Epigenetic Regulation of Immune Responses in Endocrine-Related Cancers and Its Role in Immunotherapy
Simple Summary
Abstract
1. Introduction
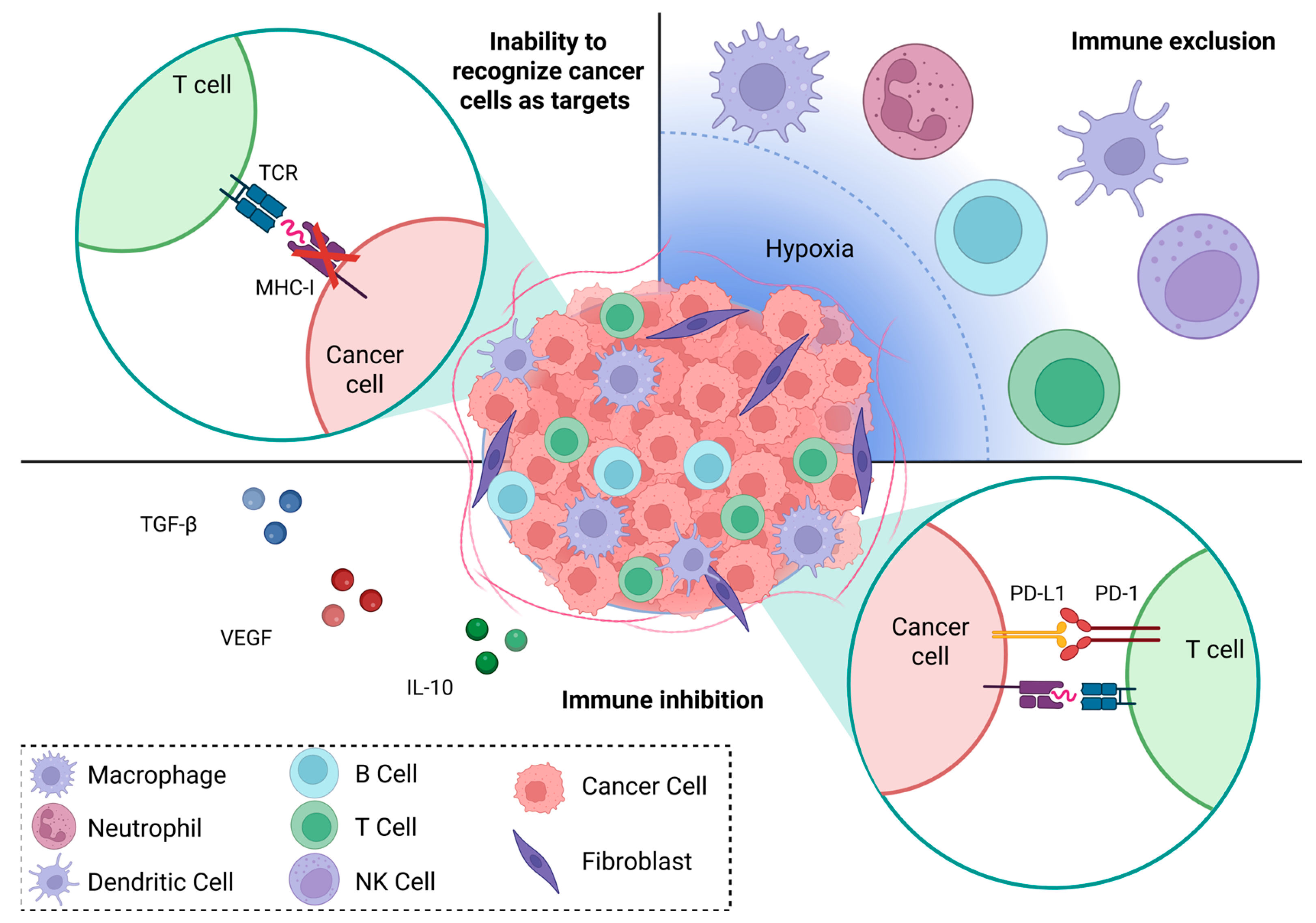
2. Immune Landscape of Endocrine-Related Cancers
3. The Rise of Epi-Drugs
4. Epigenetic Modifications Affecting Immune Responses in Cancer
4.1. Immune Recognition
4.2. Immune Exclusion
4.3. Immune Regulation—Innate Immunity
4.4. Adaptive Immunity—Checkpoint Inhibitors and Exhausted T Cells
4.5. Immune–Epigenetic–Hormonal Axis in ERCs
5. Targeting Epigenetic Dysregulation to Overcome Immunotherapy Resistance
| ERC Type | NCT Number | Epi-Drug | Epigenetic Target | Immunotherapy | Immune Target | Other Intervention | Status | Phase | References |
|---|---|---|---|---|---|---|---|---|---|
| prostate | NCT04388852 | DS3201 | EZH1/2 inhibitor | Ipilimumab | CTLA-4 | active | 1 | ||
| prostate | NCT02619253 | Vorinostat | HDACi | Pembrolizumab | PD-1 | completed | 1 | [159,161] | |
| ovarian | NCT01673217 | Decitabine | DNMTi | NY-ESO-1 vaccination | NY-ESO-1 antigen | doxorubicin | completed | 1 | [98] |
| ovarian | NCT02901899 | Guadecitabine | DNMTi | Pembrolizumab | PD-1 | completed | 2 | [160] | |
| ovarian, breast | NCT02811497 | CC-486 (oral azacitidine) | DNMTi | Durvalumab | PD-L1 | +/− vitamin C | completed | 2 | [156] |
| ovarian | NCT02900560 | Azacitidine | DNMTi | Pembrolizumab | PD-1 | terminated | 2 | [162] | |
| ovarian | NCT03206047 | Guadecitabine | DNMTi | Atezolizumab +/− NY-ESO targeting vaccine (CDX-1401) | PD-L1 +/− NY-ESO-1 antigen | completed | 1,2 | ||
| ovarian, breast | NCT03292172 | RO6870810 | BETi | atezolizumab +/− NY-ESO targeting vaccine (CDX-1401) | PD-L1 +/− NY-ESO-1 antigen | terminated | 1 | [163] | |
| ovarian | NCT04840589 | ZEN-3694 | BETi | Nivolumab +/− Ipilimumab | PD-1 +/− CTLA-4 | recruiting | 2 | ||
| ovarian | NCT02915523 | Entinostat | HDACi | Avelumab | PD-L1 | completed | 1,2 | [164] | |
| breast | NCT02453620 | Entinostat | HDACi | Nivolumab + ipilimumab | PD-1 + CTLA-4 | active, not recruiting | 1,2 | [158,165] | |
| breast | NCT02708680 | Entinostat | HDACi | atezolizumab +/− NY-ESO targeting vaccine (CDX-1401) | PD-L1 | completed | 1,2 | [157] | |
| breast | NCT02395627 | Vorinostat | HDACi | Pembrolizumab | PD-1 | anti-estrogen (tamoxifen) | terminated | 2 | [166] |
| breast | NCT02393794 | Romidepsin | HDACi | Nivolumab | PD-1 | cisplatin | unknown/completion estimated 2025 | 1,2 |
Challenges in Designing Epigenetic and Immunotherapy Combination Strategies for ERCs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-1BBL | 4-1BB ligand |
| Arg-1 | Arginase 1 |
| BC | Breast cancer |
| CAR-T | Chimeric antigen receptor-T cell |
| CCL4/5 | C-C motif chemokine ligand 4/5 |
| CD4/8/40 | Cluster of differentiation 4/8/40 |
| CSF-1 | Colony stimulating factor 1 |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CXCL9/10 | C-X-C motif chemokine ligand 9/10 |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| DNMTi | DNA methyltransferase inhibitor |
| DOT1L | Disruptor of telomeric silencing 1-like |
| dsRNA | double-stranded RNA |
| DZNep | 3-deazaneplanocin A |
| ECM | Extracellular matrix |
| ER | Estrogen receptor |
| ERC | Endocrine-related cancer |
| ERV | Endogenous retrovirus |
| EZH2 | Enhancer of Zeste homolog 2 |
| FOLR2 | Folate receptor beta |
| GATA3-AS1 | GATA binding protein 3-antisense 1 |
| H3K27me3 | Histone H3 lysine 27 trimethylation |
| H3K4 | Histone H3 lysine 4 |
| H3K79 | Histone H3 lysine 79 |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HDACi | Histone deacetylase inhibitor |
| HER | Human epidermal growth factor receptor |
| HLA-DR | Human leukocyte antigen-DR isotype |
| HMT | Histone methyltransferase |
| HOTTIP | Homebox A transcript at the distal tip |
| HR | Hormone receptor |
| ICI | Immune checkpoint inhibitor |
| IFN | Interferon |
| IFNγ | Interferon gamma |
| IL-10 | Interleukin 10 |
| lncRNA | Long non-coding RNA |
| LPS | Lipopolysaccharide |
| LSD | Lysine-specific demethylase |
| MAGE | Melanoma antigen gene |
| Malat-1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| MDSC | Myeloid derived suppressor cell |
| METTL2 | Methyltransferase 2 |
| MHC-I | Major histocompatibility complex class 1 |
| miR | MicroRNA |
| ncRNA | Non-coding ribonucleic acid |
| NK | Natural killer |
| NY-ESO-1 | New York esophageal squamous cell carcinoma 1 |
| OS | Overall survival |
| OX-40L | OX-40 ligand |
| PD-1/2 | Programmed cell death protein 1/2 |
| pDC | Plasmacytoid dendritic cells |
| PD-L1/2 | Programmed death-ligand 1/2 |
| PFS | Progression-free survival |
| PTC | Papillary thyroid cancer |
| RNA | Ribonucleic acid |
| SMARCE1 | Switch/Sucrose non-fermentable-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 |
| TAM | Tumor-associated macrophage |
| TE | Transposable element |
| TET1 | Ten-eleven translocation 1 |
| TGF-β | Transforming growth factor beta |
| Th1 | T helper 1 |
| TIL | Tumor-infiltrating lymphocyte |
| TME | Tumor microenvironment |
| TNBC | Triple negative breast cancer |
| Treg | Regulatory T cell |
| TSA | Tumor-specific antigen |
| TSHR | Thyroid-stimulating hormone receptor |
| VEGF | Vascular endothelial growth factor |
| VEGF-A | Vascular endothelial growth factor A |
| β2M | Beta-2 microglobulin |
References
- Liu, D.; Zhou, L.; Li, C.; Li, Y.; Liu, J.; Zhou, L.; Tang, J.; Xiong, W.; Wang, L. Endocrine cancer trends 1990–2021: Global disparities and health inequalities. Endocr. Relat. Cancer 2024, 31, e230363. [Google Scholar] [CrossRef]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- French, J.D. Immunotherapy for advanced thyroid cancers—Rationale, current advances and future strategies. Nat. Rev. Endocrinol. 2020, 16, 629–641. [Google Scholar] [CrossRef]
- Mitsogiannis, I.; Tzelves, L.; Dellis, A.; Issa, H.; Papatsoris, A.; Moussa, M. Prostate cancer immunotherapy. Expert Opin. Biol. Ther. 2022, 22, 577–590. [Google Scholar] [CrossRef]
