Simple Summary
Calreticulin (CALR) mutations in myeloproliferative neoplasms (MPNs) create unique opportunities for targeted therapy. The most advanced approach involves monoclonal antibodies specifically designed to recognize the CALR neoepitope, with early clinical trials showing encouraging results. Additional preclinical data derives from bispecific antibodies that redirect T cells against CALR-mutant cells, precision antibody–drug conjugates delivering cytotoxic payloads, and CAR T-cell therapies. Vaccination against CALR-derived peptides has shown immune activity but limited benefit, and its role remains uncertain. This review discusses these advances and outlines the challenges that must be overcome to translate them into routine clinical practice.
Abstract
More than a decade after its discovery, advances have been made in understanding the oncogenic role of mutant CALR in BCR::ABL1-negative myeloproliferative neoplasms (MPNs). Disease biology has proven to be distinct from other MPN subtypes, with meaningful differences that have created opportunities for therapeutic targeting of CALR-mutant clones. Among the approaches under investigation, immunotherapy has advanced furthest into clinical development and holds promise. Several strategies are now being explored, including monoclonal antibodies directed against the CALR neoepitope, T-cell–redirecting bispecific antibodies, precision antibody–drug conjugates, vaccination approaches, and CAR T-cell therapies. Early-phase clinical trials with fully human anti-CALR monoclonal antibodies (e.g., INCA033989) have shown very promising hematologic and molecular responses with manageable toxicity. In preclinical models, bispecific antibodies and CAR T-cell therapy offer additional avenues to exploit the selective cell-surface localization of mutant CALR. By contrast, vaccination strategies have so far demonstrated limited clinical efficacy, and their potential in clinical practice remains challenging. At the same time, the complexity of CALR-driven disease raises key questions, including whether anti-CALR therapies can shift treatment goals beyond thrombotic risk reduction, how best to monitor clonal burden, and how to address immune escape. In this review, we highlight the latest therapeutic advances in CALR-mutated MPNs while outlining the critical unmet needs that will shape the future of care for these patients.
1. Introduction
BCR::ABL1-negative myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are clonal hematopoietic stem cell disorders characterized by sustained myeloid proliferation and a variable risk of thrombosis, hemorrhage, and fibrotic or leukemic progression [1]. Their clonal origin was first suggested in the 1970s [2], but major advances in understanding occurred with the identification in 2005 of the JAK2 V617F mutation, present in nearly all PV cases and in a majority of ET and PMF. Soon after, activating mutations in MPL, the thrombopoietin (TPO) receptor, were identified in a smaller subset of ET and PMF [3]. A further breakthrough came in 2013 with the identification of somatic insertions and deletions in exon 9 of the CALR gene, which encodes calreticulin, occurring in 20–25% of ET and PMF patients without JAK2 or MPL mutations [4,5].
In recent years, interest in CALR mutation has steadily grown, shifting from their initial diagnostic and prognostic relevance to their potential as direct therapeutic targets. The unique biochemical features of mutant CALR, including its interaction with MPL and its immunogenic neoepitope, have opened new avenues for targeted therapies. Considering these advances, we examine the evolving significance of CALR in MPNs together with open issues for integrating immune-based strategies into the management of CALR-positive diseases.
2. Genetic and Molecular Pathways in CALR-Mutated MPNs
2.1. Structure and Function of Calreticulin
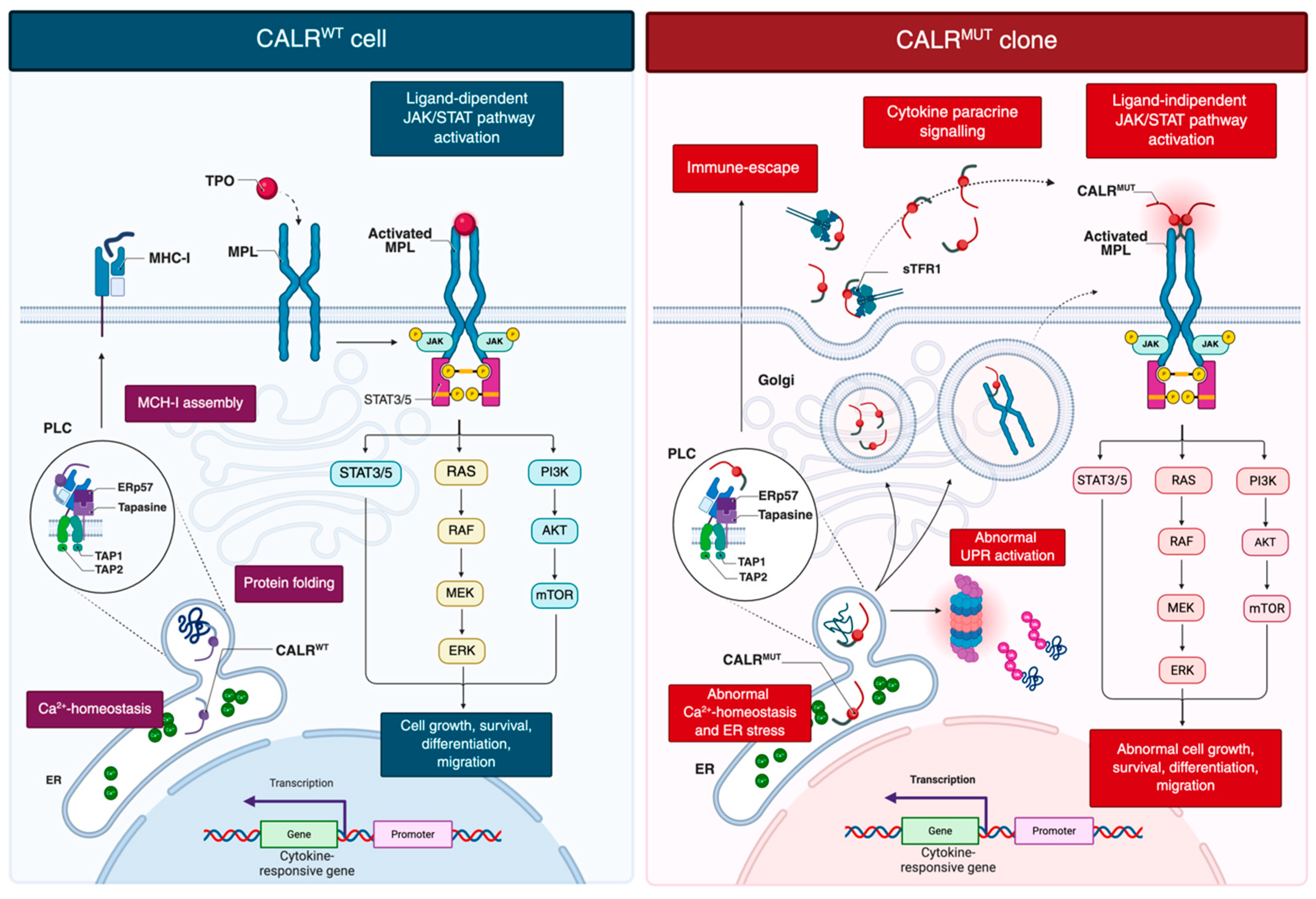
Wild-type CALR is a conserved endoplasmic reticulum (ER) lumen chaperone essential for intracellular calcium (Ca2+) homeostasis, protein quality control, and glycoprotein folding (Figure 1) [6].
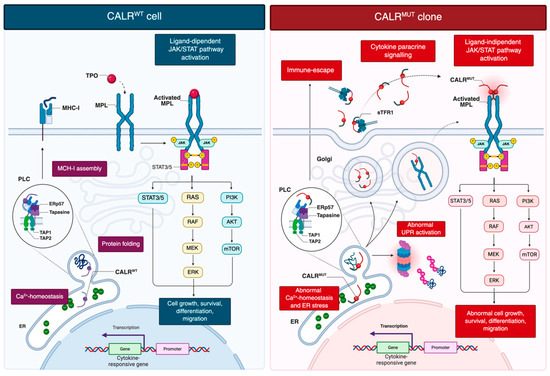
Figure 1.
Current model of oncogenic signaling in CALR-mutant clones. CALRwt contributes to protein folding, Ca2+ homeostasis in ER, and PLC-mediated MHC-I assembly. TPO-dependent activation of MPL drives ligand-mediated JAK/STAT signaling that support proliferation, survival, differentiation and migration. CALRwt assists proper MPL folding while remaining ER-retained. CALRmut lacks the C-terminal KDEL retention signal, enabling its dislocation into the Golgi and secretory pathway. The mutant C-terminus, generated by a frameshift, induces conformational changes in the N-domain, increasing accessibility to the lectin-binding pocket and facilitating aberrant interactions with immature N-glycans and the D1 domain of MPL. This interaction occurs in the ER and Golgi and allows the CALRmut-MPL complex to traffic to the cell surface, where CALRmut acts as a cytokine, inducing ligand-independent MPL dimerization and constitutive JAK/STAT activation. Additional consequences include abnormal UPR activation, disrupted Ca2+ homeostasis, and immune escape due to defective MHC-I assembly. Created in BioRender. Costa, A. (2025) https://BioRender.com/6cdrn75. Abbreviations: CALR, calreticulin; ER, endoplasmic reticulum; MHC-I, major histocompatibility complex class I; MPN, myeloproliferative neoplasm; PLC, peptide-loading complex; sTFR1, soluble transferrin receptor 1; TPO, thrombopoietin; UPR, unfolded protein response.
The CALR gene comprises nine exons and encodes a protein organized into three functional domains: an N-terminal lectin-like domain that binds glycans, a central proline-rich (P) domain that interacts with polypeptides, and a C-terminal domain ending with the ER-retention signal KDEL (Lys-Asp-Glu-Leu) [7].
Functionally, the C-terminal domain binds Ca2+ with low affinity, thereby contributing to ER calcium buffering [8]. The KDEL sequence ensures retention of CALR within the ER, where it facilitates the proper folding of N-glycosylated proteins destined for the plasma membrane, extracellular space, or other cellular compartments [9]. As a holdase chaperone, CALR preferentially binds glycosylated precursors through its lectin domain, preventing aggregation, premature oligomerization, oxidation, and degradation of misfolded proteins [10].
