Simple Summary
Sex differences play an important role in how certain cancers develop and progress. This study reviewed patterns of cancer incidence and survival between men and women, focusing on tumors of the esophagus, bladder, head and neck, lung, liver, kidney, stomach, and skin melanoma. We explored how male sex hormones (mainly testosterone) and the androgen receptor (AR) may influence cancer risk and outcomes. In some cancers, higher AR activity is linked to worse survival, suggesting that therapies targeting AR could help certain patients. However, the relationship between hormones and cancer is complex and not fully understood. More research is needed to clarify these effects and to develop treatments that consider biological sex as a factor in cancer care.
Abstract
Several major cancer types exhibit significant sex dimorphism in incidence and survival. Whether and how sex as a biological factor impacts tumorigenesis, progression, and survival warrants full investigation, as such knowledge may lead to novel, precise prevention and treatment strategies. We reviewed epidemiological and molecular data on sex differences in cancers of the esophagus, bladder, head and neck, lung, liver, kidney, stomach, and skin melanoma, as well as the potential role of androgens and androgen receptor (AR) activity in these cancers. The potential molecular mechanisms are briefly discussed. Elevated testosterone (T) levels seemed to be associated with increased liver cancer and cutaneous melanoma incidences, and with reduced esophageal cancer risk. AR activity does not always correlate with T levels in tumorigenesis and progression. Higher AR expressions are associated with poorer survival in ESCC, whereas the role of AR in the survival of HNSCC and melanoma patients is inconsistent. The molecular impact of AR in liver cancer, kidney cancer, melanoma, and lung cancer is controversial. However, AR is likely to promote tumor growth and/or progression in esophagus, bladder, head and neck, and stomach cancers, and thus is associated with poor survival. Patients diagnosed with a tumor in this latter group could potentially benefit from therapeutic approaches targeting AR. Overall, the research on sex hormone androgens and AR in these cancers is limited. Further research is needed to determine a possible U-shaped relationship of T with cancer risk, and to decipher the role of testosterone and AR in some of these tumors to facilitate our understanding of sex dimorphism and to explore novel T/AR-based treatment options.
1. Introduction
Sex disparities in cancer incidence and mortality are well-documented across various cancer types, geographic regions, age groups, and time periods. While sex hormones like androgens, estrogens, and progesterone, along with their cellular receptors, are known to causatively impact cancers of the reproductive system (mainly, breast cancer, ovarian cancer, cervical cancer, and prostate cancer), their influence on cancers originating in non-reproductive organs remains less clear [1]. Emerging research suggests that sex hormones may impact the development and progression of non-reproductive cancers through various mechanisms, including dual roles as an oncogene and as a tumor suppressor, depending on cellular environment and cell lineage [2]. For example, an AR-modulated immune response can be either pro-inflammatory or anti-inflammatory [3]. These results were demonstrated in prostate cancer, where the roles of AR were more clearly understood. Similar results were also reported in non-reproductive cancers, with less clarity [1,4], and the goal of this review is to gain a comprehensive understanding of current discoveries of testosterone/AR in a few selected cancer types.
Specifically, we summarized epidemiological data and molecular evidence from the literature, taking into consideration sex differences in cancers arising from the non-reproductive system. It is noticeable that lifelong fluctuations in sex hormone levels may also be associated with the observed sex differences in cancer incidence and mortality. Our focus will be on androgen and the androgen receptor [5].
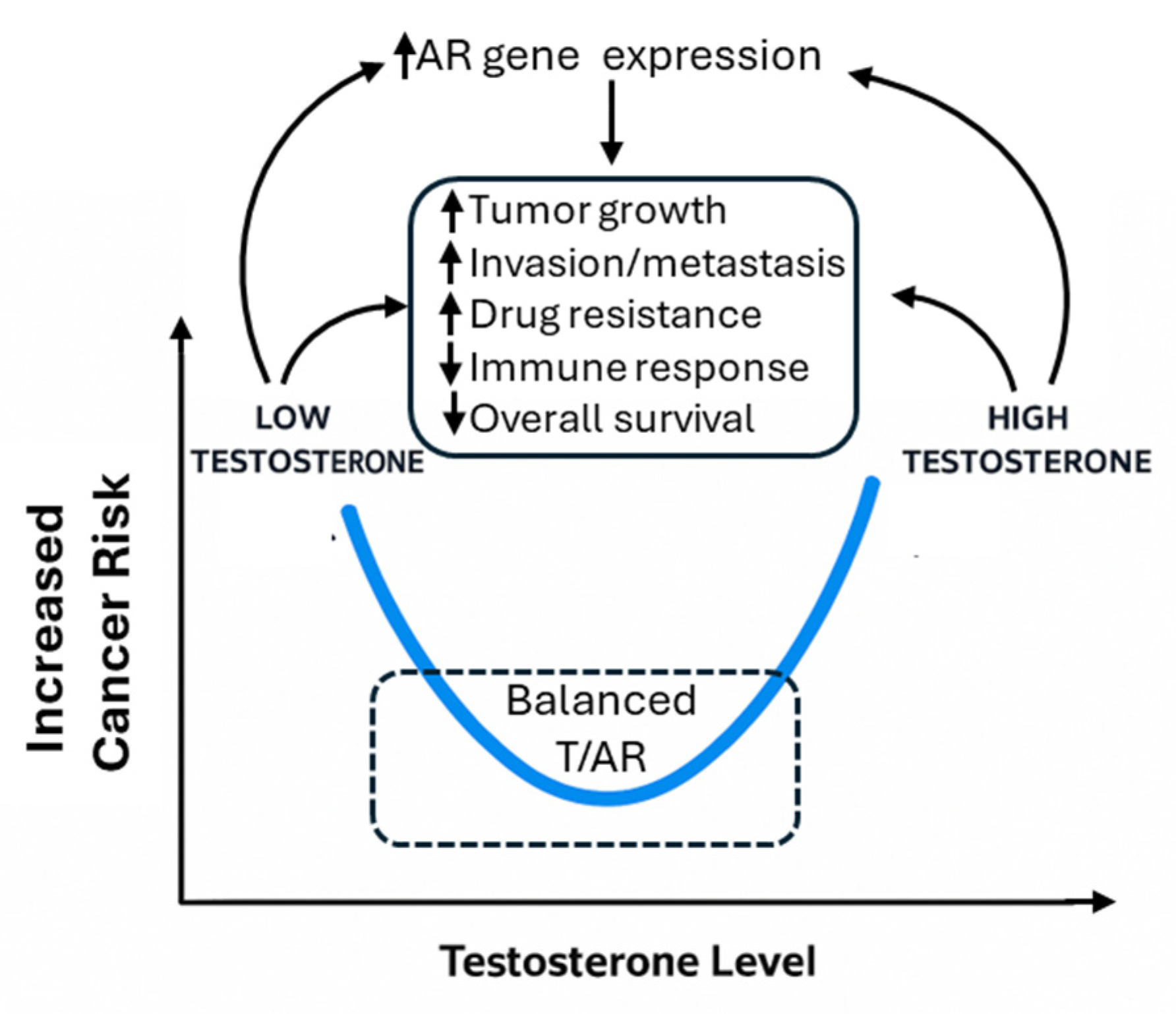
Recently, it was demonstrated that the binding patterns of AR in normal prostate cells on the target genes were significantly different as compared to tumor cells, highlighting a tumor suppressor role of AR in normal tissues, in contrast to a tumor-promoting role in cancer cells [2,6]. Correspondingly, both high and low levels of serum testosterone are linked to prostate cancer risk or worse prognosis [7]. Therefore, a U-shaped relationship was proposed to explain the complex relationship of this key sex hormone with prostate cancer [8]. In this review, we adopt this hypothesis and propose that the T/AR axis contributes to sex disparities in non-reproductive cancers not linearly, but in a context- and cancer-type-dependent manner, potentially also following a U-shaped model (Figure 1).
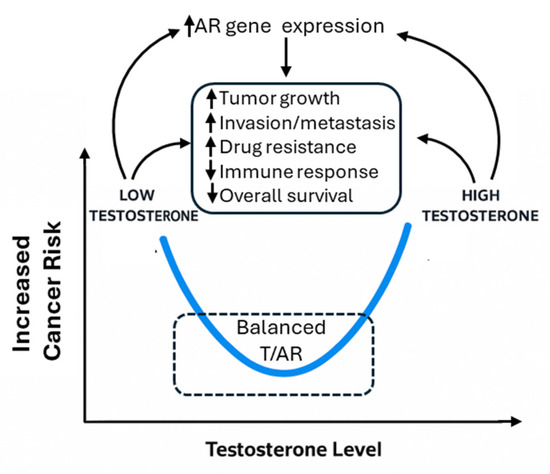
Figure 1.
Illustration of U-shaped effects of T/AR axis and their potential pathways.
2. Overview of Androgens, Androgen Receptors, and Their Roles in Disease Development
2.1. Androgens and the Androgen Receptor
Androgens, also known as male sex hormones, are referred to a group of steroid hormones that play crucial roles in the development and maintenance of male characteristics and reproductive activity [9]. Androgens include androstenedione (A4), dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), testosterone (T), and dihydrotestosterone (DHT). They are synthesized from cholesterol, mainly in the testes, ovaries, and adrenal glands [9]. While present in both males and females, males typically have higher levels of androgens [10]. T is the primary androgen present in the circulation of mature mammals [11]. Sex hormones, including T and DHT, normally bind to sex hormone binding globulin (SHBG) in the circulatory system, and the free hormones are considered to be biologically active [12].
Androgens primarily exert their effects by binding to androgen receptors (ARs), encoded by the AR gene located on Chromosome X at Xq11-Xq12 locus [13]. This ligand-dependent transcription factor belongs to a nuclear hormone receptor (NHR) superfamily classified by protein homology, which includes other Type I class nuclear hormone transcription factors, such as estrogen receptors (ERs), glucocorticoid receptors (GRs), progesterone receptors (PRs), and mineralocorticoid receptors (MRs) [14]. AR is also known by the new nomenclature as NR3C4 (nuclear receptor subfamily 3, group C, gene 4). AR and other NHRs have three primary functional domains, including a N-terminal domain (NTD), a DNA-binding domain (DBD), and a ligand-binding domain (LBD) at the C-terminus, which has been extensively reviewed elsewhere [15,16].
2.2. Androgens Exhibit Both Receptor-Dependent and -Independent Effects
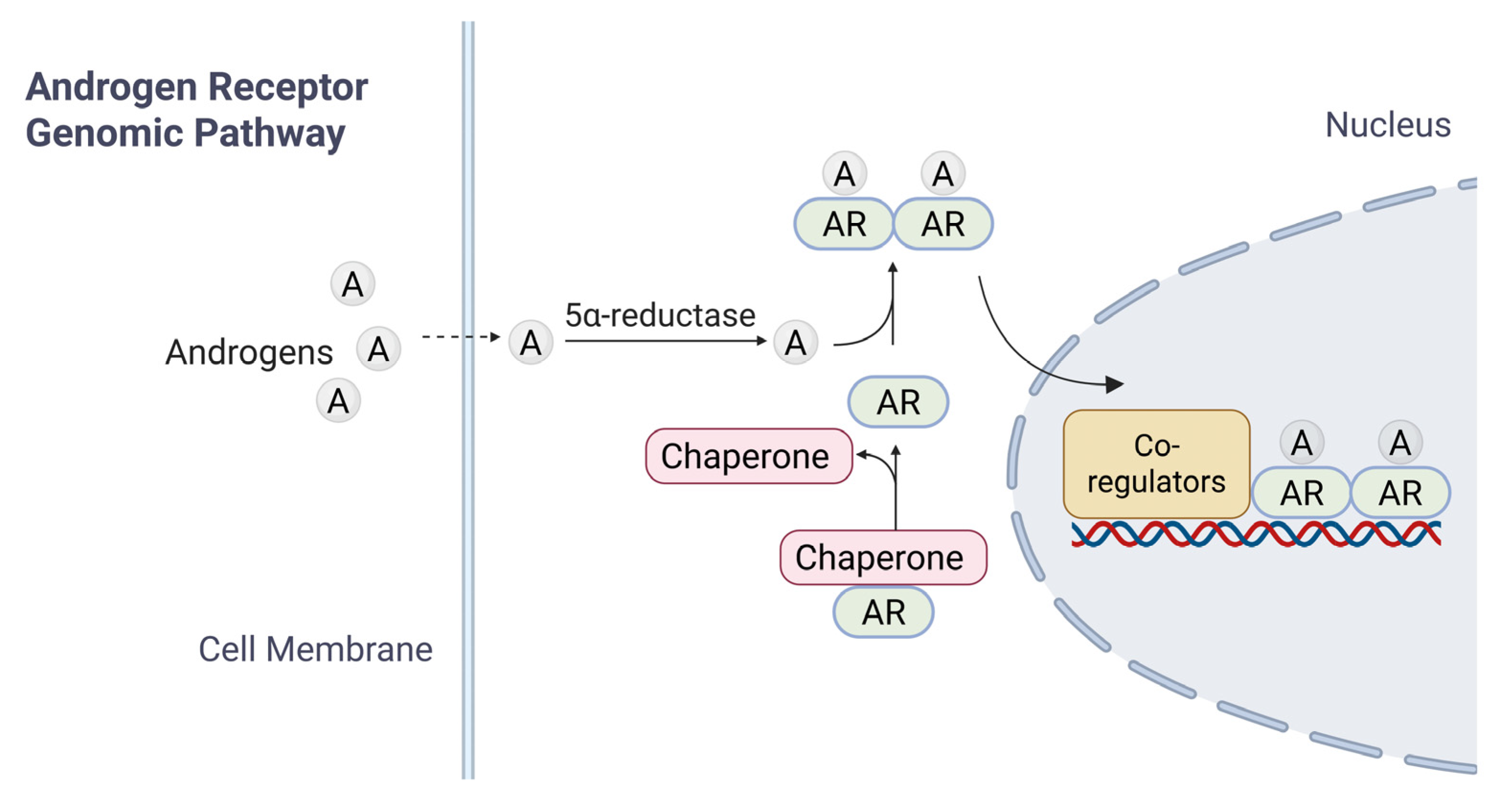
Inactive forms of AR are typically found in the cytoplasm, bound to heat shock proteins (HSPs); such an association ensures proper folding of AR [17,18]. Upon entry of androgens (such as T) into target cells by passive diffusion and binding to AR LBD, the AR undergoes a conformational change, leading to its dissociation with HSPs, homodimerization, translocation into the nucleus, and activation of AR-target genes [19] (Figure 2). Downstream signaling cascades are activated through two different mechanisms: the canonical (or genomic) pathway (Figure 2) and the non-genomic pathway [20].
