Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Oncologic Patients—A New Promise? Preliminary Results
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
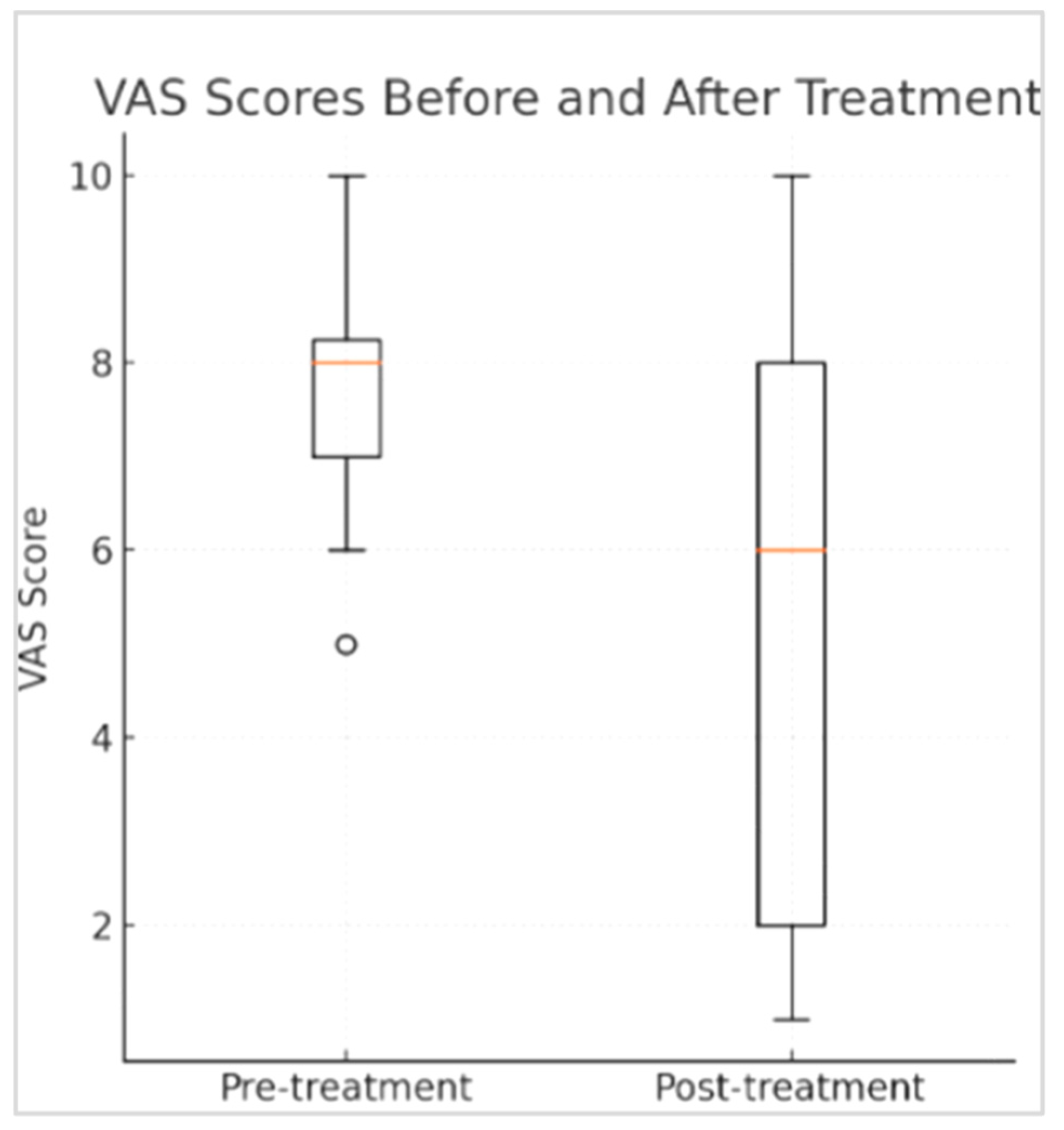
