The Role of WWOX in Cancer Progression: Mechanisms and Therapeutic Potential
Simple Summary
Abstract
1. Introduction
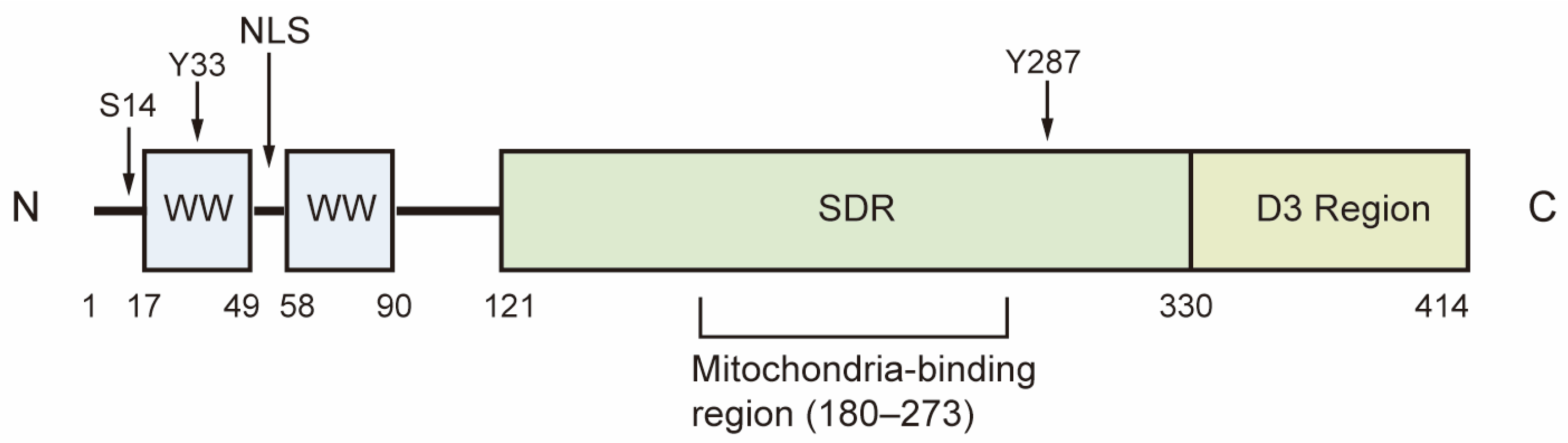
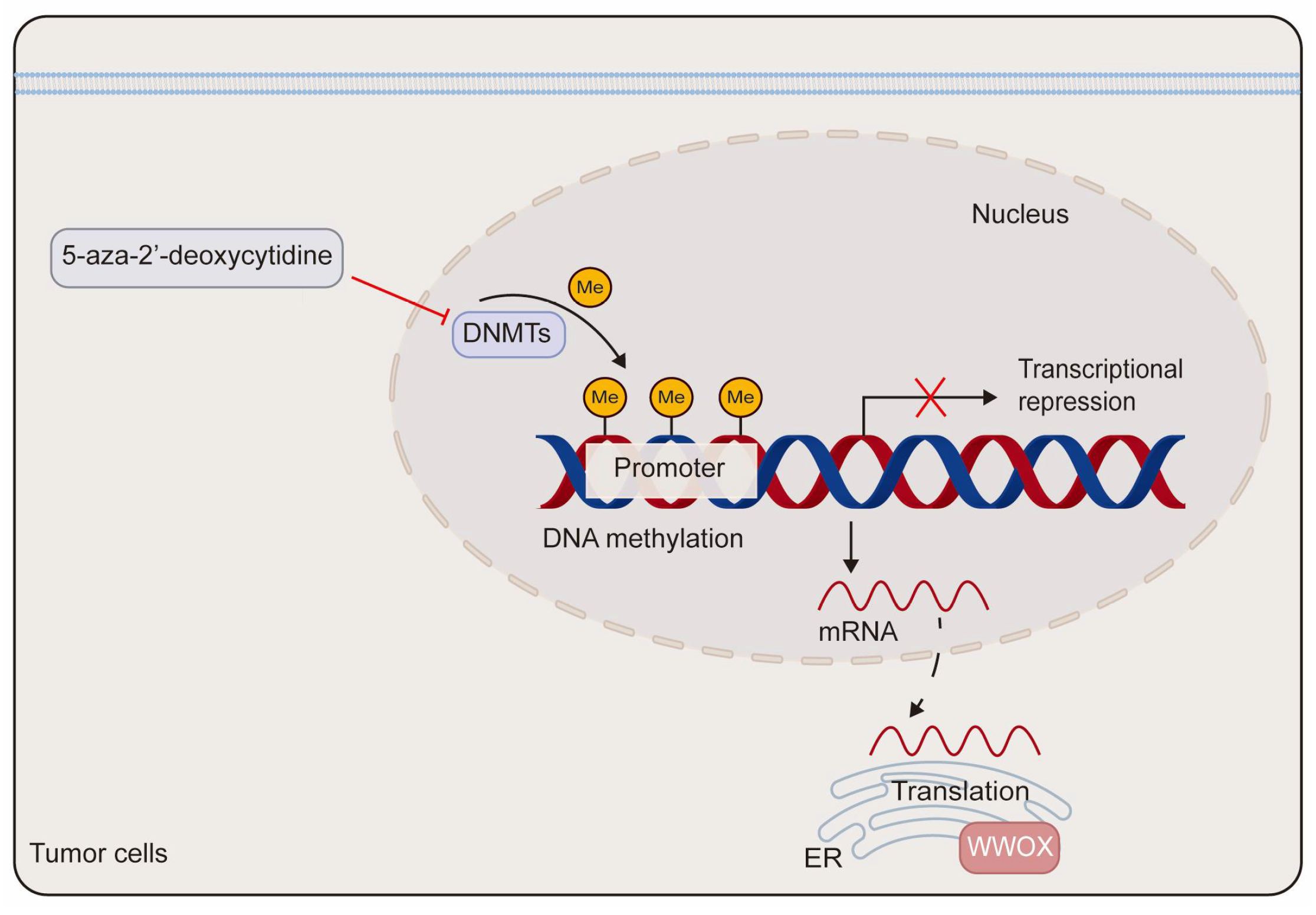
2. Epigenetic Regulation and Post-Translational Modification of WWOX
3. WWOX in Cell Proliferation and Survival
4. WWOX in Cell Invasion and Migration
5. WWOX in the Immune Response
6. WWOX in Metabolism
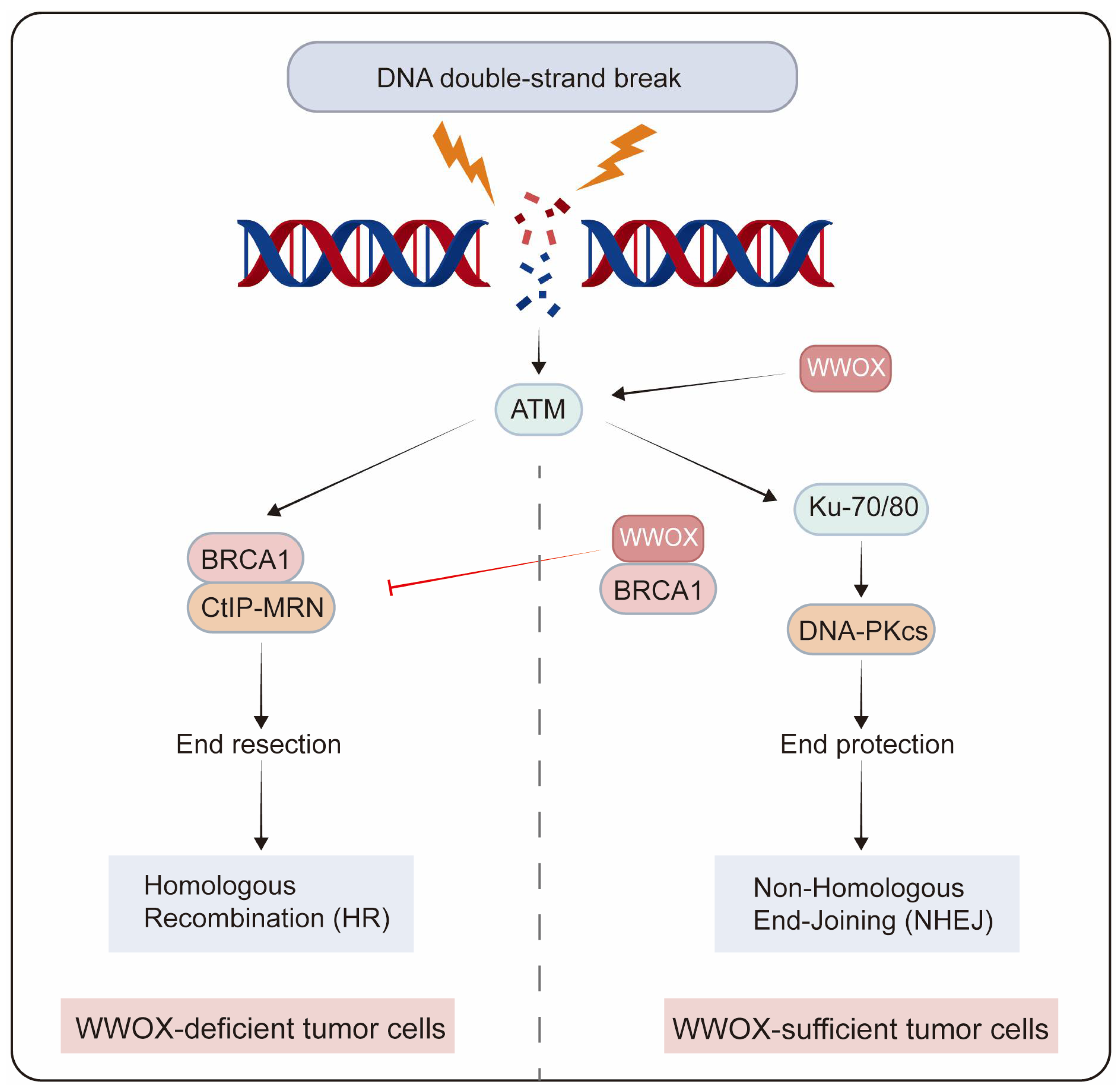
7. WWOX in Genomic Stability
8. Therapeutic Potential of WWOX in Cancer
8.1. WWOX as a Potential Tumor Biomarker
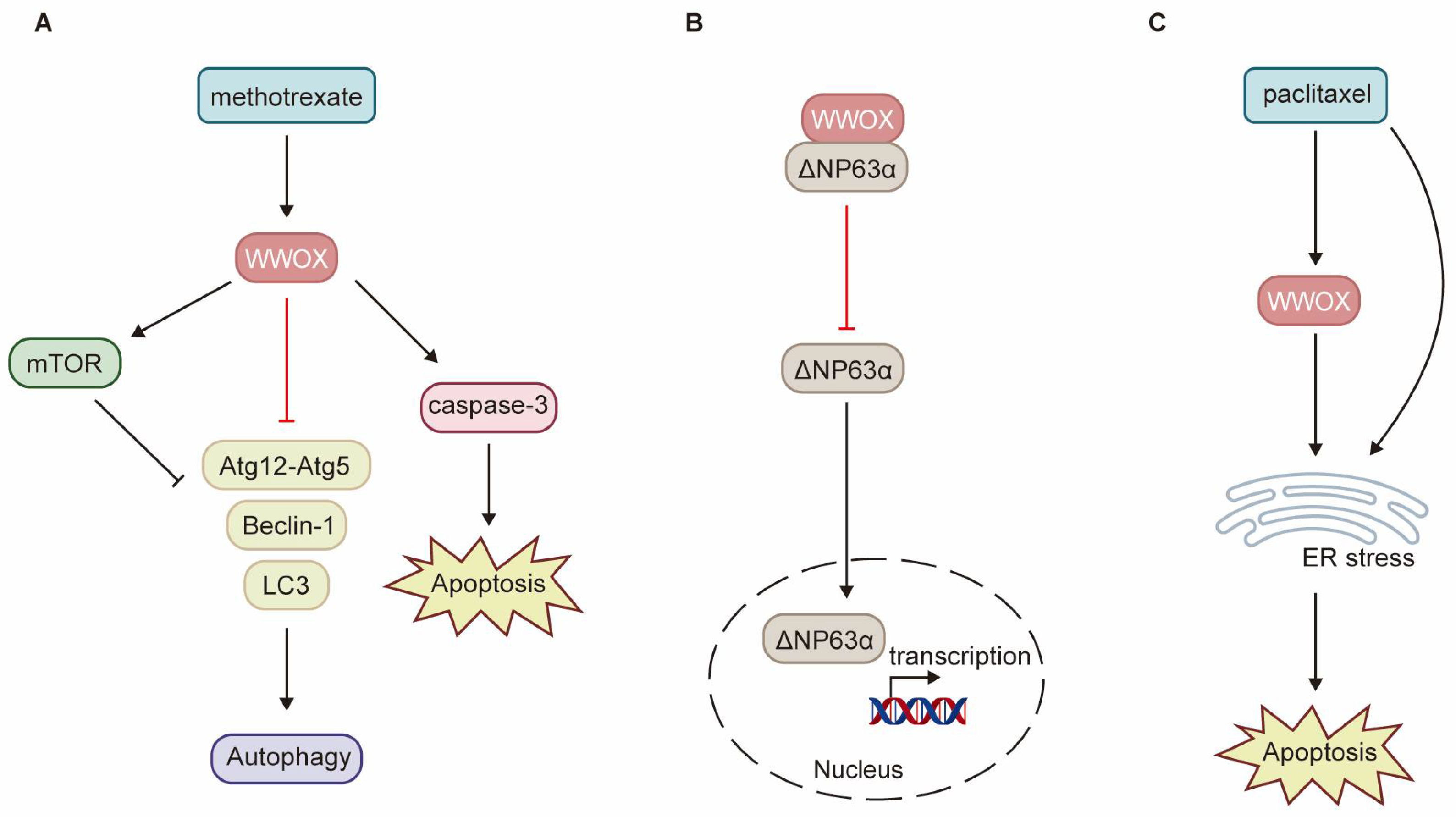
8.2. WWOX in Chemoresistance
8.3. Compounds Related to WWOX
8.4. Other WWOX Modulators
9. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WWOX | WW domain-containing oxidoreductase |
| SDR | Short-chain dehydrogenase/reductase |
| SCAR12 | Spinocerebellar ataxia, autosomal recessive-12 |
| WOREE | WWOX-related epileptic encephalopathy |
| WT | Wild-type |
| ENU | Ethyl nitrosourea |
| Zfra | Zinc finger-like protein that regulates apoptosis |
| ACK1 | Activated Cdc42-associated kinase |
| HCC | Hepatocellular carcinoma |
| Dvl2 | Dishevelled2 |
| TSN | Toosendanin |
| NRF2 | NF-E2-related factor 2 |
| AP-2γ | Activator Protein-2γ |
| YAP | Yes-associated protein |
| ErbB-4 | Erythroblastic Leukemia Viral Oncogene Homolog 4 |
| EMT | Epithelial–mesenchymal transition |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemical |
| GLUT1 | Glucose transporter 1 |
| PKM2 | Pyruvate kinase M2 |
| MEFs | Mouse embryonic fibroblasts |
| DEN | N-nitrosodiethylamine |
| DSBs | DNA double-strand breaks |
| NHEJ | Non-homologous end joining |
| HR | Homologous recombination |
| ATM | Ataxia telangiectasia-mutated |
| MERIT40 | Mediator of RAP80 Interaction and Targeting 40 kDa protein |
| ROS | Reactive oxygen species |
| MTX | Methotrexate |
| SCC | Squamous cell carcinoma |
| ER | Endoplasmic reticulum |
| EOC | Epithelial ovarian cancer |
| ABZ | Albendazole |
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 |
| FPN1 | Ferroportin 1 |
| GPX4 | Glutathione peroxidase 4 |
| HNSCC | Head and neck squamous cell carcinoma |
References
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar] [PubMed]
- Bednarek, A.K.; Keck-Waggoner, C.L.; Daniel, R.L.; Laflin, K.J.; Bergsagel, P.L.; Kiguchi, K.; Brenner, A.J.; Aldaz, C.M. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001, 61, 8068–8073. [Google Scholar] [PubMed]
- Ludes-Meyers, J.H.; Kil, H.; Bednarek, A.K.; Drake, J.; Bedford, M.T.; Aldaz, C.M. WWOX binds the specific proline-rich ligand PPXY: Identification of candidate interacting proteins. Oncogene 2004, 23, 5049–5055. [Google Scholar] [CrossRef]
- Nunez, M.I.; Ludes-Meyers, J.; Aldaz, C.M. WWOX protein expression in normal human tissues. J. Mol. Histol. 2006, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Hippo, Y.; Taniguchi, H.; Iwanari, H.; Yashiro, M.; Hirakawa, K.; Kodama, T.; Aburatani, H. An opposing view on WWOX protein function as a tumor suppressor. Cancer Res. 2003, 63, 8629–8633. [Google Scholar] [PubMed]
- Chang, N.S.; Schultz, L.; Hsu, L.J.; Lewis, J.; Su, M.; Sze, C.I. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: Potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005, 24, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Héron, D.; Nougues, M.C.; et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Mallaret, M.; Synofzik, M.; Lee, J.; Sagum, C.A.; Mahajnah, M.; Sharkia, R.; Drouot, N.; Renaud, M.; Klein, F.A.; Anheim, M.; et al. The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 2014, 137, 411–419. [Google Scholar] [CrossRef]
- Oliver, K.L.; Trivisano, M.; Mandelstam, S.A.; De Dominicis, A.; Francis, D.I.; Green, T.E.; Muir, A.M.; Chowdhary, A.; Hertzberg, C.; Goldhahn, K.; et al. WWOX developmental and epileptic encephalopathy: Understanding the epileptology and the mortality risk. Epilepsia 2023, 64, 1351–1367. [Google Scholar] [CrossRef]
