Overcoming Immune Evasion in the Prostate Tumor Microenvironment: Novel Targeted Strategies to Improve Treatment Outcomes
Simple Summary
Abstract
1. Introduction
2. Mechanisms of Immune Evasion in the Prostate Cancer Tumor Microenvironment
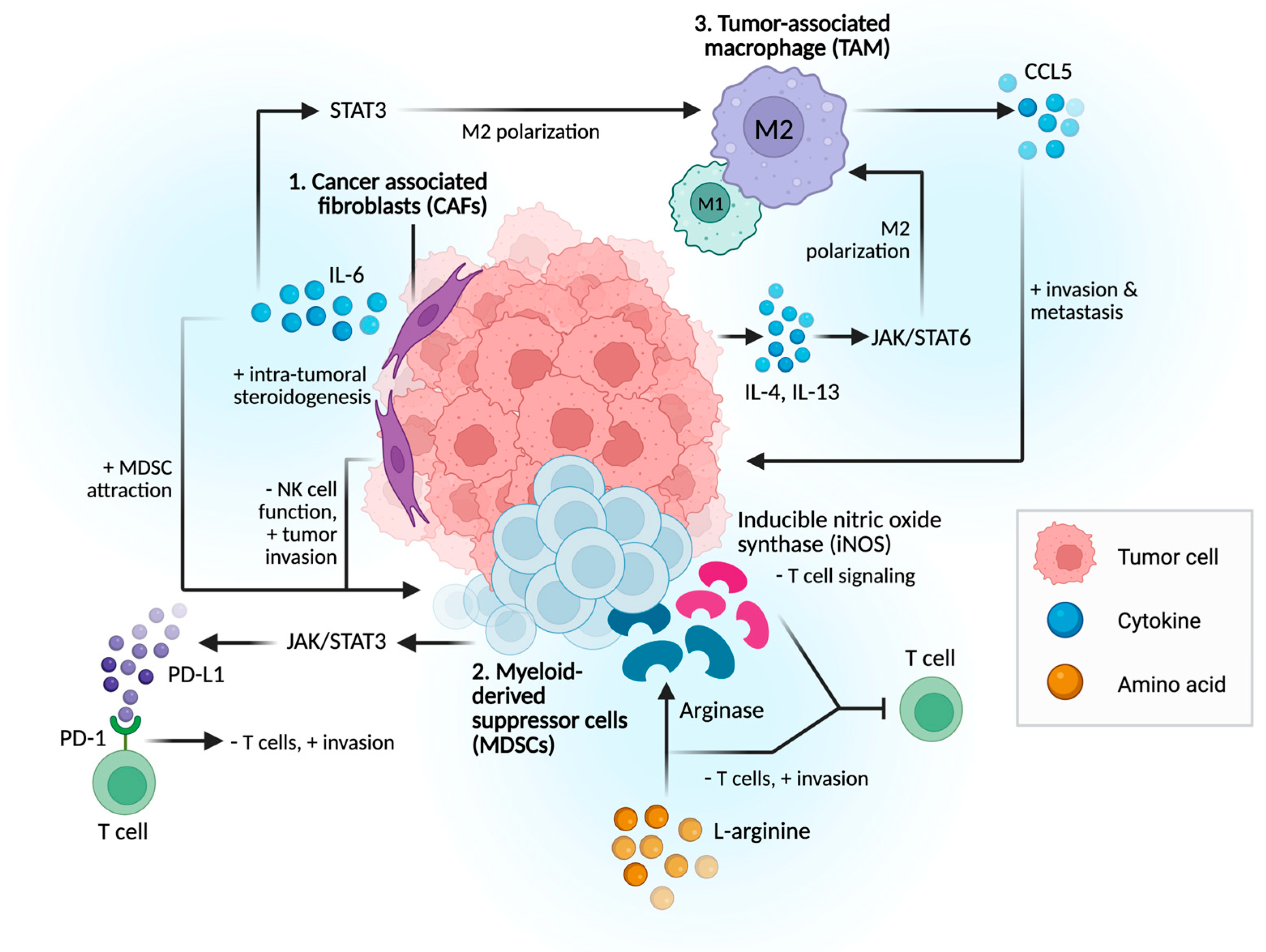
2.1. Immunosuppressive Cells and Signaling Pathways
2.1.1. Cancer-Associated Fibroblasts (CAFs)
2.1.2. Myeloid-Derived Suppressor Cells (MDSCs)
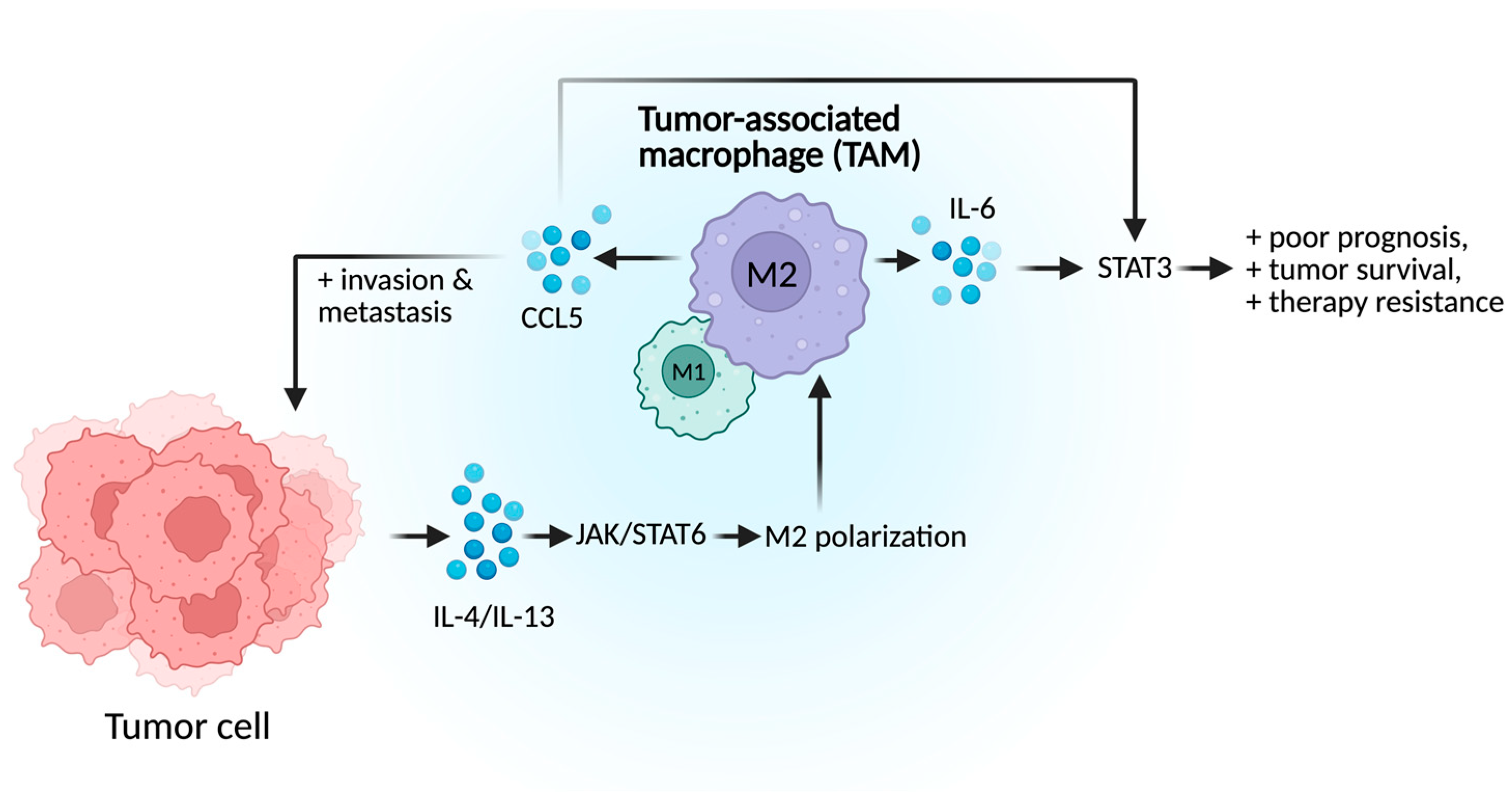
2.1.3. Tumor-Associated Macrophages (TAMs)
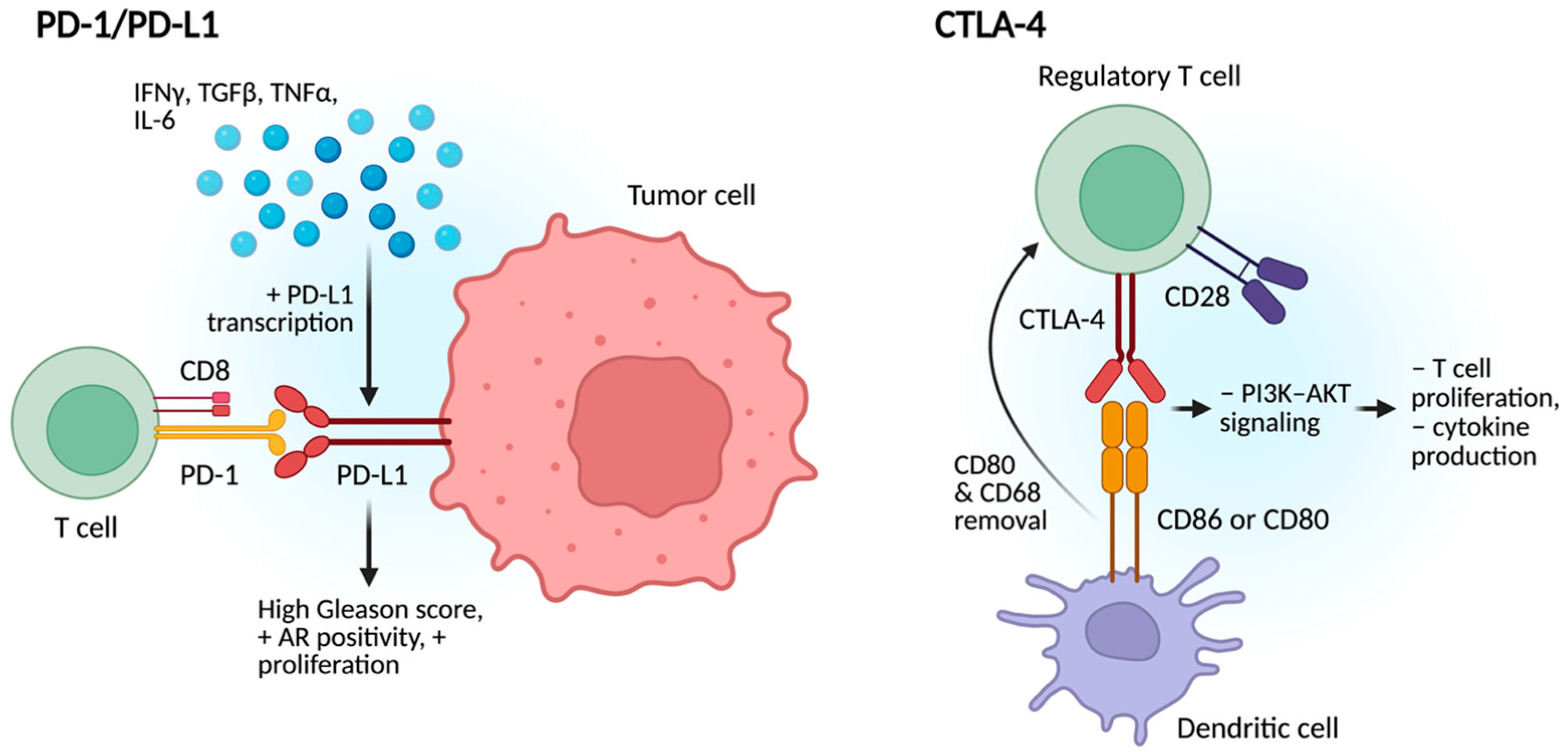
2.2. Modulation of Immune Checkpoints
2.2.1. PD-1/PD-L1
2.2.2. CTLA-4
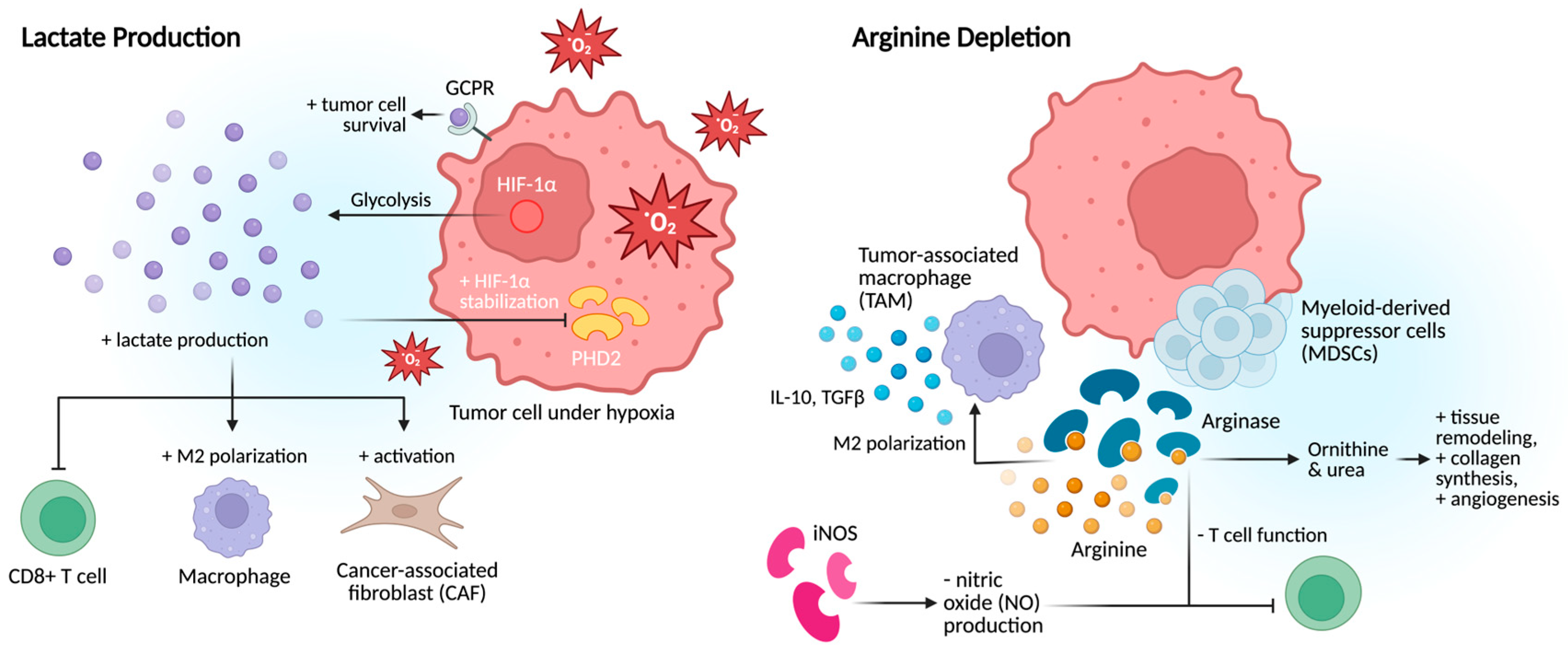
2.3. Metabolic Alterations
2.3.1. Lactate Production
2.3.2. Arginine Depletion
3. Potential Opportunities: Modulating the Tumor Microenvironment
3.1. Targeting Immunosuppressive Cells
3.1.1. Targeting CAFs
3.1.2. Targeting MDSC and TAMs
3.1.3. Targeting Metabolic Pathways
3.1.4. Modulating Immune Checkpoint Inhibitors
3.1.5. Opportunities to Change the Tumor Microenvironment: Combination Therapy
3.1.6. Chemotherapy
3.1.7. Tyrosine Kinase Inhibitor (TKIs)
3.1.8. AKT Inhibitor
3.1.9. Radioligand Therapy
3.1.10. PARP Inhibitors
3.1.11. Microbiome
3.1.12. Other Combinations
3.1.13. Key to Future Success
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | Prostate Cancer |
| ADC | Antibody–drug conjugate |
| ADT | Androgen deprivation therapy |
| APCs | Antigen-presenting cells |
| AR | Androgen receptor |
| ASS | Argininosuccinate synthase |
| BCR | Biochemical recurrence |
| CAFs | Cancer-associated fibroblasts |
| mCRPC | Metastatic castration-resistant prostate cancer |
| dMMR | Mismatch repair deficiency |
| EMT | Epithelial–Mesenchymal transition |
| ERBB | Erythroblastic leukemia viral oncogene homolog |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| PHD2 | Prolyl hydroxylase domain-containing protein 2 |
| iNOS | Inducible nitric oxide synthase |
| ICI | Immune checkpoint inhibitor |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| MDSCs | Myeloid-derived suppressor cells |
| MSI-High | Microsatellite instability-high |
| OS | Overall survival |
| PFS | Progression-free survival |
| NRG1 | Neuregulin-1 |
| ROS | Reactive oxygen species |
| PSA | Prostate-specific antigen |
| PARPi | Poly (ADP-ribose) polymerase inhibitor |
| PSMA RLT | PSMA radioligand therapy |
| TAMs | Tumor-associated macrophages |
| TKI | Tyrosine kinase inhibitor |
| TME | Tumor microenvironment |
| PSMA | Prostate-specific membrane antigen |
| PMN-MDSCs | Polymorphonuclear myeloid-derived suppressor cells |
| HER3 | Human epidermal growth factor receptor 3 |
| Tregs | Regulatory T-cells |
