Transformation and Management of Long-Bone Atypical Cartilaginous Tumours
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
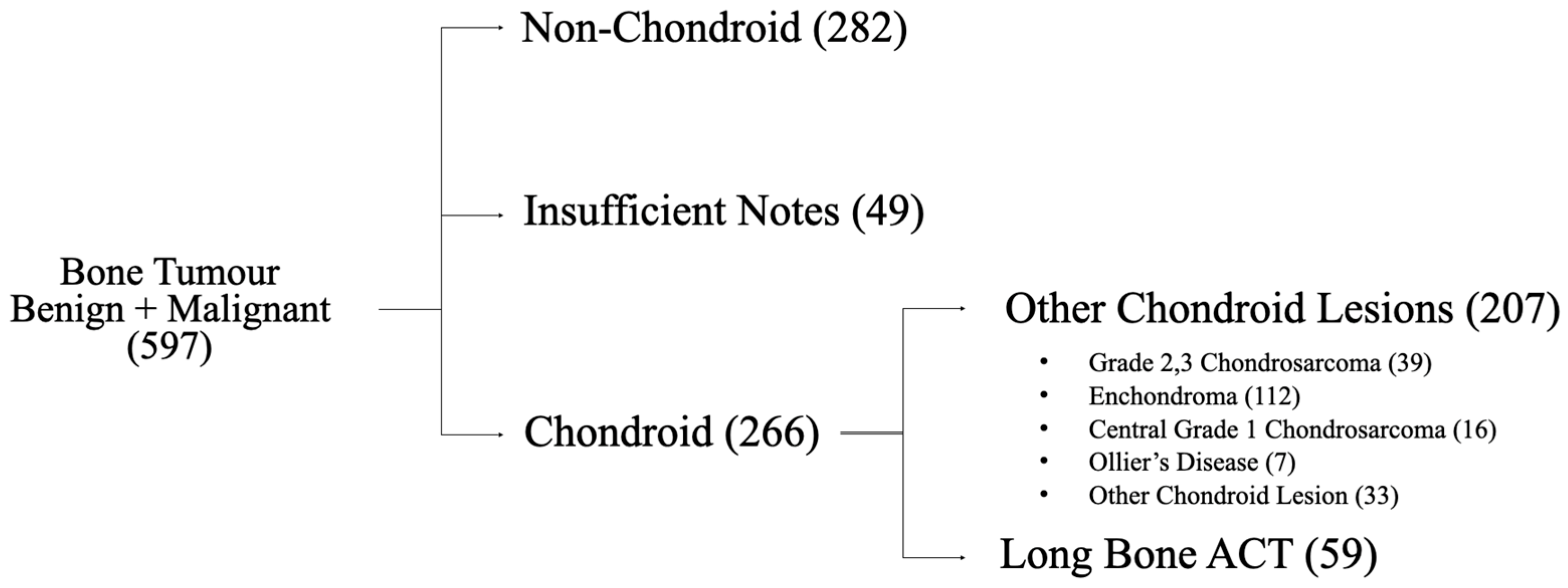
3. Results
4. Discussion
| Author | Date | Lesion Types | Patient Numbers | Mean Follow-Up (Months) | Transformation |
|---|---|---|---|---|---|
| Deckers et al. [20] | 2016 | Enchondroma + ACT | 41 | 66 | 0 |
| Kumar et al. [22] | 2016 | Enchondroma + ACT | 46 | 60 | 0 |
| Chung et al. [23] | 2018 | Enchondroma + ACT | 21 | 45 | 0 |
| Omlor et al. [24] | 2019 | Enchondroma + ACT | 153 | 88 | 0 |
| Deckers et al. [10] | 2021 | Enchondroma + ACT | 128 | 50 | 0 |
| LaPrade et al. [19] | 2023 | Enchondroma + ACT | 57 | 55 | 0 |
| Woltsche et al. [25] | 2024 | Enchondroma + ACT | 176 | 27 * | 0 |
| Scholte et al. [26] | 2024 | ACT | 117 | 41 | 0 |
| Van Den Berghe et al. [27] | 2024 | BACTIP Category I + II | 61 | 38 | 0 |
| Davies et al. [28] | 2025 | BACTIP Category I + II | 721 | 86 | 4 |
| Opper et al. [29] | 2025 | BACTIP Category I + II | 63 | 35 | 1 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | atypical cartilaginous tumour |
| CS1 | grade 1 chondrosarcoma |
| MSK | musculoskeletal |
| CLUMP | Chondroid Tumour of Uncertain Malignant Potential |
| BACTIP | Birmingham Atypical Cartilage Tumour Imaging Protocol |
References
- Bovée, J.V.M.G.; Bloem, J.L.; Flanagan, A.M.; Nielsen, G.P.; Yoshida, A. Central atypical cartilaginous tumour/chondrosarcoma, grade 1. In WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2020; Volume 3, pp. 370–372. [Google Scholar]
- Vieira da Silva, G.; Vargas, F.; Neto, P.; Serra, F.; Nelas, J.; Souto, C.; Carrapatoso, M.; Cardoso, P.; Oliveira, V. Imaging differences between enchondroma and atypical cartilaginous tumor: Insights from a reference center. J. Orthop. Rep. 2025. [Google Scholar] [CrossRef]
- Brien, E.W.; Mirra, J.M.; Kerr, R. Benign and malignant cartilage tumors of bone and joint: Their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skelet. Radiol. 1997, 26, 325–353. [Google Scholar] [CrossRef]
- Thorkildsen, J.; Taksdal, I.; Bjerkehagen, B.; Norum, O.J.; Myklebust, T.A.; Zaikova, O. Risk stratification for central conventional chondrosarcoma of bone: A novel system predicting risk of metastasis and death in the Cancer Registry of Norway cohort. J. Surg. Oncol. 2020, 121, 1115–1125. [Google Scholar] [CrossRef]
- Andreou, D.; Gilg, M.M.; Gosheger, G.; Werner, M.; Hardes, J.; Pink, D.; Leithner, A.; Tunn, P.-U.; Streitbürger, A. Metastatic Potential of Grade I Chondrosarcoma of Bone: Results of a Multi-institutional Study. Ann. Surg. Oncol. 2016, 23, 120–125. [Google Scholar] [CrossRef]