- Repudi, S.; Kustanovich, I.; Abu-Swai, S.; Stern, S.; Aqeilan, R.I. Neonatal neuronal WWOX gene therapy rescues Wwox null phenotypes. EMBO Mol. Med. 2021, 13, e14599. [Google Scholar] [CrossRef]
- Repudi, S.; Steinberg, D.J.; Elazar, N.; Breton, V.L.; Aquilino, M.S.; Saleem, A.; Abu-Swai, S.; Vainshtein, A.; Eshed-Eisenbach, Y.; Vijayaragavan, B.; et al. Neuronal deletion of Wwox, associated with WOREE syndrome, causes epilepsy and myelin defects. Brain 2021, 144, 3061–3077. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hagan, J.P.; de Bruin, A.; Rawahneh, M.; Salah, Z.; Gaudio, E.; Siddiqui, H.; Volinia, S.; Alder, H.; Lian, J.B.; et al. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology 2009, 150, 1530–1535. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.H.; Gaur, T.; Stein, G.S.; et al. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Trapasso, F.; Hussain, S.; Costinean, S.; Marshall, D.; Pekarsky, Y.; Hagan, J.P.; Zanesi, N.; Kaou, M.; Stein, G.S.; et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc. Natl. Acad. Sci. USA 2007, 104, 3949–3954. [Google Scholar] [CrossRef]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef] [PubMed]
- Janczar, S.; Nautiyal, J.; Xiao, Y.; Curry, E.; Sun, M.; Zanini, E.; Paige, A.J.; Gabra, H. WWOX sensitises ovarian cancer cells to paclitaxel via modulation of the ER stress response. Cell Death Dis. 2017, 8, e2955. [Google Scholar] [CrossRef]
- Abdeen, S.K.; Salah, Z.; Maly, B.; Smith, Y.; Tufail, R.; Abu-Odeh, M.; Zanesi, N.; Croce, C.M.; Nawaz, Z.; Aqeilan, R.I. Wwox inactivation enhances mammary tumorigenesis. Oncogene 2011, 30, 3900–3906. [Google Scholar] [CrossRef]
- Lin, J.T.; Li, H.Y.; Chang, N.S.; Lin, C.H.; Chen, Y.C.; Lu, P.J. WWOX suppresses prostate cancer cell progression through cyclin D1-mediated cell cycle arrest in the G1 phase. Cell Cycle 2015, 14, 408–416. [Google Scholar] [CrossRef]
- Gao, K.; Yin, J.; Dong, J. Deregulated WWOX is involved in a negative feedback loop with microRNA-214-3p in osteosarcoma. Int. J. Mol. Med. 2016, 38, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S.; Doherty, J.; Ensign, A.; Schultz, L.; Hsu, L.J.; Hong, Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J. Biol. Chem. 2005, 280, 43100–43108. [Google Scholar] [CrossRef]
- Chang, N.S.; Doherty, J.; Ensign, A.; Lewis, J.; Heath, J.; Schultz, L.; Chen, S.T.; Oppermann, U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem. Pharmacol. 2003, 66, 1347–1354. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Nie, K.; Peng, X.; Huo, J.; Fu, X.; Zhang, Y. WWOX-mediated p53/SAT1 and NRF2/FPN1 axis contribute to toosendanin-induced ferroptosis in hepatocellular carcinoma. Biochem. Pharmacol. 2025, 233, 116790. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.R.; Iliopoulos, D.; Semba, S.; Fabbri, M.; Druck, T.; Volinia, S.; Croce, C.M.; Morrison, C.D.; Klein, R.D.; Huebner, K. A role for the WWOX gene in prostate cancer. Cancer Res. 2006, 66, 6477–6481. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Iliopoulos, D.; Trapasso, F.; Aqeilan, R.I.; Cimmino, A.; Zanesi, N.; Yendamuri, S.; Han, S.Y.; Amadori, D.; Huebner, K.; et al. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 15611–15616. [Google Scholar] [CrossRef]
- Zhou, Y.; Shou, F.; Zhang, H.; You, Q. Adenovirus-delivered wwox inhibited lung cancer growth in vivo in a mouse model. Cancer Gene Ther. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Fabbri, M.; Druck, T.; Qin, H.R.; Han, S.Y.; Huebner, K. Inhibition of breast cancer cell growth in vitro and in vivo: Effect of restoration of Wwox expression. Clin. Cancer Res. 2007, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, W.; Li, B.; Lu, C.; Wan, X. WWOX induces apoptosis and inhibits proliferation in cervical cancer and cell lines. Int. J. Mol. Med. 2013, 31, 1139–1147. [Google Scholar] [CrossRef]
