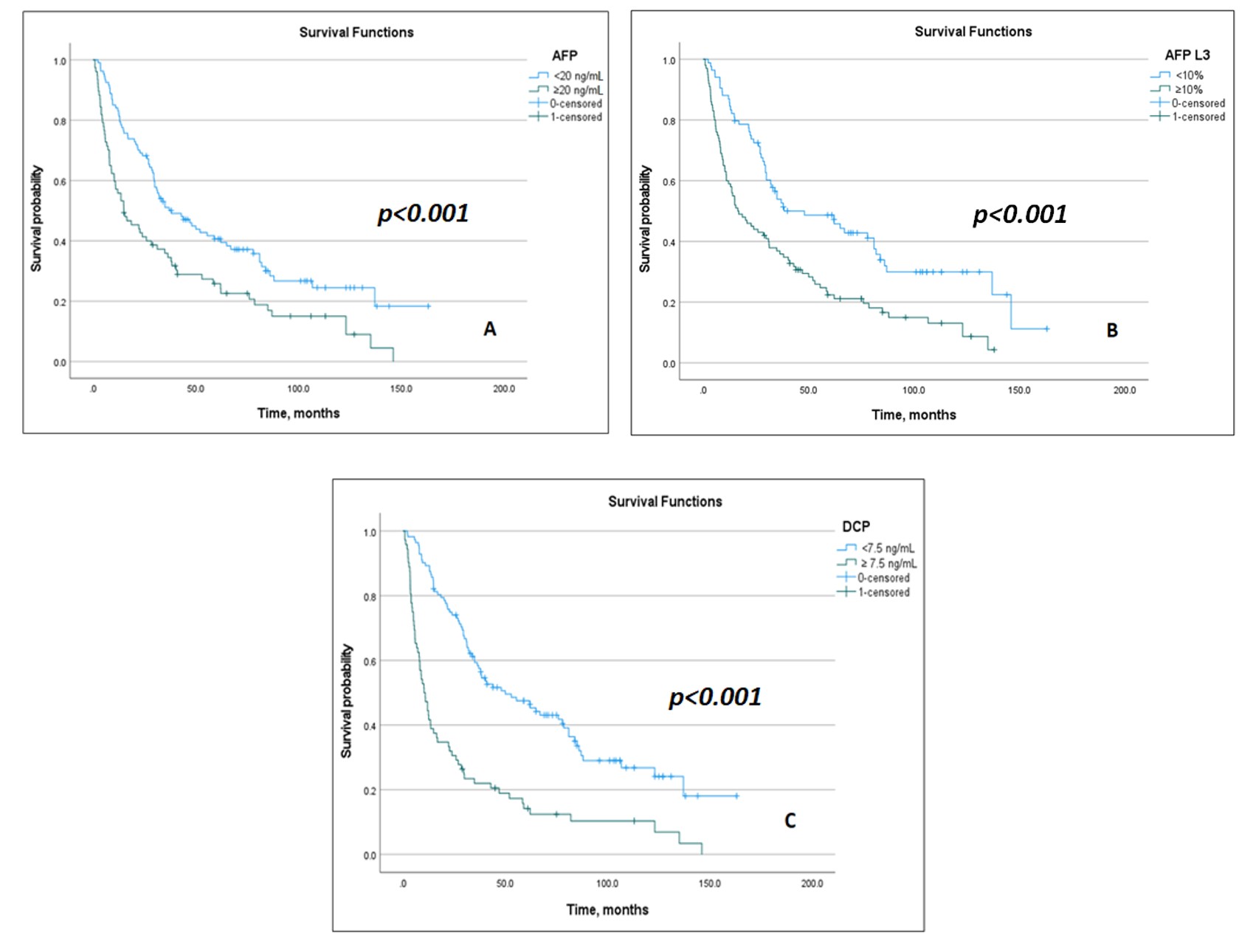
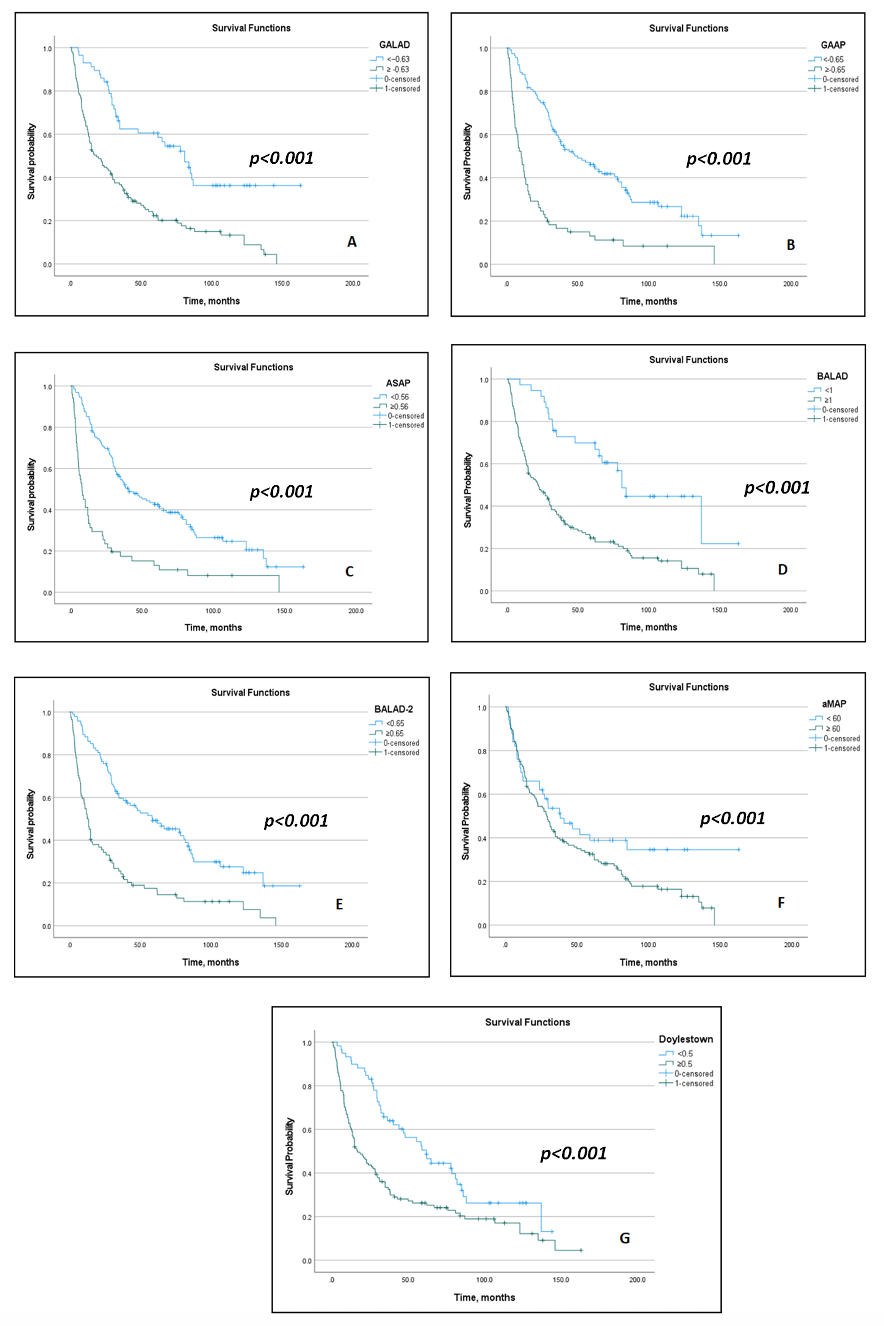
3.2. Prognostic Impact of Biomarkers and Models
3.3. Model Discrimination and Subgroup Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alfa-fetoprotein |
| AFP-L3% | Lens culnaris agglutinin-reactive AFP |
| ALBI | Albumin-bilirubin, |
| ALD | Alcohol-related liver disease |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AUROC | Area under the receiver operating characteristic curve |
| BCLC | Barcelona Clinic Liver Cancer |
| BMI | Body mass index |
| BSC | Best supportive care |
| c-index | Concordance index |
| CP | Child–Pugh |
| DCP | Des-gamma-carboxy prothrombin |
| EASL | European Association for the Study of the Liver |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| INR | International normalized ratio |
| MASLD | Metabolic-associated steatotic liver disease |
| MELD | Model for End-Stage Liver Disease |
| OS | Overall survival |
| PVT | Portal vein thrombosis |
| RFA | Radiofrequency ablation |
| TACE | Transarterial chemoembolization |
| TARE | Transarteriel radioembolization |
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Demirtas, C.O.; Ricco, G.; Ozdogan, O.C.; Baltacioglu, F.; Ones, T.; Yumuk, P.F.; Dulundu, E.; Uzun, S.; Colombatto, P.; Oliveri, F.; et al. Proposal and Validation of a Novel Scoring System for Hepatocellular Carcinomas Beyond Curability Borders. Hepatol. Commun. 2022, 6, 633–645. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Ardiri, A.; Malaguarnera, M.; Malaguarnera, G.; Bertino, N.; Calvagno, G.S. Hepatocellualar carcinoma serum markers. Semin. Oncol. 2012, 39, 410–433. [Google Scholar] [CrossRef]
- Miyaaki, H.; Nakashima, O.; Kurogi, M.; Eguchi, K.; Kojiro, M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: A histopathological study of surgically resected hepatocellular carcinoma. J. Gastroenterol. 2007, 42, 962–968. [Google Scholar] [CrossRef]
- Zhu, R.; Yang, J.; Xu, L.; Dai, W.; Wang, F.; Shen, M.; Zhang, Y.; Zhang, H.; Chen, K.; Cheng, P.; et al. Diagnostic Performance of Des-gamma-carboxy Prothrombin for Hepatocellular Carcinoma: A Meta-Analysis. Gastroenterol. Res. Pract. 2014, 2014, 529314. [Google Scholar] [CrossRef]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Pirrie, S.J.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Tayob, N.; Mehta, A.; Marrero, J.A.; El-Serag, H.; Jin, Q.; Saenz de Viteri, C.; Fobar, A.; Parikh, N.D. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 2022, 75, 541–549. [Google Scholar] [CrossRef]