- Chardin, L.; Leary, A. Immunotherapy in Ovarian Cancer: Thinking Beyond PD-1/PD-L1. Front. Oncol. 2021, 11, 795547. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Imran, K.; Iqbal, M.J.; Ahmed, M.M.; Khalid, A.; Cortés, H.; Reyes-Hernández, O.D.; Figueroa-González, G.; Leyva-Gómez, G.; Falzone, L.; Libra, M.; et al. Epigenetic dysregulation in cancer: Mechanisms, diagnostic biomarkers and therapeutic strategies. Med. Oncol. 2025, 42, 359. [Google Scholar] [CrossRef]
- Mishra, B.; Bachu, M.; Yuan, R.; Wingert, C.; Chaudhary, V.; Brauner, C.; Bell, R.; Ivashkiv, L.B. IL-10 targets IRF transcription factors to suppress IFN and inflammatory response genes by epigenetic mechanisms. Nat. Immunol. 2025, 26, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Davis, X.D.; Bohannon, J.K. TGFβ macrophage reprogramming: A new dimension of macrophage plasticity. J. Leukoc. Biol. 2024, 115, 411–414. [Google Scholar] [CrossRef]
- Chatterjee, B.; Majumder, P.; Chen, C.-C.; Wang, J.-P.; Su, P.-H.; Lai, H.-C.; Liu, C.-C.; Lin, H.-N.; Yu, C.-H.A.; Yuan, H.S.; et al. Hypoxia-induced genome-wide DNA demethylation by DNMT3A and EMT of cancer cells. Cell. Mol. Biol. Lett. 2025, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Bertrand, K.; Minhas, K.; Coomber, B.L. Induction of DNA Hypomethylation by Tumor Hypoxia. Epigenetics 2007, 2, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. High Tumor Mutation Burden and Other Immunotherapy Response Predictors in Breast Cancers: Associations and Therapeutic Opportunities. Target. Oncol. 2019, 15, 127–138. [Google Scholar] [CrossRef]
- Luo, C.; Chen, J.; Chen, L. Exploration of gene expression profiles and immune microenvironment between high and low tumor mutation burden groups in prostate cancer. Int. Immunopharmacol. 2020, 86, 106709. [Google Scholar] [CrossRef]
- Thomas, A.; Routh, E.D.; Pullikuth, A.; Jin, G.; Su, J.; Chou, J.W.; Hoadley, K.A.; Print, C.; Knowlton, N.; Black, M.A.; et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. OncoImmunology 2018, 7, e1490854. [Google Scholar] [CrossRef]
- Liao, J.; Ye, Y.; Xu, X. Comprehensive analysis of tumor mutation burden and immune microenvironment in prostate cancer. Clin. Transl. Oncol. 2022, 24, 1986–1997. [Google Scholar] [CrossRef]
- Menicali, E.; Guzzetti, M.; Morelli, S.; Moretti, S.; Puxeddu, E. Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. 2021, 11, 637826. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, J.; Wang, Z.; Zhang, Z.; Peng, J.; Wang, Y.; Hong, L. A novel tumor mutational burden-based risk model predicts prognosis and correlates with immune infiltration in ovarian cancer. Front. Immunol. 2022, 13, 943389. [Google Scholar] [CrossRef]
- Bou-Dargham, M.J.; Sha, L.; Sang, Q.-X.A.; Zhang, J. Immune landscape of human prostate cancer: Immune evasion mechanisms and biomarkers for personalized immunotherapy. BMC Cancer 2020, 20, 572. [Google Scholar] [CrossRef] [PubMed]
- Apavaloaei, A.; Hardy, M.-P.; Thibault, P.; Perreault, C. The Origin and Immune Recognition of Tumor-Specific Antigens. Cancers 2020, 12, 2607. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, V.; Marincola, F.M. Hypoxia and the phenomenon of immune exclusion. J. Transl. Med. 2021, 19, 9. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Tuong, Z.K.; Loudon, K.W.; Berry, B.; Richoz, N.; Jones, J.; Tan, X.; Nguyen, Q.; George, A.; Hori, S.; Field, S.; et al. Resolving the immune landscape of human prostate at a single-cell level in health and cancer. Cell Rep. 2021, 37, 110132. [Google Scholar] [CrossRef]
- Meagher, N.S.; Hamilton, P.; Milne, K.; Thornton, S.; Harris, B.; Weir, A.; Alsop, J.; Bisinoto, C.; Brenton, J.D.; Brooks-Wilson, A.; et al. Profiling the immune landscape in mucinous ovarian carcinoma. Gynecol. Oncol. 2022, 168, 23–31. [Google Scholar] [CrossRef]
- Gerashchenko, T.; Frolova, A.; Patysheva, M.; Fedorov, A.; Stakheyeva, M.; Denisov, E.; Cherdyntseva, N. Breast Cancer Immune Landscape: Interplay Between Systemic and Local Immunity. Adv. Biol. 2024, 8, e2400140. [Google Scholar] [CrossRef]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef]
- Ryder, M.; A Ghossein, R.; Ricarte-Filho, J.C.M.; A Knauf, J.; A Fagin, J. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr.-Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef]
- Dos Reis, F.D.; Jerónimo, C.; Correia, M.P. Epigenetic modulation and prostate cancer: Paving the way for NK cell anti-tumor immunity. Front. Immunol. 2023, 14, 1152572. [Google Scholar] [CrossRef]
- Lv, J.; Liu, C.; Chen, F.-K.; Feng, Z.-P.; Jia, L.; Liu, P.-J.; Yang, Z.-X.; Hou, F.; Deng, Z.-Y. M2-like tumour-associated macrophage—Secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021, 24, 604. [Google Scholar] [CrossRef]
- Lv, J.; Feng, Z.; Chen, F.; Liu, C.; Jia, L.; Liu, P.; Yang, C.; Hou, F.; Deng, Z. M2-like tumor-associated macrophages-secreted Wnt1 and Wnt3a promotes dedifferentiation and metastasis via activating β-catenin pathway in thyroid cancer. Mol. Carcinog. 2020, 60, 25–37. [Google Scholar] [CrossRef]
- Ramos, R.N.; Missolo-Koussou, Y.; Gerber-Ferder, Y.; Bromley, C.P.; Bugatti, M.; Núñez, N.G.; Boari, J.T.; Richer, W.; Menger, L.; Denizeau, J.; et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 2022, 185, 1189–1207.e25. [Google Scholar] [CrossRef]
- De Sanctis, F.; Bronte, V.; Ugel, S. Tumor-Induced Myeloid-Derived Suppressor Cells. In Myeloid Cells in Health and Disease: A Synthesis; Wiley: Hoboken, NJ, USA, 2017; pp. 833–856. [Google Scholar] [CrossRef]
- Christmas, B.J.; Rafie, C.I.; Hopkins, A.C.; Scott, B.A.; Ma, H.S.; Cruz, K.A.; Woolman, S.; Armstrong, T.D.; Connolly, R.M.; Azad, N.A.; et al. Entinostat Converts Immune-Resistant Breast and Pancreatic Cancers into Checkpoint-Responsive Tumors by Reprogramming Tumor-Infiltrating MDSCs. Cancer Immunol. Res. 2018, 6, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Humblin, E.; Korpas, I.; Prokhnevska, N.; Vaidya, A.; Filipescu, D.; Lu, J.; van der Heide, V.; Fraga, C.A.d.C.; Bobrowski, T.; Marks, A.; et al. The costimulatory molecule ICOS limits memory-like properties and function of exhausted PD-1+CD8+ T cells. Immunity 2025, 58, 1966–1983.e10. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Jerome, J.M.; Krieger, K.L.; Ashraf, N.; Rowley, D.R. The reactive stroma response regulates the immune landscape in prostate cancer. J. Transl. Genet. Genom. 2024, 8, 249–277. [Google Scholar] [CrossRef]