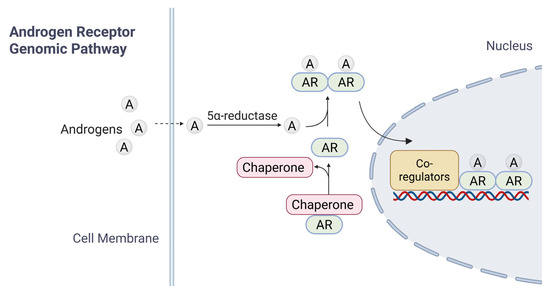
Figure 2.
A brief summary of AR signaling. The diagram illustrates the mechanisms of androgen receptor (AR) signaling in response to androgen. In the genomic pathway, androgen diffuses across the cell membrane. Androgen binds to cytoplasmic AR, which is maintained in an inactive conformation by chaperone proteins. Ligand binding induces AR activation, chaperone dissociation, and subsequent nuclear translocation. Within the nucleus, AR-Androgen complexes bind to androgen response elements (AREs) on DNA, recruiting co-regulatory proteins to modulate transcription of target genes.
In the current model for canonical AR signaling pathway, dissociation from HSP triggers the homodimerization of ligand-bound AR and its translocation into the nucleus, where dimerized AR interacts with androgen response elements (AREs) of target genes for transcriptional regulation. The modulation of gene expression governs processes such as development, homeostasis, and function of androgen-responsive tissues [14,21]. AR targets include genes regulating a range of cellular functions such as cell cycle progression (CCND1, CDK4, and CDC25A), apoptosis (BCL2 and MCL1), DNA repair and genome stability (Ku70/Ku80, BRCA1, and RAD51), immune modulation (IL-6 and PD-L1), cell invasion and tumor metastasis (MMP9, VEGF, and EPHB2), metabolism (HK2, LDHA, and ACLY) and miRNAs.
While non-genomic (rapid and extranuclear) actions of androgens have been proposed, such as direct interactions with cytoplasmic targets like Src kinase or PI3K/AKT, leading to quick cellular responses [22]. These are controversial and less relevant to the chronic processes underlying cancer disparities discussed in this review. Non-genomic signaling is typically transient and observed mainly in in vitro models at pharmacological (supra-physiological) concentrations of steroids, with limited in vivo evidence supporting its role in sustained tumorigenesis or progression in non-reproductive cancers. Moreover, many reported non-genomic effects may be artifacts of high-dose experiments, confounded by overlapping genomic actions, or non-physiological responses rather than true endogenous mechanisms [23]. Thus, this review focuses on the well-established genomic pathway, as it better aligns with the epidemiological and molecular data linking T/AR to sex disparities in cancer incidence and survival.
2.3. Role of Androgen Receptor and Testosterone in Health and Development
In health, AR mediates T and DHT effects for male differentiation and maintenance, with fetal T-AR signaling forming genitalia [24] and pubertal AR-T promoting muscle, bone, and spermatogenesis [25]. Females rely on low T-AR for ovarian and metabolic balance [25], while AR supports non-reproductive anabolism and cognition across sexes [26], potentially influencing tumor dynamics in non-reproductive sites.
Mutations disrupt AR function, causing developmental issues like Androgen Insensitivity Syndrome from loss of function, leading to virilization failure and gonadal cancer risk [27], or spinal and bulbar muscular atrophy via CAG expansions, inducing neurodegeneration [28]. Somatic mutations drive prostate cancer progression [29], with germline variants linking to cancer susceptibility [30], highlighting mutational roles in disparities. Such mutations may exacerbate male-predominant cancers by altering AR sensitivity in non-reproductive tissues.
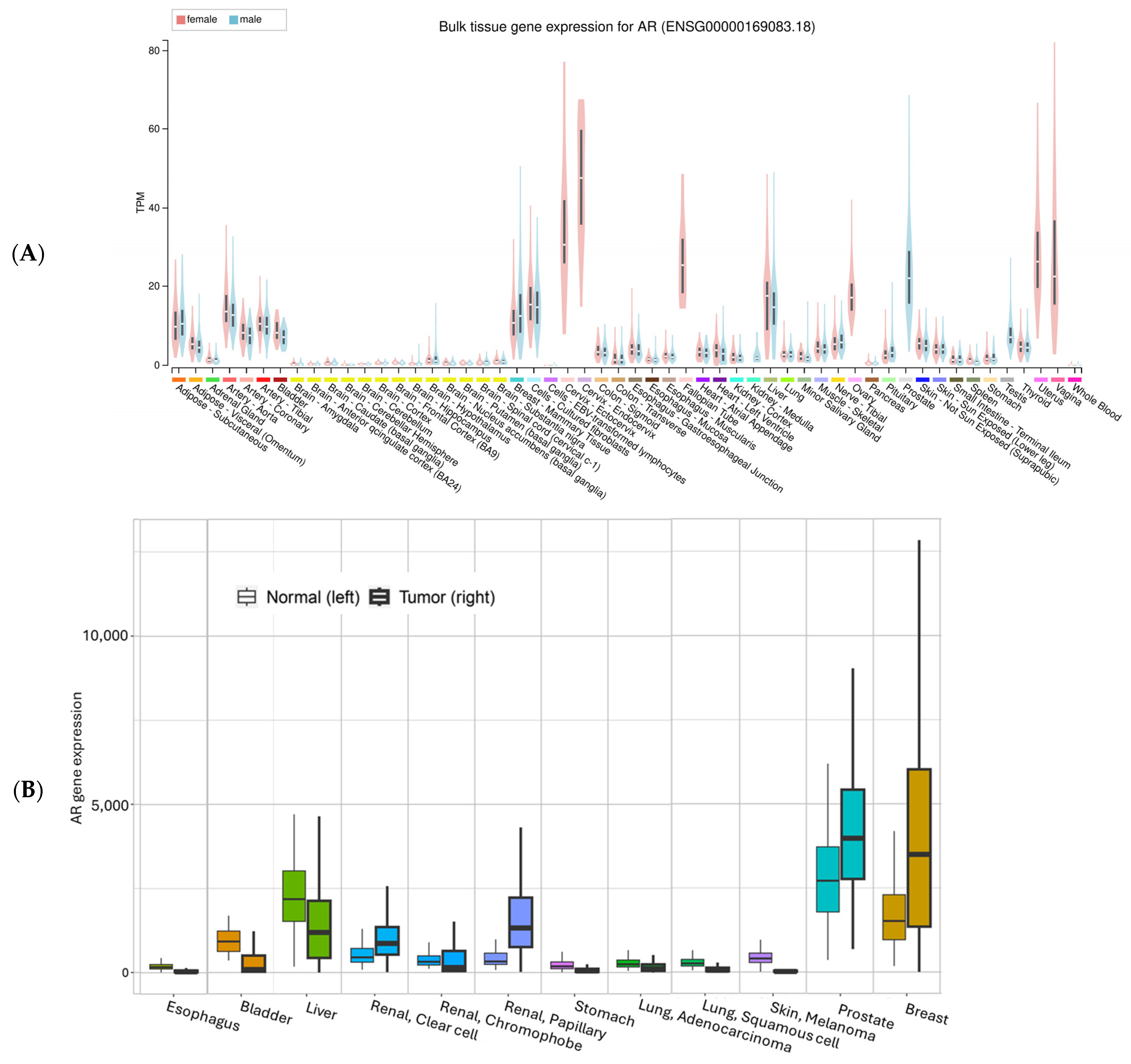
Utilizing the expression data from the GTEx project [31], we summarized AR expression in various organs/tissues (Figure 3A). The highest mRNA expression levels are found mostly in reproductive tissues such as the prostate, breast, ovary, vagina, and cervix, while the lowest expression is in brain tissues. All other tissues have various expressions. For most tissues, there seem to be comparable levels between males and females. Liver tissue unexpectedly has a level comparable to breast mammary tissues. For skin, suprapubic skin seems to have slightly higher expression than the sun-exposed lower leg tissues. For the esophagus mucosa, AR mRNA expression is low, consistent with previous IHC staining of Barrett Esophagus mucosa tissues, which showed negative staining for AR [32].
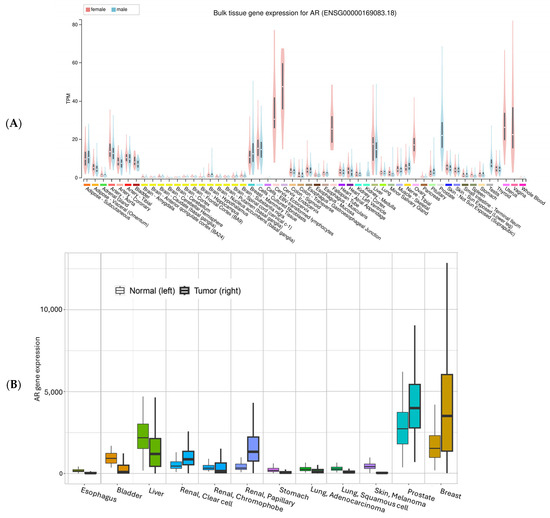
Figure 3.
AR mRNA expression in normal (A) and normal vs. tumor tissues. The gene expression in normal tissue was based on GTEx data (Genotype-Tissue Expression, with all tissues included), the normal vs. tumor expression was based on an integrated database which included TCGA (The Cancer Genome Atlas), GTEx, TARGET (Therapeutically Applicable Research to Generate Effective Treatments), and NCBI GEO data [33]. The y-axis in (B) is batch-adjusted and normalized counts. All comparisons showed significant differences (α < 0.05).
For a comparison between normal tissue and tumor tissue, we plotted the mRNA expression of AR using an online tool called TNMplot (https://tnmplot.com/analysis/ (accessed on 5 August 2025)) [33] (Figure 3B). Consistent with GTEx data, normal liver tissue and liver cancer showed relatively high AR expression, together with renal pelvis adenocarcinoma (Figure 3B). Skin data in this figure should be interpreted with caution because the tumor data are from melanoma, while the normal skin only contains a small portion of melanocytes.
2.4. Current Knowledge About Androgens and AR in Diseases
Both males and females require a balance between androgens with estrogens in the system to maintain fertility and reproductive organ function. For example, polycystic ovary syndrome (PCOS), leading to irregular menstruation and difficulty in becoming pregnant, is found in approximately 4 to 20 out of every 100 women of reproductive age. The underlying causes for PCOS may vary; PCOS is commonly characterized by elevated androgen levels produced by the ovaries to block ovulation [34]. Similarly, 20–30% of male infertility cases were found to be associated with low T levels or increased levels of luteinizing hormone (LH), leading to a decrease in sperm count [35].
An androgen imbalance may also play a crucial role in the development of cancers in reproductive organs, such as prostate cancer and androgen-sensitive breast cancer [36,37]. Notably, the role of androgens and AR signaling pathways in prostate cancer, the most frequently diagnosed cancer in American men and the second leading cause of cancer-related deaths, is the most studied [38].
Besides their roles in the reproductive system, androgens are also important for the development and maintenance of many non-reproductive physiological tissues, such as muscle mass and bone density [9,39]. Moreover, several types of cancers originated in non-reproductive tissues, such as lung cancer, bladder cancer, melanoma, and glioblastoma, have been associated with the activation of androgens/AR signaling pathways for proliferation or enhancing survival of cancer stem cells [40,41]. Although preclinical data suggested blockage of AR signaling is promising to inhibit proliferation of these tumor cells, less is known about the exact mechanism, and thus it remains a challenge in blocking androgens/AR signaling pathways in therapeutic strategies against non-reproductive cancers. For instance, a key consideration when selecting an AR antagonist for glioblastoma treatment is the ability of the drug to cross the blood–brain barrier [42]. Additionally, determining the heterogeneity of AR isoform expression is crucial for appropriately applying AR inhibition in these cancers to ensure targeted and effective treatment.
A clue to the significance of androgens in these cancers may come from a better understanding of sex differences in cancer risk. Since androgen levels and perhaps also AR levels differ between males and females [40], it is of great interest to determine whether androgens, particularly T, and AR are linked to sex-specific differences in the incidence and survival outcomes of cancers arising in non-reproductive tissues. In the next section of this review, we will outline the known involvement of T and AR in cancers originating from non-reproductive systems and attempt to understand their possible role in sex disparity.
2.5. Overview of AR in Cancer
As shown in Figure 3B, AR expression is enhanced in some cancer types and decreased in other types compared to their corresponding normal tissues, suggesting a complex role of AR, as well as tissue-specific functions of AR in cancers. AR plays a role in almost all aspects of cancer development, such as tumor cell proliferation, apoptosis, invasion, metastasis, tumor microenvironment modulation, tumor immune responses, drug resistance, and patient survival [43].
While the role of AR in initial tumorigenesis can be either oncogenic or tumor suppressive [44,45,46,47,48], in established cancer cells, AR generally stimulates cell proliferation and promotes tumor growth [49]. The oncogenic function of AR is primarily through its transcriptional activity and its association with other oncogenes such as EGFR, RAS family, and MAPK pathway, as well as cell type-specific co-regulators [49,50,51]. The tumor suppression function is partially through AR targets, such as PTEN and DNA damage response genes [52,53]. However, the same DNA damage response may also lead to drug resistance [49].
AR plays multifaceted roles in regulating cancer cell invasion and metastasis, with effects that vary across tumor types and microenvironments in cell invasion and tumor metastasis. For example, AR promotes metastasis in lung cancer by modulating the miR-23a-3p/EPHB2 signaling axis. AR was reported to increase hematogenous metastasis while suppressing lymphatic spread, mediated by differential regulation of VEGF-A and VEGF-C via miR-185-5p [54]. Mechanistically, AR can act through transcriptional regulation of metastasis-related genes and microRNAs, influencing epithelial–mesenchymal transition and angiogenesis. Therapeutically, targeting AR signaling may offer site-specific benefits, especially when combined with other clinical drugs.
The relationship between circulating androgen levels and tumor-intrinsic AR activity is quite complex. Circulating testosterone declines with age, obesity, chronic diseases, and chemotherapy [55,56,57]. Overall, hypogonadism is associated with poorer cancer survival [58,59]. Higher circulating testosterone levels could directly activate tumor AR activities, but tumors can also develop ligand-independent sustained AR activities through mechanisms such as AR mutation, gene amplification, or even intratumoral androgen synthesis [60]. In prostate cancer, both high and low serum total testosterone levels have been associated with increased risk [8], suggesting a non-linear relationship. However, blocking tumor AR activity is a standard therapeutic method. AR blockade has now been tested in a number of other cancer types, with various results [61]. Similar observations in other contexts support this complexity; for example, Di Donato et al. reported that ligand-activated AR drove melanoma invasiveness independent of systemic androgen levels [62].