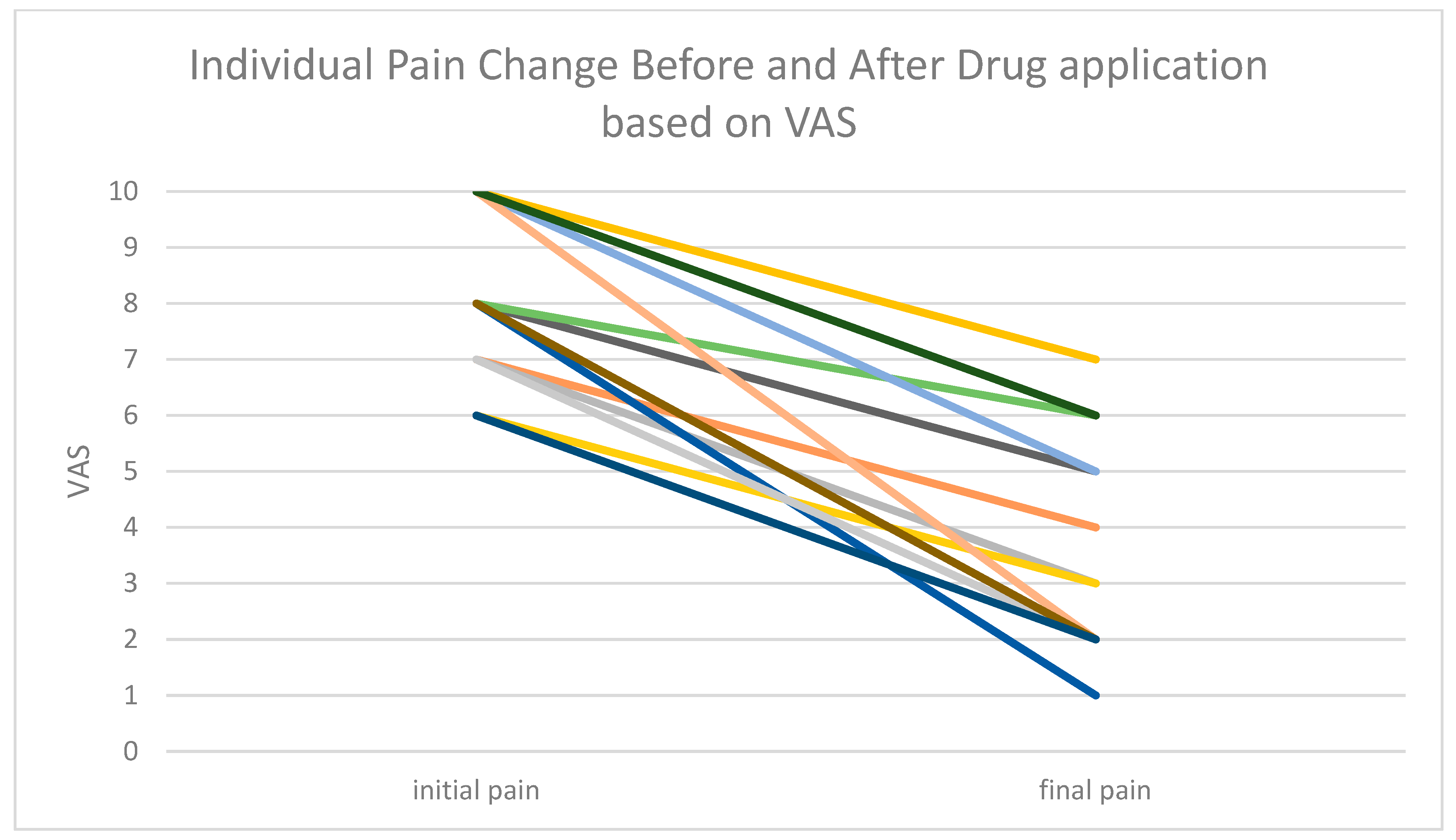
3. Results
4. Discussion
- the particle size of the applied material: there is an inverse relation between the size and penetration. The smaller the particle, the better the penetration via the skin.