- Chowdhury, S.; Veyhl, J.; Jessa, F.; Polyakova, O.; Alenzi, A.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016, 7, 32318–32328. [Google Scholar] [CrossRef]
- Bai, Y.; Niu, D.; Huang, X.; Jia, L.; Kang, Q.; Dou, F.; Ji, X.; Xue, W.; Liu, Y.; Li, Z.; et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn. Pathol. 2017, 12, 72. [Google Scholar] [CrossRef]
- Van Verschuer, V.M.; Hooning, M.J.; van Baare-Georgieva, R.D.; Hollestelle, A.; Timmermans, A.M.; Koppert, L.B.; Verhoog, L.C.; Martens, J.W.; Seynaeve, C.; van Deurzen, C.H. Tumor-associated inflammation as a potential prognostic tool in BRCA1/2-associated breast cancer. Hum. Pathol. 2015, 46, 182–190. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef]
- Varga, A.; Piha-Paul, S.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Morosky, A.; Yang, P.; Ruman, J.; Matei, D. Pembrolizumab in patients with programmed death ligand 1–positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019, 152, 243–250. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.-P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.-C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti–PD-1 antibody pembrolizumab in patients with advanced, PD-L1–positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Rugo, H.S.; Delord, J.-P.; Im, S.-A.; Ott, P.A.; Piha-Paul, S.A.; Bedard, P.L.; Sachdev, J.; Le Tourneau, C.; van Brummelen, E.M.; Varga, A.; et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Luo, H.; Meng, X.; Zhu, W.; Wang, D.; Zeng, H.; Zhang, H. The frequency and inter-relationship of PD-L1 expression and tumour mutational burden across multiple types of advanced solid tumours in China. Exp. Hematol. Oncol. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Lønning, P.; Helle, H.; Duong, N.; Ekse, D.; Aas, T.; Geisler, J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J. Steroid Biochem. Mol. Biol. 2009, 117, 31–41. [Google Scholar] [CrossRef]
- Zhao, R.; Lian, W.; Xu, Q. Sex hormones and immune regulation in ovarian cancer. Discov. Oncol. 2024, 15, 849. [Google Scholar] [CrossRef]
- Montesinos, M.d.M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chin, Y.-T.; Shih, Y.-J.; Chen, Y.-R.; Leinung, M.; Keating, K.A.; Mousa, S.A.; Davis, P.J. In tumor cells, thyroid hormone analogues non-immunologically regulate PD-L1 and PD-1 accumulation that is anti-apoptotic. Oncotarget 2018, 9, 34033–34037. [Google Scholar] [CrossRef]
- Ghisoni, E.; Benedetti, F.; Minasyan, A.; Desbuisson, M.; Cunnea, P.; Grimm, A.J.; Fahr, N.; Capt, C.; Rayroux, N.; De Carlo, F.; et al. Myeloid cell networks govern re-establishment of original immune landscapes in recurrent ovarian cancer. Cancer Cell 2025, 43, 1568–1586.e10. [Google Scholar] [CrossRef]
- Peres, L.C.; Colin-Leitzinger, C.; Sinha, S.; Marks, J.R.; Conejo-Garcia, J.R.; Alberg, A.J.; Bandera, E.V.; Berchuck, A.; Bondy, M.L.; Christensen, B.C.; et al. Racial Differences in the Tumor Immune Landscape and Survival of Women with High-Grade Serous Ovarian Carcinoma. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1006–1016. [Google Scholar] [CrossRef]
- Novysedlak, R.; Guney, M.; Al Khouri, M.; Bartolini, R.; Foley, L.K.; Benesova, I.; Ozaniak, A.; Novak, V.; Vesely, S.; Pacas, P.; et al. The Immune Microenvironment in Prostate Cancer: A Comprehensive Review. Oncology 2024, 103, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Ciarka, A.; Piątek, M.; Pęksa, R.; Kunc, M.; Senkus, E. Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Prognostic and Predictive Significance across Molecular Subtypes. Biomedicines 2024, 12, 763. [Google Scholar] [CrossRef]
- Dvir, K.; Giordano, S.; Leone, J.P. Immunotherapy in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 7517. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Guo, Y. Advances in and prospects of immunotherapy for prostate cancer. Cancer Lett. 2024, 601, 217155. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Matsubara, N.; Oudard, S.; Saad, F.; Merseburger, A.S.; Soares, A.; McGregor, B.A.; Zurawski, B.; et al. Cabozantinib plus atezolizumab in metastatic prostate cancer (CONTACT-02): Final analyses from a phase 3, open-label, randomised trial. Lancet Oncol. 2025, 26, 860–876. [Google Scholar] [CrossRef]
- Neto, A.P.; Lucchesi, H.L.; Valsecchi, V.A.; Dos, S.; Ward, L.S.; Cunha, L.L. Immuno-therapy for patients with thyroid cancer: A comprehensive appraisal. Chin. Clin. Oncol. 2024, 13, 36. [Google Scholar] [CrossRef]
- Ghisoni, E.; Morotti, M.; Sarivalasis, A.; Grimm, A.J.; Kandalaft, L.; Laniti, D.D.; Coukos, G. Immunotherapy for ovarian cancer: Towards a tailored immunophenotype-based approach. Nat. Rev. Clin. Oncol. 2024, 21, 801–817. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Vignali, P.; Proietti, A.; Ugolini, C.; Torregrossa, L.; Basolo, A.; Elisei, R.; Santini, F.; Basolo, F. Predictive Biomarkers in Thyroid Cancer. Front. Oncol. 2022, 12, 901004. [Google Scholar] [CrossRef]
- Al Aboud, H.G.; Simpson, B.; Al Aboud, N.M. Genetics, DNA Packaging; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Castro-Muñoz, L.J.; Ulloa, E.V.; Sahlgren, C.; Lizano, M.; De La Cruz-Hernández, E.; Contreras-Paredes, A. Modulating epigenetic modifications for cancer therapy (Review). Oncol. Rep. 2023, 49, 59. [Google Scholar] [CrossRef]
- Zelic, R.; Fiano, V.; Grasso, C.; Zugna, D.; Pettersson, A.; Gillio-Tos, A.; Merletti, F.; Richiardi, L. Global DNA hypomethylation in prostate cancer development and progression: A systematic review. Prostate Cancer Prostatic Dis. 2014, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Klinkebiel, D.; Barger, C.J.; Pandey, S.; Guda, C.; Miller, A.; Akers, S.N.; Odunsi, K.; Karpf, A.R. Global DNA Hypomethylation in Epithelial Ovarian Cancer: Passive Demethylation and Association with Genomic Instability. Cancers 2020, 12, 764. [Google Scholar] [CrossRef]
- Hesselink, E.N.K.; Zafon, C.; Villalmanzo, N.; Iglesias, C.; van Hemel, B.M.; Hesselink, M.S.K.; Montero-Conde, C.; Buj, R.; Mauricio, D.; A Peinado, M.; et al. Increased Global DNA Hypomethylation in Distant Metastatic and Dedifferentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 103, 397–406. [Google Scholar] [CrossRef]