| TMB | Tumor mutational burden |
| VEGF | Vascular endothelial growth factor |
| ARTA | Androgen receptor-targeted agents |
| M-MDSCs | Monocytic myeloid-derived suppressor cells |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clinicians. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagata, M.; Hachiya, T.; Wakita, H.; Ikehata, Y.; Takahashi, K.; China, T.; Shimizu, F.; Lu, J.; Jin, Y.; et al. Increased circulating polymorphonuclear myeloid-derived suppressor cells are associated with prognosis of metastatic castration-resistant prostate cancer. Front. Immunol. 2024, 15, 1372771. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Azizi, S.; Mahjoubfar, A.; Varaki, F.A.; Fayyaz, F.; Mousavian, A.-H.; Bashash, D.; Parizi, M.K.; Kasaeian, A. Efficacy and safety of immune checkpoint inhibitors for patients with prostate cancer: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1181051. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Conteduca, V.; Landriscina, M.; Procopio, A.D. Circulating myeloid-derived suppressor cells and survival in prostate cancer patients: Systematic review and meta-analysis. Prostate Cancer Prostatic Diseases 2022, 26, 41–46. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Chang, Y.; Xiao, Y.; He, Y.; Ren, S. Advances in landscape and related therapeutic targets of the prostate tumor microenvironment. Acta Biochim. Et Biophys. Sinica 2023, 55, 956–973. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Kibel, A.S.; Yu, E.Y.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vogelzang, N.J.; Corman, J.M.; Millard, F.E.; Maher, J.C.; et al. Sequencing of Sipuleucel-T and Androgen Deprivation Therapy in Men with Hormone-Sensitive Biochemically Recurrent Prostate Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2017, 23, 2451–2459. [Google Scholar] [CrossRef]
- Holl, E.K.; McNamara, M.A.; Healy, P.; Anand, M.; Concepcion, R.S.; Breland, C.D.; Dumbudze, I.; Tutrone, R.; Shore, N.; Armstrong, A.J.; et al. Prolonged PSA stabilization and overall survival following sipuleucel-T monotherapy in metastatic castration-resistant prostate cancer patients. Prostate Cancer Prostatic Dis. 2019, 22, 588–592. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Mortazavi, A.; Zhang, J. Emerging Immunotherapy Approaches for Treating Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 14347. [Google Scholar] [CrossRef]