- Nakayama, S.; Semba, S.; Maeda, N.; Aqeilan, R.I.; Huebner, K.; Yokozaki, H. Role of the WWOX gene, encompassing fragile region FRA16D, in suppression of pancreatic carcinoma cells. Cancer Sci. 2008, 99, 1370–1376. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.Y.; Melino, G.; Huebner, K.; et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Liu, Y. Upregulation of tumor suppressor WWOX promotes immune response in glioma. Cell Immunol. 2013, 285, 1–5. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, P.H.; Lin, K.J.; Cheng, Y.T.; Chang, L.C. Novel Hydrogel-Mediated Lentiviral Gene Delivery via Intravesical Administration for Bladder Cancer Treatment. Pharmaceutics 2025, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Lai, F.J.; Sheu, H.M.; Lin, Y.S.; Chang, T.H.; Jan, M.S.; Chen, S.M.; Hsu, P.C.; Huang, T.T.; Huang, T.C.; et al. WWOX suppresses autophagy for inducing apoptosis in methotrexate-treated human squamous cell carcinoma. Cell Death Dis. 2013, 4, e792. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Song, L.; Xu, Y.; Wu, Y.; Dai, C.; Wang, X.; Sun, X.; Hou, Y.; Li, W.; Zhan, X.; et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat. Commun. 2018, 9, 3486. [Google Scholar] [CrossRef]
- Yang, T.; Xu, R.; Huo, J.; Wang, B.; Du, X.; Dai, B.; Zhu, M.; Zhan, Y.; Zhang, D.; Zhang, Y. WWOX activation by toosendanin suppresses hepatocellular carcinoma metastasis through JAK2/Stat3 and Wnt/β-catenin signaling. Cancer Lett. 2021, 513, 50–62. [Google Scholar] [CrossRef]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Khawaled, S.; Nigita, G.; Distefano, R.; Oster, S.; Suh, S.S.; Smith, Y.; Khalaileh, A.; Peng, Y.; Croce, C.M.; Geiger, T.; et al. Pleiotropic tumor suppressor functions of WWOX antagonize metastasis. Signal. Transduct. Target. Ther. 2020, 5, 43. [Google Scholar] [CrossRef]
- Khawaled, S.; Suh, S.S.; Abdeen, S.K.; Monin, J.; Distefano, R.; Nigita, G.; Croce, C.M.; Aqeilan, R.I. WWOX Inhibits Metastasis of Triple-Negative Breast Cancer Cells via Modulation of miRNAs. Cancer Res. 2019, 79, 1784–1798. [Google Scholar] [CrossRef]
- Gourley, C.; Paige, A.J.; Taylor, K.J.; Ward, C.; Kuske, B.; Zhang, J.; Sun, M.; Janczar, S.; Harrison, D.J.; Muir, M.; et al. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res. 2009, 69, 4835–4842. [Google Scholar] [CrossRef]
- El-Hage, P.; Petitalot, A.; Monsoro-Burq, A.H.; Maczkowiak, F.; Driouch, K.; Formstecher, E.; Camonis, J.; Sabbah, M.; Bièche, I.; Lidereau, R.; et al. The Tumor-Suppressor WWOX and HDAC3 Inhibit the Transcriptional Activity of the β-Catenin Coactivator BCL9-2 in Breast Cancer Cells. Mol. Cancer Res. 2015, 13, 902–912. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Palamarchuk, A.; Weigel, R.J.; Herrero, J.J.; Pekarsky, Y.; Croce, C.M. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 2004, 64, 8256–8261. [Google Scholar] [CrossRef]
- Gaudio, E.; Palamarchuk, A.; Palumbo, T.; Trapasso, F.; Pekarsky, Y.; Croce, C.M.; Aqeilan, R.I. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 2006, 66, 11585–11589. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Donati, V.; Palamarchuk, A.; Trapasso, F.; Kaou, M.; Pekarsky, Y.; Sudol, M.; Croce, C.M. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005, 65, 6764–6772. [Google Scholar] [CrossRef]
- Kurek, K.C.; Del Mare, S.; Salah, Z.; Abdeen, S.; Sadiq, H.; Lee, S.H.; Gaudio, E.; Zanesi, N.; Jones, K.B.; DeYoung, B.; et al. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res. 2010, 70, 5577–5586. [Google Scholar] [CrossRef]
- Del Mare, S.; Aqeilan, R.I. Tumor Suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci. Rep. 2015, 5, 12959. [Google Scholar] [CrossRef]
- Ferguson, B.W.; Gao, X.; Zelazowski, M.J.; Lee, J.; Jeter, C.R.; Abba, M.C.; Aldaz, C.M. The cancer gene WWOX behaves as an inhibitor of SMAD3 transcriptional activity via direct binding. BMC Cancer 2013, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Husanie, H.; Abu-Remaileh, M.; Maroun, K.; Abu-Tair, L.; Safadi, H.; Atlan, K.; Golan, T.; Aqeilan, R.I. Loss of tumor suppressor WWOX accelerates pancreatic cancer development through promotion of TGFβ/BMP2 signaling. Cell Death Dis. 2022, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor suppressor WWOX regulates glucose metabolism via HIF1α modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Khalaileh, A.; Pikarsky, E.; Aqeilan, R.I. WWOX controls hepatic HIF1α to suppress hepatocyte proliferation and neoplasia. Cell Death Dis. 2018, 9, 511. [Google Scholar] [CrossRef]