- Schwab, J.H.; Wenger, D.; Unni, K.; Sim, F.H. Does local recurrence impact survival in low-grade chondrosarcoma of the long bones? Clin. Orthop. Relat. Res. 2007, 462, 175–180. [Google Scholar] [CrossRef] [PubMed]
- van Praag (Veroniek), V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; van der Geest, I.C.; Bramer, J.A.; Schaap, G.R.; Jutte, P.C.; Bart Schreuder, H.W.; Ploegmakers, J.J.W.; et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Stomp, W.; Reijnierse, M.; Kloppenburg, M.; de Mutsert, R.; Bovée, J.V.M.G.; den Heijer, M.; Bloem, J.L. Prevalence of cartilaginous tumours as an incidental finding on MRI of the knee. Eur. Radiol. 2015, 25, 3480–3487. [Google Scholar] [CrossRef]
- Jeys, L.M.; Thorkildsen, J.; Kurisunkal, V.; Puri, A.; Ruggieri, P.; Houdek, M.T.; Boyle, R.A.; Ebeid, W.; Botello, E.; Morris, G.V.; et al. Controversies in orthopaedic oncology: Attempting international consensus. Bone Jt. J. 2024, 106-B, 425–429. [Google Scholar] [CrossRef]
- Deckers, C.; de Rooy, J.W.J.; Flucke, U.; Schreuder, H.W.B.; Dierselhuis, E.F.; van der Geest, I.C.M. Midterm MRI Follow-Up of Untreated Enchondroma and Atypical Cartilaginous Tumors in the Long Bones. Cancers 2021, 13, 4093. [Google Scholar] [CrossRef]
- Campanacci, D.A.; Scoccianti, G.; Franchi, A.; Roselli, G.; Beltrami, G.; Ippolito, M.; Caff, G.; Frenos, F.; Capanna, R. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J. Orthop. Traumatol. 2013, 14, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Patel, A.; James, S.L.; Botchu, R. A retrospective validation of an imaging protocol for the management of solitary central cartilage tumours of the proximal humerus and around the knee. Clin. Radiol. 2019, 74, 962–971. [Google Scholar] [CrossRef]
- Deckers, C.; Steyvers, M.J.; Hannink, G.; Schreuder, H.W.B.; de Rooy, J.W.J.; Van Der Geest, I.C.M. Can MRI differentiate between atypical cartilaginous tumors and high-grade chondrosarcoma? A systematic review. Acta Orthop. 2020, 91, 471–478. [Google Scholar] [CrossRef]
- Murphey, M.D.; Flemming, D.J.; Boyea, S.R.; Bojescul, J.A.; Sweet, D.E.; Temple, H.T. Enchondroma versus chondrosarcoma in the appendicular skeleton: Differentiating features. RadioGraphics 1998, 18, 1213–1237. [Google Scholar] [CrossRef]
- Schwartz, H.S.; Zimmerman, N.B.; Simon, M.A.; Wroble, R.R.; Millar, E.A.; Bonfiglio, M. The malignant potential of enchondromatosis. J. Bone Jt. Surg. Am. 1987, 69, 269–274. [Google Scholar] [CrossRef]
- Gassert, F.G.; Breden, S.; Neumann, J.; Gassert, F.T.; Bollwein, C.; Knebel, C.; Lenze, U.; von Eisenhart-Rothe, R.; Mogler, C.; Makowski, M.R.; et al. Differentiating Enchondromas and Atypical Cartilaginous Tumors in Long Bones with Computed Tomography and Magnetic Resonance Imaging. Diagnostics 2022, 12, 2186. [Google Scholar] [CrossRef]