- Kim, Y.; Ko, J.Y.; Kong, H.K.; Lee, M.; Chung, W.; Lim, S.; Son, D.; Oh, S.; Park, J.W.; Kim, D.Y.; et al. Hypomethylation of ATP1A1 Is Associated with Poor Prognosis and Cancer Progression in Triple-Negative Breast Cancer. Cancers 2024, 16, 1666. [Google Scholar] [CrossRef]
- Burdelski, C.; Ruge, O.M.; Melling, N.; Koop, C.; Simon, R.; Steurer, S.; Sauter, G.; Kluth, M.; Hube-Magg, C.; Minner, S.; et al. HDAC1 overexpression independently predicts biochemical recurrence and is associated with rapid tumor cell proliferation and genomic instability in prostate cancer. Exp. Mol. Pathol. 2015, 98, 419–426. [Google Scholar] [CrossRef]
- Müller, B.M.; Jana, L.; Kasajima, A.; Lehmann, A.; Prinzler, J.; Budczies, J.; Winzer, K.-J.; Dietel, M.; Weichert, W.; Denkert, C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer—Overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 2013, 13, 215. [Google Scholar] [CrossRef]
- Giaginis, C.; Alexandrou, P.; Delladetsima, I.; Giannopoulou, I.; Patsouris, E.; Theocharis, S. Clinical significance of histone deacetylase (HDAC)-1, HDAC-2, HDAC-4, and HDAC-6 expression in human malignant and benign thyroid lesions. Tumor Biol. 2013, 35, 61–71. [Google Scholar] [CrossRef]
- Hayashi, A.; Horiuchi, A.; Kikuchi, N.; Hayashi, T.; Fuseya, C.; Suzuki, A.; Konishi, I.; Shiozawa, T. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int. J. Cancer 2010, 127, 1332–1346. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Hu, X.; Zhang, Q.; Sun, T.; Cui, S.; Wang, S.; Ouyang, Q.; Yin, Y.; Geng, C.; et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 806–815. [Google Scholar] [CrossRef]
- Kaminskas, E.; Farrell, A.; Abraham, S.; Baird, A.; Hsieh, L.-S.; Lee, S.-L.; Leighton, J.K.; Patel, H.; Rahman, A.; Sridhara, R.; et al. Approval Summary: Azacitidine for Treatment of Myelodysplastic Syndrome Subtypes. Clin. Cancer Res. 2005, 11, 3604–3608. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized Controlled Trial of Azacitidine in Patients with the Myelodysplastic Syndrome: A Study of the Cancer and Leukemia Group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Zacchi, F.; Reni, A.; Rota, M.; Palmerio, S.; Menis, J.; Zivi, A.; Milleri, S.; Milella, M. Progresses and Pitfalls of Epigenetics in Solid Tumors Clinical Trials. Int. J. Mol. Sci. 2024, 25, 11740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, W.; Hu, X.; Sun, T.; Cui, S.; Wang, S.; Ouyang, Q.; Yin, Y.; Geng, C.; Tong, Z.; et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer: A long-term safety and overall survival update from the randomised, double-blind, placebo-controlled, phase 3 trial. Transl. Breast Cancer Res. 2023, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A.; Ismail-Khan, R.R.; Melichar, B.; Lichinitser, M.; Munster, P.N.; Klein, P.M.; Cruickshank, S.; Miller, K.D.; Lee, M.J.; Trepel, J.B. Randomized Phase II, Double-Blind, Placebo-Controlled Study of Exemestane with or Without Entinostat in Postmenopausal Women With Locally Recurrent or Metastatic Estrogen Receptor-Positive Breast Cancer Progressing on Treatment With a Nonsteroidal Aromatase Inhibitor. J. Clin. Oncol. 2013, 31, 2128–2135. [Google Scholar] [CrossRef]
- Connolly, R.M.; Zhao, F.; Miller, K.D.; Lee, M.-J.; Piekarz, R.L.; Smith, K.L.; Brown-Glaberman, U.A.; Winn, J.S.; Faller, B.A.; Onitilo, A.A.; et al. E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor–Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2021, 39, 3171–3181. [Google Scholar] [CrossRef]
- Rana, Z.; Diermeier, S.; Hanif, M.; Rosengren, R.J. Understanding Failure and Improving Treatment Using HDAC Inhibitors for Prostate Cancer. Biomedicines 2020, 8, 22. [Google Scholar] [CrossRef]
- Varun, D.; Haque, M.; Jackson-Oxley, J.; Thompson, R.; Kumari, A.A.; Woodcock, C.L.; Harris, A.E.; Madhusudan, S.; Rakha, E.; Rutland, C.S.; et al. Epigenetic Therapies in Endocrine-Related Cancers: Past Insights and Clinical Progress. Cancers 2025, 17, 2418. [Google Scholar] [CrossRef]
- Kong, Y.; Rose, C.M.; Cass, A.A.; Williams, A.G.; Darwish, M.; Lianoglou, S.; Haverty, P.M.; Tong, A.-J.; Blanchette, C.; Albert, M.L.; et al. Transposable element expression in tumors is associated with immune infiltration and increased antigenicity. Nat. Commun. 2019, 10, 5228. [Google Scholar] [CrossRef]
- Rycaj, K.; Plummer, J.B.; Yin, B.; Li, M.; Garza, J.; Radvanyi, L.; Ramondetta, L.M.; Lin, K.; Johanning, G.L.; Tang, D.G.; et al. Cytotoxicity of Human Endogenous Retrovirus K–Specific T Cells toward Autologous Ovarian Cancer Cells. Clin. Cancer Res. 2015, 21, 471–483. [Google Scholar] [CrossRef]
- Reis, B.S.; Jungbluth, A.A.; Frosina, D.; Holz, M.; Ritter, E.; Nakayama, E.; Ishida, T.; Obata, Y.; Carver, B.; Scher, H.; et al. Prostate Cancer Progression Correlates with Increased Humoral Immune Response to a Human Endogenous Retrovirus GAG Protein. Clin. Cancer Res. 2013, 19, 6112–6125. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human Endogenous Retrovirus K Triggers an Antigen-Specific Immune Response in Breast Cancer Patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Liu, J.; Rycaj, K.; Huang, M.; Tsai, K.; Rosen, D.G.; Chen, D.; Lu, D.W.; Barnhart, K.F.; Johanning, G.L. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer 2006, 120, 81–90. [Google Scholar] [CrossRef]
- Stricker, E.; Peckham-Gregory, E.C.; Lai, S.Y.; Sandulache, V.C.; Scheurer, M.E. Targeted Variant Assessments of Human Endogenous Retroviral Regions in Whole Genome Sequencing Data Reveal Retroviral Variants Associated with Papillary Thyroid Cancer. Microorganisms 2024, 12, 2435. [Google Scholar] [CrossRef] [PubMed]
- Grundy, E.E.; Diab, N.; Chiappinelli, K.B. Transposable element regulation and expression in cancer. FEBS J. 2021, 289, 1160–1179. [Google Scholar] [CrossRef]
- Panda, A.; de Cubas, A.A.; Stein, M.; Riedlinger, G.; Kra, J.; Mayer, T.; Smith, C.C.; Vincent, B.G.; Serody, J.S.; Beckermann, K.E.; et al. Endogenous retrovirus expression is associated with response to immune checkpoint pathway in clear cell renal cell carcinoma. J. Clin. Investig. 2018, 3, e121522. [Google Scholar] [CrossRef]