CALR also plays a key role as an extracellular “eat-me” signal expressed on the surface of stressed or apoptotic cells. Once externalized, CALR binds to phosphatidylserine and functions as a damage-associated molecular pattern (DAMP), thereby promoting macrophage-mediated phagocytosis [11]. The P domain of CALR has been proposed to serve as a molecular bridge to phagocytic receptors, including low-density lipoprotein receptor-related protein 1 (LRP1) and C1q, enhancing its pro-phagocytic function [12]. In parallel, activated macrophages have been shown to secrete CALR, which binds to viable target cells and marks them for clearance through a mechanism of programmed cell removal (PrCR) [13].
2.2. Advances in Molecular Mechanisms and Oncogenic Transformation of CALR-Mutated MPNs
Since their discovery in 2013, more than fifty distinct CALR mutations have been identified. All are located within exon 9 and produce a +1 base pair (bp) frameshift [5]. Based on structural features and the extent of acidic domain loss, they are categorized as type 1 mutations (52-bp deletion; CALRDel52), with complete loss of the acidic C-terminal region, or type 2 mutations (5-bp insertion; CALRIns5), with partial retention of the acidic domain [4,5]. The remaining 20% are designated as type 1-like or type 2-like mostly according to structural predictions based on α-helical content. Mutants with higher predicted α-helical content are considered type 2-like, whereas those with lower content resemble type 1 [14,15,16].
Structural studies have provided insight into the abnormal interaction between mutant CALR and MPL. Neither the intrinsic chaperone activity of CALR nor its polypeptide-binding domain is necessary for MPL activation. Instead, three features are critical: specific N-glycosylation sites on MPL, the lectin-binding ability of mutant CALR, and the positively charged residues in its novel C-terminal tail generated by the frameshift [17,18,19,20]. Replacing these basic residues with neutral glycine, or partially deleting the mutant tail, reduces the CALR transforming capacity without affecting MPL binding, indicating that receptor binding and activation are distinct processes [21].
Mutations in CALR have shown to affect key aspects of cellular biology and immune regulation (Figure 1) [22,23,24]. Loss of the ER-retention KDEL sequence directs mutant CALR into the secretory pathway, enabling pathological MPL activation [4,5]. The altered C-terminal tail triggers an N-domain conformational change, exposing the N-glycan binding pocket for recognition of immature N-glycans on MPL [25]. Direct electrostatic interactions are also thought to occur between the positively charged residues in the mutant C-terminal domain and negatively charged residues within the D1 domain of MPL [25]. This interaction, occurring in the ER and Golgi, facilitates trafficking of the CALR–MPL complex to the cell surface, where mutant CALR acts as a cytokine mimic, promoting ligand-independent MPL dimerization and sustained JAK/STAT activation [19,26]. Additional data indicate that homomultimerization of mutant CALR, driven by its aberrant C-terminal sequence, is necessary for full receptor activation [25,27]. Mutant CALR was also detected in plasma of MPN patients [28]. Pecquet et al. [28] investigated its biological role and demonstrated a correlation between soluble mutant CALR (smCALR) levels and mutational burden. They further showed that the prolonged half-life of smCALR results from its complex formation with soluble transferrin receptor 1 (sTFR1). Functionally, smCALR probably binds to and activates MPL on megakaryocyte progenitor cells, promoting ligand-independent proliferation and sensitizing neighboring cells to CALR-mediated signaling.
2.3. Clonal Dynamics and Open Challenges
Over the past decade, preclinical models and genomic analyses have provided critical insights into clonal evolution, tracing the trajectory of hematopoietic stem cells (HSCs) from early subclinical expansion to overt disease [29]. In particular, investigations into self-renewal dynamics and somatic mutation–based lineage tracing have shown that JAK2 V617F mutations may arise decades before diagnosis [30], and in some cases even in utero [31]. A striking parallel has been reported for CALR in a unique case involving monozygotic twins who developed CALR-mutated MF in adulthood. Both individuals harbored the same somatic CALR mutation, likely acquired in one twin and transmitted to the other through transplacental hematopoietic cell sharing. Subsequent clonal divergence was evidenced by the presence of a TET2 mutation in only one twin [32]. Mathematical modeling studies suggest that, unlike JAK2, CALR mutations may arise later in life but confers a stronger proliferative advantage, thereby accelerating clonal expansion [33]. Interestingly, some evidence showed that the proportion of mutated cells is not equally distributed within the progenitors, with CALR-mutated HSCs biased towards myeloid differentiation and CALR-mutated cells heavily enriched in megakaryocyte progenitors [23,34].
However, translational research on CALR remains limited by several constraints inherent to current models, including species-specific differences, reliance on ectopic overexpression, and limited phenotypic penetrance [35]. Transgenic mouse models, including CalrDel52;Calr+/+ and heterozygous knock-in lines, typically develop thrombocytosis without progression to fibrosis [36,37]. Homozygous models exhibit more pronounced features, such as marked thrombocytosis, reduced bone marrow erythropoiesis, splenomegaly, and extramedullary hematopoiesis, but lack a competitive repopulating advantage in transplantation assays [36,38,39]. More recently, it has also been shown that HSCs with CALR haploinsufficiency display MPN-like features, including reduced bone marrow erythropoiesis and splenic extramedullary hematopoiesis; the combination of CALRDel52 and CALR haploinsufficiency restores the self-renewal capacity of mutant HSCs, eliminating the competition from wild-type cells and aiding the expansion of mutant clones towards MPN [40].
Recently, CRISPR/Cas9 editing has enabled the targeted introduction of CALR mutations directly into the endogenous mouse locus, generating in-frame deletions of 19, 52, or 61 bp. However, these models also exhibit only mild thrombocytosis and limited JAK/STAT activation [41,42]. To overcome these limitations, a novel humanized knock-in model was developed in 2023, in which common CALR mutations were introduced into healthy human HSCs using CRISPR/Cas9 [35]. Consistent with earlier findings, CALR-mutated HSCs did not show immediate proliferative advantage, suggesting that additional transcriptional or microenvironmental events are required to drive transformation. Interestingly, some mice transplanted with human CALR-mutated HSCs developed marrow fibrosis and splenomegaly, potentially reflecting a greater sensitivity of human MPL to TPO compared with its murine counterpart [35]. These findings support the concept that CALR-driven transformation is not cell-autonomous but instead depends on a complex interplay between the driver mutation, intrinsic transcriptional programs, and extrinsic environmental signals. Nonetheless, further analyses are needed to fully understand the oncogenic dynamics of CALR and to overcome the limitations of current models.
3. Immunotherapeutic Strategies in CALR-Mutated MPNs
3.1. Anti-CALR Monoclonal Antibodies
The advent of immunotherapy has transformed the treatment landscape of hematologic malignancies. Among these, monoclonal antibodies (MoAbs) occupy a central role due to their ability to selectively target malignant cells, limiting off-target toxicity and enabling durable responses even in relapsed or refractory disease [43]. While well established in lymphoproliferative disorders and acute leukemias, their application in MPNs is more recent, yet they represent the most advanced immunotherapeutic approach in both preclinical and early clinical development (Table 1).

Table 1.
Ongoing Phase I clinical trials in MPN patients with documented CALR exon 9 mutation.
The cell-surface localization of mutant CALR makes it an attractive target. The first preclinical evidence, reported in 2020, described B3, a murine chimeric MoAb recognizing the mutant CALR C-terminal domain [44]. In CALRDel52 ET murine model, B3 reduced bone marrow megakaryocyte counts and improved thrombocytosis. Subsequently, Achyutuni et al. [38] treated homozygous CALRDel52 transgenic mice with a murine IgG2a antibody directed against the human CALR neoantigen, achieving a rapid decline in platelet counts after five doses over 2.5 days, with rebound to baseline within 24 h after discontinuation.
In 2022, two independent groups advanced this concept. Mughal et al. [45] generated antibodies targeting internal peptides within the mutant CALR C-terminal, defining key epitope. In parallel, Tvorogov et al. [46] developed 4D7, an IgG2α MoAb specific for a C-terminal sequence common to type 1 and type 2 CALR mutations. 4D7 selectively disrupted the mutant CALR-MPL interaction, attenuated hyperactivation of the JAK/STAT pathway, suppressed thrombopoietin-independent megakaryocytic proliferation, and inhibited differentiation of CALR-mutated CD34+ cells, prolonging survival in xenograft models, including ruxolitinib-resistant disease.