3. An Overview of Sex Disparities in Cancers in Non-Reproductive Organs
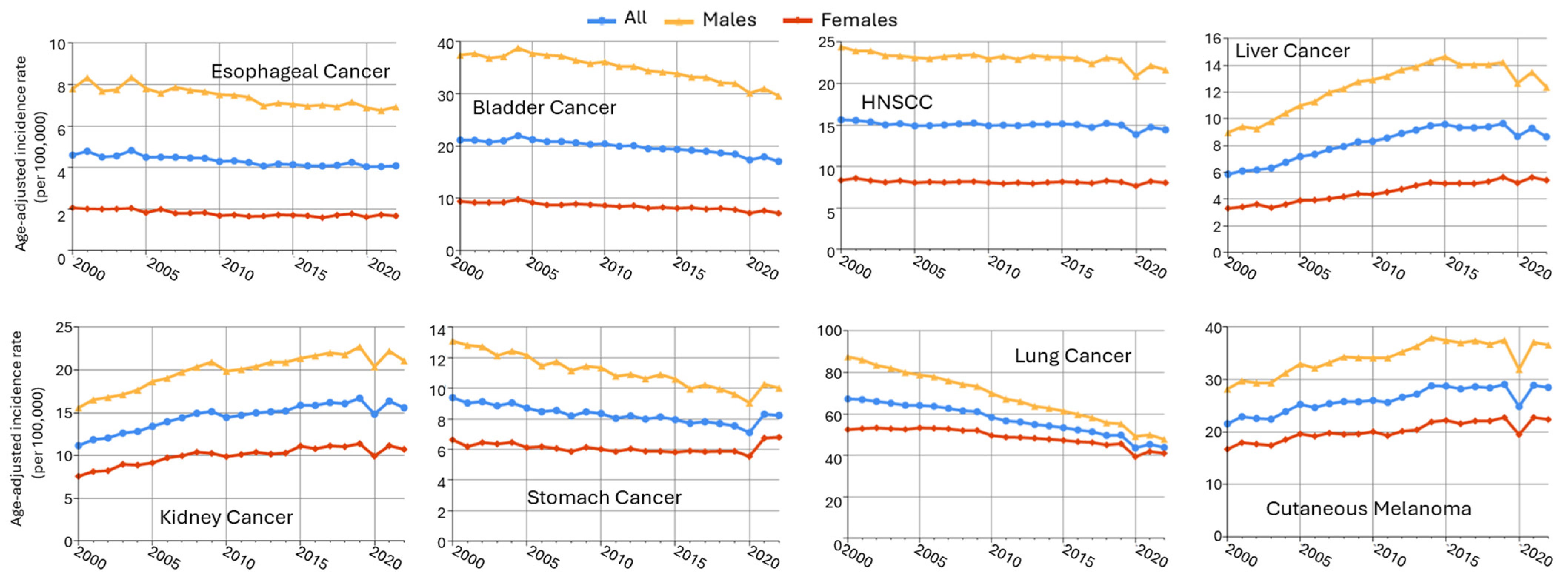
Male predominance is found in multiple cancers in non-reproductive systems, including esophageal cancer, bladder cancer, head and neck cancer, liver cancer, oral larynx cancer, kidney cancer, and stomach cancer (Table 1). Using the data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (downloaded by SEERStat9.0.41 software), the age-standardized incidence rates (ASRs) in men and women from 2000 to 2022 are plotted in Figure 4. Esophageal (SEER site code C15) and bladder cancers (C67) exhibited the greatest sex differences, more than fourfold of ASR in males as compared to females (M/F ratio), followed by head and neck cancer (C00–C14 and C32, with an M/F ratio of 2.84), liver cancer (C22), kidney cancer (C64.9), stomach cancer (C16), and cutaneous melanoma (C44). Major cancer types with an M/F ratio > 1.5 are included in this review, as well as highly prevalent lung cancer, which had an M/F ratio of 1.37.

Table 1.
Male to female incidence ratios (Data: SEER 17 registries, 2000–2022 *).
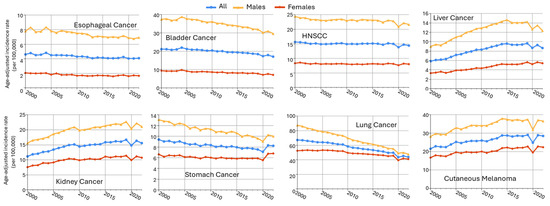
Figure 4.
Sex disparities in age-adjusted cancer incidence rates across multiple cancer types (SEER 17 data, 2000–2022). Trends in age-adjusted cancer incidence rates (per 100,000 person-years) from 2000 to 2022 for males (orange), females (red), and all (blue) patients across 8 cancer types. Data were obtained from the SEER Research Limited-Field Database (17 registries; November 2024 submission). Rates were age-adjusted to the 2000 U.S. standard population.
3.1. Esophageal Cancer
Esophageal cancer incidence is 6.3 per 100,000 person-years globally and exhibits a substantial sex difference [63,64] (Table 1). Its 5-year relative survival rate is only 15–20% [65]. The incidence and histological types of esophageal cancer vary by region. Esophageal squamous cell carcinoma (ESCC) is more prevalent in developing countries, while increasing cases of esophageal adenocarcinoma (EAC) have been observed in recent years in developed nations, including the United States and Western Europe [63,64].
It is estimated that 22,070 new cases of esophageal cancer will be diagnosed in the United States in 2025, and about 80% of them will be in males [66]. This trend is also revealed by data obtained through SEER from 2000 to 2022, with the incidence rates for esophageal cancer of 7.4 per 100,000 for males and 1.8 per 100,000 for females (Figure 4, Table 1), resulting in a male-to-female incidence rate ratio of approximately 4.16 (Table 1). The odds for EAC and ESCC are estimated to be 7–10 and 3–4 times higher in males than in females, respectively [64]. The established risk factors, like obesity, gastroesophageal reflux disease (GERD), or smoking, cannot fully explain the male predominance, implicating androgens as a contributing factor [67,68].
Whether sex hormone levels are associated with esophageal cancer remains inconclusive. One study showed that higher concentrations of DHEA but not T or DHT were associated with reduced risk of EAC [69]. A separate nested case–control study showed that higher T levels or higher T/E2 (testosterone/estradiol) ratios were associated with a decreased risk of EAC [70] (Table 2). A meta-study combined these two studies validated the association of T with EAC [70], which was not confirmed in a later meta-study with five studies [71] (Table 2). A potential confounder is perhaps BMI, as it is known that testosterone levels decrease with increased BMI, and after adjusting for BMI, the association of T with EAC is no longer significant (Table 2).

Table 2.
Testosterone and SHBG association with EAC.
AR showed varied expression levels in ESCC cell lines and tumors from patients [72], and higher expression was found in invasive ESCC tumors [73] (Table 3). Consequently, high tumor AR levels were associated with poor patient survival, especially for smoker patients, consistent with the finding that AR promoted tumor cell proliferation as well as invasion [72]. A second study validated the association of high tumor AR levels with poor ESCC patient survival [74].

Table 3.
AR expression and co-regulators/targets.
The mechanism through which AR acts in ESCC is not fully understood. Existing evidence shows that AR may exert its effects by upregulating matrix metalloproteinase 2 (MMP2) and activating the AKT signaling pathway [73], or through a feedback loop via pro-inflammatory cytokine interleukin-6 (IL-6), concomitant with activation of the STAT3 oncogenic signaling [72]. A ChIP-seq analysis indicated that GATA3 was a key co-regulator of AR in ESCC, and DUSP4 and FOSB were the key negatively regulated targets of AR [74]. Lower expression of DUSP4 or FOSB seemed to be associated with worse disease-free survival in patients [74]. A more recent study of this same ChIP-Seq experiment identified an additional AR target gene in ESCC: UGT2B15 (uridine diphosphate glucuronosyltransferase family 2 member B15), with AP-1 as an important co-regulator [75]. Inhibiting both AP-1 and AR exhibited strong effects in inhibiting cell invasion [75]. The AR targets and co-regulators are listed in Table 3.
Although cellular T or DHT is normally believed to upregulate AR levels, whether this regulation occurs in esophageal cancers remains uncertain. In ESCC and EAC, lower circulating T levels have been associated with increased cancer risk, whereas higher AR expression is observed in more advanced tumors and correlates with poorer survival [73]. An extensive investigation is warranted into the roles of T, DHT, and AR function in esophageal cancers.
3.2. Bladder Cancer
Bladder cancer is one of the top non-reproductive cancers showing a male-predominant pattern [76]. As shown in Table 1, the incidence rates for bladder cancer were 34.5 per 100,000 for males and 8.4 per 100,000 for females, indicating a 4.1 times higher incidence rate in men as compared to women. Following this trend, there will be 65,080 new cases in men and 19,790 new cases in women diagnosed with bladder cancer in 2025 [66]. A recent meta-analysis based on nine previous studies showed that nulliparous women and women with early menopause exhibited a significantly higher risk of bladder cancer [136], suggesting a link with sex hormones [137]. Conversely, women are more likely to be diagnosed with advanced stages of the disease [138,139]. Furthermore, female sex is independently associated with disease recurrence and worse cancer-specific survival [140,141].
The poorer outcomes for women in bladder cancer were partly attributable to diagnostic delays, as hematuria in women is frequently misattributed to urinary tract infections, resulting in later-stage diagnoses [142]. Moreover, women are less likely to receive guideline-concordant treatments such as neoadjuvant chemotherapy and radical cystectomy, even when matched for disease stage and comorbidities [142]. The survival gap is most pronounced in advanced-stage disease, where female patients demonstrate significantly lower overall survival than their male counterparts. The underlying biology and genomics mechanism may be related to sex hormone signaling, but this requires further investigation [143].
Tobacco usage is the strongest risk factor for bladder cancer, accounting for 50–65% of all cases [144]. Smoking was assumed to be a major cause of sex differences in bladder cancer, as there were more male smokers in general. However, when smoking intensity was stratified, men consistently showed higher incidence rates than women in each stratum, suggesting that smoking alone is insufficient to explain the sex disparity [145]. Zhu and Zhao summarized various factors that may impact the sex differences in bladder cancer, including biological differences and occupational differences [139]. Together with the above-mentioned survival disadvantage in women, it is vitally important to understand the mechanism behind the sex difference in bladder cancer incidence and survival.
Epidemiological research has investigated the association between androgens and bladder cancer. A recent case–control study in China, involving 147 male bladder cancer patients and 154 healthy controls, reported that cancer patients exhibited higher serum total T levels [146]. However, a study using the large UK Biobank data did not find such an association, either in men or post-menopausal women [111]. A two-sample Mendelian randomization (MR) study also found no relationship between genetically predicted T levels and bladder cancer risk in men [147] (Table 4).

Table 4.
Effect of androgens on cancer incidence.
Higher AR levels were found in bladder tumors compared to normal bladder mucosa, and they were higher in males compared to females [77]. The potential role of AR in bladder cancer development was described as early as 1975. In this mouse study, male mice showed a higher incidence of chemically induced bladder tumors compared to female mice [78]. Bladder tumors were absent in AR knockout mice, which underscored the pivotal role of AR in carcinogen-induced bladder carcinogenesis [78]. A mouse model with urothelial-specific AR knockout showed that DNA damage was reduced and TP53 was increased in AR-negative cells as compared to wild-type urothelial cells [46]. Another experiment with AR knockdown showed reduced levels of CD24, a key driver for metastasis [79]. Supportive of this finding, in a cohort of 10,720 male patients with bladder cancer, patients who received 5α-reductase inhibitors, which decrease the AR activity by reducing DHT levels, exhibited improved disease-specific survival [80]. Furthermore, androgen depletion in AR-positive human bladder cancer cells in vitro, or castration in mice, inhibited tumor cell growth, as did the AR knockdown [81,82]. AR expression was detected in only a subset of bladder cancer cell lines and bladder tumors [77,83]. Some bladder tumors contained little androgen, with nearly undetectable DHT and castration-level of T [84]. Consistent with a tumor suppressor role of androgens and/or AR in bladder cancer, the absence of AR expression was associated with recurrence in non-muscle-invasive bladder cancer [85,86]. Still, a meta-analysis revealed that AR levels were positively correlated with the tumor grade of bladder cancer, but not with susceptibility or tumor stage [87]. Whether the possible tumor suppressor role of AR and/or androgen explains the disadvantage of female survival in bladder cancer warrants further investigation.
In terms of molecular mechanisms, multiple downstream targets or signaling cascades of AR may be involved, such as EGFR, AKT, matrix metalloproteinases, UDP-glucuronosyltransferases (UGTs), and β-catenin/WNT pathway [88,89,90], which were extensively reviewed by Li et al. [77]. A recent study suggested that AR-regulated circular RNA activated KRAS signaling, thereby enhancing bladder cancer invasion and chemoresistance [91]. Of particular interest regarding sex differences is the GATA3 gene, a suggested prognostic biomarker for bladder cancer [92,93]. GATA3 expression is significantly higher in bladder tissues of female mice compared to male mice, and its expression was increased by orchiectomy in males but decreased by ovariectomy in females [94]. Loss of GATA3 in a subset of bladder cancer, especially in high-grade tumors, was correlated with higher AR activity in males [94].
3.3. Head and Neck Cancer
Head and neck cancer ranks as the seventh most common cause of cancer-induced mortality, encompassing a group of malignancies from the anatomical sites of the upper aerodigestive tract, the paranasal sinuses, and the salivary glands [101], with major subtypes including head and neck squamous cell carcinoma (HNSCC), oral squamous cell carcinoma (OSCC), oropharyngeal squamous cell carcinoma (OPSCC), and so on. HNSCC, the most prevalent subtype (accounting for over 90% of all cases) [102], has a five-year survival rate, less than 50% in advanced stages, and males have up to a fivefold higher risk in all ethnic groups when compared to females [103,104]. The SEER data revealed incidence rates for HNC of 23.0 per 100,000 for males and 8.1 per 100,000 for females, resulting in an M/F ratio of approximately 2.8 (Figure 4, Table 1). In 2025, it is estimated that approximately 59,660 new cases of cancer in the oral cavity and pharynx will be diagnosed in the United States, and 42,500 of them will be men, which is two to three times higher than that for women [66]. The major risk factors for head and neck cancer include tobacco smoking, chronic alcohol consumption, and infection with human papillomavirus [105].
To date, large-scale prospective studies have not demonstrated a clear association between T levels and the overall incidence of head and neck cancers (HNC). For instance, one study that examined sex hormone levels across multiple cancer types found no significant link between T and cancers of the oral cavity, pharynx, or larynx [153]. However, hormonal factors may not be ignored because a pooled analysis of 11 case–control studies indicated that women who had used hormone replacement therapy (HRT) experienced approximately a 42% reduction in the odds of developing HNC (OR ≈ 0.58) compared to those who had never used HRT [154].