- Topham, J.T.; Titmuss, E.; Pleasance, E.D.; Williamson, L.M.; Karasinska, J.M.; Culibrk, L.; Lee, M.K.-C.; Mendis, S.; Denroche, R.E.; Jang, G.-H.; et al. Endogenous Retrovirus Transcript Levels Are Associated with Immunogenic Signatures in Multiple Metastatic Cancer Types. Mol. Cancer Ther. 2020, 19, 1889–1897. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef]
- Soldi, R.; Ghosh Halder, T.; Weston, A.; Thode, T.; Drenner, K.; Lewis, R.; Kaadige, M.R.; Srivastava, S.; Daniel Ampanattu, S.; Rodriguez Del Villar, R.; et al. The novel reversible LSD1 inhibitor SP-2577 promotes anti-tumor immunity in SWItch/Sucrose-NonFermentable (SWI/SNF) complex mutated ovarian cancer. PLoS ONE 2020, 15, e0235705. [Google Scholar] [CrossRef]
- Lian, Y.; Sang, M.; Ding, C.; Zhou, X.; Fan, X.; Xu, Y.; Lü, W.; Shan, B. Expressions of MAGE-A10 and MAGE-A11 in breast cancers and their prognostic significance: A retrospective clinical study. J. Cancer Res. Clin. Oncol. 2011, 138, 519–527. [Google Scholar] [CrossRef]
- Heninger, E.; Krueger, T.E.; Thiede, S.M.; Sperger, J.M.; Byers, B.L.; Kircher, M.R.; Kosoff, D.; Yang, B.; Jarrard, D.F.; McNeel, D.G.; et al. Inducible expression of cancer-testis antigens in human prostate cancer. Oncotarget 2016, 7, 84359–84374. [Google Scholar] [CrossRef]
- Sang, M.; Wu, X.; Fan, X.; Sang, M.; Zhou, X.; Zhou, N. MultipleMAGE-Agenes as surveillance marker for the detection of circulating tumor cells in patients with ovarian cancer. Biomarkers 2013, 19, 34–42. [Google Scholar] [CrossRef]
- Gunda, V.; Cogdill, A.P.; Bernasconi, M.J.; Wargo, J.A.; Parangi, S. Potential role of 5-Aza-2′-deoxycytidine induced MAGE-A4 expression in immunotherapy for anaplastic thyroid cancer. Surgery 2013, 154, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Menendez, L.; Walker, D.; Matyunina, L.V.; Dickerson, E.B.; Bowen, N.J.; Polavarapu, N.; Benigno, B.B.; McDonald, J.F. Identification of candidate methylation-responsive genes in ovarian cancer. Mol. Cancer 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Adair, S.J.; Hogan, K.T. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol. Immunother. 2008, 58, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Szender, J.B.; Papanicolau-Sengos, A.; Eng, K.H.; Miliotto, A.J.; Lugade, A.A.; Gnjatic, S.; Matsuzaki, J.; Morrison, C.D.; Odunsi, K. NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer. Gynecol. Oncol. 2017, 145, 420–425. [Google Scholar] [CrossRef]
- Odunsi, K.; Matsuzaki, J.; James, S.R.; Mhawech-Fauceglia, P.; Tsuji, T.; Miller, A.; Zhang, W.; Akers, S.N.; Griffiths, E.A.; Miliotto, A.; et al. Epigenetic Potentiation of NY-ESO-1 Vaccine Therapy in Human Ovarian Cancer. Cancer Immunol. Res. 2014, 2, 37–49. [Google Scholar] [CrossRef]
- Fosså, A.; Berner, A.; Fosså, S.D.; Hernes, E.; Gaudernack, G.; Smeland, E.B. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate 2004, 59, 440–447. [Google Scholar] [CrossRef]
- Nakada, T.; Noguchi, Y.; Satoh, S.; Ono, T.; Saika, T.; Kurashige, T.; Gnjatic, S.; Ritter, G.; Chen, Y.-T.; Stockert, E.; et al. NY-ESO-1 mRNA expression and immunogenicity in advanced prostate cancer. Cancer Immunol. 2003, 3, 10. [Google Scholar]
- Karbach, J.; Kiselicki, D.; Brand, K.; Wahle, C.; Sinelnikov, E.; Gustavus, D.; Hoffmeister, H.; Prisack, H.-B.; Atmaca, A.; Jäger, E. Tumor-infiltrating lymphocytes mediate complete and durable remission in a patient with NY-ESO-1 expressing prostate cancer. J. Immunother. Cancer 2023, 11, e005847. [Google Scholar] [CrossRef]
- Gunda, V.; Frederick, D.T.; Bernasconi, M.J.; Wargo, J.A.; Parangi, S. A Potential Role for Immunotherapy in Thyroid Cancer by Enhancing NY-ESO-1 Cancer Antigen Expression. Thyroid® 2014, 24, 1241–1250. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishigami, S.; Kijima, Y.; Funasako, Y.; Hirata, M.; Okumura, H.; Shinchi, H.; Koriyama, C.; Ueno, S.; Yoshinaka, H.; et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer 2011, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; LoPresti, J.S.; Epstein, A.L. MHC Class I Loss Is a Frequent Mechanism of Immune Escape in Papillary Thyroid Cancer That Is Reversed by Interferon and Selumetinib Treatment. In Vitro Clin. Cancer Res. 2014, 20, 6034–6044. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Villabona, L.; Bergfeldt, K.; Carlson, J.W.; Ferrone, S.; Kiessling, R.; Seliger, B.; Masucci, G.V. Correlation of HLA-A02* genotype and HLA class I antigen down-regulation with the prognosis of epithelial ovarian cancer. Cancer Immunol. Immunother. 2012, 61, 1243–1253. [Google Scholar] [CrossRef]
- Luker, A.J.; Graham, L.J.; Smith, T.M.; Camarena, C.; Zellner, M.P.; Gilmer, J.-J.S.; Damle, S.R.; Conrad, D.H.; Bear, H.D.; Martin, R.K. The DNA methyltransferase inhibitor, guadecitabine, targets tumor-induced myelopoiesis and recovers T cell activity to slow tumor growth in combination with adoptive immunotherapy in a mouse model of breast cancer. BMC Immunol. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Nixon, M.J.; Gonzalez-Ericsson, P.I.; Sanchez, V.; Opalenik, S.R.; Li, H.; Zahnow, C.A.; Nickels, M.L.; Liu, F.; Tantawy, M.N.; et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat. Commun. 2018, 9, 248. [Google Scholar] [CrossRef]
- Qin, Y.; Vasilatos, S.N.; Chen, L.; Wu, H.; Cao, Z.; Fu, Y.; Huang, M.; Vlad, A.M.; Lu, B.; Oesterreich, S.; et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene 2018, 38, 390–405. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef]
- Ji, X.; Guo, D.; Ma, J.; Yin, M.; Yu, Y.; Liu, C.; Zhou, Y.; Sun, J.; Li, Q.; Chen, N.; et al. Epigenetic Remodeling Hydrogel Patches for Multidrug-Resistant Triple-Negative Breast Cancer. Adv. Mater. 2021, 33, 2100949. [Google Scholar] [CrossRef]
- Giannakakis, A.; Karapetsas, A.; Dangaj, D.; Lanitis, E.; Tanyi, J.; Coukos, G.; Sandaltzopoulos, R. Overexpression of SMARCE1 is associated with CD8+ T-cell infiltration in early stage ovarian cancer. Int. J. Biochem. Cell Biol. 2014, 53, 389–398. [Google Scholar] [CrossRef]
- Hey, J.; Halperin, C.; Hartmann, M.; Mayer, S.; Schönung, M.; Lipka, D.B.; Scherz-Shouval, R.; Plass, C. DNA methylation landscape of tumor-associated macrophages reveals pathways, transcription factors and prognostic value relevant to triple-negative breast cancer patients. Int. J. Cancer 2022, 152, 1226–1242. [Google Scholar] [CrossRef]