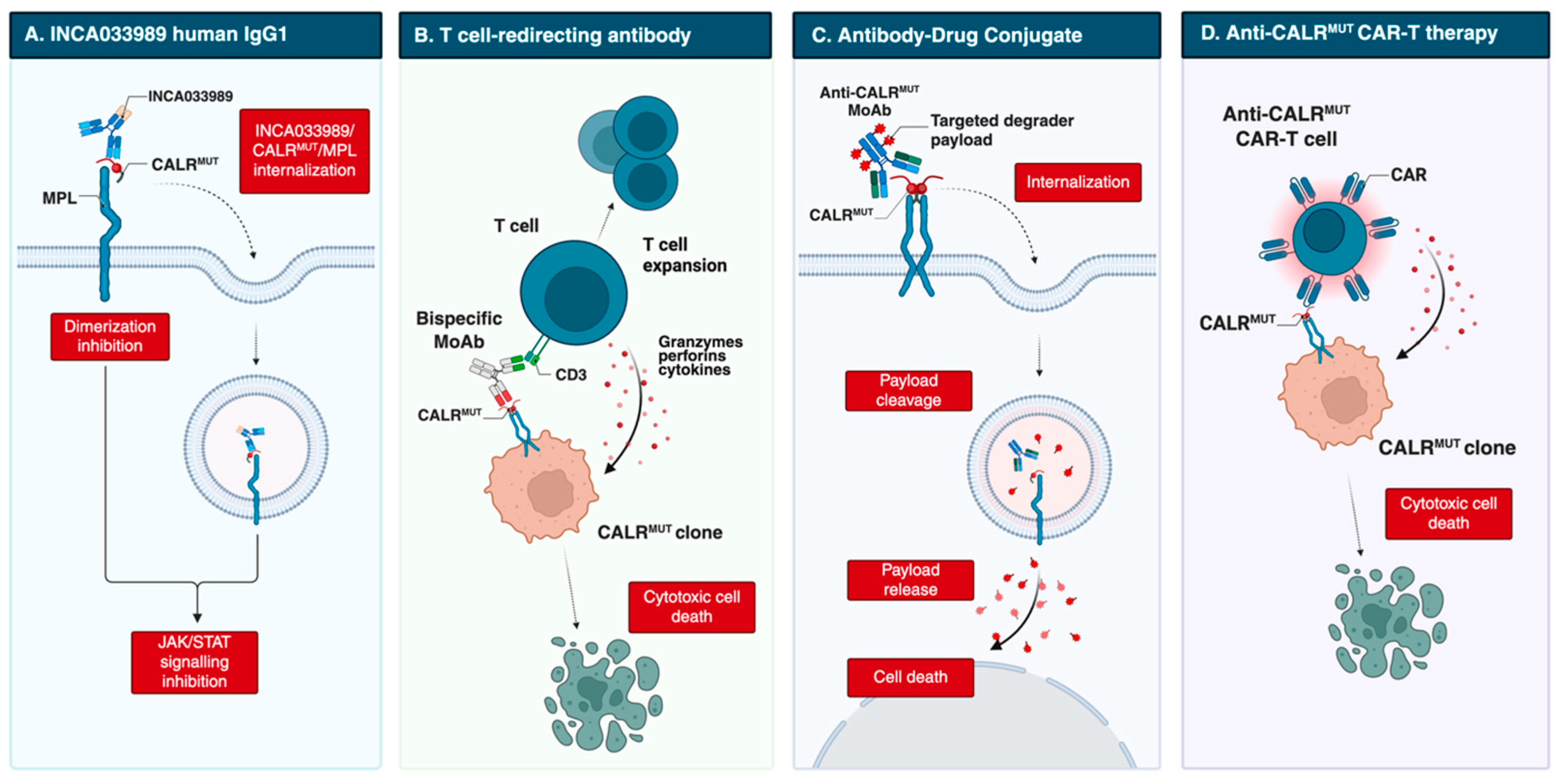
However, these antibodies were generated in non-human species and had incompletely characterized mechanisms of action. A major advance came with INCA033989, developed by Reis et al. [47]. This fully human IgG1 MoAb harbors an N297A Fc mutation that abolishes Fc-mediated effector functions (Figure 2). INCA033989 binds recombinant CALRDel52 (Kd 1.75 nM) and CALRIns5 (Kd 6.78 nM), with preferential binding to CALRDel52, likely reflecting epitope exposure differences in the CALRDel52-MPL complex. The MoAb/CALR/MPL complex relies on dynamin-dependent endocytosis; whether mutant CALR or antibody binding affects MPL recycling remains unclear. Functionally, INCA033989 inhibited pSTAT3/pSTAT5 in CALR-mutated MF CD34+ cells in a dose-dependent manner, without affecting wild-type, JAK2-mutated, or MPL-mutated cells. In a CALR-mutated ET murine model, it induced hematologic and molecular responses by selectively targeting mutant hematopoietic stem cells while sparing normal hematopoiesis. In a CALRDel52/TP53 Q317 double-mutant post-MPN AML xenograft, it selectively targeted mutant cells and prolonged survival [48].
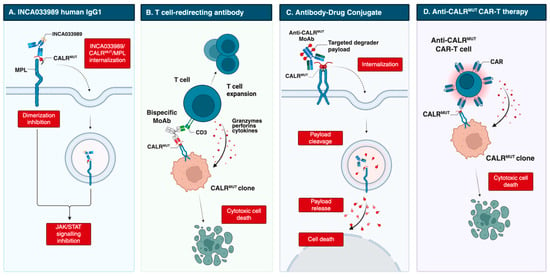
Figure 2.
Emerging therapeutic strategies targeting mutant CALR, including direct MoAb, T-cell redirecting bispecific Ab, antibody-drug conjugate and anti-CALR CAR-T cell therapy. Created in BioRender. Costa, A. (2025) https://BioRender.com/vwehboc. Abbreviations: CAR-T, chimeric antigen receptor T cell; MoAb, monoclonal antibody.
To date, two phase 1 clinical trials are underway for INCA033989, specifically the INCA33989-101 (NCT05936359, ex-US) and INCA3989-192 (NCT06034002, US). Both are multicenter, open-label, first-in-human studies with a phase 1a dose-escalation stage in high-risk MF or ET patients harboring exon 9 CALR mutations, followed by phase 1b expansion and phase 1c crossover with ruxolitinib in MF patients with suboptimal response. Preliminary data in high-risk ET (n = 49) treated with doses ranging from 24 mg to 2500 mg administered intravenously every two weeks show encouraging activity without dose-limiting toxicities. As per protocol design, hydroxyurea (HU) or anagrelide as cytoreductive therapy was allowed, while prohibited medications included interferon, thalidomide, busulfan or lenalidomide, requiring a washout period of 5 half-live prior to study enrollment. A rapid reduction in platelet count was reported, with hematologic responses achieved within 4 weeks, an overall response rate (ORR) of 79%, and complete responses (CR) in 66% of patients. Reductions in variant allele frequency (VAF) were observed in 88% of patients, with 25% achieving a ≥50% reduction after 12 weeks of treatment. Most adverse events were grade 1–2, with transient, asymptomatic grade 3 events mainly consisting of lipase elevations [49]. Early dose-dependent normalization of platelet counts and reductions in clonal burden were observed; definitive results are awaited.
Additional antibodies are designed to recruit immune effector cells, mediating antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). In 2023, Kuchnio et al. [50] reported JNJ-88549968, a bispecific T-cell–redirecting antibody selectively targeting MPN clones. This agent induced specific binding and T-cell activation against CALR-mutated cells in vitro, including autologous assays, and in xenograft models, regardless of mutation subtype; it is now in a phase 1 trial (NCT06150157).
Another T-cell–redirecting antibody, INCA035784, is a fully human IgG1 with a silenced Fc domain. Unlike INCA033989, it binds an N-domain region of mutant CALR conserved across C-terminal mutations [51]. It does not recognize wild-type CALR exposed on the cell surface after doxorubicin-induced stress, and binding is unaffected by soluble CALR. In vitro, it induces dose-dependent T-cell activation and cytotoxicity against CALR-mutated CD34+ cells from both type 1 and type 2 cases.
Finally, precision antibody–drug conjugates (pADCs) have been explored to deliver cytotoxic payloads selectively to malignant cells. One approach couples degraders of SMARCA2/4 or CDK9 to an anti-CALR MoAb, leveraging dysregulation of the SWI/SNF chromatin-remodeling complex, which governs gene expression programs essential for hematopoietic stem cell maintenance [52,53,54]. Dual degradation disrupts aberrant SWI/SNF function, inducing cytotoxicity in progenitors and restoring normal hematopoiesis. Preclinical data show selective binding to mutant cells, inhibition of megakaryocytic growth and differentiation in patient-derived CALR-mutated samples, and durable disease regression. Further studies are ongoing [52].
3.2. Vaccination Strategies Against Exon 9 CALR Mutations and Immune Checkpoint Inhibition
Therapeutic cancer vaccines aim to elicit immune responses directly against tumor-specific antigens [55]. In MPNs, exon 9 CALR mutations generate unique neoantigens, making them attractive targets. Early studies showed that patient-derived T cells could recognize and eliminate autologous CALR-mutated cells ex vivo, but their activity was weaker than that of healthy donor T cells stimulated with the same peptides [56,57]. To optimize immunogenicity, Gigoux et al. [58] used in silico prediction to identify mutant CALR neoepitopes with strong MHC-I binding; however, these alleles were underrepresented in CALR-mutated compared to JAK2-mutated cases, potentially restricting vaccine applicability.
Clinically, the CALRlong36 peptide vaccine was evaluated in the phase 1 trial NCT03566446 [59]. Ten CALR-mutated MPN patients received 15 doses over one year. Ex vivo T-cell responses emerged in 8 patients, yet no hematologic or molecular responses were achieved. Attempts to enhance efficacy through combinatorial approaches have so far been unsuccessful. In NCT05444530, dual CALR/JAK2 vaccination was combined with the CTLA-4 inhibitor ipilimumab in 14 patients [60]. Mutant CALR-specific immune responses were observed in 35.7%, but no reduction in allele burden occurred, leading to early trial discontinuation.
3.3. CAR T-Cell Therapy Targeting Mutant CALR
Preclinical efforts have also extended to CAR T-cell therapy. Schueller et al. [61] showed that murine CALR-mutant-specific CAR-T cells could target Ba/F3-hMPL cells expressing human CALRDel52 and prolong survival in xenografts, although no remissions were achieved in immunocompetent chimeric mice. More recently, Rampotas et al. [62] reported a CALR-directed CAR T-cell strategy capable of selectively eradicating CALR-mutated human cell lines, irrespective of expression levels. In NGS xenografts harboring CALR-mutated AML cells, this approach reduced leukemic burden and improved survival. In ex vivo assays using CD34+ cells from MPN patients, depletion ranged from 40% to 90% with limited off-target toxicity toward JAK2 V617F samples.
4. Targeting CALR in the Era of Precision Medicine: Issues to Be Addressed
From the earliest observations it became clear that CALR-mutated MPNs display distinctive clinical features compared with other driver mutations. Indeed, CALR-mutated patients, as first reported by Klampfl and Nangalia [4,5], are younger and characteristically show marked thrombocytosis accompanied by relatively lower hemoglobin and leukocyte counts when compared with JAK2-mutated cases. In ET, CALR mutations further delineate a subgroup with reduced thrombotic risk [63]. While survival in ET appears independent of driver mutation status [64,65], type 1 CALR mutations in MF are consistently associated with improved outcomes [66,67,68]. These findings have informed the evolution of prognostic scoring systems [69]. It is important to note, however, that CALR attenuate but do not eliminate the adverse prognostic impact of high molecular risk (HMR) mutations and unfavorable karyotype [70]. These clinical and prognostic insights raise key considerations for the development of therapies targeting CALR-mutated clones.