- The carrier ingredients: There are several materials that are known for their capability for enhancing the penetration, e.g., ethanol.
- The formulation of the cream: as depicted above, there have been several compositions in the past with different concentrations of the same compounds. The Level of absorption was different every time up to complete failure to penetrate the skin and reach the desired goals.
- The integrity of the skin prior to the treatment can affect the absorption; the more damage to the skin, the higher the absorption.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanbayashi, Y.; Hosokawa, T. Peripheral Neuropathies Especially in Cancer Patients: Focus on Chemotherapy-induced Peripheral Neuropathies and Post-Herpetic Neuralgia. Int. J. Cancer Res. Prev. 2015, 8, 489–501. [Google Scholar]
- Addington, J.; Freimer, M. Chemotherapy-induced peripheral neuropathy: An update on the current understanding. F1000Res 2016, 5, 1466. [Google Scholar] [CrossRef]
- Grisold, W.; Cavaletti, G.; Windebank, A.J. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-oncology 2012, 14 (Suppl. 4), iv45–iv54. [Google Scholar] [CrossRef]
- Fradkin, M.; Batash, R.; Elmaleh, S.; Debi, R.; Schaffer, P.; Schaffer, M.; Asna, N. Management of Peripheral Neuropathy Induced by Chemotherapy. Curr. Med. Chem. 2019, 26, 4698–4708. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Le Cesne, A. A role for maintenance therapy in managing sarcoma. Cancer Treat. Rev. 2012, 38, 368–378. [Google Scholar] [CrossRef]
- In, G.K.; Hu, J.S.; Tseng, W.W. Treatment of advanced, metastatic soft tissue sarcoma: Latest evidence and clinical considerations. Ther. Adv. Med. Oncol. 2017, 9, 533–550. [Google Scholar] [CrossRef]
- Frisk, P.; Stålberg, E.; Strömberg, B.; Jakobson, Å. Painful peripheral neuropathy after treatment with high-dose ifosfamide. Med. Pediatr. Oncol. 2001, 37, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.M.; Connolly, S.; Latoufis, C.; Eagle, K.; Ash, C.M.; Fowler, C.; Souhami, R.L. Long-Term Neurotoxicity of Chemotherapy in Adolescents and Young Adults Treated for Bone and Soft Tissue Sarcomas. Sarcoma 1998, 2, 97–105. [Google Scholar] [CrossRef]
- Gordon-Williams, R.; Farquhar-Smith, P. Recent advances in understanding chemotherapy-induced peripheral neuropathy. F1000Res 2020, 9, 177. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef]
- Jones, K.F.; Wechsler, S.; Zulewski, D.; Wood, L. Pharmacological and Nonpharmacological Management of Chemotherapy-Induced Peripheral Neuropathy: A Scoping Review of Randomized Controlled Trials. J. Palliat. Med. 2022, 25, 964–995. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Y.; Yang, H.; Pei, Z.; Molassiotis, A. Evaluating the safety, feasibility, and efficacy of non-invasive neuromodulation techniques in chemotherapy-induced peripheral neuropathy: A systematic review. Eur. J. Oncol. Nurs. 2022, 58, 102124. [Google Scholar] [CrossRef]
- Schaffer, M.; Bonel, H.; Sroka, R.; Schaffer, P.; Busch, M.; Reiser, M.; Dühmke, E. Effects of 780 nm diode laser irradiation on blood microcirculation: Preliminary findings on time-dependent T1-weighted contrast-enhanced magnetic resonance imaging (MRI). J. Photochem. Photobiol. B Biol. 2000, 54, 55–60. [Google Scholar] [CrossRef]