- Guan, M.C.; Zhang, S.Y.; Ding, Q.; Li, N.; Fu, T.T.; Zhang, G.X.; He, Q.Q.; Shen, F.; Yang, T.; Zhu, H. The Performance of GALAD Score for Diagnosing Hepatocellular Carcinoma in Patients with Chronic Liver Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 949. [Google Scholar] [CrossRef]
- Villa, E.; Donghia, R.; Baldaccini, V.; Tedesco, C.C.; Shahini, E.; Cozzolongo, R.; Ascari, S.; Pesole, P.L.; Coletta, S.; Critelli, R.M.; et al. GALAD outperforms aMAP and ALBI for predicting HCC in patients with compensated advanced chronic liver disease: A 12-year prospective study. Hepatol. Commun. 2023, 7, e0262. [Google Scholar] [CrossRef]
- Liu, M.; Wu, R.; Liu, X.; Xu, H.; Chi, X.; Wang, X.; Zhan, M.; Wang, B.; Peng, F.; Gao, X.; et al. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J. Hepatocell. Carcinoma 2020, 7, 219–232. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Cai, W.; Ming, A.; Li, H. Validation and update of a multivariable prediction model for the identification and management of patients at risk for hepatocellular carcinoma. Clin. Proteom. 2021, 18, 21. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Devarajan, K.; Singal, A.G.; Marrero, J.A.; Dai, J.; Feng, Z.; Rinaudo, J.A.; Srivastava, S.; Evans, A.; Hann, H.W.; et al. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev. Res. 2016, 9, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Osaki, Y.; Oka, H.; Urano, F.; Kudo, M.; Matsunaga, T. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin. Gastroenterol. Hepatol. 2006, 4, 1528–1536. [Google Scholar] [CrossRef]
- Fox, R.; Berhane, S.; Teng, M.; Cox, T.; Tada, T.; Toyoda, H.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Johnson, P.J. Biomarker-based prognosis in hepatocellular carcinoma: Validation and extension of the BALAD model. Br. J. Cancer 2014, 110, 2090–2098. [Google Scholar] [CrossRef]
- Cagnin, S.; Donghia, R.; Martini, A.; Pesole, P.L.; Coletta, S.; Shahini, E.; Boninsegna, G.; Biasiolo, A.; Pontisso, P.; Giannelli, G. Galad Score as a Prognostic Marker for Patients with Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 16485. [Google Scholar] [CrossRef]
- Jitpraphawan, O.; Ruamtawee, W.; Treewatchareekorn, M.; Sethasine, S. Diagnostic and prognostic performances of GALAD score in staging and 1-year mortality of hepatocellular carcinoma: A prospective study. World J. Gastroenterol. 2024, 30, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Ohama, H.; Hiraoka, A.; Tada, F.; Kato, K.; Fukunishi, Y.; Yanagihara, E.; Kato, M.; Saneto, H.; Izumoto, H.; Ueki, H.; et al. Usefulness of aMAP Risk Score for Predicting Recurrence after Curative Treatment for Hepatocellular Carcinoma within Milan Criteria. Oncology 2023, 101, 575–583. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Endo, Y.; Ratti, F.; Marques, H.P.; Cauchy, F.; Lam, V.; Poultsides, G.A.; Popescu, I.; Alexandrescu, S.; Martel, G.; et al. The aMAP score predicts long-term outcomes after resection of hepatocellular carcinoma: A multi-institutional analysis. HPB 2024, 26, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yin, D.X.; Diao, Y.K.; Wang, M.D.; Wang, X.M.; Zeng, Y.Y.; Chen, Z.; Liu, H.; Chen, F.J.; Li, Y.C.; et al. Prognostic Value of the ASAP Score for Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma: A Multicenter Analysis of 1,239 Patients. J. Clin. Exp. Hepatol. 2025, 15, 102497. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; et al. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Kagebayashi, C.; Yamaguchi, I.; Akinaga, A.; Kitano, H.; Yokoyama, K.; Satomura, M.; Kurosawa, T.; Watanabe, M.; Kawabata, T.; Chang, W.; et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal. Biochem. 2009, 388, 306–311. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, Y.; Li, Z.; Guo, P.; Zeng, J.; Dong, X.; Hu, E.; Qiu, L.; Cai, Z.; Chen, G.; et al. Des-gamma-carboxy Prothrombin in hepatocellular carcinoma post-operative recurrence risk evaluation. Commun. Med. 2025, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.S.; Li, P.J.; Kotwani, P.; Shui, A.M.; Yao, F.; Mehta, N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J. Hepatol. 2023, 79, 1469–1477. [Google Scholar] [CrossRef]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886 e876. [Google Scholar] [CrossRef]
- Toyoda, H.; Tada, T.; Johnson, P.J.; Izumi, N.; Kadoya, M.; Kaneko, S.; Kokudo, N.; Ku, Y.; Kubo, S.; Kumada, T.; et al. Validation of serological models for staging and prognostication of HCC in patients from a Japanese nationwide survey. J. Gastroenterol. 2017, 52, 1112–1121. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Negron-Ocasio, G.M.; Mara, K.C.; Prasai, K.; Abdallah, M.A.; Ahn, K.S.; Yang, J.D.; Addissie, B.D.; Giama, N.H.; Harmsen, W.S.; et al. BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: A North American cohort study. HPB 2021, 23, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Hiraoka, A.; Ochi, H.; Tsuji, K.; Tajiri, K.; Tani, J.; Tada, T.; Okubo, T.; Atsukawa, M.; Hirooka, M.; et al. Prognostic Significance of the BALAD Serological Model in Systemic Therapies for Hepatocellular Carcinoma: A Personalized Approach to the Prediction of Survival Benefit. JCO Clin. Cancer Inform. 2025, 9, e2400175. [Google Scholar] [CrossRef]
- Debes, J.D.; Romagnoli, P.A.; Prieto, J.; Arrese, M.; Mattos, A.Z.; Boonstra, A.; on behalf of the ESCALON Consortium. Serum Biomarkers for the Prediction of Hepatocellular Carcinoma. Cancers 2021, 13, 1681. [Google Scholar] [CrossRef]
