Astragalin Attenuates Bone Destruction and the Progression of Bone Metastasis in Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Cells and Treatment
2.2. In Vitro Osteoclastogenesis Assays
2.3. Quantitative Real-Time PCR
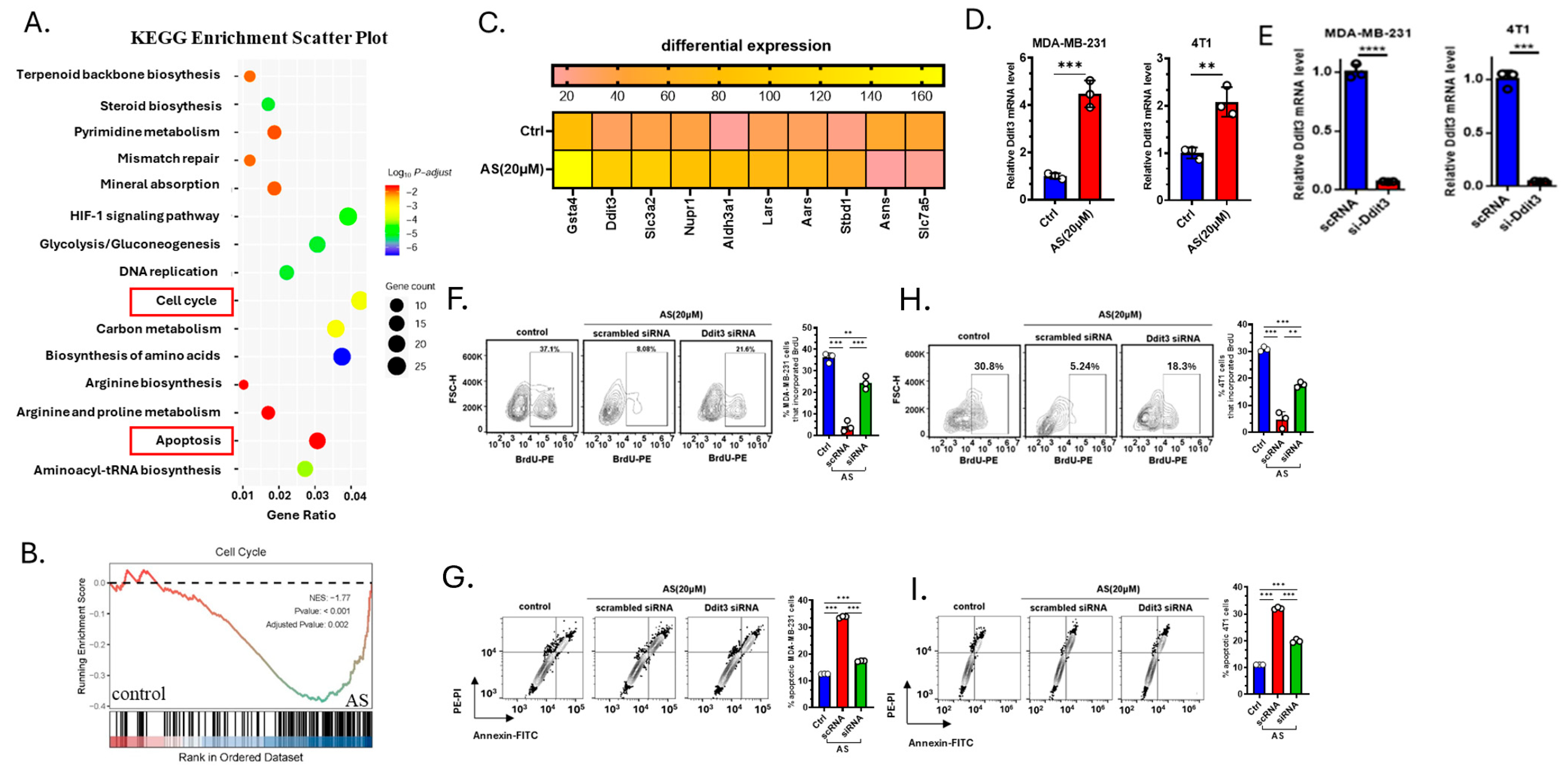
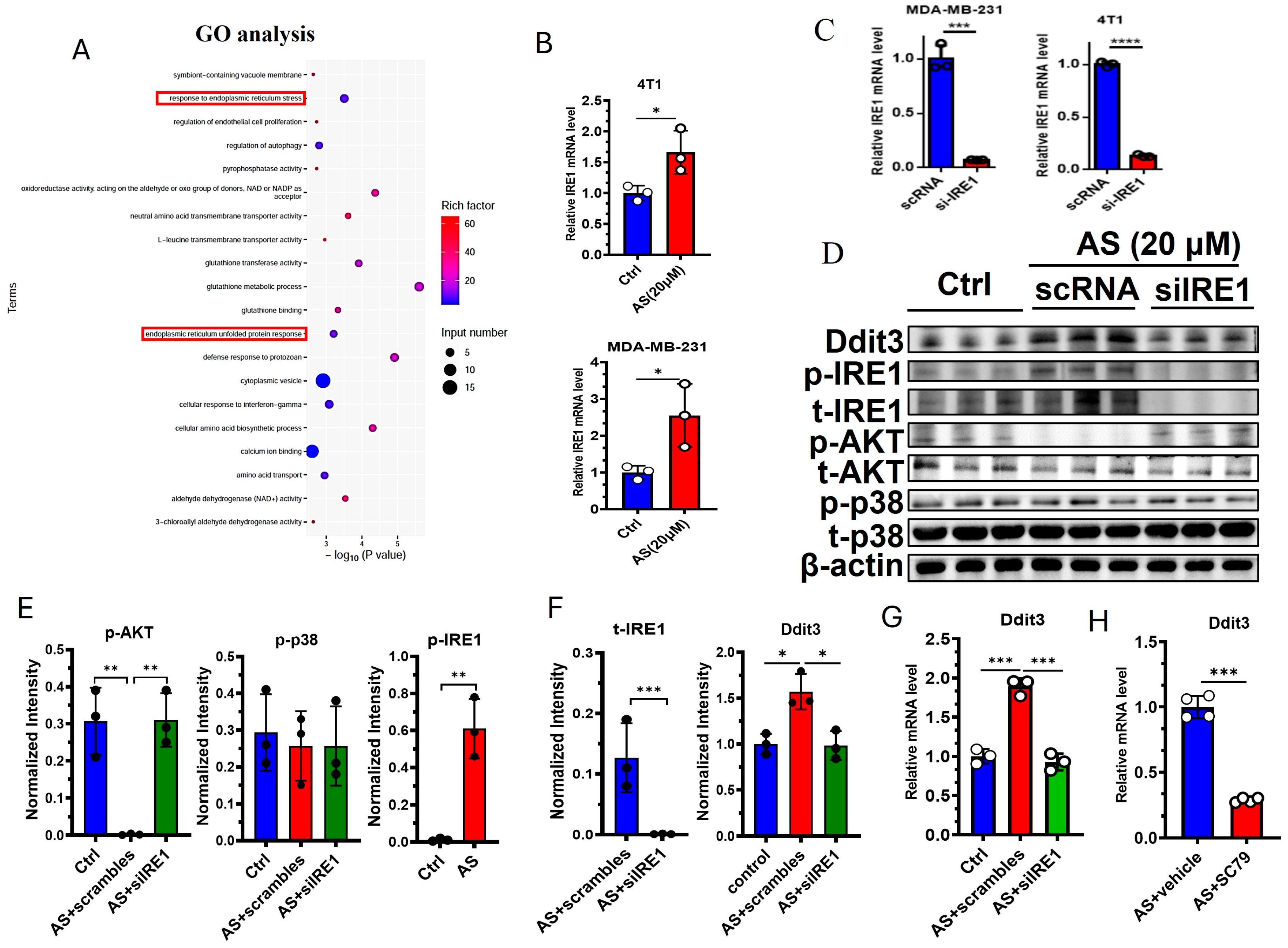
2.4. Application of Small Interfering RNA (siRNA)
2.5. Western Blotting
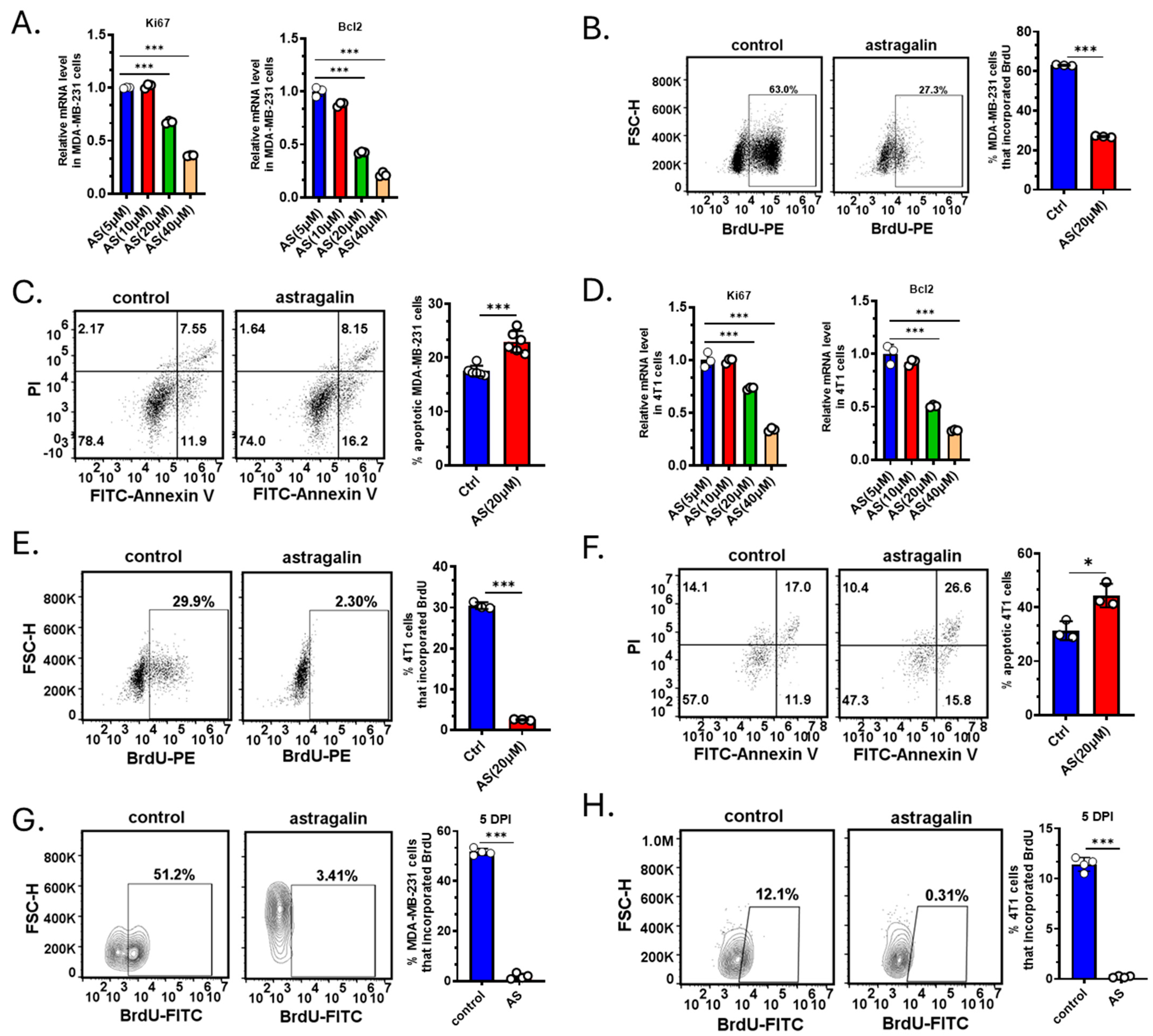
2.6. In Vitro Proliferation Assay and Apoptosis Assay
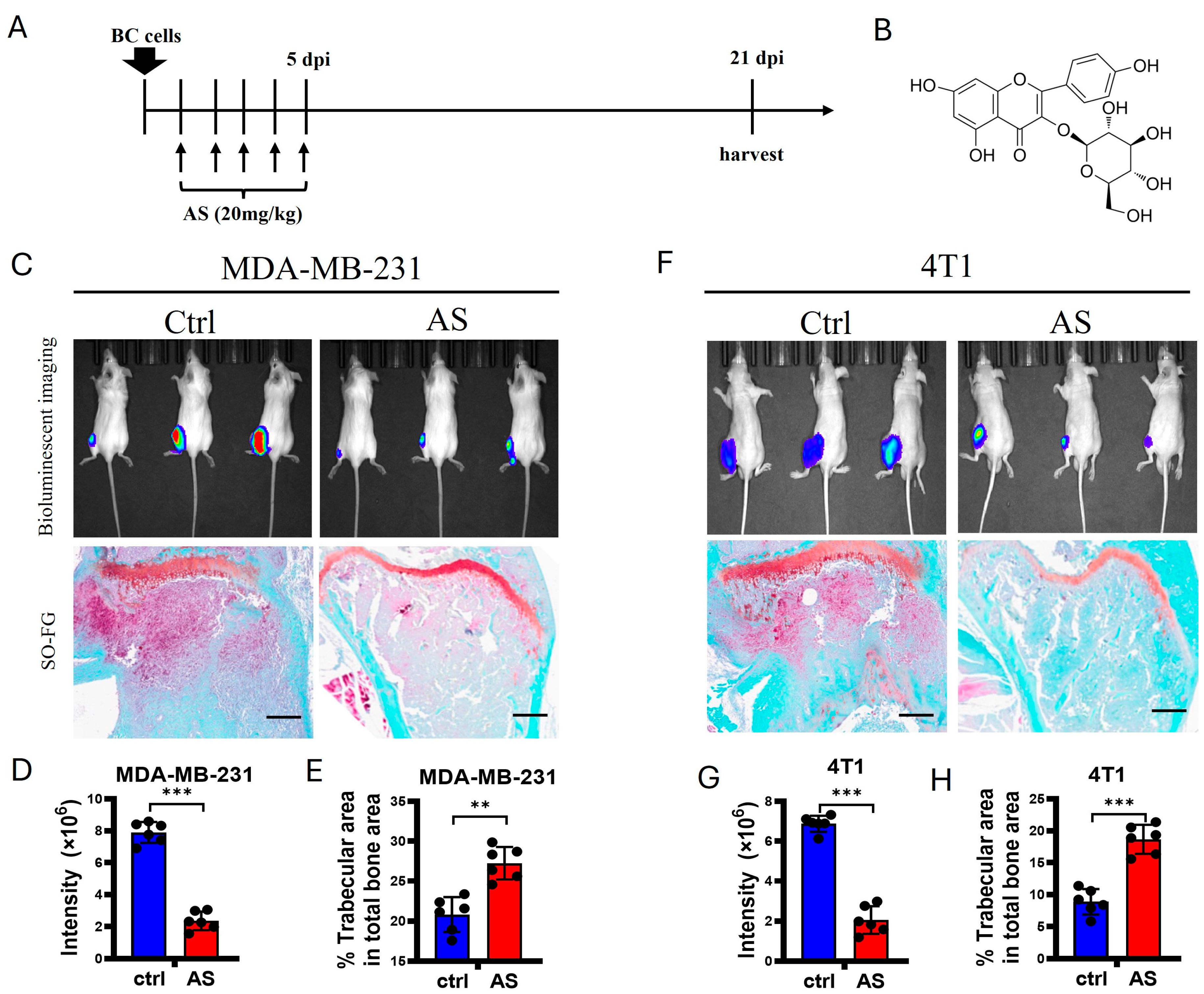
2.7. Animal Experiments
2.8. Bioluminescence Imaging (BLI)
2.9. M. CT Analysis
2.10. Transcriptome
2.11. Histochemical Analysis
2.12. Statistical Analysis
3. Results
3.1. AS Inhibited the Progression of Bone Metastasis in BC
3.2. AS Inhibited the Growth of BC Cells Both In Vitro and In Vivo
3.3. AS Inhibited the Growth of BC Cells by Upregulating Ddit3
3.4. The IRE1-AKT Pathway Regulates the Expression of Ddit3 in BC Cells
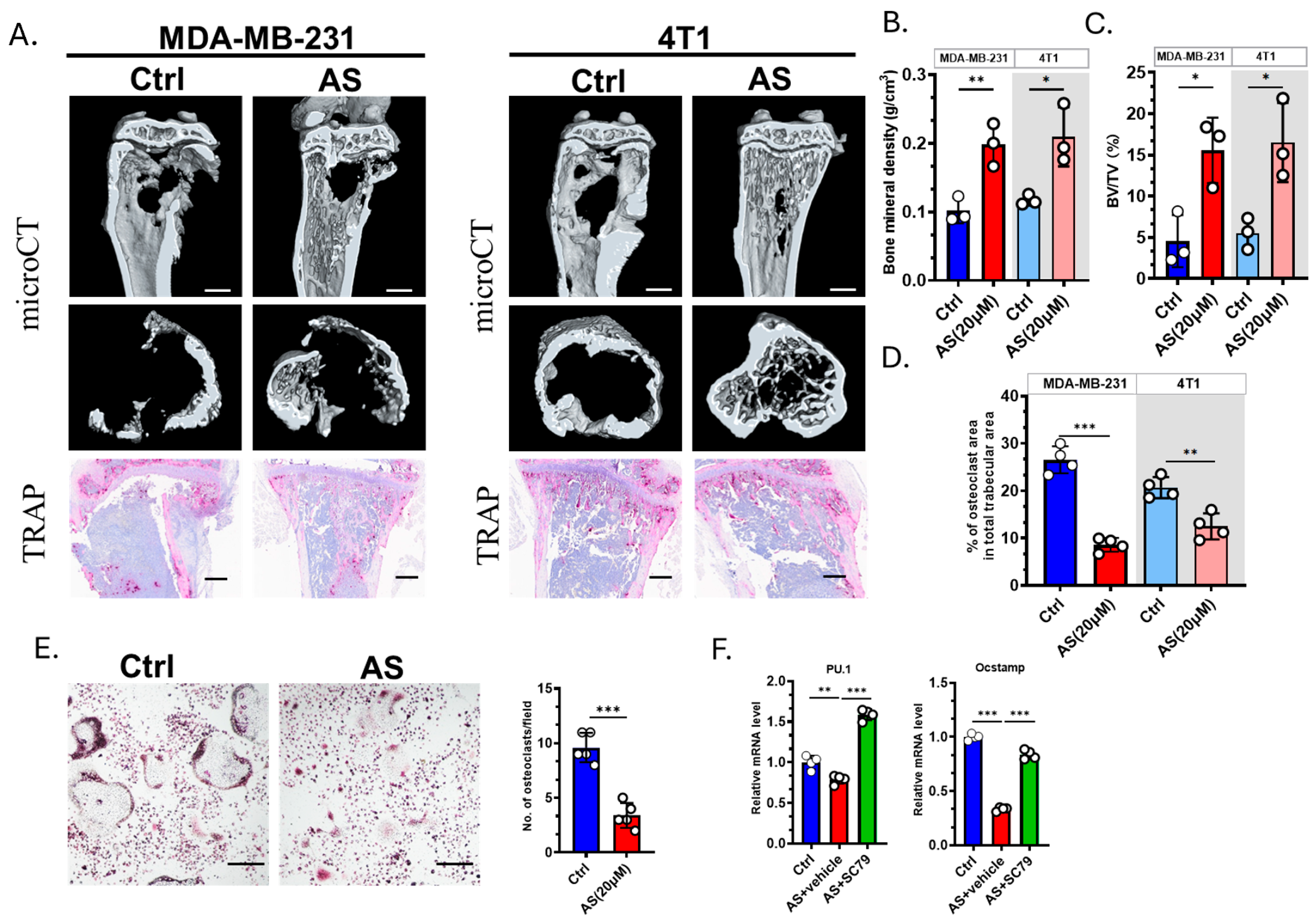
3.5. AS Inhibited Osteoclastogenesis in the Bone Metastasis of BC
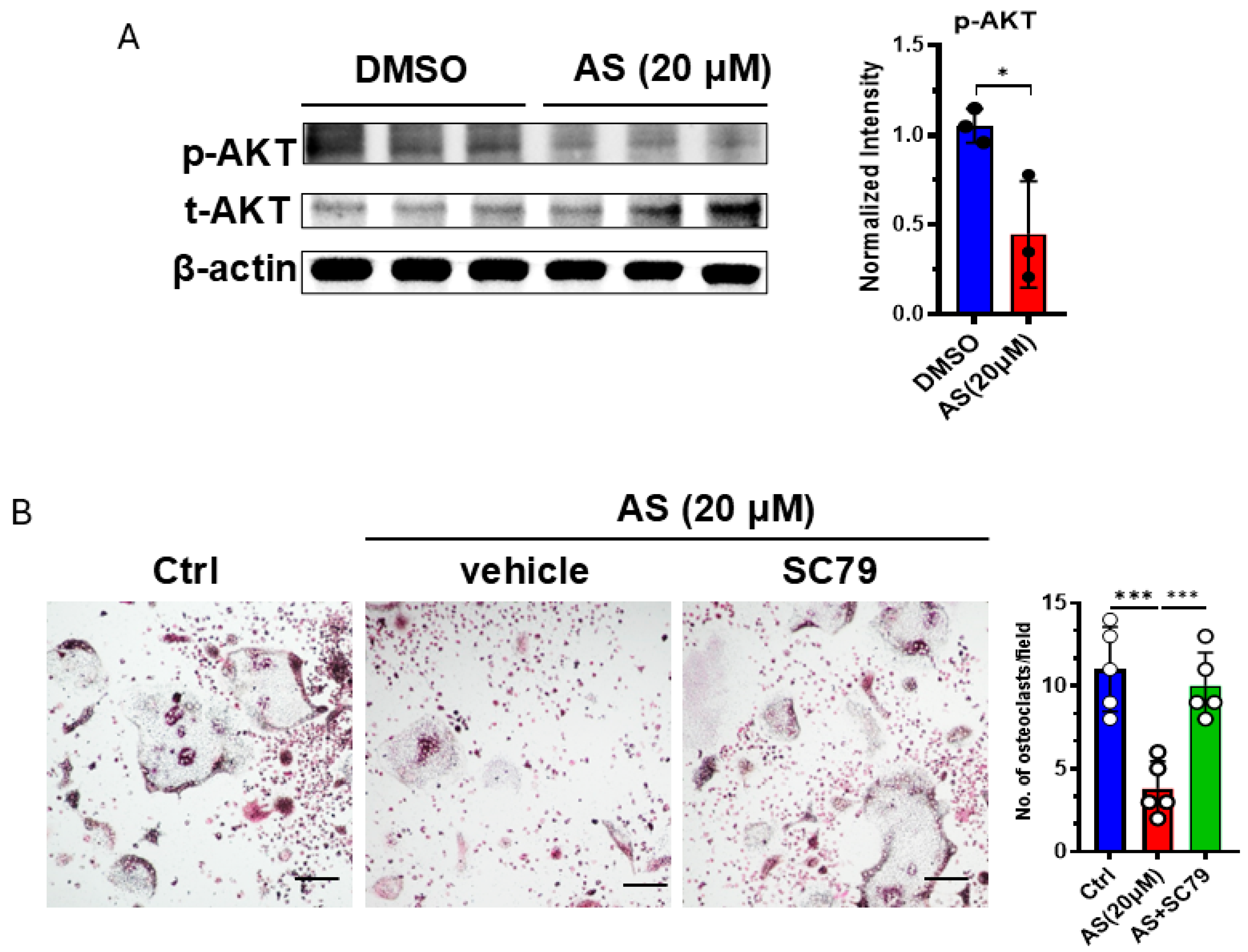
3.6. AS Inhibited Osteoclastogenesis Partly by Inhibiting the AKT Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Zhang, S.; Liao, X.; Li, M.; Chen, S.; Li, X.; Wu, X.; Yang, M.; Tang, M.; Hu, Y.; et al. Circular RNA circIKBKB promotes breast cancer bone metastasis through sustaining NF-kappaB/bone remodeling factors signaling. Mol. Cancer 2021, 20, 98. [Google Scholar] [CrossRef]