- Teng, C.; Egger, S.; Blinman, P.L.; Vardy, J.L. Evaluating laser photobiomodulation for chemotherapy-induced peripheral neuropathy: A randomised phase II trial. Support. Care Cancer 2022, 31, 52. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, L.; de Souza, M.O.; Sassaron, L.A.; Ezequiel, T.d.S.; Carvalho, R.L.; Boas, V.F.V.; de Rezende, L.F. Influence of photobiomodulation on sensory symptoms, balance, and gait speed in chemotherapy-induced peripheral neuropathy. Support. Care Cancer 2025, 33, 355. [Google Scholar] [CrossRef] [PubMed]
- Argenta, P.A.; Ballman, K.V.; Geller, M.A.; Carson, L.F.; Ghebre, R.; Mullany, S.A.; Teoh, D.G.; Winterhoff, B.J.; Rivard, C.L.; Erickson, B.K. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: A randomized, sham-controlled clinical trial. Gynecol. Oncol. 2017, 144, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Joy, L.; Jolien, R.; Marithé, C.; Stijn, E.; Laura, S.; Hilde, L.; Sandra, B.; Wendy, N.; Ruth, H.; Liesbeth, R.; et al. The use of photobiomodulation therapy for the prevention of chemotherapy-induced peripheral neuropathy: A randomized, placebo-controlled pilot trial (NEUROLASER trial). Support. Care Cancer 2022, 30, 5509–5517. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Zhang, S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin. Oncol. 2021, 48, 193–207. [Google Scholar] [CrossRef]
- Li, Y.; Lustberg, M.B.; Hu, S. Emerging Pharmacological and Non-Pharmacological Therapeutics for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. Cancers 2021, 13, 766. [Google Scholar] [CrossRef]
- Crichton, M.; Yates, P.M.; Agbejule, O.A.; Spooner, A.; Chan, R.J.; Hart, N.H. Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2403. [Google Scholar] [CrossRef]
- Streckmann, F.; Elter, T.; Lehmann, H.C.; Baurecht, H.; Nazarenus, T.; Oschwald, V.; Koliamitra, C.; Otten, S.; Draube, A.; Heinen, P.; et al. Preventive Effect of Neuromuscular Training on Chemotherapy-Induced Neuropathy: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1046–1053. [Google Scholar] [CrossRef]
- Chen, X.; Gan, Y.; Au, N.P.B.; Ma, C.H.E. Current understanding of the molecular mechanisms of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2024, 17, 1345811. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef]
- Park, S.B.; Cetinkaya-Fisgin, A.; A Argyriou, A.; Höke, A.; Cavaletti, G.; Alberti, P. Axonal degeneration in chemotherapy-induced peripheral neurotoxicity: Clinical and experimental evidence. J. Neurol. Neurosurg. Psychiatry 2023, 94, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Ollodart, J.; Steele, L.R.; Romero-Sandoval, E.A.; Strowd, R.E.; Shiozawa, Y. Contributions of neuroimmune interactions to chemotherapy-induced peripheral neuropathy development and its prevention/therapy. Biochem. Pharmacol. 2024, 222, 116070. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Pozzi, E.; Terribile, G.; Cherchi, L.; Di Girolamo, S.; Sancini, G.; Alberti, P. Ion Channel and Transporter Involvement in Chemotherapy-Induced Peripheral Neurotoxicity. Int. J. Mol. Sci. 2024, 25, 6552. [Google Scholar] [CrossRef]
- Brandolini, L.; D’Angelo, M.; Antonosante, A.; Cimini, A.; Allegretti, M. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef]
- Calcutt, N.A. Diabetic neuropathy and neuropathic pain: A (con)fusion of pathogenic mechanisms? Pain 2020, 161 (Suppl. 1), S65–S86. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.; Di Salvo, J.; Epifani, R.; Piacevole, A.; Tagliaferri, G.; et al. Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef]