A possible interplay between HPV positivity and AR signaling has been suggested in HNSCC. Mohamed et al. (2018) observed that HPV-positive OPSCC (oropharyngeal squamous cell carcinoma) tumors were associated with increased AR expression as compared to HPV-negative tumors [155], and oropharyngeal tumors were more likely to express sex hormone receptors, including AR and ERa, as compared to other sites in HNSCC [155,156]. However, the biology of OPSCC may differ from the rest of HNSCC.
The significant sex difference in HNSCC has prompted hypotheses of a synergistic interplay among smoking, alcohol consumption, and sex hormones [157]. Tobacco synergizes with AR, as smoke components upregulate AR expression and activity, boosting HNSCC cell proliferation via EGFR/AKT [158], explaining male bias from higher smoking rates [157]. Alcohol shows limited direct AR synergy, often suppressing T via metabolism or estrogen conversion [159], but may indirectly enhance AR signaling through Golgi fragmentation in models [160], potentially cooperating with inflammation in HNC, though evidence is sparse [161]. Future studies should clarify AR–alcohol crosstalk in HNC cohorts.
AR expression varies in HNSCC at different sites; for example, the AR mRNA level was upregulated in laryngeal squamous cell carcinoma [100,162] (Table 3). Upregulation of AR and a greater distribution into cytoplasm (≥20%) were associated with poor progression in OSCC [97], consistent with in vitro studies, which demonstrated that AR suppression reduced tumor cell growth by inducing apoptosis [157]. Consistent with these results, an immunohistochemical study showed that when more than 20% of OSCC tumor cells showed cytoplasmic AR staining, epithelial Ki67, lymphocyte VEGF, and macrophage MMP9 were all significantly positively associated with AR levels, while epithelial HIF1b and macrophage VEGF were negatively associated with AR levels in metastatic tumors [98]. In AR+ OSCC cell line SCC9, DHT induced AR expression and activation, which led to enhanced cell migration and aggressiveness by induction of EGFR and AKT [50]. This signal pathway and effect were absent in AR-cell lines, underscoring the direct involvement of the AR gene [50]. AR also cooperated with co-activator RBAP48 (Retinoblastoma-associated protein 48), a protein highly expressed in OSCC, to activate AR targets. Both AR and RABP48 at higher levels were associated with worse survival of patients [99]. AR co-regulators and targets are summarized in Table 3. Hence, AR was suggested to be a prognostic biomarker for OSCC and a promising target for therapeutic intervention in OSCC.
Salivary gland cancers (SGCs) are relatively rare and usually diagnosed in older people (>60 years) [163,164,165]. Among SGCs, salivary duct carcinoma (SDC) is a highly aggressive subtype, with AR positivity observed in around 75–89% of cases [96,166,167]. Hence, it was not a surprise that there were case reports showing that SDCs were responsive to androgen-deprivation therapy [168,169]. However, a Phase II clinical trial with Enzalutamide in AR+ SGC patients failed to meet the endpoint expectations [170]. The exact mechanism of AR function in SGC is largely unknown. It was reported that AR overexpression in SGC might be through the increased copy number of chromosome X or TP53 mutation [171]. Genomic analysis revealed that AR might interact with transcription factor FOXA1 (Forkhead box protein A1) to activate the downstream target genes [166,171,172].
3.4. Liver Cancer
Liver cancer, primarily hepatocellular carcinoma (HCC), is a significant global health concern, ranking as the third leading death-causing cancer in 2022 [107]. It was reported to have 2–4 M/F ratios in the age-standardized incidence rates across all regions in the world [173,174]. In the SEER data, the incidence rate for liver cancer was 12.8 per 100,000 for males and 4.7 per 100,000 for females, with an M/F ratio of 2.8 (Table 1). Despite this constant observation, the reason underlying the sex disparity remains unclear. Risk factors for liver cancer include viral hepatitis, aflatoxin exposure, alcohol consumption, diabetes, and obesity. However, it is hard to explain the consistency of sex disparity since the prevalence of these factors is changing over time in different populations [175]. This led to the hypothesis that the biological differences between males and females, such as sex hormones, may also play a critical role in liver cancer.
Multiple prospective studies have reported positive associations between T and liver cancer incidence. The UK Biobank study showed that total T and SHBG, but not free T, were significantly associated with liver cancer in men, but not in women [111]. A meta-analysis of 29 prospective studies found that higher T levels were associated with elevated risk of liver cancer, with a stronger effect in men [71]. An earlier prospective cohort study observed that men with higher total T had an increased risk of early mortality after cancer diagnosis, while women showed the same effect only in the highest quintile [176]. In an early diethylnitrosamine (DEN)-induced liver cancer mouse model, castration reduced both tumor incidence and tumor size in male mice [177]. Taking it together, current studies suggest that elevated T levels may be linked to an increased risk of liver cancer as well as mortality.
AR expression was found in both normal liver tissue and HCC, with an overall higher expression in tumor regions [178,179]. In one report, AR expression levels were positively correlated with the recurrence rate of HCC and negatively correlated with the survival rate, indicating AR as a promoter in liver cancer [108]. These results correspond with the above-mentioned results of T levels. Furthermore, within similar serum T levels, the absence of AR postponed or lessened the development of carcinogen-induced HCC [109]. However, loss of AR in mice bearing HCC led to more metastasis [110], corresponding to another report where a patient with higher AR expression in tumors showed better overall and disease-specific survival [180]. Taken together, it was suggested that AR played a dual role in liver cancer: a promoter in triggering tumorigenesis and a suppressor at advanced stages [110]. The controversial results regarding AR’s impact on survival outcome warrant further clarification [181].
The molecular studies suggested several instances of AR signaling in HCC (summarized in Table 3): upregulating EZH2 expression which correlates with tumor progression and poor prognosis [182]; promoting arachidonic acid metabolism and angiogenic tumor microenvironment in AFP (alpha-fetoprotein)-negative HCC [183]; and serving as a molecular docking target for metformin, resulting in lower AR at protein level and enhanced ferroptosis [180]. HBV (hepatitis B virus) increased AR expression, which in turn, upregulated BIRC7, IGFBP3, and NTSR1, leading to increased proliferation of HCC cells [184]. It was also suggested that AR (together with the estrogen receptor) interacts with HBV and HCV (hepatitis C virus) in a sex-specific manner, leading to sex-differentiated immune response, inflammatory damage, liver fibrosis, and carcinogenesis [185]. In addition, the polymorphism of AR (number of CAG repeats) affects liver cancer differently in males and females, which also likely contributes to sex disparities of HCC. Yu et al. reported that shorter AR alleles (i.e., fewer repeats) conferred a higher risk for HCC in men with HBV, whereas higher incidence rates were found in HBV female carriers with longer AR alleles [186].
3.5. Kidney Cancer
Since the 1970s, the global incidence of kidney cancer has been increasing [187]. In 2025, the National Cancer Institute estimated approximately 80,980 new cases in the United States, with 52,410 male cases [66]. Renal cell carcinoma (RCC) is a leading cause of cancer death, ranking sixth among men and eighth among women [188,189]. As shown in Figure 4 and Table 1, incidences of kidney cancer were 20.1 per 100,000 for males and 10.0 per 100,000 for females, resulting in an M/F ratio of 2.0. This male predominance is consistent across various age groups, time periods, and geographic locations [112,190,191]. The reasons behind these sex differences are multifaceted, involving both biological and environmental factors. Established modifiable risk factors, such as smoking and hypertension, contribute significantly to RCC incidence, and hormonal influences, particularly the role of androgens and the AR, have been implicated in RCC development and progression [113,192]. In particular, by upregulating 20-HETE production, which elevates renal vascular tone and blood pressure, androgen potentially creates a pro-tumorigenic environment in the kidney [114].
Epidemiological studies on the association between androgens and kidney cancer risk are limited. Two UK Biobank studies showed no significant association between baseline T (total or free) and risk of kidney cancer in either sex [111,193]. RCC is the predominant form of kidney cancer, with clear cell RCC (ccRCC) being the most common subtype [115,194,195]. AR expression was positively correlated with tumor-originated vasculogenesis in ccRCC patients [116], suggesting a tumor-promoting role, which was validated in in vitro experiments, possibly through modulating the lncRNA-TANAR/TWIST1 signaling pathway [116]. Consistently, AR suppressed the expression of a circular RNA named circHIAT1 by downregulating its host gene, resulting in enhanced ccRCC cell migration and invasion [117]. In contrast, other studies showed the opposite effect of AR in ccRCC: higher expression of AR correlated with better overall survival in both males and females [118,119,189]. Higher AR expression was also correlated with male patients, lower tumor grade, and early stages of RCC [120]. Again, the role of AR in ccRCC development and progression is inconclusive: even though AR was associated with tumor vasculogenesis and invasion, higher tumor AR levels were associated with better patient survival.
3.6. Stomach Cancer
Despite a decline in both incidence and mortality rates over recent decades, gastric cancer remains one of the most lethal malignancies [123]. In the United States, it was estimated that there would be 17,720 new stomach cancer cases in 2025, among which 12,580 would be males [66]. This male-dominant trend is also exhibited by the SEER data (2000 to 2022), which shows the incidence rates of 10.9 per 100,000 for males and 6.1 per 100,000 for females (Figure 4), indicating an M/F ratio of 1.80 (Table 1).
Current epidemiologic data do not suggest a clear association between T and stomach cancer risk. One systematic review of prospective cohorts of 11 studies concluded that circulating T was not significantly associated with gastric cancer risk in either sex [124]. The UK Biobank study also revealed no significant association of total or free T levels with stomach cancer, neither in men nor in women [111]. However, one study indicated that elevated SHBG was associated with increased gastric cancer incidence in men only [71]. This association was validated in the UK Biobank study, which was not found in post-menopausal women [111]. Hence, although T alone is not associated with stomach cancer, higher SHBG seems to confer a higher risk in men.
Research has indicated that disruptions in AR homeostasis might contribute to the higher prevalence of gastric cancer in men compared to women [125]. Further research has identified AR variants in gastric cancer tissues and cell lines, implicating them in promoting tumorigenesis and metastasis [126]. Additionally, recent research has discovered that AR activation leads to increased expression of LAMA4, which in turn induces resistance to cisplatin in gastric cancer cells, highlighting a potential mechanism by which AR contributes to treatment resistance [196]. In addition, AR was frequently overexpressed in gastric cancer tissues compared with normal gastric mucosa [121,197]. Hou et al. further explained that increased AR expression, facilitated by the co-factor YAP1, drove the development of chemoresistance in gastric cancer [198]. Therefore, AP appears to contribute to chemoresistance via multiple pathways. AR overexpression was also associated with nail, beta-catenin, Twist1, and STAT3, all of which led to worse outcomes [122].
3.7. Lung Cancer
Lung cancer remains the leading cause of cancer-related deaths worldwide, accounting for approximately 1.8 million fatalities annually and remaining the top death-causing cancer in the United States [199]. The major types of lung cancer include small cell lung carcinoma (SCLC), non-small cell lung cancer (NSCLC), the latter of which comprises lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and large cell carcinoma. The male predominance in lung cancer incidence is observed in the SEER data (2000 to 2022) with an M/F ratio of 1.37 (Table 1). However, the sex difference is diminishing and even reversed in recent years (Figure 4 and [135,200]). In the United States, it is estimated that there will be 226,250 lung cancer cases in 2025, with male cases of 110,680, less than the female cases (115,970) [66]. But women are often diagnosed with lung cancer at a younger age, in earlier stages, and generally have a more favorable prognosis and better 5-year survival rates compared to men, particularly in (NSCLC) [133].
The relationship between T and lung cancer remains inconclusive. One MR study found no relationship between T and lung cancer risk in either men or women [201], whereas another MR study showed that, based on 74 SNPs, higher levels of bioavailable T were associated with a lower risk of LUSC and SCLC but not LUAD in men [202]. No association was found in serum hormone levels of total T or free T or SHBG with lung cancer in men or post-menopausal women in a large UK Biobank study [111].
AR expression has been detected in both SCLC and NSCLC, including LUAD—the most prevalent type of NSCLC in non-smokers and young people [199,203,204]. However, the role of AR in lung cancer remains unclear. In A549 cells, an NSCLC cell line with relatively high AR expression, AR and EGFR (a crucial oncogene in lung cancer) collaboratively promote proliferation by activating p38 MAPK signaling [205,206]. AR also promoted invasion and metastasis via the miR23a-3p/EPHB2 pathway [134]. Treating A549 cells with luteolin reduced AR expression and subsequently inhibited cell proliferation [207]. Similarly, prostate cancer patients receiving androgen-deprivation therapy (ADT) showed lower secondary lung cancer incidence when compared to those in the non-ADT group [150].
Consistently, exposure to androgen pathway inhibition (primarily 5-alpha reductase inhibitors, which reduce DHT levels) led to greater survival of lung cancer patients regardless of age, stage, and histology [151]. On the contrary, other reports found that the presence or level of AR was positively correlated with better survival in LUAD patients [48,152]. This hypothesis was supported by the observation that AR inhibited tumor cell invasion in NSCLC via the circular-SLCO1B7/miR-139-5p axis and reduced oncogene D52 expression [208]. It is also likely through tumor-promoting miR-224-5p, which targets AR and inhibits AR expression [48]. Of particular note, given the distinct biology of LUAD, LUSC, and SCLC, we analyzed androgen-related findings by histologic subtype; associations observed in LUAD were not assumed to generalize to LUSC or SCLC.
3.8. Melanoma
The global incidence of melanoma has been rising in recent decades and is projected to continue increasing [209]. In 2025, it is estimated that 104,960 individuals in the United States will be diagnosed with invasive melanoma, with 60,550 male and 44,410 female cases [66]. Risk factors for melanoma include UV exposure (both from sunlight and artificial sources like tanning beds), fair skin, a history of sunburns, the number of moles, a family history of melanoma, and a weakened immune system [127]. The risk increases with age, with the average age of diagnosis being 65 [127]. Meanwhile, females exhibit higher incidence rates of melanoma compared to males at a younger age, whereas males have higher incidence rates at an older age [128]. Men have a lower melanoma survival rate compared to women after adjusting for age, stage of disease, and other prognostic factors [129].
The relationship between androgens and melanoma has not been extensively investigated, and results are mixed. Based on the observation of female survival advantage in melanoma, it was suggested in 1980 that melanoma might be an androgen-dependent tumor [130]. But later studies generally did not support this hypothesis due to a lack of apparent AR expression in most normal melanocytes or melanoma cells [53].
A recent study using UK Biobank data showed that higher total T and free T levels were associated with a higher risk of melanoma in men but not in post-menopausal women [111]. This study underscores the importance of investigating sex hormone impact on melanoma risk. However, this study did not fully adjust for sun exposure, which might confound the association between T levels and melanoma [111], as it has been shown that chronic UVB exposure increased T levels in male but not in female mice [131]. Men showed peak T levels in the summer or fall months [131,132,210]. Therefore, further clarification is needed. In another study, male pattern baldness, which is often associated with high T levels, was found to have a strong association with melanoma risk [211]. However, based on the MR analysis, the authors concluded that this association is likely driven by sun exposure rather than T expression [211]. Nevertheless, there is a potential crosstalk between T and sun exposure because a fish melanoma model showed that UVB irradiation was able to rapidly up-regulate AR expression [212], consistent with UV-increased T levels in humans.