4.1. Will Anti-CALR Therapies Shift Management from Thrombotic Risk Reduction to Disease Modification?
An open question is how anti-CALR therapies might reshape current management strategies and in which clinical settings their use would be most impactful. The potential to modify the natural history of the disease by directly targeting the mutant clone raises the issue of whether therapeutic goals should move beyond thrombotic risk reduction. This is especially relevant in ET, where current management is primarily focused on reducing thrombotic risk. While HU remains the standard treatment for high-risk IPSET-thrombosis patients and intermediate-risk cases requiring cytoreduction, unmet therapeutic needs persist. Anagrelide can be considered in patients who are resistant or intolerant to HU [71], although clinically significant anemia and long-term bone marrow fibrosis, particularly in CALR-mutated cases, have raised concerns [72,73]. Busulfan and pipobroman are effective alternatives but carry potential leukemogenic risks [74]. In addition, ruxolitinib did not demonstrate superiority over HU in the MAJIC-ET trial [75]. By contrast, pegylated interferon-α (Peg-IFNα) has shown efficacy in controlling thrombocytosis, reducing spleen size, and improving symptom burden [76,77], with notable molecular responses, particularly in JAK2 V617F–mutated cases. Similar results have recently been reported for ropeginterferon α-2b (Ropeg-IFN) in patients with PV, and full results from ongoing studies in ET, including the SURPASS-ET trial, are eagerly awaited [78].
Similar issues arise in CALR-mutated MF, where management largely follows the principles applied to other driver mutations, yet distinct clinical considerations warrant attention. Pivotal trials of ruxolitinib and fedratinib have only partially addressed whether driver mutation status influences outcomes [79]. In a retrospective analysis of 29 CALR-mutated MF patients in the COMFORT-II trial, 20 received ruxolitinib and demonstrated safety, efficacy, and survival comparable to the overall cohort [80]. More recently, momelotinib was approved for MF with anemia due to its dual JAK1/JAK2 inhibition and blockade of activin A receptor type 1 (ACVR1). Among 79 JAK inhibitor-naïve patients treated with momelotinib, 14% carried type 1 CALR and 3% type 2 CALR mutations [81], with type 1 CALR patients showing significantly higher 3-, 5-, and 10-year survival even after multivariate adjustment for transplantation. Real-world data highlight additional nuances. In a cohort of 1.055 MF patients treated with ruxolitinib, 135 with CALR mutations more frequently developed anemia and increased blast percentages [82], likely reflecting later treatment initiation, higher disease burden, and longer intervals from diagnosis, which may diminish the favorable prognostic impact of CALR mutations.
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative option. Advances in technique and prognostic tools have broadened its feasibility. In 2023, Hernández-Boluda et al. [83] reported outcomes in 346 CALR-mutated MF patients undergoing HSCT, with five-year survival of 63% versus 50% in JAK2-mutated cases, though no distinction was made by CALR subtype or HMR status. More recently, Gagelmann et al. [84] analyzed clonal dynamics in 324 patients after reduced-intensity conditioning: 23% were CALR-mutated, achieving molecular clearance in 73% at day 30 and 82% at day 100, compared with 42% and 63% for JAK2-mutated cases and 54% and 100% for MPL-mutated cases, potentially explaining the superior post-transplant outcomes in CALR- and MPL-mutated patients.
Taken together, anti-CALR therapies represent an important opportunity to achieve disease modification, something rarely attainable with current approaches. As ongoing studies mature, they will clarify whether these agents can meaningfully reduce thrombotic risk, delay disease progression, and improve quality of life in patients with ET and MF.
4.2. Are We Ready for Measurable Residual Disease Monitoring?
The prospect of targeted therapy capable of eradicating the mutant clone highlights the importance of molecular monitoring, akin to measurable residual disease (MRD), to evaluate treatment efficacy over time and guide clinical decisions. Improvements in blood counts, symptoms, or organ involvement do not always correlate with molecular responses [85].
Although current management of PV and ET focuses largely on thrombo-hemorrhagic risk reduction, preventing fibrotic or leukemic progression remains a critical research goal. This is supported by evidence linking disease progression to increasing allele burden during follow-up [86]. While the JAK2 V617F VAF exhibits considerable interindividual variability in MPNs, CALR-mutated cases show a more homogeneous distribution, typically between 40% and 50% [87,88,89]. In patients with CALR VAF ≤20%, lower platelet and neutrophil counts and improved overall survival have been reported, independent of mutation subtype, age, or thrombotic history [88]. Conversely, higher allele burdens are associated with increased acquisition of additional mutations, greater risk of anemia during follow-up, and more frequent progression to fibrosis [87,89].
To date, no therapy has convincingly altered the natural history of MPNs. As previously said, IFNα has demonstrated not only complete hematologic responses but also molecular responses, outcomes not achieved with cytoreduction using anagrelide or HU [90]. However, in CALR-mutated patients, clonal reduction dynamics are variable even among molecular responders [91], and the reduction of the CALR-mutant clone with Peg-IFNα is generally less pronounced than in JAK2 V617F-mutated patients [92,93]. The MAJIC-PV trial provided evidence that molecular responses in PV patients treated with ruxolitinib correlate with improved overall and progression-free survival [94]. In addition, Guglielmelli et al. [85] prospectively measured changes in JAK2 and CALR VAF in 77 PV or ET patients treated with ruxolitinib, reporting reductions from a median of 68% to 3.5% in JAK2-mutated cases and from 49% to 4% in a CALR-mutated ET patient. Molecular responses were also observed in three CALR-mutated ET patients treated with ruxolitinib in the MAJIC-ET study [75].
Several techniques have been evaluated for monitoring allele burden, including fragment length analysis (FLA), Sanger sequencing, and next-generation sequencing (NGS) [86,91]. Jones et al. [95] compared detection limits of CALR assays using cell lines with 61 bp deletions, reporting detection limits of 10–25%, 5%, 5%, and 1.2% for Sanger sequencing, FLA, high-resolution melt analysis, and NGS, respectively. While FLA is rapid and cost-effective for diagnostic screening, it has a relatively high detection limit and is unsuitable for precise quantification of fragments of different sizes, often overestimating shorter fragments [91,96]. Similarly, Sanger sequencing, with 10–20% sensitivity, is inadequate for MRD monitoring [97]. NGS is highly sensitive but limited by cost and turnaround time, particularly for allele burdens below 1–5%. Quantitative PCR (qPCR) offers higher sensitivity and broad availability, but its application to CALR is challenged by the complex assay design required due to nucleotide repeats in exon 9. This approach has been employed to quantify type 1 and type 2 mutations, with a detection limit that generally does not exceed 0.1%, remaining suboptimal for reliable MRD monitoring [97].
In contrast, digital droplet PCR (ddPCR) overcomes the limitations of conventional PCR by partitioning the reaction into thousands of microdroplets, each analyzed individually. The low DNA copy number per droplet minimizes competition between primers, enabling highly accurate quantification of allele burden [84,86,91]. Using this approach, mutant CALR alleles can be detected down to 0.01%, providing a highly sensitive and precise tool for MRD assessment [96,98]. A clear example of its clinical utility is the monitoring of MRD post-HSCT in myelofibrosis. Early studies demonstrated that CALR mutations are cleared more rapidly and frequently post-transplant compared with MPL or JAK2 V617F mutations [99]. Patients with detectable mutations at days +100 and +180 had a significantly higher risk of relapse, irrespective of the underlying driver mutation. More recent evidence indicates that mutation clearance as early as day +30 is a strong predictor of both relapse and post-transplant survival, emphasizing the importance of timely molecular monitoring [84].
Nevertheless, defining molecular response as a marker of disease modification requires careful standardization and validation of threshold levels, which must ultimately translate into meaningful clinical benefit. Importantly, clearance of the dominant mutant clone does not necessarily equate to clearance of all clones, highlighting the need for further studies to clarify the clinical relevance of clonal monitoring in MPNs.
4.3. How to Face the Immune Escape Issue?
Accumulating evidence supports the presence of an immunosuppressive environment in CALR-mutated MPNs. While the underlying mechanisms remain incompletely understood, they appear primarily related to impaired antigen presentation and immunosuppressive remodeling of the tumor microenvironment. Impaired phagocytosis of tumor cells has been observed in CALRDel52 knock-in mice, consistent with an immunosuppressive role of CALR-mutated clones; this defect was associated with reduced T-cell activation and expansion of regulatory T cells (Tregs) [100], rather than affecting phagocytic capacity of macrophages [101]. Additional studies have shown that CALRDel52 variant drives the expansion of TGF-β1-producing erythroid progenitors and Tregs, while suppressing T-cell cytotoxicity [102].
Moreover, although CALR mutations can elicit T-cell responses, CALR-mutant-specific responses are attenuated in MPN patients compared with healthy individuals [103]. High expression of immune checkpoint receptors such as PD-1 and CTLA-4, consistent with T-cell exhaustion, has been reported. Furthermore, CALR-mutant–specific T cells from patients are predominantly CD4+, and the absence of CALR-mutant-specific CD8+ T cells may reflect impaired antigen presentation [103]. Under normal conditions, CALR participates in the peptide-loading complex (PLC) along with ERp57 and tapasin, ensuring proper assembly and high-affinity peptide loading onto major histocompatibility complex (MHC) class I molecules [104]. Proteasome-derived peptides are transported into the endoplasmic reticulum by the TAP1/TAP2 heterodimer, trimmed by ERAP1, and loaded onto MHC class I heavy chains and β2-microglobulin with the assistance of tapasin, ERp57, and CALR. Fully assembled complexes are then exported via the Golgi to the cell surface to mediate immune recognition of abnormal cells [105]. By guiding MHC class I molecules to the PLC and stabilizing peptide loading, CALR is essential for antigen presentation. Mutant CALR disrupts this process, impairs high-affinity peptide binding, and reduces MHC class I expression at the cell surface, likely facilitating immune evasion of CALR-mutated clones [106].
Collectively, these findings highlight immune escape as a major barrier in CALR-mutated MPNs, likely contributing to the limited success of T-cell-based and vaccination strategies. Continued efforts to overcome this hurdle will be essential for the development of effective therapies.