- Lanka, S.M.; Zorko, N.A.; Antonarakis, E.S.; Barata, P.C. Metastatic Castration-Resistant Prostate Cancer, Immune Checkpoint Inhibitors, and Beyond. Curr. Oncol. 2023, 30, 4246–4256. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Liu, R.; Xu, C.; Tang, H.; Zou, Y.; Cao, Q.; Huang, W. CXCL12/CXCR4 Axis Governs Treg Spatial Dominance over CD8+ T Cells via IL-2 sequestration: A Dual Therapeutic Target in Prostate Cancer. Front. Immunol. 2025, 16, 1626708. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Fang, B.; Lu, Y.; Li, X.; Wei, Y.; Ye, D.; Wei, G.; Zhu, Y. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. 2024, 28, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bedeschi, M.; Marino, N.; Cavassi, E.; Piccinini, F.; Tesei, A. Cancer-Associated Fibroblast: Role in Prostate Cancer Progression to Metastatic Disease and Therapeutic Resistance. Cells 2023, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Bonollo, F.; Thalmann, G.N.; Kruithof-de Julio, M.; Karkampouna, S. The Role of Cancer-Associated Fibroblasts in Prostate Cancer Tumorigenesis. Cancers 2020, 12, 1887. [Google Scholar] [CrossRef]
- Cao, Z.; Quazi, S.; Arora, S.; Osellame, L.D.; Burvenich, I.J.; Janes, P.W.; Scott, A.M. Cancer-associated fibroblasts as therapeutic targets for cancer: Advances, challenges, and future prospects. J. Biomed. Sci. 2025, 32, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Stultz, J.; Fong, L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef]
- Shinder, B.; Mao, N.; Zhang, Z.; Sawyers, C.L.; Carver, B.S. PD09-09 Role of Tumor Microenvironment Derived NRG1 In Androgen Resistance: Implications for a Novel Prostate Cancer Treatment Strategy. J. Urol. 2024, 211, E182–E183. [Google Scholar] [CrossRef]
- Song, H.; Lu, T.; Han, D.; Zhang, J.; Gan, L.; Xu, C.; Liu, S.; Li, P.; Zhang, K.; Hu, Z.; et al. YAP1 Inhibition Induces Phenotype Switching of Cancer-Associated Fibroblasts to Tumor Suppressive in Prostate Cancer. Cancer Res. 2024, 84, 3728–3742. [Google Scholar] [CrossRef]
- Hawley, J.E.; Pan, S.; Figg, W.D.; Lopez-Bujanda, Z.A.; Strope, J.D.; Aggen, D.H.; Dallos, M.C.; Lim, E.A.; Stein, M.N.; Hu, J.; et al. Association between immunosuppressive cytokines and PSA progression in biochemically recurrent prostate cancer treated with intermittent hormonal therapy. Prostate 2020, 80, 336–344. [Google Scholar] [CrossRef]
- Neuwirt, H.; Bouchal, J.; Kharaishvili, G.; Ploner, C.; Jöhrer, K.; Pitterl, F.; Weber, A.; Klocker, H.; Eder, I.E. Cancer-associated fibroblasts promote prostate tumor growth and progression through upregulation of cholesterol and steroid biosynthesis. Cell Commun. Signal. 2020, 18, 11. [Google Scholar] [CrossRef]