- Schrock, M.S.; Batar, B.; Lee, J.; Druck, T.; Ferguson, B.; Cho, J.H.; Akakpo, K.; Hagrass, H.; Heerema, N.A.; Xia, F.; et al. Wwox-Brca1 interaction: Role in DNA repair pathway choice. Oncogene 2017, 36, 2215–2227. [Google Scholar] [CrossRef]
- Park, D.; Gharghabi, M.; Schrock, M.S.; Plow, R.; Druck, T.; Yungvirt, C.; Aldaz, C.M.; Huebner, K. Interaction of Wwox with Brca1 and associated complex proteins prevents premature resection at double-strand breaks and aberrant homologous recombination. DNA Repair 2022, 110, 103264. [Google Scholar] [CrossRef]
- Bidany-Mizrahi, T.; Shweiki, A.; Maroun, K.; Abu-Tair, L.; Mali, B.; Aqeilan, R.I. Unveiling the relationship between WWOX and BRCA1 in mammary tumorigenicity and in DNA repair pathway selection. Cell Death Discov. 2024, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Taouis, K.; Vacher, S.; Guirouilh-Barbat, J.; Camonis, J.; Formstecher, E.; Popova, T.; Hamy, A.S.; Petitalot, A.; Lidereau, R.; Caputo, S.M.; et al. WWOX binds MERIT40 and modulates its function in homologous recombination, implications in breast cancer. Cancer Gene Ther. 2023, 30, 1144–1155. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Xu, M.; Zhou, Q.; Weng, J.; Hu, Z.; Xu, M.; Xu, W.; Yi, Y.; Shi, Y.; et al. WWOX tuning of oleic acid signaling orchestrates immunosuppressive macrophage polarization and sensitizes hepatocellular carcinoma to immunotherapy. J. Immunother. Cancer 2024, 12, e010422. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Yendamuri, S.; Trapasso, F.; Matsuyama, A.; Aqeilan, R.I.; Alder, H.; Rattan, S.; Cesari, R.; Nolli, M.L.; Williams, N.N.; et al. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin. Cancer Res. 2004, 10, 2459–2465. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Guler, G.; Han, S.Y.; Johnston, D.; Druck, T.; McCorkell, K.A.; Palazzo, J.; McCue, P.A.; Baffa, R.; Huebner, K. Fragile genes as biomarkers: Epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 2005, 24, 1625–1633. [Google Scholar] [CrossRef]
- Paige, A.J.; Taylor, K.J.; Taylor, C.; Hillier, S.G.; Farrington, S.; Scott, D.; Porteous, D.J.; Smyth, J.F.; Gabra, H.; Watson, J.E. WWOX: A candidate tumor suppressor gene involved in multiple tumor types. Proc. Natl. Acad. Sci. USA 2001, 98, 11417–11422. [Google Scholar] [CrossRef]
- Chang, N.S.; Hsu, L.J.; Lin, Y.S.; Lai, F.J.; Sheu, H.M. WW domain-containing oxidoreductase: A candidate tumor suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.J.; Cheng, C.L.; Chen, S.T.; Wu, C.H.; Hsu, L.J.; Lee, J.Y.; Chao, S.C.; Sheen, M.C.; Shen, C.L.; Chang, N.S.; et al. WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin. Cancer Res. 2005, 11, 5769–5777. [Google Scholar] [CrossRef]
- Chang, N.S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef]
- Lee, M.H.; Shih, Y.H.; Lin, S.R.; Chang, J.Y.; Lin, Y.H.; Sze, C.I.; Kuo, Y.M.; Chang, N.S. Zfra restores memory deficits in Alzheimer’s disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 189–204. [Google Scholar] [CrossRef]
- Su, W.-P.; Wang, W.-J.; Chang, J.-Y.; Ho, P.-C.; Liu, T.-Y.; Wen, K.-Y.; Kuo, H.-L.; Chen, Y.-J.; Huang, S.-S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Ho, P.-C.; Nagarajan, G.; Chen, Y.-A.; Kuo, H.-L.; Subhan, D.; Su, W.-P.; Chang, J.-Y.; Lu, C.-Y.; Chang, K.T.; et al. WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: Role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005, 65, 10514–10523. [Google Scholar] [CrossRef]