5. Conclusions
CALR mutations and their downstream pathophysiologic effects offer unique opportunities for precision targeting. Despite highly encouraging preclinical results demonstrating selectivity and low toxicity, challenges persist. While the biological rationale and preclinical evidence strongly support the development of mutant CALR-directed therapies, clinical success will require overcoming disease-intrinsic immune evasion, refining patient selection, and implementing robust strategies for clonal burden monitoring.
Author Contributions
Conceptualization, A.C. and M.B.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and M.B.; visualization, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
M.B. received honoraria from Novartis, Incyte, BMS/Celgene, Pfizer, and Abbvie. A.C. declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACVR1 | Activin A receptor type 1 |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| CAR-T | Chimeric antigen receptor T cell |
| CDC | Complement-dependent cytotoxicity |
| CR | Complete response |
| ET | Essential Thrombocytemia |
| ER | Endoplasmic reticulum |
| FLA | Fragment length analysis |
| HMR | High molecular risk |
| HSCT | Hematopoietic stem cell transplantation |
| HU | Hydroxyurea |
| MoAbs | Monoclonal antibodies |
| MPN | Myeloproliferative neoplasm |
| MHC MRD | Major histocompatibility complex Measurable residual disease |
| NGS | Next-generation sequencing |
| ORR | Overall response rate |
| pADCs | Precision antibody–drug conjugates |
| PCR | Polymerase chain reaction |
| PLC | Peptide-loading complex |
| PV | Polycythemia Vera |
| PMF | Primary Myelofibrosis |
| smCALR | Soluble mutant CALR |
| TPO | Thrombopoietin |
| Tregs | Regulatory T cells |
| VAF | Variant allele frequency |
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Adamson, J.W.; Fialkow, P.J.; Murphy, S.; Prchal, J.F.; Steinmann, L. Polycythemia Vera: Stem-Cell and Probable Clonal Origin of the Disease. N. Engl. J. Med. 1976, 295, 913–916. [Google Scholar] [CrossRef]
- Pikman, Y.; Lee, B.H.; Mercher, T.; McDowell, E.; Ebert, B.L.; Gozo, M.; Cuker, A.; Wernig, G.; Moore, S.; Galinsky, I.; et al. MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med. 2006, 3, e270. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a Multi-Process Calcium-Buffering Chaperone of the Endoplasmic Reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Houen, G.; Højrup, P.; Ciplys, E.; Gaboriaud, C.; Slibinskas, R. Structural Analysis of Calreticulin, an Endoplasmic Reticulum-Resident Molecular Chaperone. In Cellular Biology of the Endoplasmic Reticulum; Agellon, L.B., Michalak, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 13–25. ISBN 978-3-030-67696-4. [Google Scholar]
- Wijeyesakere, S.J.; Gafni, A.A.; Raghavan, M. Calreticulin Is a Thermostable Protein with Distinct Structural Responses to Different Divalent Cation Environments. J. Biol. Chem. 2011, 286, 8771–8785. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.R.; Ora, A.; Van, P.N.; Helenius, A. Transient, Lectin-Like Association of Calreticulin with Folding Intermediates of Cellular and Viral Glycoproteins. Mol. Biol. Cell 1995, 6, 1173–1184. [Google Scholar] [CrossRef]
- Schürch, P.M.; Malinovska, L.; Hleihil, M.; Losa, M.; Hofstetter, M.C.; Wildschut, M.H.E.; Lysenko, V.; Lakkaraju, A.K.K.; Maat, C.A.; Benke, D.; et al. Calreticulin Mutations Affect Its Chaperone Function and Perturb the Glycoproteome. Cell Rep. 2022, 41, 111689. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Wijeyesakere, S.J.; Bedi, S.K.; Huynh, D.; Raghavan, M. The C-terminal acidic region of calreticulin mediates phosphatidylserine binding and apoptotic cell phagocytosis. J. Immunol. 2016, 196, 3896–3909. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Chen, J.Y.; Weissman-Tsukamoto, R.; Volkmer, J.P.; Ho, P.Y.; McKenna, K.M.; Cheshier, S.; Zhang, M.; Guo, N.; Gip, P.; et al. Macrophages eat cancer cells using their own calreticulin as a guide: Roles of TLR and Btk. Proc. Natl. Acad. Sci. USA 2015, 112, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Tischer, A.; Wassie, E.A.; Finke, C.M.; Belachew, A.A.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.D. The Prognostic Advantage of Calreticulin Mutations in Myelofibrosis Might Be Confined to Type 1 or Type 1-like CALR Variants. Blood 2014, 124, 2465–2466. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.; Li, Y.; Peter Gale, R.; Song, Z.; Ai, X.; Qin, T.; Zhang, Y.; Zhang, P.; Huang, G.; et al. The Different Prognostic Impact of Type-1 or Type-1 like and Type-2 or Type-2 like CALR Mutations in Patients with Primary Myelofibrosis. Am. J. Hematol. 2016, 91, E320–E321. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Rotunno, G.; Fanelli, T.; Pacilli, A.; Brogi, G.; Calabresi, L.; Pancrazzi, A.; Vannucchi, A.M. Validation of the Differential Prognostic Impact of Type 1/Type 1-like versus Type 2/Type 2-like CALR Mutations in Myelofibrosis. Blood Cancer J. 2015, 5, e360. [Google Scholar] [CrossRef]
- Elf, S.; Abdelfattah, N.S.; Chen, E.; Perales-Patón, J.; Rosen, E.A.; Ko, A.; Peisker, F.; Florescu, N.; Giannini, S.; Wolach, O.; et al. Mutant Calreticulin Requires Both Its Mutant C-Terminus and the Thrombopoietin Receptor for Oncogenic Transformation. Cancer Discov. 2016, 6, 368–381. [Google Scholar] [CrossRef]
- Nivarthi, H.; Chen, D.; Cleary, C.; Kubesova, B.; Jäger, R.; Bogner, E.; Marty, C.; Pecquet, C.; Vainchenker, W.; Constantinescu, S.N.; et al. Thrombopoietin Receptor Is Required for the Oncogenic Function of CALR Mutants. Leukemia 2016, 30, 1759–1763. [Google Scholar] [CrossRef]
- Chachoua, I.; Pecquet, C.; El-Khoury, M.; Nivarthi, H.; Albu, R.-I.; Marty, C.; Gryshkova, V.; Defour, J.-P.; Vertenoeil, G.; Ngo, A.; et al. Thrombopoietin Receptor Activation by Myeloproliferative Neoplasm Associated Calreticulin Mutants. Blood 2016, 127, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Yang, Y.; Masubuchi, N.; Hironaka, Y.; Takei, H.; Morishita, S.; Mizukami, Y.; Kan, S.; Shirane, S.; Edahiro, Y.; et al. Activation of the Thrombopoietin Receptor by Mutant Calreticulin in CALR-Mutant Myeloproliferative Neoplasms. Blood 2016, 127, 1307–1316. [Google Scholar] [CrossRef]
- Elf, S.; Abdelfattah, N.S.; Baral, A.J.; Beeson, D.; Rivera, J.F.; Ko, A.; Florescu, N.; Birrane, G.; Chen, E.; Mullally, A. Defining the Requirements for the Pathogenic Interaction between Mutant Calreticulin and MPL in MPN. Blood 2018, 131, 782–786. [Google Scholar] [CrossRef]
- Salati, S.; Genovese, E.; Carretta, C.; Zini, R.; Bartalucci, N.; Prudente, Z.; Pennucci, V.; Ruberti, S.; Rossi, C.; Rontauroli, S.; et al. Calreticulin Ins5 and Del52 Mutations Impair Unfolded Protein and Oxidative Stress Responses in K562 Cells Expressing CALR Mutants. Sci. Rep. 2019, 9, 10558. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Kim, K.-T.; Chaligne, R.; Izzo, F.; Ang, C.; Taylor, J.; Myers, R.M.; Abu-Zeinah, G.; Brand, R.; Omans, N.D.; et al. Somatic Mutations and Cell Identity Linked by Genotyping of Transcriptomes. Nature 2019, 571, 355–360. [Google Scholar] [CrossRef]
- Jutzi, J.S.; Marneth, A.E.; Jiménez-Santos, M.J.; Hem, J.; Guerra-Moreno, A.; Rolles, B.; Bhatt, S.; Myers, S.A.; Carr, S.A.; Hong, Y.; et al. CALR-Mutated Cells Are Vulnerable to Combined Inhibition of the Proteasome and the Endoplasmic Reticulum Stress Response. Leukemia 2023, 37, 359–369. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Nédélec, A.; Derenne, A.; Şulea, T.A.; Pecquet, C.; Chachoua, I.; Vertenoeil, G.; Tilmant, T.; Petrescu, A.-J.; Mazzucchelli, G.; et al. Oncogenic CALR Mutant C-Terminus Mediates Dual Binding to the Thrombopoietin Receptor Triggering Complex Dimerization and Activation. Nat. Commun. 2023, 14, 1881. [Google Scholar] [CrossRef]
- Pecquet, C.; Chachoua, I.; Roy, A.; Balligand, T.; Vertenoeil, G.; Leroy, E.; Albu, R.-I.; Defour, J.-P.; Nivarthi, H.; Hug, E.; et al. Calreticulin Mutants as Oncogenic Rogue Chaperones for TpoR and Traffic-Defective Pathogenic TpoR Mutants. Blood 2019, 133, 2669–2681. [Google Scholar] [CrossRef]