Another MR study using instrumental T variants in the whole population without separating susceptibility by sex showed no causal association between T with melanoma risk [213]. This study failed to account for the markedly different genetic determinants of T between sexes [12], rendering the conclusion highly unreliable.
More recent molecular studies have shown AR expression in melanoma cell lines and tumors [214,215]. AR exhibits a seemingly controversial role in melanoma development and survival. Morvillo et al. demonstrated that androgens significantly stimulated proliferation in a human melanoma cell line expressing an atypical form of AR, while treatment with the androgen antagonist flutamide reversed these effects [216]. Min et al. also showed that AR suppression reduced melanoma tumor cells’ proliferation by increasing DNA damage and activating a STING-dependent inflammatory response [53]. Further, AR signaling was reported to drive melanoma invasiveness through tumorigenic fucosylation or the MITF/AXL axis [217,218]. Specifically, AR activated transcription of the FUT4 gene, which, in turn, fucosylated L1CAM at the adherence junctions, leading to increased metastasis [217]. AR also increased melanoma invasion through regulating the miRNA-539-3p/USP13/MITF/AXL signals. AR functioned through binding to AREs in the promoter region of the host gene for miR-539-3p and activated its expression [218]. In this study, AR+ melanoma patients were shown to have worse overall survival [218]. Another study did not find the association of baseline AR levels with patient outcomes [41], but they found that AR signaling was induced by BRAF/MEK inhibitors, and blocking this increase in AR improved patient responses to the targeted therapy [41]. These results, however, somewhat contrast with the epidemiological findings where patients in the TCGA dataset with higher AR levels showed better overall survival [40]. Previous publications also showed that lower T levels were associated with poorer cancer survival [219], particularly in hypogonadism patients [58,59]. A mouse study revealed that castration increased melanoma tumor burden, but supplementing T reversed it [220]. It was then suggested that extreme T levels were potentially harmful. Such non-linear observations were also reported in prostate cancer [8,221]. Based on these conflicting observations, we propose that a non-linear T/AR axis also exists in melanoma, as shown in Figure 1.
In melanoma, AR seemed to be heavily involved in the regulation of the tumor microenvironment and immune responses. A genomics study using the TCGA-SKCM data revealed that increased AR signal was significantly associated with exhaustion of CD8+ T cells, and blocking AR signaling could synergize with T cell-based cancer immunotherapy [4]. Inhibiting AR activities was shown to increase immune cell abundances and was associated with higher IFNγ pathway activity, leading to better response to ICI treatment [222].
4. Summary and Conclusions
The effect of androgens (including SHBG) is summarized in Table 4. No solid evidence shows an association of T levels with head and neck cancer, bladder cancer, kidney cancer, or lung cancer. Evidence may suggest a sex-specific link of T or SHBG with liver cancer, stomach cancer, and melanoma, with higher T associated with elevated risk. Expression levels of AR seem to tell a different story (Table 5): although there are controversies, inhibition of AR at the cellular level generally resulted in decreased cell proliferation. However, in liver cancer, kidney cancer, melanoma, and lung cancer, higher tumor AR levels are apparently associated with better patient survival.

Table 5.
Effects of AR in tumor progression and patient survival.
Given all the above mixed results, we found a previous hypothesis regarding a U-shaped relationship of T with prostate cancer is interesting [8] (Figure 1). Specifically, Salonia et al. found that when serum total T was grouped into low, middle, and high levels, both low and high levels were associated with a higher risk of prostate cancer [8]. This may imply that appropriate levels of T are key in maintaining tissue homeostasis, including the non-reproductive tissues. Too high or too low levels of T may be associated with cancer risk. When only linear regression, or regression assuming a linear relationship, is used, the results can be mixed. While high levels of T apparently stimulate AR activities through ligand binding, evidence also showed that low levels of T increased AR protein stability in a compensatory manner [224]. Hence, it is likely that T/AR activities also follow a U-shaped model as described in Figure 1.
Furthermore, T acts in concert with other sex hormones such as estrogens and progesterone in physiological functions. Thus, examining the combined impact of sex hormones, such as T/E2 ratios, may be more informative for understanding the roles of sex hormones in cancer development. Although genetics play an important role in determining sex hormone levels, diet, supplements, and exercise can all tune hormone levels and therefore make interventions possible [225]. Additionally, many agonists and antagonists of AR have been developed and used in cancer treatment, and these methods can potentially be applied to cancers such as bladder, esophagus, head and neck, and stomach.
A major limitation of the existing clinical literature is the lack of consistent adjustment for key confounding variables such as age, BMI, smoking, alcohol intake, and comorbid conditions such as diabetes and metabolic syndrome, which are all known to influence circulating testosterone and AR activity [55,56]. Much more epidemiological work is needed in this area to obtain consistent and convincing results.
In summary, this review focuses on non-reproductive cancers with higher incidence in males, highlighting differences in T levels and potential AR roles in affecting tumorigenesis, progression, treatment, and outcome. Despite mounting evidence of AR’s involvement in these cancers, the precise contribution of T/AR in sex disparities, cancer biology, prognosis, treatment responses, and survival is largely unclear. Closing this knowledge gap may provide novel therapeutic methods, as targeting AR signaling presents a promising yet intricate avenue. To advance our understanding of T/AR’s role and improve AR-targeted therapies, future research should 1) delve into epidemiological and genomic data to characterize T/AR and other sex hormones in a sex-specific manner, thereby guiding mechanistic studies, and 2) identify biomarkers that can stratify patients most likely to benefit from AR-targeted interventions, thus maximizing treatment efficacy.
Author Contributions
Conceptualization, F.L.-S.; methodology, F.L.-S. and J.L.; software, J.L. and J.Z.; validation, F.L.-S., J.F. and R.N.; formal analysis, F.L.-S., J.L. and J.Z.; investigation, F.L.-S., J.L. and J.Z.; resources, F.L.-S.; data curation, F.L.-S., J.L. and J.Z.; writing—original draft preparation, F.L.-S., J.L. and J.Z.; writing—review and editing, F.L.-S., J.F. and R.N.; visualization, J.L. and J.Z.; supervision, F.L.-S.; project administration, F.L.-S.; funding acquisition, F.L.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH/NIEHS, grant number R21ES034142 to F.L.-S. and 1R01NS131106-01A1 to R.N.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original datasets are publicly available from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Özdemir, B.C.; Dotto, G.P. Sex hormones and anticancer immunity. Clin. Cancer Res. 2019, 25, 4603–4610. [Google Scholar] [CrossRef]
- Chen, X.; Augello, M.A.; Liu, D.; Lin, K.; Hakansson, A.; Sjostrom, M.; Khani, F.; Deonarine, L.D.; Liu, Y.; Travascio-Green, J.; et al. Canonical androgen response element motifs are tumor suppressive regulatory elements in the prostate. Nat. Commun. 2024, 15, 10675. [Google Scholar] [CrossRef]
- Ainslie, R.J.; Simitsidellis, I.; Kirkwood, P.M.; Gibson, D.A. RISING STARS: Androgens and immune cell function. J. Endocrinol. 2024, 261, e230398. [Google Scholar] [CrossRef]
- Yang, C.; Jin, J.; Yang, Y.; Sun, H.; Wu, L.; Shen, M.; Hong, X.; Li, W.; Lu, L.; Cao, D.; et al. Androgen receptor-mediated CD8+ T cell stemness programs drive sex differences in antitumor immunity. Immunity 2022, 55, 1268–1283.e1269. [Google Scholar] [CrossRef]
- Vesper, H.W.; Wang, Y.; Vidal, M.; Botelho, J.C.; Caudill, S.P. Serum Total Testosterone Concentrations in the US Household Population from the NHANES 2011–2012 Study Population. Clin. Chem. 2015, 61, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Augello, M.A.; Liu, D.; Deonarine, L.D.; Robinson, B.D.; Huang, D.; Stelloo, S.; Blattner, M.; Doane, A.S.; Wong, E.W.P.; Chen, Y.; et al. CHD1 Loss Alters AR Binding at Lineage-Specific Enhancers and Modulates Distinct Transcriptional Programs to Drive Prostate Tumorigenesis. Cancer Cell 2019, 35, 603–617.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sakamoto, S.; Yamada, Y.; Sato, K.; Tamura, T.; Shibata, H.; Sazuka, T.; Imamura, Y.; Ichikawa, T. The Role of Serum Testosterone in the Pathogenesis and Treatment of Prostate Cancer: A Review Based on the Clinical Evidence. Int. J. Urol. 2025, 32, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Abdollah, F.; Capitanio, U.; Suardi, N.; Briganti, A.; Gallina, A.; Colombo, R.; Ferrari, M.; Castagna, G.; Rigatti, P.; et al. Serum sex steroids depict a nonlinear u-shaped association with high-risk prostate cancer at radical prostatectomy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3648–3657. [Google Scholar] [CrossRef]
- Alemany, M. The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. Int. J. Mol. Sci. 2022, 23, 11952. [Google Scholar] [CrossRef]
- Hammes, R.S.; Levin, R.E. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Nutsch, L.V.; Will, G.R.; Tobiansky, J.D.; Reilly, P.M.; Gore, C.A.; Dominguez, M.J. Age-related changes in sexual function and steroid-hormone receptors in the medial preoptic area of male rats. Horm. Behav. 2017, 96, 4–12. [Google Scholar] [CrossRef]
- Selby, C. Sex hormone binding globulin: Origin, function and clinical significance. Ann. Clin. Biochem. 1990, 27 Pt 6, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Goss, S.J.; Lubahn, D.B.; Joseph, D.R.; Wilson, E.M.; French, F.S.; Willard, H.F. Androgen receptor locus on the human X chromosome: Regional localization to Xq11-12 and description of a DNA polymorphism. Am. J. Human Genet. 1989, 44, 264–269. [Google Scholar]
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Culig, Z.; Santer, F.R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014, 33, 413–427. [Google Scholar] [CrossRef]
- Jaattela, M. Heat shock proteins as cellular lifeguards. Ann. Med. 1999, 31, 261–271. [Google Scholar] [CrossRef]
- Sullivan, W.P.; Vroman, B.T.; Bauer, V.J.; Puri, R.K.; Riehl, R.M.; Pearson, G.R.; Toft, D.O. Isolation of steroid receptor binding protein from chicken oviduct and production of monoclonal antibodies. Biochemistry 1985, 24, 4214–4222. [Google Scholar] [CrossRef]
- Srinivas-Shankar, U.; Wu, C.F. Drug Insight: Testosterone preparations. Nat. Clin. Pract. Urol. 2006, 3, 653–665. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Srivastava, S.K.; Pai, S.; Singh, S.; Singh, A.P. Therapies Targeted to Androgen Receptor Signaling Axis in Prostate Cancer: Progress, Challenges, and Hope. Cancers 2019, 12, 51. [Google Scholar] [CrossRef]
- Koochekpour, S. Androgen receptor signaling and mutations in prostate cancer. Asian J. Androl. 2010, 12, 639–657. [Google Scholar] [CrossRef]
- Leung, J.K.; Sadar, M.D. Non-Genomic Actions of the Androgen Receptor in Prostate Cancer. Front. Endocrinol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Simons, S.S., Jr. What goes on behind closed doors: Physiological versus pharmacological steroid hormone actions. Bioessays 2008, 30, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Matsumoto, T.; Sakari, M.; Okada, M.; Yokoyama, A.; Takahashi, S.; Kouzmenko, A.; Kato, S. The androgen receptor in health and disease. Annu. Rev. Physiol. 2013, 75, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, E.R.; Miles, M.C.; Fuxjager, M.J. Evolution of the androgen receptor: Perspectives from human health to dancing birds. Mol. Cell. Endocrinol. 2020, 499, 110577. [Google Scholar] [CrossRef]
- McPhaul, M.J.; Marcelli, M.; Zoppi, S.; Griffin, J.E.; Wilson, J.D. Genetic basis of endocrine disease. 4. The spectrum of mutations in the androgen receptor gene that causes androgen resistance. J. Clin. Endocrinol. Metab. 1993, 76, 17–23. [Google Scholar] [CrossRef]
- Gottlieb, B.; Beitel, L.K.; Nadarajah, A.; Paliouras, M.; Trifiro, M. The androgen receptor gene mutations database: 2012 update. Hum. Mutat. 2012, 33, 887–894. [Google Scholar] [CrossRef]
- Brinkmann, A.O.; Jenster, G.; Ris-Stalpers, C.; van der Korput, J.A.; Bruggenwirth, H.T.; Boehmer, A.L.; Trapman, J. Androgen receptor mutations. J. Steroid Biochem. Mol. Biol. 1995, 53, 443–448. [Google Scholar] [CrossRef][Green Version]
- Shukla, G.C.; Plaga, A.R.; Shankar, E.; Gupta, S. Androgen receptor-related diseases: What do we know? Andrology 2016, 4, 366–381. [Google Scholar] [CrossRef]