- Xie, B.; Zen, Q.; Wang, X.; He, X.; Xie, Y.; Zhang, Z.; Li, H. ACK1 promotes hepatocellular carcinoma progression via downregulating WWOX and activating AKT signaling. Int. J. Oncol. 2015, 46, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Bar-Mag, T.; Huang, H.; Kim, T.; Salah, Z.; Abdeen, S.K.; Sudol, M.; Reichmann, D.; Sidhu, S.; Kim, P.M.; et al. Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks. J. Biol. Chem. 2014, 289, 8865–8880. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tong, J.; Lin, X.; Han, Q.; Huang, H. Effect of the WWOX gene on the regulation of the cell cycle and apoptosis in human ovarian cancer stem cells. Mol. Med. Rep. 2015, 12, 1783–1788. [Google Scholar] [CrossRef]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced by the tumor suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef][Green Version]
- Chou, P.Y.; Lin, S.R.; Lee, M.H.; Schultz, L.; Sze, C.I.; Chang, N.S. A p53/TIAF1/WWOX triad exerts cancer suppression but may cause brain protein aggregation due to p53/WWOX functional antagonism. Cell Commun. Signal. 2019, 17, 76. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chiang, M.F.; Lin, S.R.; Lee, M.H.; He, H.; Chou, P.Y.; Chen, S.J.; Chen, Y.A.; Yang, L.Y.; Lai, F.J.; et al. TIAF1 self-aggregation in peritumor capsule formation, spontaneous activation of SMAD-responsive promoter in p53-deficient environment, and cell death. Cell Death Dis. 2012, 3, e302. [Google Scholar] [CrossRef]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef]
- Hsu, L.J.; Schultz, L.; Hong, Q.; Van Moer, K.; Heath, J.; Li, M.Y.; Lai, F.J.; Lin, S.R.; Lee, M.H.; Lo, C.P.; et al. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Shen, J.; Wang, Y.; Xu, Y.; Wang, Y.; Han, Z.; Li, X. Upregulation of the putative oncogene COTE1 contributes to human hepatocarcinogenesis through modulation of WWOX signaling. Int. J. Oncol. 2014, 45, 719–731. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.V.; Colella, A.; Dayan, S.; Chen, Q.; Choo, A.; Jacob, R.; Price, G.; Venter, D.; Richards, R.I. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum. Mol. Genet. 2011, 20, 497–509. [Google Scholar] [CrossRef]
- Richards, R.I.; Choo, A.; Lee, C.S.; Dayan, S.; O’Keefe, L. WWOX, the chromosomal fragile site FRA16D spanning gene: Its role in metabolism and contribution to cancer. Exp. Biol. Med. 2015, 240, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.; O’Keefe, L.V.; Choo, A.; Richards, R.I. Common chromosomal fragile site FRA16D tumor suppressor WWOX gene expression and metabolic reprograming in cells. Genes Chromosomes Cancer 2013, 52, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Reinfeld, B.I.; Rathmell, W.K.; Kim, T.K.; Rathmell, J.C. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell. Mol. Immunol. 2022, 19, 46–58. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Dioletis, E.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Stärkel, P.; et al. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1α Transactivation in Steatohepatitis. Cell Metab. 2018, 27, 339–350.e333. [Google Scholar] [CrossRef]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell. Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Cheng, H.C.; Huang, P.H.; Lai, F.J.; Jan, M.S.; Chen, Y.L.; Chen, S.Y.; Chen, W.L.; Hsu, C.K.; Huang, W.; Hsu, L.J. Loss of fragile WWOX gene leads to senescence escape and genome instability. Cell Mol. Life Sci. 2023, 80, 338. [Google Scholar] [CrossRef]
- Donati, V.; Fontanini, G.; Dell’Omodarme, M.; Prati, M.C.; Nuti, S.; Lucchi, M.; Mussi, A.; Fabbri, M.; Basolo, F.; Croce, C.M.; et al. WWOX expression in different histologic types and subtypes of non-small cell lung cancer. Clin. Cancer Res. 2007, 13, 884–891. [Google Scholar] [CrossRef]
