Impact of Response Assessment Intervals on Survival and Economic Burden in Long-Term Responders to Immunotherapy for Advanced Non-Small-Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
2.2. Collection of Variables
2.3. Classification of Response Assessment Strategy
2.4. PFS Stratification
2.5. Economic Analysis and Modeling Process
2.6. Statistical Analysis
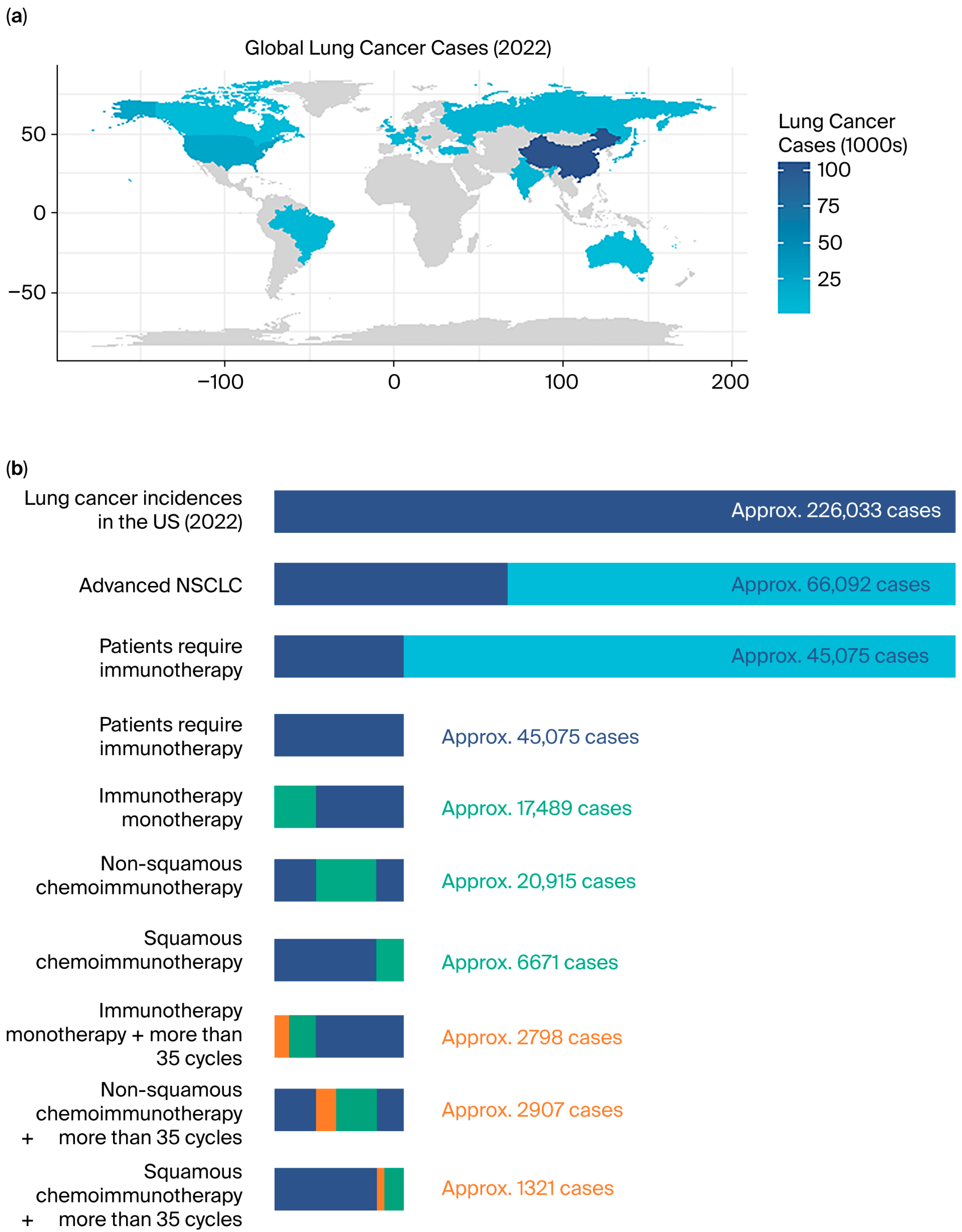
3. Results
3.1. Patient Features
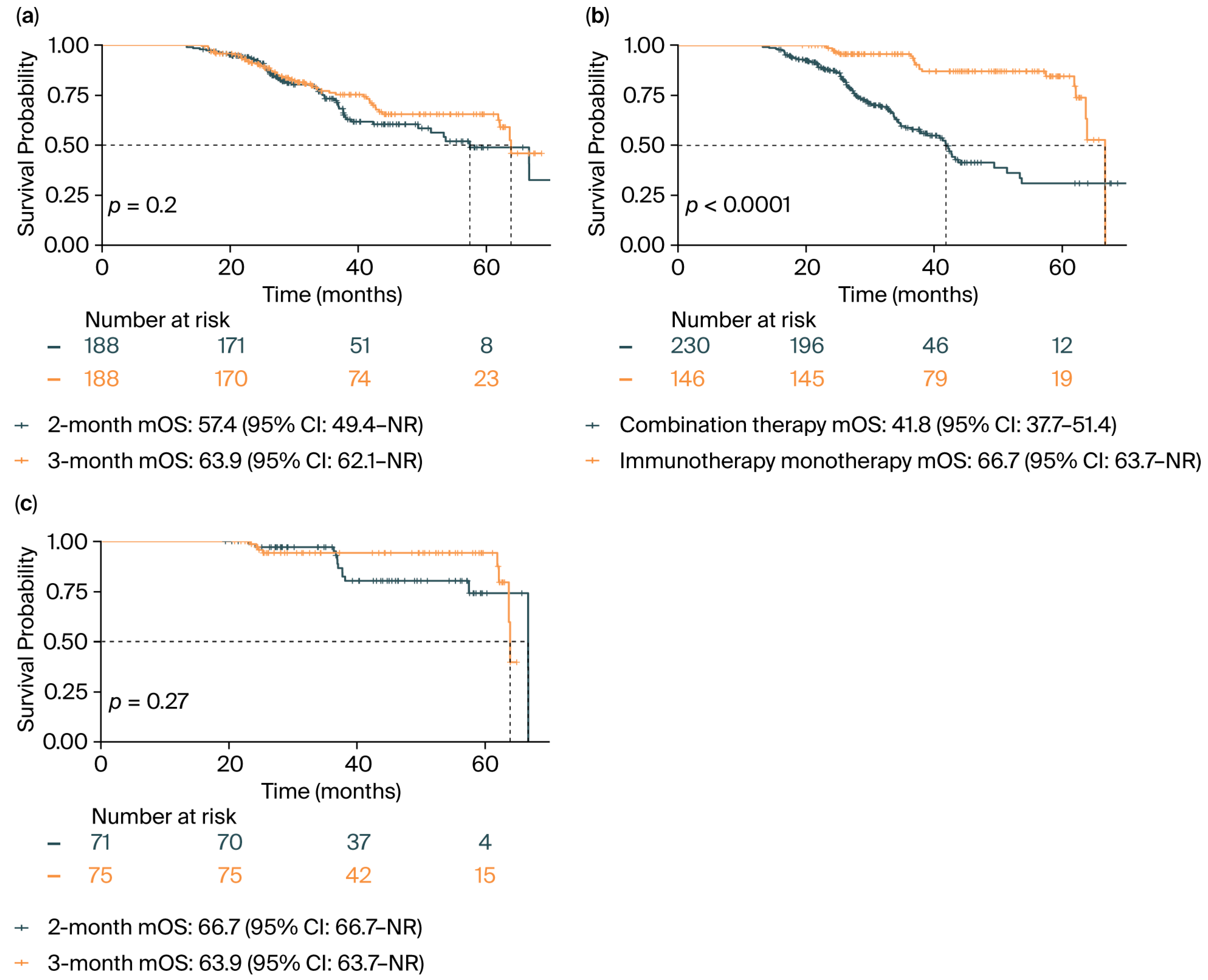
3.2. Survival Analysis
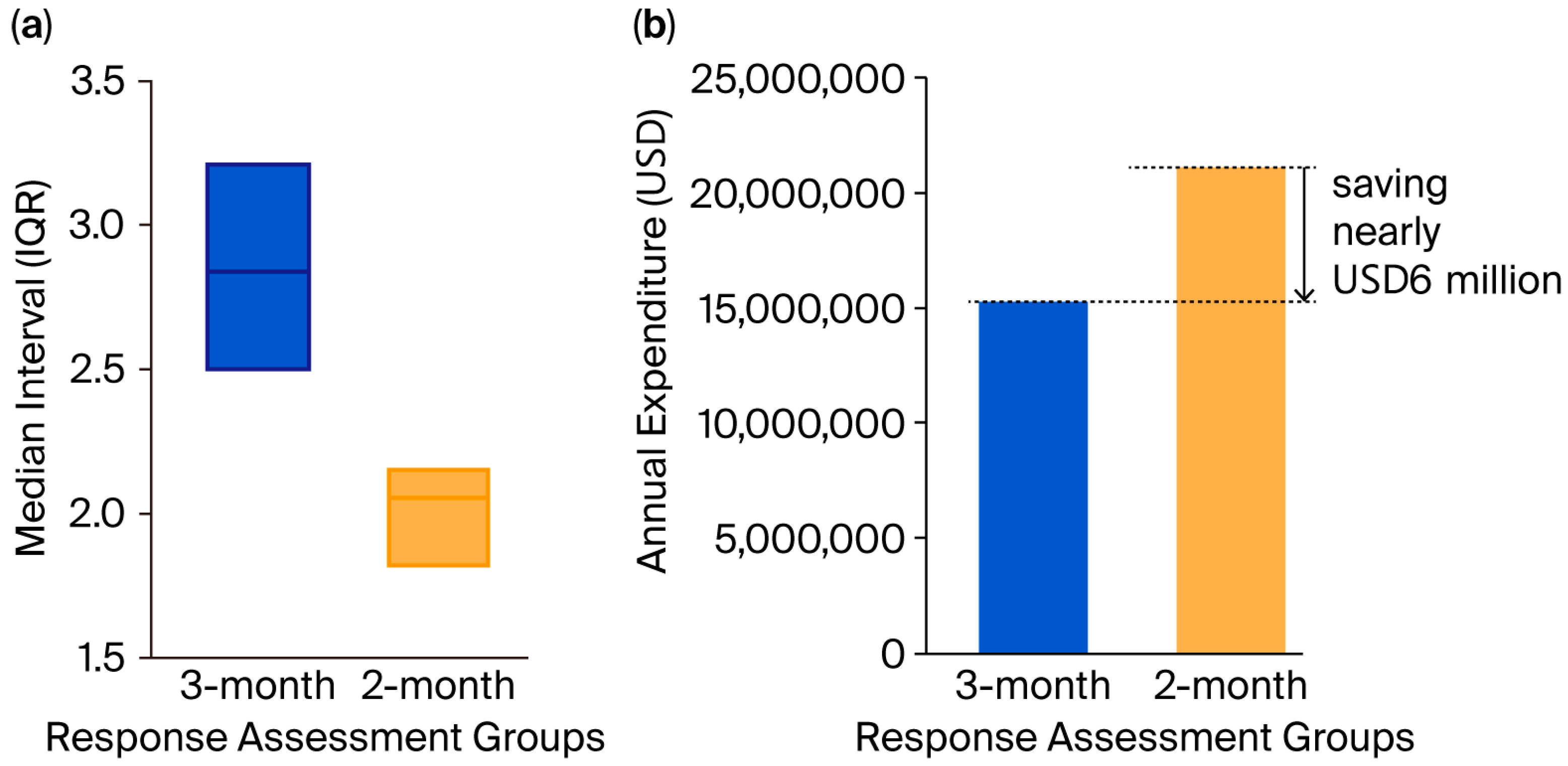
3.3. Economic Cost Analysis and the Extrapolation Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| PFS | Progression-free survival |
| OS | Overall survival |
| PSM | Propensity score matching |
| CMA | Cost minimization analysis |
| HR | Hazard ratio |
| CI | Confidence interval |
| SMD | Standardized mean difference |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death ligand 1 |
| ICIs | Immune checkpoint inhibitors |
| TPS | Tumor proportion score |
| NCCN | National Comprehensive Cancer Network |
| ECOG-PS | Eastern Cooperative Oncology Group Performance Status |
| HIS | Health Information System |
| RECIST | The Response Evaluation Criteria In Solid Tumors |
| WHO | World Health Organization |
| irAEs | Immune-related adverse events |
| CBC | Complete blood count |
| CMP | Comprehensive metabolic panel |
| TSH | Thyroid-stimulating hormone |
| FT4 | Free thyroxine |
| mOS | Median OS |
| IQR | Interquartile range |
| NR | Not reached |
| The US | The United States |
| TMB | Tumor mutational burden |
| SCNAs | Somatic copy number alterations |