- Tian, X.; Wang, T.; Shen, H.; Wang, S. Tumor microenvironment. histone modifications, and myeloid-derived suppressor cells. Cytokine Growth Factor Rev. 2023, 74, 108–121. [Google Scholar] [CrossRef]
- Kashyap, V.; Ahmad, S.; Nilsson, E.M.; Helczynski, L.; Kenna, S.; Persson, J.L.; Gudas, L.J.; Mongan, N.P. The lysine specific demethylase-1 (LSD1/KDM1A) regulates VEGF-A expression in prostate cancer. Mol. Oncol. 2013, 7, 555–566. [Google Scholar] [CrossRef]
- Moufarrij, S.; Srivastava, A.; Gomez, S.; Hadley, M.; Palmer, E.; Austin, P.T.; Chisholm, S.; Diab, N.; Roche, K.; Yu, A.; et al. Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer. Sci. Rep. 2020, 10, 3470. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, M.-J.; Lee, S.; Tomita, S.; Chumsri, S.; Cruickshank, S.; Ordentlich, P.; Trepel, J.B. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: Correlative analysis of ENCORE 301, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. OncoImmunology 2016, 5, e1219008. [Google Scholar] [CrossRef]
- Stone, M.L.; Chiappinelli, K.B.; Li, H.; Murphy, L.M.; Travers, M.E.; Topper, M.J.; Mathios, D.; Lim, M.; Shih, I.-M.; Wang, T.-L.; et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc. Natl. Acad. Sci. USA 2017, 114, E10981–E10990. [Google Scholar] [CrossRef]
- Belk, J.A.; Daniel, B.; Satpathy, A.T. Epigenetic regulation of T cell exhaustion. Nat. Immunol. 2022, 23, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Han, Y.; Gu, T.; Wang, R.; Si, X.; Kong, D.; Zhao, P.; Wang, X.; Li, J.; Zhai, X.; et al. Class I HDAC inhibitors enhance antitumor efficacy and persistence of CAR-T cells by activation of the Wnt pathway. Cell Rep. 2024, 43, 114065. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.; Bhargava, R.K.; Ahn, R.; Bahna, S.; Kang, N.H.; Lacoul, A.; Niles, L.P. HDAC inhibitor M344 suppresses MCF-7 breast cancer cell proliferation. Biomed. Pharmacother. 2012, 66, 232–236. [Google Scholar] [CrossRef] [PubMed]
- De Vos, L.; Dietrich, J.; Strieth, S.; Bootz, F.; Dietrich, D.; Franzen, A. PD-1, CTLA4, PD-L1 and PD-L2 DNA Methylation in Papillary Thyroid Carcinoma. Immunotherapy 2020, 12, 903–920. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Gaire, B.; Zou, Y.; Uddin, M.M.; Vancurova, I. IFNγ-induced PD-L1 expression in ovarian cancer cells is regulated by JAK1, STAT1 and IRF1 signaling. Cell. Signal. 2022, 97, 110400. [Google Scholar] [CrossRef]
- Wang, H.; Fu, C.; Du, J.; Wang, H.; He, R.; Yin, X.; Li, H.; Li, X.; Wang, H.; Li, K.; et al. Enhanced histone H3 acetylation of the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for PD-L1 expression in drug-resistant cancer cells. J. Exp. Clin. Cancer Res. 2020, 39, 29. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, L.; Wang, M.; Xu, B.; Lyu, R.; Shi, Y.G.; Tan, L. TET2 Inhibits PD-L1 Gene Expression in Breast Cancer Cells through Histone Deacetylation. Cancers 2021, 13, 2207. [Google Scholar] [CrossRef]
- Darvin, P.; Nair, V.S.; Elkord, E. PD-L1 Expression in Human Breast Cancer Stem Cells Is Epigenetically Regulated through Posttranslational Histone Modifications. J. Oncol. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.J.; Christenson, J.L.; Greene, L.I.; O’NEill, K.I.; Williams, M.M.; Gordon, M.A.; Nemkov, T.; D’ALessandro, A.; Degala, G.D.; Shin, J.; et al. Reversal of Triple-Negative Breast Cancer EMT by miR-200c Decreases Tryptophan Catabolism and a Program of Immunosuppression. Mol. Cancer Res. 2019, 17, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Janzer, A.; Becker, A.; Zimmer, A.; Schüle, R.; Buettner, R.; Kirfel, J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis 2009, 31, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, S.; Sedukhina, A.S.; Nakagawa, Y.; Maeda, I.; Kubota, M.; Ohnuma, S.; Tsugawa, K.; Ohta, T.; Roche-Molina, M.; Bernal, J.A.; et al. LSD1 Overexpression Is Associated with Poor Prognosis in Basal-Like Breast Cancer, and Sensitivity to PARP Inhibition. PLoS ONE 2015, 10, e0118002. [Google Scholar] [CrossRef]
- Tu, W.J.; McCuaig, R.D.; Tan, A.H.Y.; Hardy, K.; Seddiki, N.; Ali, S.; Dahlstrom, J.E.; Bean, E.G.; Dunn, J.; Forwood, J.; et al. Targeting Nuclear LSD1 to Reprogram Cancer Cells and Reinvigorate Exhausted T Cells via a Novel LSD1-EOMES Switch. Front. Immunol. 2020, 11, 1228. [Google Scholar] [CrossRef]
- Kong, L.-L.; Man, D.-M.; Wang, T.; Zhang, G.-A.; Cui, W. Downregulation of LSD1 suppresses the proliferation, tumorigenicity and invasion of papillary thyroid carcinoma K1 cells. Oncol. Lett. 2016, 11, 2475–2480. [Google Scholar] [CrossRef][Green Version]
- Bally, A.P.R.; Neeld, D.K.; Lu, P.; Majumder, P.; Tang, Y.; Barwick, B.G.; Wang, Q.; Boss, J.M. PD-1 Expression during Acute Infection Is Repressed through an LSD1–Blimp-1 Axis. J. Immunol. 2020, 204, 449–458. [Google Scholar] [CrossRef]
- Liu, J.; He, D.; Cheng, L.; Huang, C.; Zhang, Y.; Rao, X.; Kong, Y.; Li, C.; Zhang, Z.; Liu, J.; et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene 2020, 39, 3939–3951. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Messina, E.; Sanavia, T.; Mundo, L.; Farinella, F.; Lazzi, S.; Megiorni, F.; Ceccarelli, S.; Pontecorvi, P.; Marampon, F.; et al. MiR-200c-3p Contrasts PD-L1 Induction by Combinatorial Therapies and Slows Proliferation of Epithelial Ovarian Cancer through Downregulation of β-Catenin and c-Myc. Cells 2021, 10, 519. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J. Cell. Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef]
- Cioffi, M.; Trabulo, S.M.; Vallespinos, M.; Raj, D.; Kheir, T.B.; Lin, M.-L.; Begum, J.; Baker, A.-M.; Amgheib, A.; Saif, J.; et al. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget 2017, 8, 21609–21625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, N.; Song, P.; Fu, Y.; Ren, Y.; Li, Z.; Wang, J. LncRNA GATA3-AS1 facilitates tumour progression and immune escape in triple-negative breast cancer through destabilization of GATA3 but stabilization of PD-L1. Cell Prolif. 2020, 53, e12855. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef] [PubMed]