- Patel, R.; Fleming, J.; Mui, E.; Loveridge, C.; Repiscak, P.; Blomme, A.; Harle, V.; Salji, M.; Ahmad, I.; Teo, K.; et al. Sprouty2 loss-induced IL6 drives castration-resistant prostate cancer through scavenger receptor B1. EMBO Mol. Med. 2018, 10, e8347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell. 2020, 38, 279–296.e9. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Miranda, S.; Riisnaes, R.; Gurel, B.; D’Ambrosio, M.; Vasciaveo, A.; Crespo, M.; Ferreira, A.; Brina, D.; Troiani, M.; et al. HER3 Is an Actionable Target in Advanced Prostate Cancer. Cancer Res. 2021, 81, 6207–6218. [Google Scholar] [CrossRef]
- Matos, A.; Carvalho, M.; Bicho, M.; Ribeiro, R. Arginine and Arginases Modulate Metabolism, Tumor Microenvironment and Prostate Cancer Progression. Nutrients 2021, 13, 4503. [Google Scholar] [CrossRef]
- Siemińska, I.; Baran, J. Myeloid-Derived Suppressor Cells as Key Players and Promising Therapy Targets in Prostate Cancer. Front. Oncol. 2022, 12, 862416. [Google Scholar] [CrossRef]
- Koinis, F.; Xagara, A.; Chantzara, E.; Leontopoulou, V.; Aidarinis, C.; Kotsakis, A. Myeloid-Derived Suppressor Cells in Prostate Cancer: Present Knowledge and Future Perspectives. Cells 2021, 11, 20. [Google Scholar] [CrossRef]
- Hellsten, R.; Lilljebj√∂rn, L.; Johansson, M.; Leandersson, K.; Bjartell, A. The STAT3 inhibitor galiellalactone inhibits the generation of MDSC-like monocytes by prostate cancer cells and decreases immunosuppressive and tumorigenic factors. Prostate 2019, 79, 1611–1621. [Google Scholar] [CrossRef]
- He, S.; Zheng, L.; Qi, C. Myeloid-derived Suppressor Cells (MDSCs) in the Tumor Microenvironment and Their Targeting in Cancer Therapy. Mol. Cancer 2025, 24, 5. [Google Scholar] [CrossRef]
- Bakhtiarvand, V.K.; Akbari, K.R.-A.; Jalali, S.A.; Hojjat-Farsangi, M.; Jeddi-Tehrani, M.; Shokri, F.; Shabani, M. Myeloid-derived Suppressor Cells (MDSCs) Depletion by Cabozantinib Improves the Efficacy of anti-HER2 antibody-based Immunotherapy in a 4T1-HER2 Murine Breast Cancer Model. Int. Immunopharmacol. 2022, 113, 109470. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Y.; Rao, D.; Wang, T.; Lei, Z.; Chen, X.; Zhang, B.; Li, Y.; Liu, B.; Xia, L.; et al. Myeloid-derived suppressor cells in cancer: Therapeutic targets to overcome tumor immune evasion. Exp. Hematol. Oncol. 2024, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Lei, Q.-Y.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef]
- Gu, Q.; Qi, A.; Wang, N.; Zhou, Z.; Zhou, X. Macrophage dynamics in prostate cancer: Molecular to therapeutic insights. Biomed. Pharmacother. 2024, 177, 117002. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef]
- Ma, J.; Shayiti, F.; Ma, J.; Wei, M.; Hua, T.; Zhang, R.; Su, J.; Chen, P. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol. Int. 2021, 45, 2054–2062. [Google Scholar] [CrossRef]