| ASCO | American Society of Clinical Oncology |
| QALYs | Quality-adjusted life years |
| CUA | Cost–utility analysis |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cagle, P.T.; Allen, T.C.; Olsen, R.J. Lung Cancer Biomarkers: Present Status and Future Developments. Arch. Pathol. Lab. Med. 2013, 137, 1191–1198. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.-S.; Ciuleanu, T.-E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non–Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.; Kudaba, I.; Wu, Y.-L.; Lopes, G.; Kowalski, D.M.; Turna, H.Z.; Caglevic, C.; Zhang, L.; Karaszewska, B.; Laktionov, K.K.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients with Non–Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J. Clin. Oncol. 2023, 41, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, D.; Zhu, X. Cancer Immunotherapy: Pros, Cons and Beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, H.; Hai, J.; Socinski, M.A.; Lim, E.; Chen, H.; Stebbing, J. Impact of PD-L1 Expression, Driver Mutations and Clinical Characteristics on Survival after Anti-PD-1/PD-L1 Immunotherapy versus Chemotherapy in Non-Small-Cell Lung Cancer: A Meta-Analysis of Randomized Trials. OncoImmunology 2018, 7, e1396403. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis from KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non–Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Migliorino, M.R.; Santo, A.; Romano, G.; Cortinovis, D.; Galetta, D.; Alabiso, O.; Cartenì, G.; Vari, S.; Fasola, G.; Pazzola, A.; et al. Economic Burden of Patients Affected by Non-Small Cell Lung Cancer (NSCLC): The LIFE Study. J. Cancer Res. Clin. Oncol. 