- Consortium, G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Nordenstedt, H.; Younes, M.; El-Serag, H.B. Expression of androgen receptors in Barrett esophagus. J. Clin. Gastroenterol. 2012, 46, 251–252. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Pundir, C.; Deswal, R.; Narwal, V.; Dang, A. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Human Reprod. Sci. 2020, 13, 261. [Google Scholar] [CrossRef]
- Dohle, G.R.; Smit, M.; Weber, R.F. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and androgen receptor signaling in prostate tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef]
- Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen Receptor in Breast Cancer-Clinical and Preclinical Research Insights. Molecules 2020, 25, 358. [Google Scholar] [CrossRef]
- Xiong, X.; Qiu, S.; Yi, X.; Xu, H.; Lei, H.; Liao, D.; Bai, S.; Peng, G.; Wei, Q.; Ai, J.; et al. Efficacy and safety of bipolar androgen therapy in mCRPC after progression on abiraterone or enzalutamide: A systematic review. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 4.e19–4.e28. [Google Scholar] [CrossRef]
- Hartgens, F.; Kuipers, H. Effects of Androgenic-Anabolic Steroids in Athletes. Sports Med. 2004, 34, 513–554. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khatib, J.; Chiu, C.-Y.; Lin, J.; Patel, S.T.; Liu-Smith, F. Tumor Androgen Receptor Protein Level Is Positively Associated with a Better Overall Survival in Melanoma Patients. Genes 2023, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Vellano, C.P.; White, M.G.; Andrews, M.C.; Chelvanambi, M.; Witt, R.G.; Daniele, J.R.; Titus, M.; McQuade, J.L.; Conforti, F.; Burton, E.M.; et al. Androgen receptor blockade promotes response to BRAF/MEK-targeted therapy. Nature 2022, 606, 797–803. [Google Scholar] [CrossRef]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef]
- Dotto, G.P.; Buckinx, A.; Ozdemir, B.C.; Simon, C. Androgen receptor signalling in non-prostatic malignancies: Challenges and opportunities. Nat. Rev. Cancer 2025, 25, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; Jindal, S.; Hui, M.; Finlay-Schultz, J.; Ebrahimie, E.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 2021, 27, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ligr, M.; Li, Y.; Zou, X.; Daniels, G.; Melamed, J.; Peng, Y.; Wang, W.; Wang, J.; Ostrer, H.; Pagano, M.; et al. Tumor suppressor function of androgen receptor coactivator ARA70alpha in prostate cancer. Am. J. Pathol. 2010, 176, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.W.; Hsu, I.; Xu, D.; Miyamoto, H.; Liang, L.; Wu, X.R.; Shyr, C.R.; Chang, C. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am. J. Pathol. 2013, 182, 1811–1820. [Google Scholar] [CrossRef]
- Matye, D.; Leak, J.; Woolbright, B.L.; Taylor, J.A., 3rd. Preclinical models of bladder cancer: BBN and beyond. Nat. Rev. Urol. 2024, 21, 723–734. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Sun, Q.; Liu, X.; Wu, Z.; Wang, X.; Fang, W.; Ma, Z. miR-224-5p-enriched exosomes promote tumorigenesis by directly targeting androgen receptor in non-small cell lung cancer. Mol. Ther. Nucleic Acids 2021, 23, 1217–1228. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. Are we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- Liu, X.; Qing, S.; Che, K.; Li, L.; Liao, X. Androgen receptor promotes oral squamous cell carcinoma cell migration by increasing EGFR phosphorylation. OncoTargets Ther. 2019, 12, 4245–4252. [Google Scholar] [CrossRef]
- Heemers, H.V.; Tindall, D.J. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.F.; Cai, L.; Zheng, D.; et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef]
- Ma, M.; Ghosh, S.; Tavernari, D.; Katarkar, A.; Clocchiatti, A.; Mazzeo, L.; Samarkina, A.; Epiney, J.; Yu, Y.R.; Ho, P.C.; et al. Sustained androgen receptor signaling is a determinant of melanoma cell growth potential and tumorigenesis. J. Exp. Med. 2021, 218, e20201137. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, Y.; Ma, X.; Gao, Y.; Li, X.; Niu, Y.; Zhang, X.; Chang, C. Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat. Commun. 2017, 8, 918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, X.; Li, Y.; Cao, D.; Luo, C.; Zhang, Q.; Zhang, S.; Jiao, Y. Age-related testosterone decline: Mechanisms and intervention strategies. Reprod. Biol. Endocrinol. 2024, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, V.; Jones, T.H. Testosterone and the metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Marshall, H.; Smith, I.L.; Greenfield, D.M.; Swain, J.; Best, E.; Ashton, J.; Brown, J.M.; Huddart, R.; Coleman, R.E.; et al. Testosterone replacement in young male cancer survivors: A 6-month double-blind randomised placebo-controlled trial. PLoS Med. 2019, 16, e1002960. [Google Scholar] [CrossRef]
- Burney, B.O.; Garcia, J.M. Hypogonadism in male cancer patients. J. Cachexia Sarcopenia Muscle 2012, 3, 149–155. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Hui, D.; Nooruddin, Z.I.; Dalal, S.; Dev, R.; Freer, G.; Roberts, L.; Palmer, J.L.; Bruera, E. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: A preliminary report. J. Pain Symptom Manag. 2010, 39, 1016–1024. [Google Scholar] [CrossRef]
- Li, C.; Cheng, D.; Li, P. Androgen receptor dynamics in prostate cancer: From disease progression to treatment resistance. Front. Oncol. 2025, 15, 1542811. [Google Scholar] [CrossRef]
- AR Blockers Augment Other Cancer Treatments. Cancer Discov. 2022, 12, OF2. [CrossRef]
- Di Donato, M.; Cristiani, C.M.; Capone, M.; Garofalo, C.; Madonna, G.; Passacatini, L.C.; Ottaviano, M.; Ascierto, P.A.; Auricchio, F.; Carbone, E.; et al. Role of the androgen receptor in melanoma aggressiveness. Cell Death Dis. 2025, 16, 34. [Google Scholar] [CrossRef]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, G.H.; Thrift, P.A.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e642. [Google Scholar] [CrossRef] [PubMed]
- Stabellini, N.; Chandar, A.K.; Chak, A.; Barda, A.J.; Dmukauskas, M.; Waite, K.; Barnholtz-Sloan, J.S. Sex differences in esophageal cancer overall and by histological subtype. Sci. Rep. 2022, 12, 5248. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Krasna, M. Overview of esophageal cancer. Ann. Cardiothorac. Surg. 2017, 6, 131–136. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Li, B.; Due, S.L.; Hussey, D.J.; Watson, D.I. Androgens and esophageal cancer: What do we know? World J. Gastroenterol. 2015, 21, 6146–6156. [Google Scholar] [CrossRef]
- Rutegard, M.; Lagergren, P.; Nordenstedt, H.; Lagergren, J. Oesophageal adenocarcinoma: The new epidemic in men? Maturitas 2011, 69, 244–248. [Google Scholar] [CrossRef]
- Petrick, J.L.; Hyland, P.L.; Caron, P.; Falk, R.T.; Pfeiffer, R.M.; Dawsey, S.M.; Abnet, C.C.; Taylor, P.R.; Weinstein, S.J.; Albanes, D.; et al. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Esophageal/Gastric Cardia Adenocarcinoma Among Men. J. Natl. Cancer Inst. 2019, 111, 34–41. [Google Scholar] [CrossRef]
- Xie, S.H.; Ness-Jensen, E.; Rabbani, S.; Langseth, H.; Gislefoss, R.E.; Mattsson, F.; Lagergren, J. Circulating Sex Hormone Levels and Risk of Esophageal Adenocarcinoma in a Prospective Study in Men. Am. J. Gastroenterol. 2020, 115, 216–223. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Lagergren, J.; Li, S.; Li, J.; Zhou, Z.; Hu, Z.; Xie, S.H. Circulating Sex Hormone Levels and Risk of Gastrointestinal Cancer: Systematic Review and Meta-Analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2023, 32, 936–946. [Google Scholar] [CrossRef]
- Dong, H.; Xu, J.; Li, W.; Gan, J.; Lin, W.; Ke, J.; Jiang, J.; Du, L.; Chen, Y.; Zhong, X.; et al. Reciprocal androgen receptor/interleukin-6 crosstalk drives oesophageal carcinoma progression and contributes to patient prognosis. J. Pathol. 2017, 241, 448–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Zhong, X.; Cheng, C. Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2. Tumour Biol. 2015, 36, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, H.; Zhu, X.; Gong, T.; Li, X.; Hankey, W.; Wang, H.; Chen, Z.; Wang, Q.; Liu, Z. The oncogenomic function of androgen receptor in esophageal squamous cell carcinoma is directed by GATA3. Cell Res. 2021, 31, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, F.; Gao, W.; Gong, T.; Chen, H.; Liu, Z. Androgen Receptor/AP-1 Activates UGT2B15 Transcription to Promote Esophageal Squamous Cell Carcinoma Invasion. Cancers 2023, 15, 5719. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.-P.; Quan, C.; Zu, X.; Ou, Z.; Tsai, Y.-C.; Messing, E.; Yeh, S.; Chang, C. The androgen receptor in bladder cancer. Nat. Rev. Urol. 2023, 20, 560–574. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Miyamoto, H. Androgen Receptor Signaling in Bladder Cancer. Cancers 2017, 9, 20. [Google Scholar] [CrossRef]
- Bertram, J.S.; Craig, A.W. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur. J. Cancer (1965) 1972, 8, 587–594. [Google Scholar] [CrossRef]
- Overdevest, J.B.; Knubel, K.H.; Duex, J.E.; Thomas, S.; Nitz, M.D.; Harding, M.A.; Smith, S.C.; Frierson, H.F.; Conaway, M.; Theodorescu, D. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc. Natl. Acad. Sci. USA 2012, 109, E3588–E3596. [Google Scholar] [CrossRef]
- Makela, V.J.; Kotsar, A.; Tammela, T.L.J.; Murtola, T.J. Bladder Cancer Survival of Men Receiving 5alpha-Reductase Inhibitors. J. Urol. 2018, 200, 743–748. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yang, Z.; Chen, Y.T.; Ishiguro, H.; Uemura, H.; Kubota, Y.; Nagashima, Y.; Chang, Y.J.; Hu, Y.C.; Tsai, M.Y.; et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007, 99, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; O’Connell, M.J.; Miyamoto, H.; Huang, J.; Yao, J.L.; Messing, E.M.; Reeder, J.E. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: A role for thrombospondin-1. BMC Urol. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Takeuchi, A.; Yokomizo, A.; Kashiwagi, E.; Tatsugami, K.; Kuroiwa, K.; Naito, S. Androgen receptor signaling regulates cell growth and vulnerability to doxorubicin in bladder cancer. J. Urol. 2012, 188, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, K.; Mathlin, J.; Lamminen, T.; Laiho, A.; Hakkinen, M.R.; Auriola, S.; Elo, L.L.; Bostrom, P.J.; Poutanen, M.; Taimen, P. Profiling steroid hormone landscape of bladder cancer reveals depletion of intratumoural androgens to castration levels: A cross-sectional study. EBioMedicine 2024, 108, 105359. [Google Scholar] [CrossRef]
- McBeth, L.; Grabnar, M.; Selman, S.; Hinds, T.D., Jr. Involvement of the Androgen and Glucocorticoid Receptors in Bladder Cancer. Int. J. Endocrinol. 2015, 2015, 384860. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Cormio, L.; Carrieri, G.; Calo, B.; Russo, D.; Menin, A.; Pastore, A.L.; Greco, F.; Bozzini, G.; Galfano, A.; et al. Role of androgen receptor expression in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. Histol. Histopathol. 2020, 35, 423–432. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Li, P.; Liu, L.; Li, C.; Zu, X. Expression and clinical significance of androgen receptor in bladder cancer: A meta-analysis. Mol. Clin. Oncol. 2017, 7, 919–927. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Izumi, K.; Ishiguro, H.; Ye, B.; Li, F.; Miyamoto, H. Androgen activates β-catenin signaling in bladder cancer cells. Endocr. -Relat. Cancer 2013, 20, 293–304. [Google Scholar] [CrossRef]
- Lin, C.; Yin, Y.; Stemler, K.; Humphrey, P.; Kibel, S.A.; Mysorekar, U.I.; Ma, L. Constitutive β-Catenin Activation Induces Male-Specific Tumorigenesis in the Bladder Urothelium. Cancer Res. 2013, 73, 5914–5925. [Google Scholar] [CrossRef]
- Izumi, K.; Zheng, Y.; Hsu, J.W.; Chang, C.; Miyamoto, H. Androgen receptor signals regulate UDP-glucuronosyltransferases in the urinary bladder: A potential mechanism of androgen-induced bladder carcinogenesis. Mol. Carcinog. 2013, 52, 94–102. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Ou, Z.; Yeh, S.; Huang, C.P.; You, B.; Tsai, Y.C.; Sheu, T.J.; Zu, X.; Chang, C. Androgen receptor-regulated circFNTA activates KRAS signaling to promote bladder cancer invasion. EMBO Rep. 2020, 21, e48467. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Monteiro, F.L.; Direito, I.; Amado, F.; Afreixo, V.; Santos, L.L.; Helguero, L.A. Association Between Estrogen Receptors and GATA3 in Bladder Cancer: A Systematic Review and Meta-Analysis of Their Clinicopathological Significance. Front. Endocrinol. 2021, 12, 684140. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Jubber, I.; Lloyd, K.; Cumberbatch, M.; Griffin, J. Gene of the month: GATA3. J. Clin. Pathol. 2023, 76, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ishiguro, H.; Kawahara, T.; Miyamoto, Y.; Izumi, K.; Miyamoto, H. GATA3 in the urinary bladder: Suppression of neoplastic transformation and down-regulation by androgens. Am. J. Cancer Res. 2014, 4, 461–473. [Google Scholar]
- Qin, C.; Lu, Y.; Zhang, H.; Zhang, Z.; Xu, W.; Wen, S.; Gao, W.; Wu, Y. Biological roles and clinical significance of estrogen and androgen receptors in head and neck cancers. J. Cancer 2022, 13, 2189–2199. [Google Scholar] [CrossRef]
- Dalin, M.G.; Watson, P.A.; Ho, A.L.; Morris, L.G. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 2017, 9, 17. [Google Scholar] [CrossRef]
- Tomasovic-Loncaric, C.; Fucic, A.; Andabak, A.; Andabak, M.; Ceppi, M.; Bruzzone, M.; Vrdoljak, D.; Vucicevic-Boras, V. Androgen Receptor as a Biomarker of Oral Squamous Cell Carcinoma Progression Risk. Anticancer Res. 2019, 39, 4285–4289. [Google Scholar] [CrossRef]