- Shastri, A.; Al Aiyan, A.; Kishore, U.; Farrugia, M.E. Immune-Mediated Neuropathies: Pathophysiology and Management. Int. J. Mol. Sci. 2023, 24, 7288. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Goñi, E.; Caballero-Ávila, M.; Querol, L. Antibodies in Autoimmune Neuropathies: What to Test, How to Test, Why to Test. Neurology 2024, 103, e209725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. Ganglioside GM1 and the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 9558. [Google Scholar] [CrossRef]
- Barton, D.L.; Wos, E.J.; Qin, R.; Mattar, B.I.; Green, N.B.; Lanier, K.S.; Bearden, J.D.; Kugler, J.W.; Hoff, K.L.; Reddy, P.S.; et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support. Care Cancer 2010, 19, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.; Umutoni, F.; Hassan, M.A.; Wamburu, M.W.; Turner, R.; Patton, J.S.; Chen, X.; Lei, W. Chemotherapy-Induced Peripheral Neuropathy: A Recent Update on Pathophysiology and Treatment. Life 2024, 14, 991. [Google Scholar] [CrossRef]
- King, K.M.; Myers, A.M.; Soroka-Monzo, A.J.; Tuma, R.F.; Tallarida, R.J.; Walker, E.A.; Ward, S.J. Single and combined effects of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 2017, 174, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.C.; Blanton, H.; Dancel, C.L.; Castro-Piedras, I.; Rorabaugh, B.R.; Morgan, D.J.; Guindon, J. Chronic Administration of Cannabinoid Agonists ACEA, AM1241, and CP55,940 Induce Sex-Specific Differences in Tolerance and Sex Hormone Changes in a Chemotherapy-Induced Peripheral Neuropathy. J. Pharmacol. Exp. Ther. 2024, 391, 258–271. [Google Scholar] [CrossRef] [PubMed]
- D’aNdre, S.; McAllister, S.; Nagi, J.; Giridhar, K.V.; Ruiz-Macias, E.; Loprinzi, C. Topical Cannabinoids for Treating Chemotherapy-Induced Neuropathy: A Case Series. Integr. Cancer Ther. 2021, 20, 15347354211061739. [Google Scholar] [CrossRef]
- Cheah, I.; Hunter, J.; Gelissen, I.; Chan, W.-J.J.; Harnett, J.E. Adverse events associated with the use of cannabis-based products in people living with cancer: A systematic scoping review. Support. Care Cancer 2024, 33, 40. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Abreu, D.; Galván, S.; Federico, M.; Martínez-Sánchez, G.; Ramallo-Fariña, Y.; Antonelli, C.; Benítez, G.; Rey-Baltar, D.; Jorge, I.J.; et al. Long-term improvement by ozone treatment in chronic pain secondary to chemotherapy-induced peripheral neuropathy: A preliminary report. Front. Physiol. 2022, 13, 935269. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Abreu, D.; Galván-Ruiz, S.; Federico, M.; Cánovas-Molina, A.; Ramallo-Fariña, Y.; Antonilli, C.; Benítez, G.; Fabelo, H.; García-Lourve, C.; et al. Long-Term Effects of Ozone Treatment in Patients with Persistent Numbness and Tingling Secondary to Chemotherapy-Induced Peripheral Neuropathy. A Retrospective Study. Integr. Cancer Ther. 2025, 24, 15347354241307038. [Google Scholar] [CrossRef]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. Biomed. Pharmacother. 2022, 147, 112671. [Google Scholar] [CrossRef]
- Smith, E.M.L. Alliance A221805: Duloxetine to prevent oxaliplatin-induced chemotherapy-induced peripheral neuropathy (CIPN)—A randomized, double-blind, placebo-controlled phase II study. J. Clin. Oncol. 2025, 43 (Suppl. 16), 12010. [Google Scholar] [CrossRef]
| Chemotherapy Drug | Main Indication | Neuropathy Symptoms | Clinical Impact |
|---|---|---|---|
| Platinum agents (Oxaliplatin, Cisplatin, Carboplatin) | Colorectal, Pancreatic, and anal cancer, Uterus, Ovarian, lymphoma, osteosarcoma | Sensory neuropathy, parasthesia, cold sensitivity, numbness | May require discontinuation or dose reduction |