2017, 143, 783–791. [Google Scholar] [CrossRef]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. Non–Small Cell Lung Cancer, Version 4.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 249–274. [Google Scholar] [CrossRef]
- Welch, H.G.; Dossett, L.A. Routine Surveillance for Cancer Metastases—Does It Help or Harm Patients? N. Engl. J. Med. 2025, 392, 1667–1670. [Google Scholar] [CrossRef]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v1–v27. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-F.; Wang, L.; Wu, N.; Li, J.-L.; Hui, Z.-G.; Liu, S.-M.; Yang, B.-Y.; Gao, S.-G.; Ren, J.-S.; Huang, H.-Y.; et al. Clinical Characteristics and Medical Service Utilization of Lung Cancer in China, 2005–2014: Overall Design and Results from a Multicenter Retrospective Epidemiologic Survey. Lung Cancer 2019, 128, 91–100. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The Prevalence of EGFR Mutation in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, C.; Ding, W.; Zhang, Z.; Qiu, X.; Mu, D.; Zhang, H.; Xi, Y.; Zhou, J.; Ma, L.; et al. Prevalence of ROS1 Fusion in Chinese Patients with Non-small Cell Lung Cancer. Thorac. Cancer 2019, 10, 47–53. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Frigola, J.; Navarro, A.; Carbonell, C.; Callejo, A.; Iranzo, P.; Cedrés, S.; Martinez-Marti, A.; Pardo, N.; Saoudi-Gonzalez, N.; Martinez, D.; et al. Molecular Profiling of Long-term Responders to Immune Checkpoint Inhibitors in Advanced Non-small Cell Lung Cancer. Mol. Oncol. 2021, 15, 887–900. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Sisó-Almirall, A. Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Ann. Intern. Med. 2024, 177, ITC17–ITC32. [Google Scholar] [CrossRef]