- He, H.; Ji, B.; Jia, Z.; Zhang, Y.; Wang, X.; Tao, X.; Liu, Y.; Jiang, J. A Practical Model is Equivalent to the BALAD or BALAD-2 Score in Predicting Long-term Survival after Hepatectomy in Chinese Patients with Hepatocellular Carcinoma. J. Cancer 2021, 12, 1474–1482. [Google Scholar] [CrossRef]
- Liang, J.; Li, P.Y.; Norman, J.; Lauzon, M.; Yeo, Y.H.; Trivedi, H.; Ayoub, W.S.; Kuo, A.; Friedman, M.L.; Sankar, K.; et al. Development and validation of a biomarker index for HCC treatment response. Hepatol. Commun. 2024, 8, e0466. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Negron-Ocasio, G.M.; Chaiteerakij, R.; Addissie, B.D.; Mohamed, E.A.; Mara, K.C.; Harmsen, W.S.; Theobald, J.P.; Peters, B.E.; Balsanek, J.G.; et al. Model combining pre-transplant tumor biomarkers and tumor size shows more utility in predicting hepatocellular carcinoma recurrence and survival than the BALAD models. World J. Gastroenterol. 2018, 24, 1321–1331. [Google Scholar] [CrossRef]
| HCC (n = 186) | |
|---|---|
| Age, median (IQR), years | 65(37–88) |
Gender, n (%)
| 139 (74.7) 47 (25.3) |
| Body-mass index, median (IQR), kg/m 2 | 28.3 (16.5–45.7) |
| Diabetes mellitus, n (%) | 73 (39.2) |
| Hypertension, n (%) | 63 (33.9) |
| Hyperlipidemia, n (%) | 26 (14.0) |
Etiology, n (%)
| 92 (49.5) 54 (29.0) 26 (14.0) 3 (1.6) 3 (1.6) 5 (2.7) 3 (1.6) |
| Ascites, n (%) | 65 (34.9) |
| Varices, n (%) | 75 (40.3) |
| Variceal bleeding history, n (%) | 17 (9.1) |
| CTP score, median (IQR) | 6 (5–12) |
| MELD score, median (IQR) | 10 (6–28) |
| ALBI score, median (IQR) | −2.41 (−4.39–1.03) |
| Albumin, median (IQR), gr/dL | 3.8 (2.1–6.4) |
| INR, median (IQR) | 1.2 (0.9–3.5) |
| Total bilirubin, median (IQR), mg/dL | 1.1 (0.2–12.8) |
| Alanine aminotransferase, median (IQR), U/L | 37 (10–258) |
| Alkaline phosphatase, median (IQR), U/L | 127 (36–642) |
| Gamma-glutamyl transferase, median (IQR), U/L | 84 (11–1612) |
| Platelet count, median (IQR), ×1000/m3 | 144 (36–838) |
| Creatinine, median (IQR), mg/dL | 0.8 (0.4–4.4) |
| Sodium, median (IQR), mEq/L | 138 (121–145) |
| HCC (n = 186) | |
|---|---|
| Tumor size, median (IQR), mm | 40 (10–162) |
Number of tumors, n (%)
| 113 (60.8) 22 (11.8) 17 (9.1) 30 (16.1) 4 (2.2) |
ECOG, n (%)
| 162 (87.2) 21 (11.3) 1 (0.5) 2 (1.0) |
| Lymph node involvement, n (%) | 27 (14.5) |
| Portal vein thrombosis, n (%) | 20 (10.8) |
| Vascular invasion, n (%) | 32 (17.2) |
| Extrahepatic metastasis, n (%) | 7 (3.8) |
BCLC stage, n (%)
| 15 (8.1) 69 (37.1) 69 (37.1) 24 (12.9) 9 (4.8) |
HCC treatment, n (%)
| 19 (10.2) 52 (28.0) 61 (32.8) 11 (5.9) 7 (3.8) 1 (0.5) 1 (0.5) 34 (18.3) |