- Batelja-Vuletic, L.; Tomasovic-Loncaric, C.; Ceppi, M.; Bruzzone, M.; Fucic, A.; Krstanac, K.; Boras Vucicevic, V. Comparison of Androgen Receptor, VEGF, HIF-1, Ki67 and MMP9 Expression between Non-Metastatic and Metastatic Stages in Stromal and Tumor Cells of Oral Squamous Cell Carcinoma. Life 2021, 11, 336. [Google Scholar] [CrossRef]
- Wang, X.; Yan, G.; Li, H.; Wang, C.; Kang, Y.; Wang, S.; Liu, W.; Lin, L.; Zou, R.; Zeng, K.; et al. RBAP48 facilitates the oral squamous cell carcinoma process in an androgen receptor-dependent and independent manners. Commun. Biol. 2025, 8, 829. [Google Scholar] [CrossRef]
- Sygut, D.; Bien, S.; Ziolkowska, M.; Sporny, S. Immunohistochemical expression of androgen receptor in salivary gland cancers. Pol. J. Pathol. Off. J. Pol. Soc. Pathol. 2008, 59, 205–210. [Google Scholar]
- Mehanna, H.; Paleri, V.; West, C.M.; Nutting, C. Head and neck cancer—Part 1: Epidemiology, presentation, and prevention. BMJ 2010, 341, c4684. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, S.J.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Keir, A.J.; Whiteside, J.O.; Winter, C.S.; Maitra, S.; Corbridge, C.R.; Cox, J.G. Outcomes in Squamous Cell Carcinoma with Advanced Neck Disease. Ann. R. Coll. Surg. Engl. 2007, 89, 703–708. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O. The androgen receptor as an emerging target in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 91–99. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Nagasue, N.; Yu, L.; Yukaya, H.; Kohno, H.; Nakamura, T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: Impact on intrahepatic recurrence after hepatic resection. Br. J. Surg. 1995, 82, 542–547. [Google Scholar] [CrossRef]
- Ma, W.L.; Hsu, C.L.; Wu, M.H.; Wu, C.T.; Wu, C.C.; Lai, J.J.; Jou, Y.S.; Chen, C.W.; Yeh, S.; Chang, C. Androgen Receptor Is a New Potential Therapeutic Target for the Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 135, 947–955.e945. [Google Scholar] [CrossRef]
- Ma, W.-L.; Lai, H.-C.; Yeh, S.; Cai, X.; Chang, C. Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocr. -Relat. Cancer 2014, 21, R165–R182. [Google Scholar] [CrossRef]
- Watts, E.L.; Perez-Cornago, A.; Knuppel, A.; Tsilidis, K.K.; Key, T.J.; Travis, R.C. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. Int. J. Cancer 2021, 149, 573–584. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, L.; Zhu, G.; Liang, L.; Guan, Z.; Chang, L.; Chen, Y.; Yeh, S.; Chang, C. ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2alpha/VEGF signaling pathway. Cancer Res. 2014, 74, 4420–4430. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Roman, R.J. Androgens and hypertension: Role in both males and females? Hypertension 2011, 57, 681–682. [Google Scholar] [CrossRef]
- Moch, H.; Gasser, T.; Amin, M.B.; Torhorst, J.; Sauter, G.; Mihatsch, M.J. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: A swiss experience with 588 tumors. Cancer 2000, 89, 604–614. [Google Scholar] [CrossRef]
- You, B.; Sun, Y.; Luo, J.; Wang, K.; Liu, Q.; Fang, R.; Liu, B.; Chou, F.; Wang, R.; Meng, J.; et al. Androgen receptor promotes renal cell carcinoma (RCC) vasculogenic mimicry (VM) via altering TWIST1 nonsense-mediated decay through lncRNA-TANAR. Oncogene 2021, 40, 1674–1689. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Tao, W.; Fei, X.; Chang, C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017, 394, 1–12. [Google Scholar] [CrossRef]
- Langner, C.; Ratschek, M.; Rehak, P.; Schips, L.; Zigeuner, R. Steroid hormone receptor expression in renal cell carcinoma: An immunohistochemical analysis of 182 tumors. J. Urol. 2004, 171, 611–614. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, L.; Li, L.; Dang, Q.; Song, W.; Yeh, S.; He, D.; Chang, C. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology 2014, 83, 510.e19–510.e24. [Google Scholar] [CrossRef]
- Yuan, P.; Ge, Y.; Liu, X.; Wang, S.; Ye, Z.; Xu, H.; Chen, Z. The Association of Androgen Receptor Expression with Renal Cell Carcinoma Risk: A Systematic Review and Meta-Analysis. Pathol. Oncol. Res. 2020, 26, 605–614. [Google Scholar] [CrossRef]
- Wang, R.; Xu, X.Y.; Zhu, H.; Liang, X.; Li, X.; Jia, M.X.; Wang, Q.H.; Wang, H.Y.; Li, X.X.; Zhao, G.J. Androgen Receptor Promotes Gastric Carcinogenesis via Upregulating Cell Cycle-Related Kinase Expression. J. Cancer 2019, 10, 4178–4188. [Google Scholar] [CrossRef]
- Soleymani Fard, S.; Yazdanbod, M.; Sotoudeh, M.; Bashash, D.; Mahmoodzadeh, H.; Saliminejad, K.; Mousavi, S.A.; Ghaffari, S.H.; Alimoghaddam, K. Prognostic and Therapeutic Significance of Androgen Receptor in Patients with Gastric Cancer. OncoTargets Ther. 2020, 13, 9821–9837. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, M.; Hua, M.; Wang, Z.; Ying, Y.; Zhang, Z.; Zeng, S.; Wang, H.; Xu, C. Association between testosterone and cancers risk in women: A two-sample Mendelian randomization study. Discov. Oncol. 2023, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wan, H.; Lin, Y.; Xie, X.; Li, Z.; Tan, G. Androgen receptor may be responsible for gender disparity in gastric cancer. Med. Hypotheses 2013, 80, 672–674. [Google Scholar] [CrossRef]
- Xia, N.; Cui, J.; Zhu, M.; Xing, R.; Lu, Y. Androgen receptor variant 12 promotes migration and invasion by regulating MYLK in gastric cancer. J. Pathol. 2019, 248, 304–315. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, S.J.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Liu, F.; Bessonova, L.; Taylor, H.T.; Ziogas, A.; Meyskens, L.F.; Anton-Culver, H. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigment Cell Melanoma Res. 2013, 26, 128–135. [Google Scholar] [CrossRef]
- Nosrati, A.; Wei, M.L. Sex disparities in melanoma outcomes: The role of biology. Arch. Biochem. Biophys. 2014, 563, 42–50. [Google Scholar] [CrossRef]
- Rampen, F.H.; Mulder, J.H. Malignant melanoma: An androgen-dependent tumour? Lancet 1980, 1, 562–564. [Google Scholar] [CrossRef]
- Parikh, R.; Sorek, E.; Parikh, S.; Michael, K.; Bikovski, L.; Tshori, S.; Shefer, G.; Mingelgreen, S.; Zornitzki, T.; Knobler, H.; et al. Skin exposure to UVB light induces a skin-brain-gonad axis and sexual behavior. Cell Rep. 2021, 36, 109579. [Google Scholar] [CrossRef]
- Zornitzki, T.; Tshori, S.; Shefer, G.; Mingelgrin, S.; Levy, C.; Knobler, H. Seasonal Variation of Testosterone Levels in a Large Cohort of Men. Int. J. Endocrinol. 2022, 2022, 6093092. [Google Scholar] [CrossRef]
- Grant, L.; Banerji, S.; Murphy, L.; Dawe, D.E.; Harlos, C.; Myal, Y.; Nugent, Z.; Blanchard, A.; Penner, C.R.; Qing, G.; et al. Androgen Receptor and Ki67 Expression and Survival Outcomes in Non-small Cell Lung Cancer. Horm. Cancer 2018, 9, 288–294. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.W.; Yu, W.W. Androgen Receptor Promotes Lung Cancer Metastasis by Modifying the miR23a-3p/EPHB2 Pathway. Curr. Med. Sci. 2024, 44, 954–963. [Google Scholar] [CrossRef]
- Ragavan, M.V.; Patel, M.I. Understanding sex disparities in lung cancer incidence: Are women more at risk? Lung Cancer Manag. 2020, 9, LMT34. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Mo, Q.; Shen, H.; Wang, S.; Liu, B. Reproductive and hormonal factors and bladder cancer risk: A prospective study and meta-analysis. Aging 2020, 12, 14691–14698. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dao, C.A.; Henderson, K.D.; Sullivan-Halley, J.; Ma, H.; West, D.; Xiang, Y.B.; Gago-Dominguez, M.; Stern, M.C.; Castelao, J.E.; Conti, D.V.; et al. Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1156–1170. [Google Scholar] [CrossRef]
- Scosyrev, E.; Noyes, K.; Feng, C.; Messing, E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009, 115, 68–74. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, H. Sexual dimorphism in bladder cancer: A review of etiology, biology, diagnosis, and outcomes. Front. Pharmacol. 2023, 14, 1326627. [Google Scholar] [CrossRef]
- May, M.; Bastian, P.J.; Brookman-May, S.; Fritsche, H.M.; Tilki, D.; Otto, W.; Bolenz, C.; Gilfrich, C.; Trojan, L.; Herrmann, E.; et al. Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urol. Oncol. 2013, 31, 1141–1147. [Google Scholar] [CrossRef]
- Tilki, D.; Svatek, R.S.; Karakiewicz, P.I.; Isbarn, H.; Reich, O.; Kassouf, W.; Fradet, Y.; Novara, G.; Fritsche, H.M.; Bastian, P.J.; et al. Characteristics and outcomes of patients with pT4 urothelial carcinoma at radical cystectomy: A retrospective international study of 583 patients. J. Urol. 2010, 183, 87–93. [Google Scholar] [CrossRef]
- Toren, P.; Wilkins, A.; Patel, K.; Burley, A.; Gris, T.; Kockelbergh, R.; Lodhi, T.; Choudhury, A.; Bryan, R.T. The sex gap in bladder cancer—A missing link in bladder cancer care? Nat. Rev. Urol. 2024, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.C. Mind the gap: What is driving the survival disparity between the sexes in bladder cancer? Cancer 2016, 122, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Krabbe, L.M.; Svatek, R.S.; Shariat, S.F.; Messing, E.; Lotan, Y. Bladder cancer risk: Use of the PLCO and NLST to identify a suitable screening cohort. Urol. Oncol. 2015, 33, 65.e19–65.e25. [Google Scholar] [CrossRef]
- Tan, W.; Gao, L.; Yuan, Y.; Huang, H.; Li, Y.; Gou, Y.; Hu, Z. Relationship between testosterone and male bladder cancer. Sci. Rep. 2023, 13, 12881. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, Y. Causal Associations Between the Presence of Prostate Cancer or Testosterone Levels and Bladder Cancer Risk: A Mendelian Randomization Study. Clin. Genitourin. Cancer 2025, 23, 102334. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Feng, J.F. A novel inflammation-based prognostic score for patients with esophageal squamous cell carcinoma: The c-reactive protein/prognostic nutritional index ratio. Oncotarget 2016, 7, 62123–62132. [Google Scholar] [CrossRef]
- Zahra, M.; Saady, M.; Azzazy, H.M.E. Molecular mechanisms of sex hormones in Hepatocellular Carcinoma: A systematic review. Mol. Biol. Rep. 2025, 52, 975. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, G.H.; Jung, J.; Noh, T.I. Secondary Cancer after Androgen Deprivation Therapy in Prostate Cancer: A Nationwide Study. World J. Mens. Health 2025, 43, 123–133. [Google Scholar] [CrossRef]
- Harlos, C.; Musto, G.; Lambert, P.; Ahmed, R.; Pitz, M.W. Androgen pathway manipulation and survival in patients with lung cancer. Horm. Cancer 2015, 6, 120–127. [Google Scholar] [CrossRef]
- Berardi, R.; Morgese, F.; Santinelli, A.; Onofri, A.; Biscotti, T.; Brunelli, A.; Caramanti, M.; Savini, A.; De Lisa, M.; Ballatore, Z.; et al. Hormonal receptors in lung adenocarcinoma: Expression and difference in outcome by sex. Oncotarget 2016, 7, 82648–82657. [Google Scholar] [CrossRef]
- Chang, J.; Wu, Y.; Zhou, S.; Tian, Y.; Wang, Y.; Tian, J.; Song, W.; Dong, Y.; Li, J.; Zhao, Z.; et al. Genetically predicted testosterone and cancers risk in men: A two-sample Mendelian randomization study. J. Transl. Med. 2022, 20, 573. [Google Scholar] [CrossRef]
- Hashim, D.; Sartori, S.; La Vecchia, C.; Serraino, D.; Maso, L.D.; Negri, E.; Smith, E.; Levi, F.; Boccia, S.; Cadoni, G.; et al. Hormone factors play a favorable role in female head and neck cancer risk. Cancer Med. 2017, 6, 1998–2007. [Google Scholar] [CrossRef]
- Mohamed, H.; Aro, K.; Jouhi, L.; Makitie, A.; Remes, S.; Haglund, C.; Atula, T.; Hagstrom, J. Expression of hormone receptors in oropharyngeal squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. 2018, 275, 1289–1300. [Google Scholar] [CrossRef]
- CP, D.E.O.N.; Brito, H.O.; RMG, D.A.C.; Brito, L.M.O. Is There a Role for Sex Hormone Receptors in Head-and-neck Cancer? Links with HPV Infection and Prognosis. Anticancer Res. 2021, 41, 3707–3716. [Google Scholar] [CrossRef]
- Conkas, J.; Sabol, M.; Ozretic, P. ‘Toxic Masculinity’: What Is Known about the Role of Androgen Receptors in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 3766. [Google Scholar] [CrossRef]
- Shiota, M.; Ushijima, M.; Imada, K.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Eto, M. Cigarette smoking augments androgen receptor activity and promotes resistance to antiandrogen therapy. Prostate 2019, 79, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.G.; Ngugi, A.K.; Twine, R.; Bottomley, C.; Kamuyu, G.; Gomez-Olive, F.X.; Connor, M.D.; Collinson, M.A.; Kahn, K.; Tollman, S.; et al. Prevalence and risk factors for active convulsive epilepsy in rural northeast South Africa. Epilepsy Res. 2014, 108, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Manca, S.; Frisbie, C.P.; LaGrange, C.A.; Casey, C.A.; Riethoven, J.M.; Petrosyan, A. The Role of Alcohol-Induced Golgi Fragmentation for Androgen Receptor Signaling in Prostate Cancer. Mol. Cancer Res. 2019, 17, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Bando, M.; Takashi, T.; Sugiuchi, M.; Hyodo, M.; Mishima, Y.; Kuroda, M.; Mori, H.; Kuroda, A.; Yumoto, H.; et al. Umami taste sensitivity is associated with food intake and oral environment in subjects with diabetes. J. Med. Investig. JMI 2023, 70, 241–250. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, J.; Zhou, J.; Xu, Y.; Wang, J. Sex-related hormone receptor in laryngeal squamous cell carcinoma: Correlation with androgen estrogen-a and prolactin receptor expression and influence of prognosis. Acta Otolaryngol. 2018, 138, 66–72. [Google Scholar] [CrossRef]
- Cruz, A.; Magalhães, H.; Pereira, F.F.; Dinis, J.; Vieira, C. A 10-year review of primary major salivary gland cancers. Ecancermedicalscience 2020, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.M.; Sharma, A.; Schmitt, C.N.; Johnson, T.J.; Ferris, L.R.; Duvvuri, U.; Kim, S. A 20-Year Review of 75 Cases of Salivary Duct Carcinoma. JAMA Otolaryngol. -Head Neck Surg. 2016, 142, 489. [Google Scholar] [CrossRef] [PubMed]