- Anton, A.; Hutchinson, R.; Hovens, C.M.; Christie, M.; Ryan, A.; Gibbs, P.; Costello, A.; Peters, J.; Neeson, P.J.; Corcoran, N.M.; et al. An immune suppressive tumor microenvironment in primary prostate cancer promotes tumor immune escape. Nie D, ed. PLoS ONE 2024, 19, e0301943. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 5: Epigenetic Regulation of PD-L1. Int. J. Mol. Sci. 2021, 22, 12314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, G.; Xie, S.; Jiang, W.; Chen, X.; Chu, M.; Hu, X.; Wang, Z.-W. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol. Ther. 2021, 29, 1958–1969. [Google Scholar] [CrossRef]
- Kwek, S.S.; Cha, E.; Fong, L. Unmasking the Immune Recognition of Prostate Cancer with CTLA4 Blockade. Nat. Rev. Cancer 2012, 12, 289–297. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Schildberg Frank, A.; Klein Sarah, R.; Freeman Gordon, J.; Sharpe Arlene, H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef]
- Chetta, P.; Sriram, R.; Zadra, G. Lactate as Key Metabolite in Prostate Cancer Progression: What Are the Clinical Implications? Cancers 2023, 15, 3473. [Google Scholar] [CrossRef]
- Sharma, U.; Jagannathan, N.R. Metabolism of Prostate Cancer by Magnetic Resonance Spectroscopy (MRS). Biophys. Rev. 2020, 12, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yu, S.-L.; Xie, Z.-X.; Zhuang, R.-L.; Peng, S.-R.; Wang, Q.; Gao, Z.; Li, B.-H.; Xie, J.-J.; Huang, H.; et al. Cancer-associated fibroblasts promote enzalutamide resistance and PD-L1 expression in prostate cancer through CCL5-CCR5 paracrine axis. iScience 2024, 27, 109674. [Google Scholar] [CrossRef]
- Clark, K.C.; Wu, Y.; Taylor, R.A.; Daly, R.J. Novel Therapeutic Targets and Biomarkers Associated with Prostate Cancer-Associated Fibroblasts (CAFs). Crit. Rev. Oncog. 2022, 27, 1–24. [Google Scholar] [CrossRef]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Crabb, S.J.; Pacey, S.; Coyle, V.; Danson, S.; Villacampa, G.; Chandran, K.; Paschalis, A.; Riisnaes, R.; Ferreira, A.; et al. A phase (Ph) I/II trial of abiraterone acetate in combination with tildrakizumab (anti-IL23 monoclonal antibody) in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS5105. [Google Scholar] [CrossRef]
- Guan, W.; Li, F.; Zhao, Z.; Zhang, Z.; Hu, J.; Zhang, Y. Tumor-Associated Macrophage Promotes the Survival of Cancer Cells upon Docetaxel Chemotherapy via the CSF1/CSF1R-CXCL12/CXCR4 Axis in Castration-Resistant Prostate Cancer. Genes 2021, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.V.; Amend, S.R.; Pienta, K.J. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for Macrophages in the Tumor Microenvironment. Mol. Cancer 2019, 18, 94. [Google Scholar] [CrossRef]