| AFP, median (IQR), ng/ml | 14.3 (0.7–200,000.0) |
| AFP-L3, median (IQR), % | 12.1 (0.5–94.4) |
| DCP, median (IQR), ng/mL | 3.3 (0.1–14980.0) |
| GALAD score, median (IQR) | 1.44 (−5.53–16.67) |
| BALAD score, median (IQR) | 2.00 (0.00–5.00) |
| BALAD-2 score, median (IQR) | 0.45 (−2.39–5094.05) |
| ASAP, median (IQR) | −2.11 (−7.22–9.76) |
| GAAP, median (IQR) | −1.58 (−7.62–10.43) |
| Doylestown algorithm, median (IQR) | 0.73 (0.02–1.00) |
| aMAP, median (IQR) | 63.41 (19.79–80.17) |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| p-Value | Beta-Coefficient | aHR | 95% CI | p-Value | |
| AFP | <0.001 | 0.000 | 1.000 + | 1.000–1.000 | <0.001 * |
| AFP-L3 | <0.001 | 0.010 | 1.009 + | 1.002–1.017 | 0.015 * |
| DCP | <0.001 | 0.000 | 1.000 ˆ | 1.000–1.000 | 0.002 * |
| GALAD | <0.001 | 0.095 | 1.074 # | 1.025–1.126 | 0.003 |
| ASAP | <0.001 | 0.160 | 1.115 † | 1.036–1.200 | 0.004 * |
| GAAP | <0.001 | 0.164 | 1.090 † | 1.013–1.173 | 0.021 * |
| BALAD | <0.001 | 0.249 | 1.307 # | 1.093–1.563 | 0.003 * |
| BALAD-2 | <0.001 | 0.000 | 1.000 # | 1.000–1.001 | 0.007 * |
| DOYLESTOWN | <0.001 | 0.974 | 1.605 ± | 0.732–3.521 | 0.238 |
| AMAP | 0.048 | 0.242 | 1.015 º | 0.989–1.042 | 0.264 |
| Whole cohort (n = 186) | |||||
| 1st-Year AUROC | 2nd-Year AUROC | 3rd-Year AUROC | 5th-Year AUROC | c-İndex | |
| AFP | 0.766 (0.684–0.848) | 0.738 (0.664–0.812) | 0.677 (0.600–0.755) | 0.584 (0.445–0.723) | 0.665 |
| AFP-L3 | 0.741 (0.664–0.818) | 0.701 (0.624–0.777) | 0.631 (0.550–0.713) | 0.593 (0.444–0.742) | 0.637 |
| DCP | 0.794 (0.721–0.866) | 0.781 (0.715–0.847) | 0.739 (0.667–0.810) | 0.702 (0.565–0.839) | 0.703 |
| ALBI | 0.731 (0.653–0.809) | 0.787 (0.720–0.853) | 0.784 (0.714–0.855) | 0.608 (0.461–0.755) | 0.702 |
| CPS | 0.654 (0.563–0.744) | 0.690 (0.612–0.769) | 0.696 (0.618–0.774) | 0.633 (0.473–0.793) | 0.634 |
| MELD | 0.626 (0.529–0.723) | 0.635 (0.553–0.718) | 0.626 (0.538–0.703) | 0.507 (0.349–0.664) | 0.594 |
| GALAD | 0.804 (0.730–0.878) | 0.801 (0.736–0.866) | 0.731 (0.659–0.804) | 0.647 (0.502–0.792) | 0.709 |
| ASAP | 0.820 (0.749–0.892) | 0.809 (0.745–0.872) | 0.756 (0.685–0.826) | 0.660 (0.513–0.807) | 0.721 |
| GAAP | 0.807 (0.735–0.880) | 0.813 (0.751–0.875) | 0.761 (0.692–0.831) | 0.673 (0.520–0.827) | 0.720 |
| BALAD | 0.801 (0.732–0.871) | 0.799 (0.735–0.863) | 0.733 (0.659–0.806) | 0.675 (0.539–0.811) | 0.705 |
| BALAD-2 | 0.827 (0.764–0.890) | 0.846 (0.791–0.901) | 0.781 (0.713–0.850) | 0.716 (0.561–0.872) | 0.737 |
| DOYLESTOWN | 0.789 (0.711–0.868) | 0.790 (0.722–0.858) | 0.733 (0.660–0.807) | 0.648 (0.480–0.816) | 0.690 |
| AMAP | 0.471 (0.374–0.568) | 0.596 (0.510–0.681) | 0.605 (0.522–0.687) | 0.502 (0.347–0.657) | 0.546 |