- Steuer, E.C.; Hanna, J.G.; Viswanathan, K.; Bates, E.J.; Kaka, S.A.; Schmitt, C.N.; Ho, L.A.; Saba, F.N. The evolving landscape of salivary gland tumors. CA A Cancer J. Clin. 2023, 73, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Dalin, G.M.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, A.L.; Lee, K.-W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef]
- Dong, S.; Li, M.; Zhang, Z.; Feng, B.; Ding, W.; Chang, J.; Liu, F. Clinical diagnosis, treatment, and survival analysis of 61 cases of salivary duct carcinoma: A retrospective study. PeerJ 2025, 13, e19626. [Google Scholar] [CrossRef]
- Locati, L.D.; Quattrone, P.; Bossi, P.; Marchiano, A.V.; Cantu, G.; Licitra, L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14, 1327–1328. [Google Scholar] [CrossRef]
- Jaspers, H.C.; Verbist, B.M.; Schoffelen, R.; Mattijssen, V.; Slootweg, P.J.; van der Graaf, W.T.; van Herpen, C.M. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, e473–e476. [Google Scholar] [CrossRef]
- Ho, A.L.; Foster, N.R.; Zoroufy, A.J.; Campbell, J.D.; Worden, F.; Price, K.; Adkins, D.; Bowles, D.W.; Kang, H.; Burtness, B.; et al. Phase II Study of Enzalutamide for Patients With Androgen Receptor-Positive Salivary Gland Cancers (Alliance A091404). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 4240–4249. [Google Scholar] [CrossRef]
- Locati, L.D.; Perrone, F.; Cortelazzi, B.; Lo Vullo, S.; Bossi, P.; Dagrada, G.; Quattrone, P.; Bergamini, C.; Potepan, P.; Civelli, E.; et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck 2016, 38, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.M.; Hickey, E.T.; Knudsen, E.K. FOXA1: Master of steroid receptor function in cancer. EMBO J. 2011, 30, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, J.C.; Vignat, J.; Laversanne, M.; Mcglynn, A.K.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Ajayi, F.; Jan, J.; Singal, A.G.; Rich, N.E. Racial and Sex Disparities in Hepatocellular Carcinoma in the USA. Curr. Hepatol. Rep. 2020, 19, 462–469. [Google Scholar] [CrossRef]
- Orsted, D.D.; Nordestgaard, B.G.; Bojesen, S.E. Plasma testosterone in the general population, cancer prognosis and cancer risk: A prospective cohort study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 712–718. [Google Scholar] [CrossRef]
- Nakatani, T.; Roy, G.; Fujimoto, N.; Asahara, T.; Ito, A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn. J. Cancer Res. Gann 2001, 92, 249–256. [Google Scholar] [CrossRef]
- Montgomery, E.J.; Xing, E.; Campbell, M.J.; Li, P.K.; Blachly, J.S.; Tsung, A.; Coss, C.C. Constitutively Active Androgen Receptor in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Nagasue, N.; Ito, A.; Yukaya, H.; Ogawa, Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology 1985, 89, 643–647. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Z.; Zhang, J.; Xu, Y.; Zhang, M.; Dai, Z.; Zhu, J.; Zheng, S. Identification of Androgen Receptor as a Molecular Docking Target for Survival and Response to Metformin-Induced Ferroptosis in Liver Cancer. Cancer Rep. 2025, 8, e70245. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Wu, Q.; Zhang, X.; Lin, S.; Ran, W.; Zhu, L.; Tang, C.; Wang, X. Sex steroid axes in determining male predominance in hepatocellular carcinoma. Cancer Lett. 2023, 555, 216037. [Google Scholar] [CrossRef]
- Song, H.; Yu, Z.; Sun, X.; Feng, J.; Yu, Q.; Khan, H.; Zhu, X.; Huang, L.; Li, M.; Mok, M.T.S.; et al. Androgen receptor drives hepatocellular carcinogenesis by activating enhancer of zeste homolog 2-mediated Wnt/beta-catenin signaling. EBioMedicine 2018, 35, 155–166. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Wang, H.; Li, S.; Miao, Z.; Yang, J.; Zhang, Y.; Lei, K.; Wu, Y.; Kang, Y.; et al. Androgen receptor promotes arachidonic acid metabolism and angiogenic microenvironment in AFP-negative hepatocellular carcinoma. Nat. Commun. 2025, 16, 6451. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, L.; Pu, R.; Liu, W.; Cai, S.; Wang, R.; Shi, Y.; Li, Z.; Zhang, Z.; Li, Z.; et al. Androgen receptor-induced molecules and androgen contribute synergistically to male-predominance of hepatocellular carcinoma. iScience 2024, 27, 110519. [Google Scholar] [CrossRef]
- Ruggieri, A.; Gagliardi, M.C.; Anticoli, S. Sex-Dependent Outcome of Hepatitis B and C Viruses Infections: Synergy of Sex Hormones and Immune Responses? Front. Immunol. 2018, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Cheng, S.W.; Lin, M.W.; Yang, S.Y.; Liaw, Y.F.; Chang, H.C.; Hsiao, T.J.; Lin, S.M.; Lee, S.D.; Chen, P.J.; et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J. Natl. Cancer Inst. 2000, 92, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- King, S.C.; Pollack, L.A.; Li, J.; King, J.B.; Master, V.A. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J. Urol. 2014, 191, 1665–1670. [Google Scholar] [CrossRef]
- Zhao, H.; Leppert, J.T.; Peehl, D.M. A Protective Role for Androgen Receptor in Clear Cell Renal Cell Carcinoma Based on Mining TCGA Data. PLoS ONE 2016, 11, e0146505. [Google Scholar] [CrossRef]
- Li, P.; Znaor, A.; Holcatova, I.; Fabianova, E.; Mates, D.; Wozniak, M.B.; Ferlay, J.; Scelo, G. Regional geographic variations in kidney cancer incidence rates in European countries. Eur. Urol. 2015, 67, 1134–1141. [Google Scholar] [CrossRef]
- Scelo, G.; Li, P.; Chanudet, E.; Muller, D.C. Variability of Sex Disparities in Cancer Incidence over 30 Years: The Striking Case of Kidney Cancer. Eur. Urol. Focus. 2018, 4, 586–590. [Google Scholar] [CrossRef]
- Guan, Z.; Li, C.; Fan, J.; He, D.; Li, L. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT --> NF-kappaB --> CXCL5 signaling. Sci. Rep. 2016, 6, 37085. [Google Scholar] [CrossRef]
- Peila, R.; Arthur, R.S.; Rohan, T.E. Association of Sex Hormones with Risk of Cancers of the Pancreas, Kidney, and Brain in the UK Biobank Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Leibovich, B.C.; Lohse, C.M.; Crispen, P.L.; Boorjian, S.A.; Thompson, R.H.; Blute, M.L.; Cheville, J.C. Histological Subtype is an Independent Predictor of Outcome for Patients With Renal Cell Carcinoma. J. Urol. 2010, 183, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Morgans, A.K.; Edwards, T.L.; Barocas, D.A.; Chang, S.S.; Duke Herrell, S.; Penson, D.F.; Resnick, M.J.; Smith, J.A.; Clark, P.E. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. 2016, 117, 260–265. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Wei, S.; Li, X.; Dang, Y.; Zhang, W.; Zhang, G. LAMA4 activated by Androgen receptor induces the cisplatin resistance in gastric cancer. Biomed. Pharmacother. 2020, 124, 109667. [Google Scholar] [CrossRef]
- Kominea, A.; Konstantinopoulos, P.A.; Kapranos, N.; Vandoros, G.; Gkermpesi, M.; Andricopoulos, P.; Artelaris, S.; Savva, S.; Varakis, I.; Sotiropoulou-Bonikou, G.; et al. Androgen receptor (AR) expression is an independent unfavorable prognostic factor in gastric cancer. J. Cancer Res. Clin. Oncol. 2004, 130, 253–258. [Google Scholar] [CrossRef]
- Hou, J.; Pan, T.; Li, F.; Sang, Q.; Wu, X.; Li, J.; Yu, B.; Zang, M.; Zhu, Z.G.; Su, L.; et al. Androgen receptor promotes cell stemness via interacting with co-factor YAP1 in gastric cancer. Biochem. Pharmacol. 2023, 217, 115849. [Google Scholar] [CrossRef]
- Durovski, D.; Jankovic, M.; Prekovic, S. Insights into Androgen Receptor Action in Lung Cancer. Endocrines 2023, 4, 22. [Google Scholar] [CrossRef]
- May, L.; Shows, K.; Nana-Sinkam, P.; Li, H.; Landry, J.W. Sex Differences in Lung Cancer. Cancers 2023, 15, 3111. [Google Scholar] [CrossRef]
- Denos, M.; Sun, Y.Q.; Brumpton, B.M.; Li, Y.; Albanes, D.; Burnett-Hartman, A.; Campbell, P.T.; Kury, S.; Li, C.I.; White, E.; et al. Sex hormones and risk of lung and colorectal cancers in women: A Mendelian randomization study. Sci. Rep. 2024, 14, 23891. [Google Scholar] [CrossRef]
- Feng, Z.; Fu, J.; Wang, K.; Yang, J.; Jiang, X.; Wu, Q. Causal relationship between hormone levels and lung cancer: A Mendelian randomization study. Front. Endocrinol. 2025, 16, 1462531. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.X.; Yeap, B.B. Dihydrotestosterone and cancer risk. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.P.; Fung, L.F.; Wang, E.; Chow, W.S.; Chiu, S.W.; Lam, W.K.; Ho, K.K.; Ma, E.S.; Wan, T.S.; Chung, L.P. Chromosomal aberrations of primary lung adenocarcinomas in nonsmokers. Cancer 2003, 97, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.; Hofmann, J.; Schilli, M.; Wegmann, B.; Klotz, U.; Wedel, S.; Virmani, A.K.; Wollmer, E.; Branscheid, D.; Gazdar, A.F.; et al. Steroid-hormone receptors in cell lines and tumor biopsies of human lung cancer. Int. J. Cancer 1996, 67, 357–364. [Google Scholar] [CrossRef]
- Recchia, A.G.; Musti, A.M.; Lanzino, M.; Panno, M.L.; Turano, E.; Zumpano, R.; Belfiore, A.; Ando, S.; Maggiolini, M. A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclinD1 expression in prostate and lung cancer cells. Int. J. Biochem. Cell Biol. 2009, 41, 603–614. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Liang, P.; Sun, M.; Li, T.; Shen, Z.; Sha, S. Luteolin inhibits A549 cells proliferation and migration by down-regulating androgen receptors. Eur. J. Med. Res. 2023, 28, 353. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Li, M.; Zhou, W.; Wang, R.; Xiao, Y. Androgen receptor suppresses lung cancer invasion and increases cisplatin response via decreasing TPD52 expression. Int. J. Biol. Sci. 2023, 19, 3709–3725. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Granata, A.R.M.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Seasonal Changes of Serum Gonadotropins and Testosterone in Men Revealed by a Large Data Set of Real-World Observations Over Nine Years. Front. Endocrinol. 2019, 10, 914. [Google Scholar] [CrossRef]
- Ong, J.S.; Seviiri, M.; Dusingize, J.C.; Wu, Y.; Han, X.; Shi, J.; Olsen, C.M.; Neale, R.E.; Thompson, J.F.; Saw, R.P.M.; et al. Uncovering the complex relationship between balding, testosterone and skin cancers in men. Nat. Commun. 2023, 14, 5962. [Google Scholar] [CrossRef]
- Mitchell, D.L.; Fernandez, A.A.; Garcia, R.; Paniker, L.; Lin, K.; Hanninen, A.; Zigelsky, K.; May, M.; Nuttall, M.; Lo, H.H.; et al. Acute exposure to ultraviolet-B radiation modulates sex steroid hormones and receptor expression in the skin and may contribute to the sex bias of melanoma in a fish model. Pigment Cell Melanoma Res. 2014, 27, 408–417. [Google Scholar] [CrossRef]
- Luo, P.; Guo, R.; Gao, D.; Zhang, Q. Causal relationship between sex hormones and cutaneous melanoma: A two-sample Mendelian randomized study. Melanoma Res. 2024, 34, 408–418. [Google Scholar] [CrossRef]
- Tadokoro, T.; Itami, S.; Hosokawa, K.; Terashi, H.; Takayasu, S. Human genital melanocytes as androgen target cells. J. Investig. Dermatol. 1997, 109, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Morvillo, V.; Luthy, I.A.; Bravo, A.I.; Capurro, M.I.; Portela, P.; Calandra, R.S.; Mordoh, J. Androgen receptors in human melanoma cell lines IIB-MEL-LES and IIB-MEL-IAN and in human melanoma metastases. Melanoma Res. 2002, 12, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Morvillo, V.; Luthy, I.A.; Bravo, A.I.; Capurro, M.I.; Donaldson, M.; Quintans, C.; Calandra, R.S.; Mordoh, J. Atypical androgen receptor in the human melanoma cell line IIB-MEL-J. Pigment Cell Res. 1995, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Adhikari, E.; Lester, D.K.; Fang, B.; Johnson, J.O.; Tian, Y.; Mockabee-Macias, A.T.; Izumi, V.; Guzman, K.M.; White, M.G.; et al. Androgen drives melanoma invasiveness and metastatic spread by inducing tumorigenic fucosylation. Nat. Commun. 2024, 15, 1148. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, Z.; Sun, Y.; Yeh, S.; Wang, X.; Long, J.; Chang, C. Androgen receptor promotes melanoma metastasis via altering the miRNA-539-3p/USP13/MITF/AXL signals. Oncogene 2017, 36, 1644–1654. [Google Scholar] [CrossRef]
- Xu, P.; Choi, E.; White, K.; Yafi, F.A. Low Testosterone in Male Cancer Patients and Survivors. Sex Med. Rev. 2021, 9, 133–142. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Regis, L.; Planas, J.; Celma, A.; de Torres, I.M.; Ferrer, R.; Morote, J. Behavior of total and free serum testosterone as a predictor for the risk of prostate cancer and its aggressiveness. Actas Urol. Esp. 2015, 39, 573–581. [Google Scholar] [CrossRef]
- Shen, B.; Mei, J.; Xu, R.; Cai, Y.; Wan, M.; Zhou, J.; Ding, J.; Zhu, Y. B7-H3 is associated with the armored-cold phenotype and predicts poor immune checkpoint blockade response in melanoma. Pathol. Res. Pract. 2024, 256, 155267. [Google Scholar] [CrossRef]
- Samarkina, A.; Youssef, M.K.; Ostano, P.; Ghosh, S.; Ma, M.; Tassone, B.; Proust, T.; Chiorino, G.; Levesque, M.P.; Goruppi, S.; et al. Androgen receptor is a determinant of melanoma targeted drug resistance. Nat. Commun. 2023, 14, 6498. [Google Scholar] [CrossRef]
- Gregory, C.W.; Johnson, R.T., Jr.; Mohler, J.L.; French, F.S.; Wilson, E.M. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001, 61, 2892–2898. [Google Scholar]
- He, Z.; Rankinen, T.; Leon, A.S.; Skinner, J.S.; Tchernof, A.; Bouchard, C. Plasma Steroids and Cardiorespiratory Fitness Response to Regular Exercise. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).