| Taxanes (Paclitaxel, Docetaxel) | Breast, Ovarian, Uterine, and Prostate cancers | Dose-dependent sensory neuropathy, higher incidence in paclitaxel | Long-lasting; major cause of treatment modification |
| Vinca Alkaloids (Vincristine, Vinblastine) | Lymphoma, Leukemia, and Sarcoma | Severe sensory and motor neuropathy | Neurotoxicity even at low doses. Can limit therapy |
| Proteasome inhibitors (Bortezomib) | Multiple myeloma | Painful sensory neuropathy, reduced with subcutan. administration | Can compromise treatment adherence and affect quality of life |
| Immunomodulators (Thalidomide, Lenalidomide) | Multiple Myeloma | Thalidomide poses high risk of chronic sensory neuropathy. Lower risk in case of Lenalidomide | Limit long-term use. High impact on daily function |
| Anthracyclines and alakalying agents (Doxorubicin) | Soft tissue sarcoma, bone sarcoma | Risk increases with high cumulative doses. Delayed neuropathy is possible | Nearly 50% develop CIPN within 8 months |
| Other agents (Docetaxal, Gemcitabine, Dacarbazine) | Advanced/metastatic sarcoma | Neuropathy varies by regimen | Cumulative burden when combined with other drugs |
| Treatment | Agents | Efficacy | Adverse Effects/Limitations |
|---|---|---|---|
| Pharmacological | Amiytriptyline, anticonvulsants, Ketamine, Cannabinoids, Opioids | Some pain and paresthesia relief, mostly symptomatic, not curative | Sedeation, dependency, systematic side effects |
| Topical | Capsaicin, Lidocaine, Opoid gels | Useful in refractory cases, can improve localized pain | Local irritation, limited durability |
| Non-pharmacological | Physical therapy, Exercise, Acupuncture | Improve function and pain relief in some RCT | Evidence heterogeneous, inconsistent recommendations |
| Neuromodulation | TENS, neurofeedback techniques | Under investigation | Limited data. |
| Photobiomodulation/low level laser | 630/850 nm light | RCTs show improved sensory sensation, gait, and balance, with durability up to 12 weeks | Heterogeneous protocols, small cohorts; no consensus or recommended guidelines. |
| Preventive strategies | PBM applied during chemotherapy. | May reduce severity of symptoms and improve quality of life. | Preliminary data only; not included in ASCO guidelines. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batash, R.; Asna, N.; Ali, S.H.; Charkovsky, T.; Asali, M.; Pelles, S.; Schaffer, M. Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Oncologic Patients—A New Promise? Preliminary Results. Cancers 2025, 17, 3321. https://doi.org/10.3390/cancers17203321
Batash R, Asna N, Ali SH, Charkovsky T, Asali M, Pelles S, Schaffer M. Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Oncologic Patients—A New Promise? Preliminary Results. Cancers. 2025; 17(20):3321. https://doi.org/10.3390/cancers17203321
Chicago/Turabian StyleBatash, Ron, Noam Asna, Sara HaJ Ali, Tatiana Charkovsky, Murad Asali, Sharon Pelles, and Moshe Schaffer. 2025. "Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Oncologic Patients—A New Promise? Preliminary Results" Cancers 17, no. 20: 3321. https://doi.org/10.3390/cancers17203321
APA StyleBatash, R., Asna, N., Ali, S. H., Charkovsky, T., Asali, M., Pelles, S., & Schaffer, M. (2025). Management of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in Oncologic Patients—A New Promise? Preliminary Results. Cancers, 17(20), 3321. https://doi.org/10.3390/cancers17203321