| BCLC | 0.788 (0.719–0.857) | 0.749 (0.678–0.820) | 0.696 (0.619–0.773) | 0.568 (0.405–0.732) | 0.677 |
| Viral etiology (n = 126) | |||||
| 1st-year AUROC | 2nd-year AUROC | 3rd-year AUROC | 5th-year AUROC | c-index | |
| AFP | 0.770 (0.668–0.871) | 0.744 (0.654–0.833) | 0.647 (0.548–0.745) | 0.640 (0.482–0.798) | 0.648 |
| AFP-L3 | 0.752 (0.654–0.850) | 0.701 (0.608–0.795) | 0.600 (0.496–0.703) | 0.594 (0.390–0.798) | 0.621 |
| DCP | 0.837 (0.760–0.913) | 0.793 (0.714–0.872) | 0.719 (0.628–0.810) | 0.685 (0.543–0.827) | 0.683 |
| ALBI | 0.757 (0.654–0.859) | 0.792 (0.708–0.877) | 0.800 (0.719–0.881) | 0.638 (0.465–0.812) | 0.717 |
| CPS | 0.652 (0.535–0.769) | 0.686 (0.587–0.786) | 0.704 (0.610–0.798) | 0.654 (0.475–0.834) | 0.633 |
| MELD | 0.636 (0.509–0.762) | 0.628 (0.524–0.732) | 0.610 (0.508–0.712) | 0.548 (0.353–0.743) | 0.591 |
| GALAD | 0.819 (0.730–0.908) | 0.803 (0.724–0.882) | 0.695 (0.601–0.788) | 0.658 (0.504–0.812) | 0.685 |
| ASAP | 0.845 (0.766–0.924) | 0.807 (0.728–0.886) | 0.714 (0.632–0.811) | 0.644 (0.496–0.792) | 0.694 |
| GAAP | 0.822 (0.736–0.908) | 0.810 (0.732–0.887) | 0.721 (0.632–0.811) | 0.661 (0.508–0.813) | 0.690 |
| BALAD | 0.828 (0.751–0.906) | 0.802 (0.725–0.879) | 0.703 (0.610–0.798) | 0.650 (0.469–0.831) | 0.692 |
| BALAD-2 | 0.846 (0.774–0.918) | 0.853 (0.788–0.918) | 0.767 (0.683–0.852) | 0.758 (0.596–0.920) | 0.726 |
| DOYLESTOWN | 0.770 (0.666-.875) | 0.769 (0.681–0.857) | 0.668 (0.571–0.764) | 0.615 (0.434–0.796) | 0.645 |
| AMAP | 0.383 (0.267–0.499) | 0.536 (0.428–0.645) | 0.564 (0.461–0.668) | 0.484 (0.295–0.673) | 0.519 |
| BCLC | 0.790 (0.509–0.762) | 0.710 (0.616–0.804) | 0.674 (0.578–0.770) | 0.559 (0.366–0.751) | 0.644 |
| Non-viral etiology (n = 60) | |||||
| 1st-year AUROC | 2nd-year AUROC | 3rd-year AUROC | 5th-year AUROC | c-index | |
| AFP | 0.741 (0.592–0.890) | 0.695 (0.552–0.837) | 0.718 (0.585–0.851) | 0.493 (0.241–0.744) | 0.702 |
| AFP-L3 | 0.702 (0.563–0.840) | 0.676 (0.536–0.816) | 0.679 (0.540–0.818) | 0.608 (0.455–0.762) | 0.673 |
| DCP | 0.741 (0.599–0.883) | 0.799 (0.683–0.914) | 0.826 (0.715–0.936) | 0.733 (0.428–1.000) | 0.752 |
| ALBI | 0.669 (0.529–0.809) | 0.762 (0.637–0.887) | 0.750 (0.605–0.895) | 0.547 (0.287–0.807) | 0.659 |
| CPS | 0.649 (0.497–0.801) | 0.689 (0.550–0.829) | 0.684 (0.484–0.782) | 0.578 (0.263–0.892) | 0.620 |
| MELD | 0.602 (0.436–0.767) | 0.635 (0.490–0.781) | 0.633 (0.484–0.782) | 0.429 (0.166–0.693) | 0.580 |
| GALAD | 0.773 (0.637–0.909) | 0.796 (0.678–0.914) | 0.810 (0.697–0.923) | 0.642 (0.349–0.934) | 0.763 |
| ASAP | 0.777 (0.642–0.912) | 0.812 (0.699–0.924) | 0.838 (0.726–0.950) | 0.679 (0.315–1.000) | 0.774 |