- Venetis, K.; Piciotti, R.; Sajjadi, E.; Invernizzi, M.; Morganti, S.; Criscitiello, C.; Fusco, N. Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management. Cells 2021, 10, 1377. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Li, X.-Y.; Keller, E.T.; Yang, J.; Cho, J.-S.; Feinberg, T.Y.; Weiss, S.J. Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases. Sci. Transl. Med. 2020, 12, eaaw6143. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.; Jung, S.; Moon, A.; Noh, D.Y.; Lee, Z.H.; Kim, H.J.; Kim, H.H. A CTGF-RUNX2-RANKL Axis in Breast and Prostate Cancer Cells Promotes Tumor Progression in Bone. J. Bone Miner. Res. 2020, 35, 155–166. [Google Scholar] [CrossRef]
- Liang, M.; Ma, Q.; Ding, N.; Luo, F.; Bai, Y.; Kang, F.; Gong, X.; Dong, R.; Dai, J.; Dai, Q.; et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Wada, A.; Tsuchiya, M.; Ozaki-Honda, Y.; Kayamori, K.; Sakamoto, K.; Yamaguchi, A.; Ikeda, T. A new osteoclastogenesis pathway induced by cancer cells targeting osteoclast precursor cells. Biochem. Biophys. Res. Commun. 2019, 509, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Sadvakassova, G.; Mikolajewicz, N.; Juhas, M.; Sabirova, Z.; Tabariès, S.; Gettemans, J.; Siegel, P.M.; Komarova, S.V. Exosomal Release of L-Plastin by Breast Cancer Cells Facilitates Metastatic Bone Osteolysis. Transl. Oncol. 