- Hauptmann, M.; Byrnes, G.; Cardis, E.; Bernier, M.-O.; Blettner, M.; Dabin, J.; Engels, H.; Istad, T.S.; Johansen, C.; Kaijser, M.; et al. Brain Cancer after Radiation Exposure from CT Examinations of Children and Young Adults: Results from the EPI-CT Cohort Study. Lancet Oncol. 2023, 24, 45–53. [Google Scholar] [CrossRef]
- Yoshinaga, S.; Mabuchi, K.; Sigurdson, A.J.; Doody, M.M.; Ron, E. Cancer Risks among Radiologists and Radiologic Technologists: Review of Epidemiologic Studies. Radiology 2004, 233, 313–321. [Google Scholar] [CrossRef]
- Bosch De Basea Gomez, M.; Thierry-Chef, I.; Harbron, R.; Hauptmann, M.; Byrnes, G.; Bernier, M.-O.; Le Cornet, L.; Dabin, J.; Ferro, G.; Istad, T.S.; et al. Risk of Hematological Malignancies from CT Radiation Exposure in Children, Adolescents and Young Adults. Nat. Med. 2023, 29, 3111–3119. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer Risks Attributable to Low Doses of Ionizing Radiation: Assessing What We Really Know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- Wood, R.; Taylor-Stokes, G. Cost Burden Associated with Advanced Non-Small Cell Lung Cancer in Europe and Influence of Disease Stage. BMC Cancer 2019, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.J.; Fitch, M.I.; Loree, J.M.; Carlson, L.E.; Turner, D.; Cheung, W.Y.; Gopaul, D.; Ellis, J.; Ringash, J.; Mathews, M.; et al. Patient and Family Financial Burden