| GAAP | 0.785 (0.657–0.913) | 0.824 (0.715–0.933) | 0.850 (0.740–0.960) | 0.689 (0.311–1.000) | 0.777 |
| BALAD | 0.740 (0.602–0.877) | 0.782 (0.663–0.901) | 0.788 (0.667–0.909) | 0.733 (0.549–0.918) | 0.727 |
| BALAD-2 | 0.796 (0.676–0.916) | 0.831 (0.725–0.938) | 0.807 (0.686–0.929) | 0.637 (0.297–0.976) | 0.762 |
| DOYLESTOWN | 0.807 (0.687–0.928) | 0.814 (0.700–0.928) | 0.846 (0.730–0.963) | 0.684 (0.301–1.000) | 0.770 |
| AMAP | 0.554 (0.389–0.719) | 0.648 (0.500–0.796) | 0.647 (0.500–0.794) | 0.538 (0.234–0.841) | 0.579 |
| BCLC | 0.789 (0.668–0.909) | 0.837 (0.726–0.948) | 0.774 (0.638–0.910) | 0.575 (0.249–0.902) | 0.732 |
| Curative treated (n = 71) | |||||
| 1st-Year AUROC | 2nd-Year AUROC | 3rd-Year AUROC | 5th-Year AUROC | c-İndex | |
| AFP | 0.801 (0.594–1.000) | 0.727 (0.566–0.888) | 0.613 (0.472–0.754) | 0.579 (0.406–0.752) | 0.633 |
| AFP-L3 | 0.792 (0.630–0.953) | 0.670 (0.519–0.820) | 0.525 (0.383–0.667) | 0.533 (0.335–0.732) | 0.601 |
| DCP | 0.749 (0.574–0.923) | 0.786 (0.668–0.905) | 0.691 (0.565–0.816) | 0.559 (0.379–0.739) | 0.654 |
| ALBI | 0.676 (0.438–0.914) | 0.729 (0.578–0.880) | 0.770 (0.656–0.884) | 0.569 (0.374–0.765) | 0.689 |
| CPS | 0.652 (0.401–0.904) | 0.661 (0.493–0.829) | 0.699 (0.568–0.830) | 0.606 (0.425–0.787) | 0.616 |
| MELD | 0.604 (0.367–0.839) | 0.610 (0.460–0.760) | 0.636 (0.498–0.775) | 0.438 (0.254–0.621) | 0.577 |
| GALAD | 0.768 (0.547–0.990) | 0.769 (0.614–0.925) | 0.641 (0.501–0.781) | 0.570 (0.386–0.754) | 0.652 |
| ASAP | 0.755 (0.561–0.949) | 0.794 (0.674–0.914) | 0.673 (0.542–0.804) | 0.568 (0.391–0.746) | 0.665 |
| GAAP | 0.729 (0.513–0.945) | 0.795 (0.664–0.926) | 0.691 (0.563–0.820) | 0.572 (0.387–0.757) | 0.664 |
| BALAD | 0.832 (0.691–0.973) | 0.737 (0.609–0.864) | 0.635 (0.500–0.769) | 0.577 (0.379–0.775) | 0.652 |
| BALAD-2 | 0.865 (0.740–0.989) | 0.813 (0.709–0.917) | 0.709 (0.585–0.833) | 0.697 (0.511–0.882) | 0.698 |
| DOYLESTOWN | 0.729 (0.497–0.962) | 0.681 (0.508–0.854) | 0.614 (0.474–0.755) | 0.602 (0.397–0.8e06) | 0.603 |
| AMAP | 0.398 (0.136–0.661) | 0.652 (0.467–0.838) | 0.729 (0.592–0.866) | 0.550 (0.368–0.732) | 0.636 |
| BCLC | 0.784 (0.601–0.967) | 0.681 (0.515–0.846) | 0.637 (0.504–0.770) | 0.414 (0.228–0.600) | 0.613 |
| Non-curative treated (n = 81) | |||||
| 1st-year AUROC | 2nd-year AUROC | 3rd-year AUROC | 5th-year AUROC | c-index | |
| AFP | 0.721 (0.619–0.822) | 0.699 (0.602–0.796) | 0.653 (0.548–0.757) | 0.426 (0.257–0.596) | 0.653 |
| AFP-L3 | 0.695 (0.595–0.796) | 0.678 (0.576–0.779) | 0.655 (0.545–0.764) | 0.643 (0.467–0.818) | 0.626 |
| DCP | 0.769 (0.676–0.862) | 0.744 (0.652–0.836) | 0.740 (0.643–0.836) | 0.744 (0.387–1.000) | 0.687 |