- Ferrer-Santacreu, E.M.; Ortiz-Cruz, E.J.; González-López, J.M.; Pérez Fernández, E. Enchondroma versus Low-Grade Chondrosarcoma in Appendicular Skeleton: Clinical and Radiological Criteria. J. Oncol. 2012, 2012, 437958. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.L.; Ilaslan, H.; Bauer, T.W.; Lietman, S.A.; Joyce, M.J.; Sundaram, M. Cortical scalloping and cortical penetration by small eccentric chondroid lesions in the long tubular bones: Not a sign of malignancy? Skelet. Radiol. 2009, 38, 791–796. [Google Scholar] [CrossRef]
- LaPrade, C.M.; Andryk, L.M.; Christensen, J.L.; Neilson, J.C.; Wooldridge, A.N.; Hackbarth, D.A.; Bedi, M.; King, D.M. Natural history of intraosseous low-grade chondroid lesions of the proximal humerus. Front. Oncol. 2023, 13, 1200286. [Google Scholar] [CrossRef] [PubMed]
- Deckers, C.; Schreuder, B.H.W.; Hannink, G.; de Rooy, J.W.J.; van der Geest, I.C.M. Radiologic follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J. Surg. Oncol. 2016, 114, 987–991. [Google Scholar] [CrossRef]
- Patel, A.; Davies, A.M.; Botchu, R.; James, S. A pragmatic approach to the imaging and follow-up of solitary central cartilage tumours of the proximal humerus and knee. Clin. Radiol. 2019, 74, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Tyrrell, P.N.M.; Singh, J.; Gregory, J.; Cribb, G.L.; Cool, P. Surveillance of intramedullary cartilage tumours in long bones. Bone Jt. J. 2016, 98-B, 1542–1547. [Google Scholar] [CrossRef]
- Chung, B.M.; Hong, S.H.; Yoo, H.J.; Choi, J.Y.; Chae, H.D.; Kim, D.H. Magnetic resonance imaging follow-up of chondroid tumors: Regression vs. progression. Skelet. Radiol. 2018, 47, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Lohnherr, V.; Lange, J.; Gantz, S.; Mechtersheimer, G.; Merle, C.; Raiss, R.; Fellenberg, J.; Lehner, B. Outcome of conservative and surgical treatment of enchondromas and atypical cartilaginous tumors of the long bones: Retrospective analysis of 228 patients. BMC Musculoskelet. Disord. 2019, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Woltsche, J.N.; Smolle, M.A.; Szolar, D.; Leithner, A. Follow-up analysis of lesion characteristics of enchondromas and atypical cartilaginous tumours of the knee and shoulder region on MRI. Eur. Radiol. 2025, 35, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Scholte, C.H.J.; Van de Sande, M.A.J.; Van der Wal, R.J.P.; Broekhuis, D.; Van Langevelde, K.; Dorleijn, D.M.J. Clinical outcome of curettage in atypical cartilaginous tumors of the long bones: A descriptive cohort study. Acta Orthop. 2024, 95, 752–757. [Google Scholar] [CrossRef]