2019, 12, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Nakachi, Y.; Yokoo, T.; Ichihara, T.; Eriksson, T.; Yonemoto, Y.; Kato, T.; Ogata, H.; Fujimoto, N.; Kobayashi, Y.; et al. Murine osteoclasts secrete serine protease HtrA1 capable of degrading osteoprotegerin in the bone microenvironment. Commun. Biol. 2019, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Qiang, L.; Xu, Y.; Li, C.; Li, T.; Wang, J. Inhibition of JNK and activation of the AMPK-Nrf2 axis by corosolic acid suppress osteolysis and oxidative stress. Nitric. Oxide. 2019, 82, 12–24. [Google Scholar] [CrossRef]
- Marino, S.; Bishop, R.T.; Carrasco, G.; Logan, J.G.; Li, B.; Idris, A.I. Pharmacological Inhibition of NFκB Reduces Prostate Cancer Related Osteoclastogenesis In Vitro and Osteolysis Ex Vivo. Calcif. Tissue Int. 2019, 105, 193–204. [Google Scholar] [CrossRef]
- Gingery, A.; Bradley, E.W.; Pederson, L.; Ruan, M.; Horwood, N.J.; Oursler, M.J. TGF-β coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp. Cell Res. 2008, 314, 2725–2738. [Google Scholar] [CrossRef]