- Cacan, E. Epigenetic-mediated immune suppression of positive co-stimulatory molecules in chemoresistant ovarian cancer cells. Cell Biol. Int. 2017, 41, 328–339. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Young, M.-J.; Chang, H.-P.; Liu, C.-Y.; Lee, C.-C.; Tseng, Y.-L.; Wang, Y.-C.; Chang, W.-C.; Hung, J.-J. Estradiol-mediated inhibition of DNMT1 decreases p53 expression to induce M2-macrophage polarization in lung cancer progression. Oncogenesis 2022, 11, 25. [Google Scholar] [CrossRef]
- Jones, L.A.; Kreem, S.; Shweash, M.; Paul, A.; Alexander, J.; Roberts, C.W. Differential Modulation of TLR3- and TLR4-Mediated Dendritic Cell Maturation and Function by Progesterone. J. Immunol. 2010, 185, 4525–4534. [Google Scholar] [CrossRef]
- Jones, L.A.; Anthony, J.; Henriquez, F.L.; Lyons, R.E.; Nickdel, M.B.; Carter, K.C.; Alexander, J.; Roberts, C.W. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology 2008, 125, 59–69. [Google Scholar] [CrossRef]
- Shepherd, R.; Kim, B.; Saffery, R.; Novakovic, B. Triiodothyronine (T3) Induces Limited Transcriptional and DNA Methylation Reprogramming in Human Monocytes. Biomedicines 2022, 10, 608. [Google Scholar] [CrossRef]
- Ning, C.; Xie, B.; Zhang, L.; Li, C.; Shan, W.; Yang, B.; Luo, X.; Gu, C.; He, Q.; Jin, H.; et al. Infiltrating Macrophages Induce ERα Expression through an IL17A-mediated Epigenetic Mechanism to Sensitize Endometrial Cancer Cells to Estrogen. Cancer Res. 2016, 76, 1354–1366. [Google Scholar] [CrossRef]
- Zhang, X.; Ho, S.-M. Epigenetics meets endocrinology. J. Mol. Endocrinol. 2011, 46, R11–32. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Shi, Y.; Sandefur, C.; Meisner, L.F.; Chang, C.; Choon, A.; Reznikoff, C.R.; Bova, G.S.; Friedl, A.; Jarrard, D.F. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000, 60, 3623–3630. [Google Scholar] [PubMed]
- Yoshida, T.; Eguchi, H.; Nakachi, K.; Tanimoto, K.; Higashi, Y.; Suemasu, K.; Iino, Y.; Morishita, Y.; Hayashi, S.-I. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans-acting factors. Carcinogenesis 2000, 21, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Archey, W.B.; McEachern, K.A.; Robson, M.; Offit, K.; Vaziri, S.A.; Casey, G.; Borg, Å.; Arrick, B.A. Increased CpG methylation of the estrogen receptor gene in BRCA1-linked estrogen receptor-negative breast cancers. Oncogene 2002, 21, 7034–7041. [Google Scholar] [CrossRef][Green Version]
- Zhao, C.; Lam, E.W.-F.; Sunters, A.; Enmark, E.; De Bella, M.T.; Coombes, R.C.; Gustafsson, J.; Dahlman-Wright, K. Expression of estrogen receptor β isoforms in normal breast epithelial cells and breast cancer: Regulation by methylation. Oncogene 2003, 22, 7600–7606. [Google Scholar] [CrossRef]
- Zhu, X.; Leav, I.; Leung, Y.-K.; Wu, M.; Liu, Q.; Gao, Y.; McNeal, J.E.; Ho, S.-M. Dynamic Regulation of Estrogen Receptor-β Expression by DNA Methylation During Prostate Cancer Development and Metastasis. Am. J. Pathol. 2004, 164, 2003–2012. [Google Scholar] [CrossRef]
- Zama, A.M.; Uzumcu, M. Fetal and Neonatal Exposure to the Endocrine Disruptor Methoxychlor Causes Epigenetic Alterations in Adult Ovarian Genes. Endocrinology 2009, 150, 4681–4691. [Google Scholar] [CrossRef]
- Xing, M.; Usadel, H.; Cohen, Y.; Tokumaru, Y.; Guo, Z.; Westra, W.B.; Tong, B.C.; Tallini, G.; Udelsman, R.; Califano, J.A.; et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: A marker of malignancy and a cause of gene silencing. Cancer Res. 2003, 63, 2316–2321. [Google Scholar]
- Zhou, Q.; Atadja, P.; Davidson, N.E. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol. Ther. 2007, 6, 64–69. [Google Scholar] [CrossRef]
- Kondo, N.; Toyama, T.; Sugiura, H.; Fujii, Y.; Yamashita, H. miR-206 Expression Is Down-regulated in Estrogen Receptor α–Positive Human Breast Cancer. Cancer Res. 2008, 68, 5004–5008. [Google Scholar] [CrossRef]
- Wang, L.; Amoozgar, Z.; Huang, J.; Saleh, M.H.; Xing, D.; Orsulic, S.; Goldberg, M.S. Decitabine Enhances Lymphocyte Migration and Function and Synergizes with CTLA-4 Blockade in a Murine Ovarian Cancer Model. Cancer Immunol. Res. 2015, 3, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.C.; Knudson, K.M.; Lee, K.L.; Hamilton, D.H.; Hodge, J.W.; Figg, W.D.; Ordentlich, P.; Jones, F.R.; Rabizadeh, S.; Soon-Shiong, P.; et al. Cooperative Immune-Mediated Mechanisms of the HDAC Inhibitor Entinostat, an IL15 Superagonist, and a Cancer Vaccine Effectively Synergize as a Novel Cancer Therapy. Clin. Cancer Res. 2020, 26, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Yau, H.L.; Chakravarthy, A.; Wang, B.; Shen, S.Y.; Ettayebi, I.; Ishak, C.A.; Bedard, P.L.; Razak, A.A.; Hansen, A.R.; et al. An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J. Immunother. Cancer 2020, 8, e000883. [Google Scholar] [CrossRef] [PubMed]
- O’SHaughnessy, J.; Moroose, R.L.; Babu, S.; Baramidze, K.; Chan, D.; Leitner, S.P.; Nemsadze, G.; Ordentlich, P.; Quaranto, C.; Meyers, M.L.; et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J. Clin. Oncol. 2020, 38, 1014. [Google Scholar] [CrossRef]
- Torres, E.R.; Danilova, L.; Tandurella, J.; Gonzalez, E.; Baugh, A.; Al-Zubeidy, B.; Hopkins, A.; Murray, J.; Downs, M.; Kagohara, L.; et al. Sequencing analysis reveals evidence of immune activation in advanced HER2 negative breast cancer responders treated with entinostat + nivolumab + ipilimumab. Res. Sq. 2025, 3, 6580687. [Google Scholar] [CrossRef]
- Pili, R.; Quinn, D.I.; Adra, N.; Logan, T.; Colligan, S.; Burney, H.N.; Hahn, N.M. A Phase I/IB, Open Label, Dose Finding Study to Evaluate Safety, Pharmacodynamics and Efficacy of Pembrolizumab in Combination With Vorinostat in Patients With Advanced Prostate, Renal or Urothelial Carcinoma. Cancer Med. 2025, 14, e70725. [Google Scholar] [CrossRef]
- Chen, S.; Xie, P.; Cowan, M.; Huang, H.; Cardenas, H.; Keathley, R.; Tanner, E.J.; Fleming, G.F.; Moroney, J.W.; Pant, A.; et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J. Clin. Investig. 2022, 132, e158800. [Google Scholar] [CrossRef]