- Chaudagar, K.; Hieromnimon, H.M.; Khurana, R.; Labadie, B.; Hirz, T.; Mei, S.; Hasan, R.; Shafran, J.; Kelley, A.; Apostolov, E.; et al. Reversal of lactate and PD-1-mediated macrophage immunosuppression controls growth of PTEN/p53-deficient prostate cancer. Clin. Cancer Res. 2023, 29, 1952–1968. [Google Scholar] [CrossRef]
- Che, J.; Liu, Y.; Liu, Y.; Song, J.; Cui, H.; Feng, D.; Tian, A.; Zhang, Z.; Xu, Y. The application of emerging immunotherapy in the treatment of prostate cancer: Progress, dilemma and promise. Front. Immunol. 2025, 16, 1544882. [Google Scholar] [CrossRef]
- Barata, P.; Agarwal, N.; Nussenzveig, R.; Gerendash, B.; Jaeger, E.; Hatton, W.; Ledet, E.; Lewis, B.; Layton, J.; Babiker, H.; et al. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-H) detected by circulating tumor DNA. J. Immunother. Cancer 2020, 8, e001065. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell. 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, W.; Dong, B.; Xin, Z.; Ji, Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Xue, W. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022, 12, 4965–4979. [Google Scholar] [CrossRef]
- E Ward, J.; Karrison, T.; Chatta, G.; Hussain, M.; Shevrin, D.; Szmulewitz, R.Z.; O’DOnnell, P.H.; Stadler, W.M.; Posadas, E.M. A randomized, phase II study of pazopanib in castrate-sensitive prostate cancer: A University of Chicago Phase II Consortium/Department of Defense Prostate Cancer Clinical Trials Consortium study. Prostate Cancer Prostatic Dis. 2011, 15, 87–92. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Matsubara, N.; Oudard, S.; Saad, F.; Merseburger, A.S.; Soares, A.; McGregor, B.A.; Zurawski, B.; et al. CONTACT-02: Phase 3 study of cabozantinib (C) plus atezolizumab (A) vs second novel hormonal therapy (NHT) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2024, 42 (Suppl. S4). [Google Scholar] [CrossRef]
- Ganta, T.; Anker, J.F.; Miller, E.; Joshi, H.; Tsao, C.K.; Oh, W.K. Characterization of exceptional responses in patients with metastatic castration-resistant prostate cancer treated with cabozantinib and immune checkpoint inhibitors. Clin. Genitourin. Cancer 2025, 23, 102336. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.B.; Wang, S.M.; Wu, C.B.; Wang, J.M.; Xu, L.B.; Wan, S.B.; Zhang, X.B.; Hou, Y.; Xia, Y.; Xu, L.; et al. Role of the AKT signaling pathway in regulating tumor-associated macrophage polarization and in the tumor microenvironment: A review. Medicine 2025, 104, e41379. [Google Scholar] [CrossRef] [PubMed]
- Nahar, T.A.; Bantounou, M.A.; Savin, I.; Chohan, N.; Kumar, N.S.; Ghose, A.; McEwan, I.J. Efficacy and Safety of Combination AKT and Androgen Receptor Signaling Inhibition in Metastatic Castration-Resistant Prostate Cancer: Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2024, 22, 102244. [Google Scholar] [CrossRef]