- Wang, Q.; Mo, J.; Zhao, C.; Huang, K.; Feng, M.; He, W.; Wang, J.; Chen, S.; Xie, Z.; Ma, J.; et al. Raddeanin A suppresses breast cancer-associated osteolysis through inhibiting osteoclasts and breast cancer cells. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, M.; Yu, Q.-F.; Yang, P.-F.; Zhang, H.-D.; Sun, Y.-H.; Zhang, Z.-F.; Gao, Y.-F. Puerarin Prevents LPS-Induced Osteoclast Formation and Bone Loss via Inhibition of Akt Activation. Biol. Pharm. Bull. 2016, 39, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-J.; Kuo, P.-L.; Hou, M.-F.; Hung, J.-Y.; Chang, F.-R.; Hsu, Y.-C.; Huang, Y.-F.; Tsai, E.-M.; Hsu, Y.-L. Wedelolactone inhibits breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR signaling. Int. J. Oncol. 2014, 46, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, W.Y.; Xie, J.; Wang, Z.L.; Wen, Y.L.; Zhao, C.C.; Tao, L.; Li, L.F.; Tian, Y.; Sheng, J. Astragalin Inhibits the Proliferation and Migration of Human Colon Cancer HCT116 Cells by Regulating the NF-κB Signaling Pathway. Front. Pharmacol. 2021, 12, 639256. [Google Scholar] [CrossRef]
- Xu, G.; Yu, B.; Wang, R.; Jiang, J.; Wen, F.; Shi, X. Astragalin flavonoid inhibits proliferation in human lung carcinoma cells mediated via induction of caspase-dependent intrinsic pathway, ROS production, cell migration and invasion inhibition and targeting JAK/STAT signalling pathway. Cell. Mol. Biol. 2021, 67, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, L.; Chen, J.; Cui, T.; Liao, W. Astragalin