- Pili, R.; Quinn, D.I.; Albany, C.; Adra, N.; Logan, T.F.; Greenspan, A.; Budka, J.; Damayanti, N.; Green, M.A.; Fletcher, J.W.; et al. Immunomodulation by HDAC inhibition: Results from a phase Ib study with vorinostat and pembrolizumab in metastatic urothelial, renal, and prostate carcinoma patients. J. Clin. Oncol. 2019, 37, 2572. [Google Scholar] [CrossRef]
- Landon, B.V.; Kaleka, G.; Balan, A.; Boland, J.L.; Cherry, C.; Pereira, G.; Zahnow, C.; Winterhoff, B.; Baylin, S.; Velculescu, V.E.; et al. Abstract 7551: Combined epigenetic therapy and immune checkpoint blockade drive reshaping of the tumor microenvironment of platinum resistant ovarian cancer. Cancer Res. 2024, 84, 7551. [Google Scholar] [CrossRef]
- Marbach, D.; Brouer-Visser, J.; Brennan, L.; Wilson, S.; Davydov, I.I.; Staedler, N.; Duarte, J.; Quetglas, I.M.; Nüesch, E.; Cañamero, M.; et al. Immune modulation in solid tumors: A phase 1b study of RO6870810 (BET inhibitor) and atezolizumab (PD-L1 inhibitor). BMC Cancer 2025, 25, 500. [Google Scholar] [CrossRef] [PubMed]
- Cadoo, K.A.; Meyers, M.L.; Burger, R.A.; Armstrong, D.K.; Penson, R.T.; Gordon, M.S.; Fleming, G.F.; Moroney, J.W.; Hamilton, E.P.; Duska, L.R.; et al. A phase II randomized study of avelumab plus entinostat versus avelumab plus placebo in patients (pts) with advanced epithelial ovarian cancer (EOC). J. Clin. Oncol. 2019, 37, 5511. [Google Scholar] [CrossRef]
- Torres, E.R.; Ho, W.J.; Danilova, L.; Tandurella, J.A.; Leatherman, J.; Rafie, C.; Wang, C.; Brufsky, A.; LoRusso, P.; Chung, V.; et al. Entinostat, nivolumab and ipilimumab for women with advanced HER2-negative breast cancer: A phase Ib trial. Nat. Cancer 2024, 5, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Terranova-Barberio, M.; Pawlowska, N.; Dhawan, M.; Moasser, M.; Chien, A.J.; Melisko, M.E.; Rugo, H.; Rahimi, R.; Deal, T.; Daud, A.; et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat. Commun. 2020, 11, 3584. [Google Scholar] [CrossRef]
- Oktyabri, D.; Ishimura, A.; Tange, S.; Terashima, M.; Suzuki, T. DOT1L histone methyltransferase regulates the expression of BCAT1 and is involved in sphere formation and cell migration of breast cancer cell lines. Biochimie 2016, 123, 20–31. [Google Scholar] [CrossRef]
- Nassa, G.; Salvati, A.; Tarallo, R.; Gigantino, V.; Alexandrova, E.; Memoli, D.; Sellitto, A.; Rizzo, F.; Malanga, D.; Mirante, T.; et al. Inhibition of histone methyltransferase DOT1L silences ERα gene and blocks proliferation of antiestrogen-resistant breast cancer cells. Sci. Adv. 2019, 5, eaav5590. [Google Scholar] [CrossRef]
- Dong, F.; Shao, L.; Gong, R.; Shen, L.; Wang, L. Histone methyltransferase DOT1L promotes myeloid-derived suppressor cell activation to ameliorate colitis by modulating JAK2/STAT3 signaling. Mol. Cell. Toxicol. 2025, 1–12. [Google Scholar] [CrossRef]
- Scheer, S.; Runting, J.; Bramhall, M.; Russ, B.; Zaini, A.; Ellemor, J.; Rodrigues, G.; Ng, J.; Zaph, C. The Methyltransferase DOT1L Controls Activation and Lineage Integrity in CD4+ T Cells during Infection and Inflammation. Cell Rep. 2020, 33, 108505. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Zhang, Y.; Huai, W.; Zhou, Q.; Xu, S.; Chen, X.; Li, N.; Cao, X. Methyltransferase Dot1l preferentially promotes innate IL-6 and IFN-β production by mediating H3K79me2/3 methylation in macrophages. Cell. Mol. Immunol. 2018, 17, 76–84. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Wu, C.-Y.; Wang, X.-Y.; Deng, J.; Cao, W.-J.; Tang, Y.-Z.; Wan, C.-C.; Chen, Z.-T.; Zhan, W.-Y.; Shan, H.; et al. Targeting inflammatory macrophages rebuilds therapeutic efficacy of DOT1L inhibition in hepatocellular carcinoma. Mol. Ther. 2022, 31, 105–118. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, P.; Mou, Y.; Song, Y.; Qin, Y. Targeting LSD1 in tumor immuno-therapy: Rationale, challenges and potential. Front. Immunol. 2023, 14, 1214675. [Google Scholar] [CrossRef]
- Bailey, C.P.; Figueroa, M.; Gangadharan, A.; Lee, D.A.; Chandra, J. Scaffolding LSD1 Inhibitors Impair NK Cell Metabolism and Cytotoxic Function Through Depletion of Glutathione. Front. Immunol. 2020, 11, 2196. [Google Scholar] [CrossRef]
- Gu, T.; Vasilatos, S.N.; Yin, J.; Qin, Y.; Zhang, L.; Davidson, N.E.; Huang, Y. Restoration of TFPI2 by LSD1 inhibition suppresses tumor progression and potentiates antitumor immunity in breast cancer. Cancer Lett. 2024, 600, 217182. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, N.; Zhu, H.; Liu, M.; Hao, J.; Wang, S.; Guo, T.; Mamun, M.; Pang, J.; Liu, Q.; et al. Unlocking the dual role of LSD1 in tumor immunity: Innate and adaptive pathways. Theranostics 2024, 14, 7054–7071. [Google Scholar] [CrossRef] [PubMed]
- Rosborough, B.R.; Castellaneta, A.; Natarajan, S.; Thomson, A.W.; Turnquist, H.R. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J. Leukoc. Biol. 2012, 91, 701–709. [Google Scholar] [CrossRef]
- Wang, H.-F.; Ning, F.; Liu, Z.-C.; Wu, L.; Li, Z.-Q.; Qi, Y.-F.; Zhang, G.; Wang, H.-S.; Cai, S.-H.; Du, J. Histone deacetylase inhibitors deplete myeloid-derived suppressor cells induced by 4T1 mammary tumors in vivo and in vitro. Cancer Immunol. Immunother. 2016, 66, 355–366. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyuz, E.M.; Gultekin, M.; Ramage, J.M.; Spendlove, I.; Jackson, A.M.; Adhikaree, J.; Malecka, A.A. Epigenetic Regulation of Immune Responses in Endocrine-Related Cancers and Its Role in Immunotherapy. Cancers 2025, 17, 3342. https://doi.org/10.3390/cancers17203342
Akyuz EM, Gultekin M, Ramage JM, Spendlove I, Jackson AM, Adhikaree J, Malecka AA. Epigenetic Regulation of Immune Responses in Endocrine-Related Cancers and Its Role in Immunotherapy. Cancers. 2025; 17(20):3342. https://doi.org/10.3390/cancers17203342
Chicago/Turabian StyleAkyuz, Evren M., Meryem Gultekin, Judith M. Ramage, Ian Spendlove, Andrew M. Jackson, Jason Adhikaree, and Anna A. Malecka. 2025. "Epigenetic Regulation of Immune Responses in Endocrine-Related Cancers and Its Role in Immunotherapy" Cancers 17, no. 20: 3342. https://doi.org/10.3390/cancers17203342
APA StyleAkyuz, E. M., Gultekin, M., Ramage, J. M., Spendlove, I., Jackson, A. M., Adhikaree, J., & Malecka, A. A. (2025). Epigenetic Regulation of Immune Responses in Endocrine-Related Cancers and Its Role in Immunotherapy. Cancers, 17(20), 3342. https://doi.org/10.3390/cancers17203342