- Araki, M.; Yang, Y.; Imai, M.; Mizukami, Y.; Kihara, Y.; Sunami, Y.; Masubuchi, N.; Edahiro, Y.; Hironaka, Y.; Osaga, S.; et al. Homomultimerization of Mutant Calreticulin Is a Prerequisite for MPL Binding and Activation. Leukemia 2019, 33, 122–131. [Google Scholar] [CrossRef]
- Pecquet, C.; Papadopoulos, N.; Balligand, T.; Chachoua, I.; Tisserand, A.; Vertenoeil, G.; Nédélec, A.; Vertommen, D.; Roy, A.; Marty, C.; et al. Secreted Mutant Calreticulins as Rogue Cytokines in Myeloproliferative Neoplasms. Blood 2023, 141, 917–929. [Google Scholar] [CrossRef]
- Hormoz, S.; Sankaran, V.G.; Mullally, A. Evolution of Myeloproliferative Neoplasms from Normal Blood Stem Cells. Haematologica 2025, 110, 840–849. [Google Scholar] [CrossRef]
- Van Egeren, D.; Escabi, J.; Nguyen, M.; Liu, S.; Reilly, C.R.; Patel, S.; Kamaz, B.; Kalyva, M.; DeAngelo, D.J.; Galinsky, I.; et al. Reconstructing the Lineage Histories and Differentiation Trajectories of Individual Cancer Cells in Myeloproliferative Neoplasms. Cell Stem Cell 2021, 28, 514–523.e9. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Lee, J.; Mitchell, E.; Moore, L.; Baxter, E.J.; Hewinson, J.; Dawson, K.J.; Menzies, A.; Godfrey, A.L.; Green, A.R.; et al. Life Histories of Myeloproliferative Neoplasms Inferred from Phylogenies. Nature 2022, 602, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sousos, N.; Leathlobhair, M.N.; Karali, C.S.; Louka, E.; Bienz, N.; Royston, D.; Clark, S.-A.; Hamblin, A.; Howard, K.; Mathews, V.; et al. In utero origin of myelofibrosis presenting in adult monozygotic twins. Nat. Med. 2022, 28, 1207–1211. [Google Scholar] [CrossRef]
- Hermange, G.; Rakotonirainy, A.; Bentriou, M.; Tisserand, A.; El-Khoury, M.; Girodon, F.; Marzac, C.; Vainchenker, W.; Plo, I.; Cournède, P.-H. Inferring the Initiation and Development of Myeloproliferative Neoplasms. Proc. Natl. Acad. Sci. USA 2022, 119, e2120374119. [Google Scholar] [CrossRef]
- Olschok, K.; Han, L.; de Toledo, M.A.S.; Böhnke, J.; Graßhoff, M.; Costa, I.G.; Theocharides, A.; Maurer, A.; Schüler, H.M.; Buhl, E.M.; et al. CALR frameshift mutations in MPN patient-derived iPSCs accelerate maturation of megakaryocytes. Stem Cell Rep. 2021, 16, 2768–2783. [Google Scholar] [CrossRef] [PubMed]
- Foßelteder, J.; Pabst, G.; Sconocchia, T.; Schlacher, A.; Auinger, L.; Kashofer, K.; Beham-Schmid, C.; Trajanoski, S.; Waskow, C.; Schöll, W.; et al. Human Gene-Engineered Calreticulin Mutant Stem Cells Recapitulate MPN Hallmarks and Identify Targetable Vulnerabilities. Leukemia 2023, 37, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Prins, D.; Park, H.J.; Grinfeld, J.; Gonzalez-Arias, C.; Loughran, S.; Dovey, O.M.; Klampfl, T.; Bennett, C.; Hamilton, T.L.; et al. Mutant Calreticulin Knockin Mice Develop Thrombocytosis and Myelofibrosis without a Stem Cell Self-Renewal Advantage. Blood 2018, 131, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Shide, K.; Kameda, T.; Yamaji, T.; Sekine, M.; Inada, N.; Kamiunten, A.; Akizuki, K.; Nakamura, K.; Hidaka, T.; Kubuki, Y.; et al. Calreticulin Mutant Mice Develop Essential Thrombocythemia That Is Ameliorated by the JAK Inhibitor Ruxolitinib. Leukemia 2017, 31, 1136–1144. [Google Scholar] [CrossRef]
- Achyutuni, S.; Nivarthi, H.; Majoros, A.; Hug, E.; Schueller, C.; Jia, R.; Varga, C.; Schuster, M.; Senekowitsch, M.; Tsiantoulas, D.; et al. Hematopoietic Expression of a Chimeric Murine-Human CALR Oncoprotein Allows the Assessment of Anti-CALR Antibody Immunotherapies in Vivo. Am. J. Hematol. 2021, 96, 698–707. [Google Scholar] [CrossRef]
- Benlabiod, C.; Cacemiro, M.d.C.; Nédélec, A.; Edmond, V.; Muller, D.; Rameau, P.; Touchard, L.; Gonin, P.; Constantinescu, S.N.; Raslova, H.; et al. Calreticulin Del52 and Ins5 Knock-in Mice Recapitulate Different Myeloproliferative Phenotypes Observed in Patients with MPN. Nat. Commun. 2020, 11, 4886. [Google Scholar] [CrossRef]
- Shide, K.; Kameda, T.; Kamiunten, A.; Ozono, Y.; Tahira, Y.; Yokomizo-Nakano, T.; Kubota, S.; Ono, M.; Ikeda, K.; Sekine, M.; et al. Calreticulin Haploinsufficiency Augments Stem Cell Activity and Is Required for Onset of Myeloproliferative Neoplasms in Mice. Blood 2020, 136, 106–118. [Google Scholar] [CrossRef]
- Shide, K.; Kameda, T.; Kamiunten, A.; Oji, A.; Ozono, Y.; Sekine, M.; Honda, A.; Kitanaka, A.; Akizuki, K.; Tahira, Y.; et al. Mice with Calr Mutations Homologous to Human CALR Mutations Only Exhibit Mild Thrombocytosis. Blood Cancer J. 2019, 9, 42. [Google Scholar] [CrossRef]
- Balligand, T.; Achouri, Y.; Pecquet, C.; Gaudray, G.; Colau, D.; Hug, E.; Rahmani, Y.; Stroobant, V.; Plo, I.; Vainchenker, W.; et al. Knock-in of Murine Calr Del52 Induces Essential Thrombocythemia with Slow-Rising Dominance in Mice and Reveals Key Role of Calr Exon 9 in Cardiac Development. Leukemia 2020, 34, 510–521. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in Hematologic Malignancies: Achievements, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2023, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Araki, M.; Imai, M.; Mori, Y.; Horino, M.; Ogata, S.; Yoshikawa, S.; Taguchi, T.; Masubuchi, N.; Mabuchi, Y.; et al. Therapeutic Potential of an Antibody Targeting the Cleaved Form of Mutant Calreticulin in Myeloproliferative Neoplasms. Blood 2020, 136, 9–10. [Google Scholar] [CrossRef]
- Mughal, F.P.; Bergmann, A.C.; Huynh, H.U.B.; Jørgensen, S.H.; Mansha, I.; Kesmez, M.; Schürch, P.M.; Theocharides, A.P.A.; Hansen, P.R.; Friis, T.; et al. Production and Characterization of Peptide Antibodies to the C-Terminal of Frameshifted Calreticulin Associated with Myeloproliferative Diseases. Int. J. Mol. Sci. 2022, 23, 6803. [Google Scholar] [CrossRef]
- Tvorogov, D.; Thompson-Peach, C.A.; Foßelteder, J.; Dottore, M.; Stomski, F.; Onnesha, S.A.; Lim, K.; Moretti, P.A.; Pitson, S.M.; Ross, D.M.; et al. Targeting Human CALR-mutated MPN Progenitors with a Neoepitope-directed Monoclonal Antibody. EMBO Rep. 2022, 23, e52904. [Google Scholar] [CrossRef]
- Reis, E.S.; Buonpane, R.; Celik, H.; Marty, C.; Lei, A.; Jobe, F.; Rupar, M.; Zhang, Y.; DiMatteo, D.; Awdew, R.; et al. Selective Targeting of Mutated Calreticulin by the Monoclonal Antibody INCA033989 Inhibits Oncogenic Function of MPN. Blood 2024, 144, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Rosa, E.; Evrard, M.; Pegliasco, J.; Chedeville, A.; Blampey, Q.; Aid, Z.; Mercher, T.; Legros, L.; Fouquet, G.; et al. Efficacy of INCA033989 in Chronic and Advanced Forms of CALRdel52 and CALRins5 Myeloproliferative Neoplasms (MPN) Models. HemaSphere 2024, 8 (Suppl. S1), 1795–1796. [Google Scholar]
- Mascarenhas, J.; Ali, H.; Yacoub, A.; Jain, T.; Chee, L.; Gupta, V.; Harrison, C.; Kiladjian, J.-J.; Mesa, R.; Shomali, W.; et al. INCA33989 Is a Novel, First in Class, Mutant Calreticulin-Specific Monoclonal Antibody That Demonstrates Safety and Efficacy in Patients with Essential Thrombocythemia. Presented at the Annual Meeting of the European Hematology Association, Milano, Italy, 12–15 June 2025. [Google Scholar]
- Kuchnio, A.; Samakai, E.; Hug, E.; Balmaña, M.; Janssen, L.; Amorim, R.; Cornelissen, I.; Majoros, A.; Broux, M.; Taneja, I.; et al. Discovery of JNJ-88549968, a Novel, First-in-Class CALRmutxCD3 T-Cell Redirecting Antibody for the Treatment of Myeloproliferative Neoplasms. Blood 2023, 142, 1777. [Google Scholar] [CrossRef]
- Pandey, V.; Wang, L.-C.; Kulkarni, A.; Hendriks, L.; Steevels, T.; Merenich, D.; Guan, J.; Ren, E.; Langalia, N.; Fiedorczuk, K.; et al. INCA035784, a novel, equipotent T cell-redirecting antibody for patients with myeloproliferative neoplasms carrying different types of calreticulin mutations. HemaSphere 2025, 9 (Suppl. S1), 258–259. [Google Scholar]
- Fultang, N.; Schwab, A.; Chandratre, S.; Johnson, I.; Karwoski, J.; Bhagwat, N.; Zou, Y.; Buesking, A.; Foroutan, M.; Chen, C.; et al. Discovery of First-in-Class Precision Antibody Drug Conjugates Targeting Mutant Calreticulin for the Treatment of Myeloproliferative Neoplasms. HemaSphere 2025, 9 (Suppl. S1), 256–257. [Google Scholar]