- Mao, N.; Lee, Y.S.; Salsabeel, N.; Zhang, Z.; Li, D.; Kaur, H.; Chen, X.; Chang, Q.; Mehta, S.; Barnes, J.; et al. Uncoupling of Akt and mTOR signaling drives resistance to Akt inhibition in PTEN loss prostate cancers. Sci. Adv. 2025, 11, eadq3802. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Taheriazam, A.; Daneii, P.; Hassanpour, A.; Kakavand, A.; Rezaei, S.; Hejazi, E.S.; Aboutalebi, M.; Gholamrezaie, H.; Saebfar, H.; et al. Targeting PI3K/Akt signaling in prostate cancer therapy. J. Cell Commun. Signal. 2022, 17, 423–443. [Google Scholar] [CrossRef]
- Eberlein, C.; Williamson, S.C.; Hopcroft, L.; Ros, S.; Moss, J.I.; Kerr, J.; van Weerden, W.M.; de Bruin, E.C.; Dunn, S.; Willis, B.; et al. Capivasertib Combines with Docetaxel to Enhance anti-tumour Activity through Inhibition of AKT-mediated Survival Mechanisms in Prostate Cancer. Br. J. Cancer 2024, 130, 1377–1387. [Google Scholar] [CrossRef]
- Charpentier, M.; Spada, S.; Van Nest, S.J.; Demaria, S. Radiation therapy-induced Remodeling of the Tumor Immune Microenvironment. Semin. Cancer Biol. 2022, 86 Pt 2, 737–747. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Morris, M.J.; De Bono, J.S.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). J. Clin. Oncol. 2021, 39 (Suppl. S18), LBA4. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Sartor, O. Status of PSMA-targeted Radioligand Therapy in Prostate Cancer: Current Data and Future Trials. Ther. Adv. Med. Oncol. 2023, 15, 175883592311576. [Google Scholar] [CrossRef]
- Inoue, T.; Sho Sekito Kageyama, T.; Sugino, Y.; Sasaki, T. Roles of the PARP Inhibitor in BRCA1 and BRCA2 Pathogenic Mutated Metastatic Prostate Cancer: Direct Functions and Modification of the Tumor Microenvironment. Cancers 2023, 15, 2662. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomized, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Abida, W.; Campbell, D.; Patnaik, A.; Bryce, A.H.; Shapiro, J.; Bambury, R.M.; Zhang, J.; Burke, J.M.; Castellano, D.; Font, A.; et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur. Urol. 2023, 84, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Longoria, O.; Beije, N.; de Bono, J.S. PARP Inhibitors for Prostate Cancer. Semin. Oncol. 2023, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Maria Teresa Bourlon Valdez, P.; Castro, E. Development of PARP Inhibitors in Advanced Prostate Cancer. Ther. Adv. Med. Oncol. 2024, 16, 1–15. [Google Scholar] [CrossRef]