- Van Den Berghe, T.; Delbare, F.; Candries, E.; Lejoly, M.; Algoet, C.; Chen, M.; Laloo, F.; Huysse, W.C.J.; Creytens, D.; Verstraete, K.L. A retrospective external validation study of the Birmingham Atypical Cartilage Tumour Imaging Protocol (BACTIP) for the management of solitary central cartilage tumours of the proximal humerus and around the knee. Eur. Radiol. 2024, 34, 4988–5006. [Google Scholar] [CrossRef]
- Davies, A.M.; Patel, A.; Azzopardi, C.; James, S.; Botchu, R. Birmingham atypical cartilaginous tumour imaging protocol (BACTIP) revisited. Clin. Radiol. 2025, 83, 106837. [Google Scholar] [CrossRef] [PubMed]
- Opper, S.; Saucedo, S.; Ercolano, L.; He, L. Retrospective validation of the Birmingham atypical cartilage tumor imaging protocol (BACTIP) in a single, United States tertiary care center. Skelet. Radiol. 2025, 54, 1927–1937. [Google Scholar] [CrossRef]
- Deckers, C.; van Zeijl, N.T.; van Hooff, M.L.; Veldman-Goossen, P.I.; Schreuder, H.W.B.; Dierselhuis, E.F.; van der Geest, I.C.M. Active surveillance of atypical cartilaginous tumours of bone: Short term quality of life measurements. J. Orthop. Surg. Res. 2023, 18, 208. [Google Scholar] [CrossRef]
- Akoh, C.C.; Craig, E.; Troester, A.M.; Miller, B.J. Radiographic Enchondroma Surveillance: Assessing Clinical Outcomes and Costs Effectiveness. Iowa Orthop. J. 2019, 39, 185–193. [Google Scholar]
- Gazendam, A.; Popovic, S.; Parasu, N.; Ghert, M. Chondrosarcoma: A Clinical Review. J. Clin. Med. 2023, 12, 2506. [Google Scholar] [CrossRef]
- Makar, G.S.; Udoeyo, I.F.; Bowen, T.R. Non-Operative Ttreatment of patients with Chondrosarcoma: An analysis of patients who refused cancer-directed surgery or patients contraindicated to surgery. Acta Orthop. Belg. 2024, 90, 745–758. [Google Scholar] [CrossRef]
- Altay, M.; Bayrakci, K.; Yildiz, Y.; Erekul, S.; Saglik, Y. Secondary chondrosarcoma in cartilage bone tumors: Report of 32 patients. J. Orthop. Sci. 2007, 12, 415–423. [Google Scholar] [CrossRef]
- Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J. Bone Jt. Surg. Am. 2007, 89, 2113–2123. [Google Scholar] [CrossRef]
- Yildirim, M.; Yildirim, H. CT radiomics-based machine learning model for differentiating between enchondroma and low-grade chondrosarcoma. Medicine 2024, 103, 39311. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Li, Q.; Ma, J.; Lu, C.; Zhong, Z. Computed tomography-based radiomics machine learning models for differentiating enchondroma and atypical cartilaginous tumor in long bones. RöFo-Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 2025, 197, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Gassert, F.G.; Lang, D.; Hesse, N.; Dürr, H.R.; Klein, A.; Kohll, L.; Hinterwimmer, F.; Luitjens, J.; Weissinger, S.; Peeken, J.C.; et al. A deep learning model for classification of chondroid tumors on CT images. BMC Cancer 2025, 25, 561. [Google Scholar] [CrossRef]