- Fultang, N.; Schwab, A.M.; Johnson, I.; Grego, A.; Filler, E.; Bachner, C.; Karwoski, J.; Moore, A.; Bartilomo, A.; Agarwal, A.; et al. Selective SMARCA2 Degradation Promotes Leukemic Differentiation and Synergizes with CDK9 Inhibition to Potently Induce Death in Pre-Clinical Models of Acute Myeloid Leukemia. Blood 2024, 144 (Suppl. S1), 2774. [Google Scholar] [CrossRef]
- Cantley, J.; Ye, X.; Rousseau, E.; Januario, T.; Hamman, B.D.; Rose, C.M.; Cheung, T.K.; Hinkle, T.; Soto, L.; Quinn, C.; et al. Selective PROTAC-Mediated Degradation of SMARCA2 Is Efficacious in SMARCA4 Mutant Cancers. Nat. Commun. 2022, 13, 6814. [Google Scholar] [CrossRef]
- Zaidi, N.; Jaffee, E.M.; Yarchoan, M. Recent Advances in Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2025, 25, 517–533. [Google Scholar] [CrossRef]
- Holmström, M.O.; Riley, C.H.; Svane, I.M.; Hasselbalch, H.C.; Andersen, M.H. The CALR Exon 9 Mutations Are Shared Neoantigens in Patients with CALR Mutant Chronic Myeloproliferative Neoplasms. Leukemia 2016, 30, 2413–2416. [Google Scholar] [CrossRef]
- Holmström, M.O.; Ahmad, S.M.; Klausen, U.; Bendtsen, S.K.; Martinenaite, E.; Riley, C.H.; Svane, I.M.; Kjær, L.; Skov, V.; Ellervik, C.; et al. High Frequencies of Circulating Memory T Cells Specific for Calreticulin Exon 9 Mutations in Healthy Individuals. Blood Cancer J. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gigoux, M.; Holmström, M.O.; Zappasodi, R.; Park, J.J.; Pourpe, S.; Bozkus, C.C.; Mangarin, L.M.B.; Redmond, D.; Verma, S.; Schad, S.; et al. Calreticulin Mutant Myeloproliferative Neoplasms Induce MHC-I Skewing, Which Can Be Overcome by an Optimized Peptide Cancer Vaccine. Sci. Transl. Med. 2022, 14, eaba4380. [Google Scholar] [CrossRef] [PubMed]
- Handlos Grauslund, J.; Holmström, M.O.; Jørgensen, N.G.; Klausen, U.; Weis-Banke, S.E.; El Fassi, D.; Schöllkopf, C.; Clausen, M.B.; Gjerdrum, L.M.R.; Breinholt, M.F.; et al. Therapeutic Cancer Vaccination With a Peptide Derived From the Calreticulin Exon 9 Mutations Induces Strong Cellular Immune Responses in Patients With CALR-Mutant Chronic Myeloproliferative Neoplasms. Front. Oncol. 2021, 11, 637420. [Google Scholar] [CrossRef]
- Otoukesh, S.; Psaila, B.; Kuykendall, A.; Gerds, A.T.; Abdulgawad, A.; Bose, P.; Van Bogaert, C.; Bishop, J.; Wilkinson, P.; Liu, B.; et al. A phase 1 study of VAC85135, a neoantigen vaccine regimen targeting calreticulin and JAK2 mutations, in combination with ipilimumab (IPI) in patients (PTS) with myeloproliferative neoplasms (MPNS). Presented at the Annual Meeting of the European Hematology Association, Milano, Italy, 12–15 June 2025. [Google Scholar]
- Schueller, C.; Varga, C.; Wais, T.; Höhrhan, M.; Xiong, S.; Balmanya, M.; Zagrijtschuk, O.; Kralovics, R. Targeted T Cells against Hematopoietic Cells Expressing Oncogenic Calreticulin Mutants. In Proceedings of the Annual Meeting of European Hematology Association, Milano, Italy, 12–15 June 2025. [Google Scholar]
- Rampotas, A.; Wong, Z.; Gannon, I.; Benlabiod, C.; Shen, Y.; Brierley, C.; Olijnik, A.-A.; Khan, S.; Hayder, N.; Cheung, G.W.-K.; et al. Development of a First-in-Class CAR-T Therapy Against Calreticulin-Mutant Neoplasms and Evaluation in the Relevant Human Tissue Environment. Blood 2024, 144 (Suppl. S1), 871. [Google Scholar] [CrossRef]
- Rotunno, G.; Mannarelli, C.; Guglielmelli, P.; Pacilli, A.; Pancrazzi, A.; Pieri, L.; Fanelli, T.; Bosi, A.; Vannucchi, A.M.; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014, 123, 1552–1555. [Google Scholar] [CrossRef]
- Gangat, N.; Karrar, O.; Al-Kali, A.; Begna, K.H.; Elliott, M.A.; Wolanskyj-Spinner, A.P.; Pardanani, A.; Hanson, C.A.; Ketterling, R.P.; Tefferi, A. One Thousand Patients with Essential Thrombocythemia: The Mayo Clinic Experience. Blood Cancer J. 2024, 14, 11. [Google Scholar] [CrossRef]
- Loscocco, G.G.; Gesullo, F.; Capecchi, G.; Atanasio, A.; Maccari, C.; Mannelli, F.; Vannucchi, A.M.; Guglielmelli, P. One Thousand Patients with Essential Thrombocythemia: The Florence-CRIMM Experience. Blood Cancer J. 2024, 14, 10. [Google Scholar] [CrossRef]
- Tefferi, A.; Nicolosi, M.; Mudireddy, M.; Szuber, N.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; et al. Driver Mutations and Prognosis in Primary Myelofibrosis: Mayo-Careggi MPN Alliance Study of 1095 Patients. Am. J. Hematol. 2018, 93, 348–355. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; et al. A Clinical-Molecular Prognostic Model to Predict Survival in Patients with Post Polycythemia Vera and Post Essential Thrombocythemia Myelofibrosis. Leukemia 2017, 31, 2726–2731. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.; Hanson, C.H.; Maffioli, M.; Caramazza, D.; Passamonti, F.; Pardanani, A. CALR vs JAK2 vs MPL-Mutated or Triple-Negative Myelofibrosis: Clinical, Cytogenetic and Molecular Comparisons. Leukemia 2014, 28, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Mora, B.; Bucelli, C.; Cattaneo, D.; Bellani, V.; Versino, F.; Barbullushi, K.; Fracchiolla, N.; Iurlo, A.; Passamonti, F. Prognostic and Predictive Models in Myelofibrosis. Curr. Hematol. Malig. Rep. 2024, 19, 223–235. [Google Scholar] [CrossRef]
- Szuber, N.; Lasho, T.L.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Determinants of Long-Term Outcome in Type 1 Calreticulin-Mutated Myelofibrosis. Leukemia 2019, 33, 780–785. [Google Scholar] [CrossRef]
- Alvarez-Larrán, A.; Sant’Antonio, E.; Harrison, C.; Kiladjian, J.J.; Griesshammer, M.; Mesa, R.; Ianotto, J.C.; Palandri, F.; Hernández-Boluda, J.C.; Birgegård, G.; et al. Unmet Clinical Needs in the Management of CALR-Mutated Essential Thrombocythaemia: A Consensus-Based Proposal from the European LeukemiaNet. Lancet Haematol. 2021, 8, e658–e665. [Google Scholar] [CrossRef] [PubMed]
- Bieniaszewska, M.; Sobieralski, P.; Leszczyńska, A.; Dutka, M. Anagrelide in essential thrombocythemia: Efficacy and long-term consequences in young patient population. Leuk. Res. 2022, 123, 106962. [Google Scholar] [CrossRef]
- Mazzucconi, M.G.; Baldacci, E.; Latagliata, R.; Breccia, M.; Paoloni, F.; Di Veroli, A.; Cedrone, M.; Anaclerico, B.; Villivà, N.; Porrini, R.; et al. Anagrelide in Essential Thrombocythemia (ET): Results from 150 Patients over 25 Years by the “Ph1-Negative Myeloproliferative Neoplasms Latium Group”. Eur. J. Haematol. 2020, 105, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Essential Thrombocythemia: 2024 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2024, 99, 697–718. [Google Scholar] [CrossRef]
- Harrison, C.N.; Mead, A.J.; Panchal, A.; Fox, S.; Yap, C.; Gbandi, E.; Houlton, A.; Alimam, S.; Ewing, J.; Wood, M.; et al. Ruxolitinib vs Best Available Therapy for ET Intolerant or Resistant to Hydroxycarbamide. Blood 2017, 130, 1889–1897. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Kantarjian, H.; Manshouri, T.; Luthra, R.; Estrov, Z.; Pierce, S.; Richie, M.A.; Borthakur, G.; Konopleva, M.; Cortes, J.; et al. Pegylated Interferon Alfa-2a Yields High Rates of Hematologic and Molecular Response in Patients with Advanced Essential Thrombocythemia and Polycythemia Vera. J. Clin. Oncol. 2009, 27, 5418–5424. [Google Scholar] [CrossRef]
- Kiladjian, J.-J.; Cassinat, B.; Chevret, S.; Turlure, P.; Cambier, N.; Roussel, M.; Bellucci, S.; Grandchamp, B.; Chomienne, C.; Fenaux, P. Pegylated Interferon-Alfa-2a Induces Complete Hematologic and Molecular Responses with Low Toxicity in Polycythemia Vera. Blood 2008, 112, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.A.; Gill, H.; Xiao, Z.; Komatsu, N.; Qin, A.; Tashi, T.; Zhang, L.; Jin, J.; Kirito, K.; Ohishi, K.; et al. Ropeginterferon Alfa-2b versus Anagrelide for the Treatment of Essential Thrombocythemia: Topline Results of the Phase 3 SURPASS-ET Trial. J. Clin. Oncol. 2025, 43, 6500. [Google Scholar] [CrossRef]
- Passamonti, F.; Caramazza, D.; Maffioli, M. JAK Inhibitor in CALR-Mutant Myelofibrosis. N. Engl. J. Med. 2014, 370, 1168–1169. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Rotunno, G.; Bogani, C.; Mannarelli, C.; Giunti, L.; Provenzano, A.; Giglio, S.; Squires, M.; Stalbovskaya, V.; Gopalakrishna, P.; et al. Ruxolitinib Is an Effective Treatment for CALR-Positive Patients with Myelofibrosis. Br. J. Haematol. 2016, 173, 938–940. [Google Scholar] [CrossRef]