induced selective kidney cancer cell death and these effects are mediated via mitochondrial mediated cell apoptosis, cell cycle arrest, and modulation of key tumor-suppressive miRNAs. J. Buon 2019, 24, 1245–1251. [Google Scholar] [PubMed]
- Tian, S.; Wei, Y.; Hu, H.; Zhao, H. Mixed computational-experimental study to reveal the anti-metastasis and anti-angiogenesis effects of Astragalin in human breast cancer. Comput. Biol. Med. 2022, 150, 106131. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Jang, Y.J.; Lee, K.; Kim, H.J.; Lee, J.Y.; Kim, J.M.; Park, S.; Song, J.S.; Lee, J.H.; et al. Denatonium inhibits RANKL-induced osteoclast differentiation and rescues the osteoporotic phenotype by blocking p65 signaling pathway. Mol. Med. 2024, 30, 248. [Google Scholar] [CrossRef]
- Dryer, Y.; Berghausen, J.; Creswell, K.; Glasgow, E.; Brelidze, T.I. Comparison of tumor growth assessment using GFP fluorescence and DiI labeling in a zebrafish xenograft model. Cancer Biol. Ther. 2023, 24, 2234140. [Google Scholar] [CrossRef]
- Campbell, J.P.; Merkel, A.R.; Masood-Campbell, S.K.; Elefteriou, F.; Sterling, J.A. Models of bone metastasis. J. Vis. Exp. 2012, 67, e4260. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Z.; Wang, S.-H.; Zhang, R.-H.; Lin, J.-H.; Tian, Y.-H.; Yang, Y.-Q.; Liu, J.; Ma, Y.-X. Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov. 2023, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Gambardella, C.; Conzo, G.; Ruggiero, R.; Tolone, S.; Lucido, F.S.; Iovino, F.; Fisone, F.; Brusciano, L.; Parmeggiani, D.; et al. Advanced Localization Technique for Non-Palpable Breast Cancer: Radiofrequency alone VS Combined Technique with Ultrasound. J. Clin. Med. 2023, 12, 5076. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Ruggiero, R.; Gualtieri, G.; Volpe, M.L.; Rinaldi, S.; Nesta, G.; Bogdanovich, L.; Lucido, F.S.; Tolone, S.; Parmeggiani, D.; et al. Combined LOCalizer™ and Intraoperative Ultrasound Localization: First Experience in Localization of Non-palpable Breast Cancer. Vivo 2021, 35, 1669–1676. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Ruan, J.; Shi, Z.; Cao, X.; Dang, Z.; Zhang, Q.; Zhang, W.; Wu, L.; Zhang, Y.; Wang, T. Research Progress on Anti-Inflammatory Effects and Related Mechanisms of Astragalin. Int. J. Mol. Sci. 2024, 25, 4476. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Ye, H.-J.; Zheng, C.; Jin, Z.-J.; Yuan, Y.; Weng, H.-B. Astragalin ameliorates renal injury in diabetic mice by modulating mitochondrial quality control via AMPK-dependent PGC1α pathway. Acta Pharmacol. Sin. 2023, 44, 1676–1686. [Google Scholar] [CrossRef]