Associated with Cancer Treatment in Canada: A National Study. Support. Care Cancer 2021, 29, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (n = 588) | 2-Month Group (n = 229) | 3-Month Group (n = 359) | p | SMD * | All Patients (n = 376) | 2-Month Group (n = 188) | 3-Month Group (n = 188) | p | SMD * | |
| Gender, n (%) | ||||||||||
| Female | 112 (19.0) | 41 (17.9) | 71 (19.8) | 0.648 | 0.048 | 68 (18.1) | 35 (18.6) | 33 (17.6) | 0.893 | 0.028 |
| Male | 476 (81.0) | 188 (82.1) | 288 (80.2) | 308 (81.9) | 153 (81.4) | 155 (82.4) | ||||
| Age, n (%) | ||||||||||
| ≤63 years | 298 (50.7) | 113 (49.3) | 185 (51.5) | 0.665 | 0.044 | 206 (54.8) | 96 (51.1) | 110 (58.5) | 0.178 | 0.15 |
| >63 years | 290 (49.3) | 116 (50.7) | 174 (48.5) | 170 (45.2) | 92 (48.9) | 78 (41.5) | ||||
| Histological type, n (%) | ||||||||||
| Non-squamous | 423 (71.9) | 156 (68.1) | 267 (74.4) | 0.121 | 0.138 | 271 (72.1) | 141 (75.0) | 130 (69.1) | 0.25 | 0.131 |
| Squamous | 165 (28.1) | 73 (31.9) | 92 (25.6) | 105 (27.9) | 47 (25.0) | 58 (30.9) | ||||
| PFS stratification, n (%) | ||||||||||
| ≤median PFS | 291 (49.5) | 131 (57.2) | 160 (44.6) | 0.004 | 0.255 | 183 (48.7) | 92 (48.9) | 91 (48.4) | 1 | 0.011 |
| >median PFS | 297 (50.5) | 98 (42.8) | 199 (55.4) | 193 (51.3) | 96 (51.1) | 97 (51.6) | ||||
| PD-L1 TPS, n (%) | ||||||||||
| <1% | 37 (6.3) | 11 (4.8) | 26 (7.2) | 0.028 | 0.257 | 8 (2.1) | 6 (3.2) | 2 (1.1) | 0.269 | 0.205 |
| 1–49% | 119 (20.2) | 37 (16.2) | 82 (22.8) | 75 (19.9) | 36 (19.1) | 39 (20.7) | ||||
| ≥50% | 205 (34.9) | 77 (33.6) | 128 (35.7) | 135 (35.9) | 73 (38.8) | 62 (33.0) | ||||