- Becker, S.; Markova, B.; Wiewrodt, R.; Hoffarth, S.; Hähnel, P.S.; Pleiner, S.; Schmidt, L.H.; Breitenbuecher, F.; Schuler, M. Functional and clinical characterization of the putative tumor suppressor WWOX in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Ben-David, U.; Shweiki, A.; Maly, B.; Aqeilan, R.I. Somatic loss of WWOX is associated with TP53 perturbation in basal-like breast cancer. Cell Death Dis. 2018, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chao, L.; Ma, G.; Chen, L.; Zang, Y.; Sun, J. The prognostic significance of WWOX expression in patients with breast cancer and its association with the basal-like phenotype. J. Cancer Res. Clin. Oncol. 2011, 137, 271–278. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, W.; Sun, J.; Atyah, M.; Yin, Y.; Zhang, W.; Guo, L.; Ye, Q.; Dong, Q.; Shi, Y.; et al. Low expression of WW domain-containing oxidoreductase associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Cancer Med. 2018, 7, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhou, L.; Song, W.; Wu, S.; Zhu, B.; Wang, D. Expression of ORAOV1, CD133 and WWOX correlate with metastasis and prognosis in gastric adenocarcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 8916–8924. [Google Scholar] [PubMed]
- Paige, A.J.; Zucknick, M.; Janczar, S.; Paul, J.; Mein, C.A.; Taylor, K.J.; Stewart, M.; Gourley, C.; Richardson, S.; Perren, T.; et al. WWOX tumour suppressor gene polymorphisms and ovarian cancer pathology and prognosis. Eur. J. Cancer 2010, 46, 818–825. [Google Scholar] [CrossRef]
- Huang, C.; Tian, Y.; Peng, R.; Zhang, C.; Wang, D.; Han, S.; Jiao, C.; Wang, X.; Zhang, H.; Wang, Y.; et al. Association of downregulation of WWOX with poor prognosis in patients with intrahepatic cholangiocarcinoma after curative resection. J. Gastroenterol. Hepatol. 2015, 30, 421–433. [Google Scholar] [CrossRef]
- Lin, J.T.; Tzai, T.S.; Liao, C.Y.; Wang, J.S.; Wu, T.T.; Wang, H.Y.; Wu, C.H.; Yu, C.C.; Lu, P.J. WWOX protein expression varies among RCC histotypes and downregulation of WWOX protein correlates with less-favorable prognosis in clear RCC. Ann. Surg. Oncol. 2013, 20, 193–199. [Google Scholar] [CrossRef]
- Del Mare, S.; Husanie, H.; Iancu, O.; Abu-Odeh, M.; Evangelou, K.; Lovat, F.; Volinia, S.; Gordon, J.; Amir, G.; Stein, J.; et al. WWOX and p53 Dysregulation Synergize to Drive the Development of Osteosarcoma. Cancer Res. 2016, 76, 6107–6117. [Google Scholar] [CrossRef]
- Akkawi, R.; Hidmi, O.; Haj-Yahia, A.; Monin, J.; Diment, J.; Drier, Y.; Stein, G.S.; Aqeilan, R.I. WWOX promotes osteosarcoma development via upregulation of Myc. Cell Death Dis. 2024, 15, 13. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, C.; Zhang, W.; Atyah, M.; Yin, Y.; Guo, L.; Tang, W.; Dong, Q.; Ye, Q.; Ren, N. Association of WWOX rs9926344 polymorphism with poor prognosis of hepatocellular carcinoma. J. Cancer 2018, 9, 1239–1247. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, C.L.; Wang, S.S.; Yang, C.K.; Li, J.R.; Chen, C.S.; Hung, S.C.; Chiu, K.Y.; Cheng, C.L.; Ou, Y.C.; et al. WWOX Polymorphisms as Predictors of the Biochemical Recurrence of Localized Prostate Cancer after Radical Prostatectomy. Int. J. Med. Sci. 2023, 20, 969–975. [Google Scholar] [CrossRef]
- Schirmer, M.A.; Lüske, C.M.; Roppel, S.; Schaudinn, A.; Zimmer, C.; Pflüger, R.; Haubrock, M.; Rapp, J.; Güngör, C.; Bockhorn, M.; et al. Relevance of Sp Binding Site Polymorphism in WWOX for Treatment Outcome in Pancreatic Cancer. J. Natl. Cancer Inst. 2016, 108, djv387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chang, Y.; Xia, Z.; Liu, Y.; Liu, X.; Xiong, L.; Liu, C.; Zhu, X.; Wang, M.; Qiu, L. Remote modulation of WWOX by an intronic variant associated with survival of Chinese gastric cancer patients. Int. J. Cancer 2024, 154, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Salah, Z.; Bar-mag, T.; Kohn, Y.; Pichiorri, F.; Palumbo, T.; Melino, G.; Aqeilan, R.I. Tumor suppressor WWOX binds to ΔNp63α and sensitizes cancer cells to chemotherapy. Cell Death Dis. 2013, 4, e480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Yamashita-Kanemaru, Y.; Morris, B.I.; Contursi, A.; Trajkovski, D.; Xu, J.; Patrascan, I.; Benson, J.; Evans, A.C.; Conti, A.G.; et al. Aspirin prevents metastasis by limiting platelet TXA(2) suppression of T cell immunity. Nature 2025, 640, 1052–1061. [Google Scholar] [CrossRef]