- Tefferi, A.; Pardanani, A.; Begna, K.H.; Al-Kali, A.; Hogan, W.J.; Litzow, M.R.; Ketterling, R.P.; Reichard, K.K.; Gangat, N. Calr Type 1/like Mutation in Myelofibrosis Is the Most Prominent Predictor of Momelotinib Drug Survival and Longevity without Transplant. Blood Cancer J. 2024, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Branzanti, F.; Morsia, E.; Dedola, A.; Benevolo, G.; Tiribelli, M.; Beggiato, E.; Farina, M.; Martino, B.; Caocci, G.; et al. Impact of Calreticulin Mutations on Treatment and Survival Outcomes in Myelofibrosis during Ruxolitinib Therapy. Ann. Hematol. 2025, 104, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Boluda, J.-C.; Pereira, A.; Zinger, N.; Gras, L.; Martino, R.; Paneesha, S.; Finke, J.; Chinea, A.; Rambaldi, A.; Robin, M.; et al. Allogeneic Hematopoietic Cell Transplantation in Patients with Myeloid/Lymphoid Neoplasm with FGFR1-Rearrangement: A Study of the Chronic Malignancies Working Party of EBMT. Bone Marrow Transpl. 2022, 57, 416–422. [Google Scholar] [CrossRef]
- Gagelmann, N.; Quarder, M.; Badbaran, A.; Rathje, K.; Janson, D.; Lück, C.; Richter, J.; Marquard, F.; Oechsler, S.; Massoud, R.; et al. Clearance of Driver Mutations after Transplantation for Myelofibrosis. N. Engl. J. Med. 2025, 392, 150–160. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Mora, B.; Gesullo, F.; Mannelli, F.; Loscocco, G.G.; Signori, L.; Pessina, C.; Colugnat, I.; Aquila, R.; Balliu, M.; et al. Clinical Impact of Mutated JAK2 Allele Burden Reduction in Polycythemia Vera and Essential Thrombocythemia. Am. J. Hematol. 2024, 99, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Cottin, L.; Riou, J.; Orvain, C.; Ianotto, J.C.; Boyer, F.; Renard, M.; Truchan-Graczyk, M.; Murati, A.; Jouanneau-Courville, R.; Allangba, O.; et al. Sequential Mutational Evaluation of CALR -Mutated Myeloproliferative Neoplasms with Thrombocytosis Reveals an Association between CALR Allele Burden Evolution and Disease Progression. Br. J. Haematol. 2020, 188, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Szuber, N.; Gangat, N.; Capecchi, G.; Maccari, C.; Harnois, M.; Karrar, O.; Abdelmagid, M.; Balliu, M.; Nacca, E.; et al. CALR Mutation Burden in Essential Thrombocythemia and Disease Outcome. Blood 2024, 143, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Aubin, L.; Vilas Boas, R.; Daltro De Oliveira, R.; Le Brun, V.; Divoux, M.; Rey, J.; Mansier, O.; Ianotto, J.-C.; Pastoret, C.; Desmares, A.; et al. CALR-Mutated Patients with Low Allele Burden Represent a Specific Subtype of Essential Thrombocythemia: A Study on Behalf of FIM and GBMHM. Am. J. Hematol. 2024, 99, 1001–1004. [Google Scholar] [CrossRef]
- Mroczkowska-Bękarciak, A.; Szeremet, A.; Chyrko, O.; Wróbel, T. CALR—Mutant Myeloproliferative Neoplasms: Insights from next-Generation Sequencing. J. Appl. Genet. 2025. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Giri, S.; Wang, R.; Podoltsev, N.; Williams, R.T.; Tallman, M.S.; Rampal, R.K.; Zeidan, A.M.; Stahl, M. Interferon Alpha Therapy in Essential Thrombocythemia and Polycythemia Vera—A Systematic Review and Meta-Analysis. Leukemia 2021, 35, 1643–1660. [Google Scholar] [CrossRef]
- Kjær, L.; Cordua, S.; Holmström, M.O.; Thomassen, M.; Kruse, T.A.; Pallisgaard, N.; Larsen, T.S.; de Stricker, K.; Skov, V.; Hasselbalch, H.C. Differential Dynamics of CALR Mutant Allele Burden in Myeloproliferative Neoplasms during Interferon Alfa Treatment. PLoS ONE 2016, 11, e0165336. [Google Scholar] [CrossRef]
- Czech, J.; Cordua, S.; Weinbergerova, B.; Baumeister, J.; Crepcia, A.; Han, L.; Maié, T.; Costa, I.G.; Denecke, B.; Maurer, A.; et al. JAK2V617F but Not CALR Mutations Confer Increased Molecular Responses to Interferon-α via JAK1/STAT1 Activation. Leukemia 2019, 33, 995–1010. [Google Scholar] [CrossRef]
- Stegelmann, F.; Teichmann, L.L.; Heidel, F.H.; Crodel, C.C.; Ernst, T.; Kreil, S.; Reiter, A.; Otten, S.; Schauer, S.; Körber, R.-M.; et al. Clinicohematologic and Molecular Response of Essential Thrombocythemia Patients Treated with Pegylated Interferon-α: A Multi-Center Study of the German Study Group-Myeloproliferative Neoplasms (GSG-MPN). Leukemia 2023, 37, 924–928. [Google Scholar] [CrossRef]
- Harrison, C.N.; Nangalia, J.; Boucher, R.; Jackson, A.; Yap, C.; O’Sullivan, J.; Fox, S.; Ailts, I.; Dueck, A.C.; Geyer, H.L.; et al. Ruxolitinib Versus Best Available Therapy for Polycythemia Vera Intolerant or Resistant to Hydroxycarbamide in a Randomized Trial. J. Clin. Oncol. 2023, 41, 3534–3544. [Google Scholar] [CrossRef]
- Jones, A.V.; Ward, D.; Lyon, M.; Leung, W.; Callaway, A.; Chase, A.; Dent, C.L.; White, H.E.; Drexler, H.G.; Nangalia, J.; et al. Evaluation of Methods to Detect CALR Mutations in Myeloproliferative Neoplasms. Leuk. Res. 2015, 39, 82–87. [Google Scholar] [CrossRef]
- Mansier, O.; Migeon, M.; Saint-Lézer, A.; James, C.; Verger, E.; Robin, M.; Socié, G.; Bidet, A.; Mahon, F.X.; Cassinat, B.; et al. Quantification of the Mutant CALR Allelic Burden by Digital PCR: Application to Minimal Residual Disease Evaluation after Bone Marrow Transplantation. J. Mol. Diagn. 2016, 18, 68–74. [Google Scholar] [CrossRef]
- Chi, J.; Manoloukos, M.; Pierides, C.; Nicolaidou, V.; Nicolaou, K.; Kleopa, M.; Vassiliou, G.; Costeas, P. Calreticulin Mutations in Myeloproliferative Neoplasms and New Methodology for Their Detection and Monitoring. Ann. Hematol. 2015, 94, 399–408. [Google Scholar] [CrossRef]
- Anelli, L.; Zagaria, A.; Coccaro, N.; Tota, G.; Minervini, A.; Casieri, P.; Impera, L.; Minervini, C.F.; Brunetti, C.; Ricco, A.; et al. Droplet digital PCR assay for quantifying CALR mutant allelic burden in myeloproliferative neoplasms. Ann. Hematol. 2016, 95, 1559–1560. [Google Scholar] [CrossRef] [PubMed]
- Wolschke, C.; Badbaran, A.; Zabelina, T.; Christopeit, M.; Ayuk, F.; Triviai, I.; Zander, A.; Alchalby, H.; Bacher, U.; Fehse, B.; et al. Impact of molecular residual disease post allografting in myelofibrosis patients. Bone Marrow Transpl. 2017, 52, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, L.; Loos, F.; Marty, C.; Xie, W.; Martins, I.; Lachkar, S.; Qu, B.; Waeckel-Énée, E.; Plo, I.; et al. Immunosuppression by Mutated Calreticulin Released from Malignant Cells. Mol. Cell 2020, 77, 748–760.e9. [Google Scholar] [CrossRef]
- Daitoku, S.; Takenaka, K.; Yamauchi, T.; Yurino, A.; Jinnouchi, F.; Nunomura, T.; Eto, T.; Kamimura, T.; Higuchi, M.; Harada, N.; et al. Calreticulin mutation does not contribute to disease progression in essential thrombocythemia by inhibiting phagocytosis. Exp. Hematol. 2016, 44, 817–825.e3. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Endres, C.; Hoefflin, R.; Andrieux, G.; Zwick, M.; Karantzelis, N.; Staehle, H.F.; Vinnakota, J.M.; Duquesne, S.; Mozaffari Jovein, M.; et al. Oncogenic Calreticulin Induces Immune Escape by Stimulating TGFβ Expression and Regulatory T-Cell Expansion in the Bone Marrow Microenvironment. Cancer Res. 2024, 84, 2985–3003. [Google Scholar] [CrossRef]
- Bozkus, C.C.; Roudko, V.; Finnigan, J.P.; Mascarenhas, J.; Hoffman, R.; Iancu-Rubin, C.; Bhardwaj, N. Immune Checkpoint Blockade Enhances Shared Neoantigen-Induced T Cell Immunity Directed against Mutated Calreticulin in Myeloproliferative Neoplasms. Cancer Discov. 2019, 9, 1192–1207. [Google Scholar] [CrossRef]
- Wijeyesakere, S.J.; Gagnon, J.K.; Arora, K.; Brooks, C.L.; Raghavan, M. Regulation of Calreticulin–Major Histocompatibility Complex (MHC) Class I Interactions by ATP. Proc. Natl. Acad. Sci. USA 2015, 112, E5608–E5617. [Google Scholar] [CrossRef] [PubMed]
- Brunnberg, J.; Barends, M.; Frühschulz, S.; Winter, C.; Battin, C.; de Wet, B.; Cole, D.K.; Steinberger, P.; Tampé, R. Dual Role of the Peptide-Loading Complex as Proofreader and Limiter of MHC-I Presentation. Proc. Natl. Acad. Sci. USA 2024, 121, e2321600121. [Google Scholar] [CrossRef] [PubMed]
- Desikan, H.; Kaur, A.; Pogozheva, I.D.; Raghavan, M. Effects of Calreticulin Mutations on Cell Transformation and Immunity. J. Cell. Mol. Med. 2023, 27, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).