- Pei, X.; Liu, L.; Han, Y. Advances in Human Microbiome and Prostate Cancer Research. Front. Immunol. 2025, 16, 1576679. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Bombelli, R.; Baci, D.; Mortara, L. Microbiome and Prostate Cancer: A Novel Target for Prevention and Treatment. Int. J. Mol. Sci. 2023, 24, 1511. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Yu, J. The Role of Gut Microbiota and Metabolites in Cancer Chemotherapy. J. Adv. Res. 2023, 64, 223–235. [Google Scholar] [CrossRef]
- He, J.; Wu, J.; Li, Z.; Zhao, Z.; Qiu, L.; Zhu, X.; Liu, Z.; Xia, H.; Hong, P.; Yang, J.; et al. Immunotherapy Vaccines for Prostate Cancer Treatment. Cancer Med. 2024, 13, e70294. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, S.; Wang, X. Applications of single-cell Technologies in Drug Discovery for Tumor Treatment. iScience 2024, 27, 110486. [Google Scholar] [CrossRef]
- Feng, D.-C.; Zhu, W.-Z.; Wang, J.; Li, D.-X.; Shi, X.; Xiong, Q.; You, J.; Han, P.; Qiu, S.; Wei, Q.; et al. The implications of single-cell RNA-seq analysis in prostate cancer: Unraveling tumor heterogeneity, therapeutic implications and pathways towards personalized therapy. Mil. Med. Res. 2024, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Weinstein, H.N.W.; Allegakoen, P.; Wadsworth, M.H.; Xie, J.; Yang, H.; Castro, E.A.; Lu, K.L.; Stohr, B.A.; Feng, F.Y.; et al. Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states. Nat. Commun. 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, R.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Applications of single-cell sequencing in cancer research: Progress and perspectives. J. Hematol. Oncol. 2021, 14, 91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ojo, A.; Tsao, S.; Horowitz, A.; Kyprianou, N.; Tsao, C.-K. Overcoming Immune Evasion in the Prostate Tumor Microenvironment: Novel Targeted Strategies to Improve Treatment Outcomes. Cancers 2025, 17, 3441. https://doi.org/10.3390/cancers17213441
Huang J, Ojo A, Tsao S, Horowitz A, Kyprianou N, Tsao C-K. Overcoming Immune Evasion in the Prostate Tumor Microenvironment: Novel Targeted Strategies to Improve Treatment Outcomes. Cancers. 2025; 17(21):3441. https://doi.org/10.3390/cancers17213441
Chicago/Turabian StyleHuang, Jing, Ademola Ojo, Serena Tsao, Amir Horowitz, Natasha Kyprianou, and Che-Kai Tsao. 2025. "Overcoming Immune Evasion in the Prostate Tumor Microenvironment: Novel Targeted Strategies to Improve Treatment Outcomes" Cancers 17, no. 21: 3441. https://doi.org/10.3390/cancers17213441
APA StyleHuang, J., Ojo, A., Tsao, S., Horowitz, A., Kyprianou, N., & Tsao, C.-K. (2025). Overcoming Immune Evasion in the Prostate Tumor Microenvironment: Novel Targeted Strategies to Improve Treatment Outcomes. Cancers, 17(21), 3441. https://doi.org/10.3390/cancers17213441