- Xing, F.; Geng, L.; Guan, H.; Liu, D.; Li, Y.; Zeng, L.; Chen, Y.; Tian, R.; Li, Z.; Cao, R.; et al. Astragalin mitigates inflammatory osteolysis by negatively modulating osteoclastogenesis via ROS and MAPK signaling pathway. Int. Immunopharmacol. 2022, 112, 109278. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, T.; Wang, X.; Xu, H.; Liu, Y.; Wang, Y.; Shi, Q.; Liang, Q. Astragalin Suppresses Inflammatory Responses and Bone Destruction in Mice With Collagen-Induced Arthritis and in Human Fibroblast-Like Synoviocytes. Front. Pharmacol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Li, T.; Su, L.; Lei, Y.; Liu, X.; Zhang, Y.; Liu, X. DDIT3 and KAT2A Proteins Regulate TNFRSF10A and TNFRSF10B Expression in Endoplasmic Reticulum Stress-mediated Apoptosis in Human Lung Cancer Cells. J. Biol. Chem. 2015, 290, 11108–11118. [Google Scholar] [CrossRef]
- Block, I.; Müller, C.; Sdogati, D.; Pedersen, H.; List, M.; Jaskot, A.M.; Syse, S.D.; Hansen, P.L.; Schmidt, S.; Christiansen, H.; et al. CFP suppresses breast cancer cell growth by TES-mediated upregulation of the transcription factor DDIT3. Oncogene 2019, 38, 4560–4573. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhao, J.; Yang, A.; Li, X. MLLT6/ATF2 Axis Restrains Breast Cancer Progression by Driving DDIT3/4 Expression. Mol. Cancer Res. 2024, 22, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Muhammad, T.S.T.; Tan, M.L. 14-Deoxy-11,12-didehydroandrographolide induces DDIT3-dependent endoplasmic reticulum stress-mediated autophagy in T-47D breast carcinoma cells. Toxicol. Appl. Pharmacol. 