- Yang, T.; Cheng, C.; Xu, R.; Huo, J.; Peng, X.; Chen, Y.; Liang, Y.; Su, Z.; Zhang, Y. Albendazole exerts an anti-hepatocellular carcinoma effect through a WWOX-dependent pathway. Life Sci. 2022, 310, 121086. [Google Scholar] [CrossRef]
- Hu, C.Y.; Wu, H.T.; Su, Y.C.; Lin, C.H.; Chang, C.J.; Wu, C.L. Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway. Molecules 2017, 22, 1175. [Google Scholar] [CrossRef]
- Yang, T.; Huo, J.; Xu, R.; Zhang, Y. Synergistic effect of toosendanin and regorafenib against cell proliferation and migration by regulating WWOX signaling pathway in hepatocellular carcinoma. Phytother. Res. 2021, 35, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 2021, 27, 149–164. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Y.; Yang, N.; Zhang, H.; Zhang, X.; Geng, Y.; Zhao, J.; Chen, Z.; Dong, C.; Lin, L.; et al. Apoptotic Bodies Derived from Fibroblast-Like Cells in Subcutaneous Connective Tissue Inhibit Ferroptosis in Ischaemic Flaps via the miR-339-5p/KEAP1/Nrf2 Axis. Adv. Sci. 2024, 11, e2307238. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chen, P.H.; Yu, T.J.; Lin, K.J.; Chang, L.C. WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules 2022, 27, 7388. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Wei, C.; Xia, S.; Qiao, Z.; Zhang, X.W.; Yu, B.; Zhou, J.; Wang, R. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol. Res. 2022, 186, 106534. [Google Scholar] [CrossRef]
- Liu, C.J.; Shen, W.G.; Peng, S.Y.; Cheng, H.W.; Kao, S.Y.; Lin, S.C.; Chang, K.W. miR-134 induces oncogenicity and metastasis in head and neck carcinoma through targeting WWOX gene. Int. J. Cancer 2014, 134, 811–821. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Wu, J.; Wang, Y.; Zhou, K.; Chen, W.; Chen, J.; Chen, C.; Chen, L. LncRNA WWOX-AS1 sponges miR-20b-5p in hepatocellular carcinoma and represses its progression by upregulating WWOX. Cancer Biol. Ther. 2020, 21, 927–936. [Google Scholar] [CrossRef]
- Qu, G.; Ma, Z.; Tong, W.; Yang, J. LncRNA WWOX-AS1 inhibits the proliferation, migration and invasion of osteosarcoma cells. Mol. Med. Rep. 2018, 18, 779–788. [Google Scholar] [CrossRef] [PubMed]


| Downstream Effectors | Regulation Form | Effect of Regulation | Biological Process | Related Cancer | Ref |
|---|---|---|---|---|---|
| Cyclin D1 | alters cyclin D1 expression | decreases cyclin D1 expression | cell cycle, cell proliferation | Prostate cancer, osteosarcoma | [18,19] |
| p53 | alters p53 expression, binding | increases p53 activity and expression | cell cycle, apoptosis, ferroptosis | Osteosarcoma, hepatocellular carcinoma | [19,20,21,22] |
| p21 | alters p21 expression | increases p21 expression | cell cycle | Osteosarcoma | [19] |
| caspase-3 | alters caspase-3 expression | increases caspase-3 activity and expression | apoptosis | Prostate cancer, lung cancer, breast cancer, cervical cancer, pancreatic cancer | [23,24,25,26,27,28] |
| p73 | binding | sequesters p73 in cytoplasm, inhibits p73-mediated transcription and transactivation | apoptosis | Breast cancer, osteosarcoma | [29] |
| FasL | alters FasL expression | reduces the level of FasL | apoptosis | Glioma | [30] |
| TNF-α | alters TNF-α expression | increases TNF-α expression | apoptosis | Bladder cancer | [31] |
| mTOR | binding | promotes apoptosis, inhibits autophagy | apoptosis, autophagy | Squamous cell carcinoma | [32] |
| NRF2 | binding | decreases NRF2 expression, inhibits NRF2-mediated transcription | ferroptosis | Hepatocellular carcinoma | [22] |
| STAT3 | binding | inhibits JAK2/STAT3 signaling pathway | cell growth, cell migration, metastasis | Breast cancer, melanoma | [33,34] |
| Dvl2 | binding | inhibits Wnt/β-catenin signaling pathway | EMT, cell growth | Breast cancer, hepatocellular carcinoma | [34,35,36] |
| E-cadherin | alters E-cadherin expression | increases E-cadherin expression | EMT, cell proliferation, invasion, migration | Osteosarcoma, breast cancer | [19,37] |
| ZEB1 | alters ZEB1 expression | decreases ZEB1 expression | EMT, cell proliferation, invasion, migration | Osteosarcoma | [19] |