- Gitto, S.; Annovazzi, A.; Nulle, K.; Interlenghi, M.; Salvatore, C.; Anelli, V.; Baldi, J.; Messina, C.; Albano, D.; Di Luca, F.; et al. X-rays radiomics-based machine learning classification of atypical cartilaginous tumour and high-grade chondrosarcoma of long bones. eBioMedicine 2024, 101, 105018. [Google Scholar] [CrossRef]
| Gender | |
| Female | 40 (68%) |
| Male | 19 (32%) |
| Age | |
| Mean | 50.8 year |
| ACT Location | |
| Femur | 27 |
| Humerus | 18 |
| Tibia | 6 |
| Fibula | 6 |
| Ulna | 1 |
| Radius | 1 |
| Pain | |
| Total | 52 |
| At Rest | 6 |
| On Activity | 38 |
| Both (Activity and Rest) | 8 |
| Pain: Alternative Pathology | |
| Osteoarthritis | 15 * |
| Tendinopathy | 10 |
| Trauma | 9 |
| Meniscal Tears | 5 |
| Pathological Fracture | 2 |
| Post-Fracture Imaging | 2 |
| Inflammatory Arthritis | 1 |
| Epiphysiodesis | 1 |
| Hereditary Multiple Exostosis | 1 |
| Iliotibial Band Syndrome | 1 |
| Cervical Radiculopathy | 1 |
| Shoulder Dysplasia | 1 |
| None | 7 |
| No Pain | |
| Total | 7 |
| Cancer-Related Imaging | 4 |
| Post-Fracture Imaging | 1 |
| Unclear Indication for Imaging | 2 |
| Scalloping | |
| Absent | 31 |
| <2/3 Width and Length | 7 |
| >2/3 Width | 14 |
| >2/3 Width and Length | 7 |
| Cortical Breach | |
| Yes | 2 |
| No | 57 |
| Soft Tissue Mass | |
| Yes | 0 |
| No | 59 |
| Lysis | |
| Yes | 7 |
| No | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coke, E.; Ben-Gal, O.; Mahendra, A.; Pietrzycki, J.; Vaughan, S.; Gupta, S. Transformation and Management of Long-Bone Atypical Cartilaginous Tumours. Cancers 2025, 17, 3178. https://doi.org/10.3390/cancers17193178
Coke E, Ben-Gal O, Mahendra A, Pietrzycki J, Vaughan S, Gupta S. Transformation and Management of Long-Bone Atypical Cartilaginous Tumours. Cancers. 2025; 17(19):3178. https://doi.org/10.3390/cancers17193178
Chicago/Turabian StyleCoke, Edmund, Ofir Ben-Gal, Ashish Mahendra, Julian Pietrzycki, Sarah Vaughan, and Sanjay Gupta. 2025. "Transformation and Management of Long-Bone Atypical Cartilaginous Tumours" Cancers 17, no. 19: 3178. https://doi.org/10.3390/cancers17193178
APA StyleCoke, E., Ben-Gal, O., Mahendra, A., Pietrzycki, J., Vaughan, S., & Gupta, S. (2025). Transformation and Management of Long-Bone Atypical Cartilaginous Tumours. Cancers, 17(19), 3178. https://doi.org/10.3390/cancers17193178