2016, 300, 55–69. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Li, J.; Yang, H.; Yin, Y. The Effects of Butyric Acid on the Differentiation, Proliferation, Apoptosis, and Autophagy of IPEC-J2 Cells. Curr. Mol. Med. 2020, 20, 307–317. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, Y.; Qiu, H.; Hu, X.; Chu, T. Astragalin Attenuates Bone Destruction and the Progression of Bone Metastasis in Breast Cancer. Cancers 2025, 17, 3442. https://doi.org/10.3390/cancers17213442
Yang S, Zhang Y, Qiu H, Hu X, Chu T. Astragalin Attenuates Bone Destruction and the Progression of Bone Metastasis in Breast Cancer. Cancers. 2025; 17(21):3442. https://doi.org/10.3390/cancers17213442
Chicago/Turabian StyleYang, Sizhen, Ying Zhang, Hao Qiu, Xu Hu, and Tongwei Chu. 2025. "Astragalin Attenuates Bone Destruction and the Progression of Bone Metastasis in Breast Cancer" Cancers 17, no. 21: 3442. https://doi.org/10.3390/cancers17213442
APA StyleYang, S., Zhang, Y., Qiu, H., Hu, X., & Chu, T. (2025). Astragalin Attenuates Bone Destruction and the Progression of Bone Metastasis in Breast Cancer. Cancers, 17(21), 3442. https://doi.org/10.3390/cancers17213442