| Vimentin | alters vimentin expression | decreases vimentin expression | EMT, cell proliferation, invasion, migration | Osteosarcoma | [19] |
| Slug | alters Slug expression | decreases Slug expression | EMT, cell proliferation, invasion, migration | Osteosarcoma | [19] |
| Fibronectin | alters fibronectin expression | decreases fibronectin expression | EMT, metastasis | Breast cancer | [37] |
| MYC | binding | inhibits MYC activity, decreases MYC expression | EMT, metastasis, invasion | Breast cancer | [37] |
| integrin α3 | alters integrin α3 expression | decreases integrin α3 expression | cell migration | Ovarian cancer | [38] |
| integrin α5β1 | alters integrin α5β1 expression | decreases integrin α5β1 expression | cell migration | Ovarian cancer | [38] |
| BCL9-2 | binding | inhibits BCL9-2-mediated transcription, inhibits Wnt/β-catenin signaling pathway | transcription regulation | Breast cancer | [39] |
| AP-2γ | binding | inhibits AP-2γ-mediated transcription and transactivation | transcription regulation | Breast cancer | [40] |
| c-Jun | binding | inhibits c-Jun-mediated transcription and transactivation | transcription regulation | Cervical cancer | [41] |
| ErbB-4 | binding | inhibits ErbB-4-mediated transactivation | transcription regulation | Breast cancer | [42] |
| RUNX2 | binding | inhibits RUNX2-mediated transactivation | transcription regulation | Osteosarcoma | [43,44] |
| SMAD3 | binding | inhibits SMAD3-mediated transcription, attenuates TGF-β signaling pathway | transcription regulation | Breast cancer, pancreatic ductal adenocarcinoma | [45,46] |
| HIF-1α | binding | inhibits HIF-1α-mediated transactivation, promotes HIF-1α degradation | transcription regulation, aerobic glycolysis | Breast cancer, hepatocellular carcinoma | [47,48] |
| ATM | binding | activates ATM | DNA damage repair | Breast cancer | [15] |
| BRCA1 | binding | inhibits HR, promotes NHEJ repair | DNA damage repair | Breast cancer | [49,50,51] |
| MERIT40 | binding | mitigates excessive HR activity | DNA damage repair | Breast cancer | [52] |
| NME2 | binding | inhibits NME2 acetylation | immune responses | Hepatocellular carcinoma | [53] |
| Cancer | Expression or Methylation Status | Clinical Indicator | Relevance | Independent or Not | Evidence | Ref. |
|---|---|---|---|---|---|---|
| Non-small cell lung cancer | methylation status | Poor prognosis | Negative | Not mentioned | Clinical | [84] |
| Triple-negative breast cancer | expression | RFS, OS | Positive | Independent | Clinical | [33,37] |
| Basal-like breast cancer | expression | DFS | Positive | Not | Clinical | [86] |
| Hepatocellular carcinoma | expression | RFS, OS | positive | Independent | Clinical | [87] |
| Epithelial ovarian cancer | expression | OS, PFS, response to paclitaxel | Positive, high WWOX expression-better response | Independent | Clinical | [16] |
| Gastric adenocarcinoma | expression | OS | Positive | Independent | Clinical | [88] |
| Intrahepatic cholangiocarcinoma | methylation status | OS, cumulative recurrence | Positive, negative | Independent | Clinical | [90] |
| Clear cell renal cell carcinoma | expression | Poor prognosis | Negative | Not | Clinical | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Liao, B.; Zhao, J.; Li, Y. The Role of WWOX in Cancer Progression: Mechanisms and Therapeutic Potential. Cancers 2025, 17, 3435. https://doi.org/10.3390/cancers17213435
Yang H, Liao B, Zhao J, Li Y. The Role of WWOX in Cancer Progression: Mechanisms and Therapeutic Potential. Cancers. 2025; 17(21):3435. https://doi.org/10.3390/cancers17213435
Chicago/Turabian StyleYang, Huiyao, Bin Liao, Juan Zhao, and Yongsheng Li. 2025. "The Role of WWOX in Cancer Progression: Mechanisms and Therapeutic Potential" Cancers 17, no. 21: 3435. https://doi.org/10.3390/cancers17213435
APA StyleYang, H., Liao, B., Zhao, J., & Li, Y. (2025). The Role of WWOX in Cancer Progression: Mechanisms and Therapeutic Potential. Cancers, 17(21), 3435. https://doi.org/10.3390/cancers17213435