| ALBI | 0.699 (0.600–0.798) | 0.788 (0.696–0.879) | 0.769 (0.664–0.874) | 0.516 (0.307–0.725) | 0.670 |
| CPS | 0.631 (0.523–0.739) | 0.693 (0.591–0.795) | 0.681 (0.569–0.794) | 0.564 (0.151–0.977) | 0.618 |
| MELD | 0.610 (0.497–0.723) | 0.633 (0.527–0.739) | 0.582 (0.462–0.701) | 0.521 (0.074–0.967) | 0.581 |
| GALAD | 0.762 (0.667–0.856) | 0.758 (0.688–0.848) | 0.719 (0.621–0.818) | 0.606 (0.238–0.929) | 0.687 |
| ASAP | 0.799 (0.711–0.887) | 0.776 (0.688–0.864) | 0.761 (0.666–0.855) | 0.612 (0.208–1.000) | 0.708 |
| GAAP | 0.778 (0.687–0.870) | 0.773 (0.685–0.862) | 0.751 (0.653–0.848) | 0.612 (0.168–1.000) | 0.698 |
| BALAD | 0.743 (0.648–0.837) | 0.779 (0.690–0.868) | 0.738 (0.638–0.837) | 0.713 (0.540–0.887) | 0.679 |
| BALAD-2 | 0.782 (0.696–0.868) | 0.829 (0.749–0.908) | 0.784 (0.687–0.880) | 0.622 (0.190–1.000) | 0.716 |
| DOYLESTOWN | 0.757 (0.662–0.868) | 0.782 (0.695–0.870) | 0.748 (0.648–0.847) | 0.506 (0.072–0.941) | 0.679 |
| AMAP | 0.475 (0.360–0.590) | 0.590 (0.482–0.698) | 0.523 (0.403–0.642) | 0.458 (0.093–0.824) | 0.517 |
| BCLC | 0.708 (0.610–0.806) | 0.679 (0.576–0.782) | 0.655 (0.540–0.770) | 0.364 (0.000–0.788) | 0.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirtas, C.O.; Eren, F.; Yilmaz Karadag, D.; Kaldirim Armutcuoglu, Y.; Tolu, T.; Huseyinov, J.; Ciftci, U.; Yilmaz, T.; Akin, S.; Dilber, F.; et al. BALAD-2 Emerges as the Most Accurate Prognostic Model in Hepatocellular Carcinoma: Results from a Biobank-Based Cohort Study. Cancers 2025, 17, 3457. https://doi.org/10.3390/cancers17213457
Demirtas CO, Eren F, Yilmaz Karadag D, Kaldirim Armutcuoglu Y, Tolu T, Huseyinov J, Ciftci U, Yilmaz T, Akin S, Dilber F, et al. BALAD-2 Emerges as the Most Accurate Prognostic Model in Hepatocellular Carcinoma: Results from a Biobank-Based Cohort Study. Cancers. 2025; 17(21):3457. https://doi.org/10.3390/cancers17213457
Chicago/Turabian StyleDemirtas, Coskun Ozer, Fatih Eren, Demet Yilmaz Karadag, Yasemin Kaldirim Armutcuoglu, Tugba Tolu, Javid Huseyinov, Ugur Ciftci, Tuba Yilmaz, Sehnaz Akin, Feyza Dilber, and et al. 2025. "BALAD-2 Emerges as the Most Accurate Prognostic Model in Hepatocellular Carcinoma: Results from a Biobank-Based Cohort Study" Cancers 17, no. 21: 3457. https://doi.org/10.3390/cancers17213457
APA StyleDemirtas, C. O., Eren, F., Yilmaz Karadag, D., Kaldirim Armutcuoglu, Y., Tolu, T., Huseyinov, J., Ciftci, U., Yilmaz, T., Akin, S., Dilber, F., & Ozdogan, O. C. (2025). BALAD-2 Emerges as the Most Accurate Prognostic Model in Hepatocellular Carcinoma: Results from a Biobank-Based Cohort Study. Cancers, 17(21), 3457. https://doi.org/10.3390/cancers17213457