| Unknown | 227 (38.6) | 104 (45.4) | 123 (34.3) | 158 (42.0) | 73 (38.8) | 85 (45.2) | ||||
| Immunotherapy drugs, n (%) | ||||||||||
| Camrelizumab | 119 (20.2) | 46 (20.1) | 73 (20.3) | 0.042 | 0.265 | 77 (20.5) | 43 (22.9) | 34 (18.1) | 0.841 | 0.123 |
| Pembrolizumab | 225 (38.3) | 82 (35.8) | 143 (39.8) | 148 (39.4) | 72 (38.3) | 76 (40.4) | ||||
| Tislelizumab | 92 (15.6) | 48 (21.0) | 44 (12.3) | 64 (17.0) | 31 (16.5) | 33 (17.6) | ||||
| Sintilimab | 105 (17.9) | 40 (17.5) | 65 (18.1) | 65 (17.3) | 32 (17.0) | 33 (17.6) | ||||
| Others | 47 (8.0) | 13 (5.7) | 34 (9.5) | 22 (5.9) | 10 (5.3) | 12 (6.4) | ||||
| Immunotherapy monotherapy, n (%) | ||||||||||
| No | 360 (61.2) | 147 (64.2) | 213 (59.3) | 0.274 | 0.1 | 230 (61.2) | 117 (62.2) | 113 (60.1) | 0.751 | 0.044 |
| Yes | 228 (38.8) | 82 (35.8) | 146 (40.7) | 146 (38.8) | 71 (37.8) | 75 (39.9) | ||||
| Smoking history, n (%) | ||||||||||
| No | 175 (29.8) | 61 (26.6) | 114 (31.8) | 0.218 | 0.113 | 115 (30.6) | 51 (27.1) | 64 (34.0) | 0.179 | 0.15 |
| Yes | 413 (70.2) | 168 (73.4) | 245 (68.2) | 261 (69.4) | 137 (72.9) | 124 (66.0) | ||||
| Radiotherapy history, n (%) | ||||||||||
| No | 299 (50.9) | 81 (35.4) | 218 (60.7) | <0.001 | 0.525 | 160 (42.6) | 73 (38.8) | 87 (46.3) | 0.175 | 0.151 |
| Yes | 289 (49.1) | 148 (64.6) | 141 (39.3) | 216 (57.4) | 115 (61.2) | 101 (53.7) | ||||
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | ||||||
| ≤63 years | Ref | Ref | ||||
| >63 years | 1.48 | 1.01–2.16 | 0.045 | 2.03 | 1.35–3.06 | 0.001 |
| Gender | ||||||
| Female | Ref | - | ||||
| Male | 1.23 | 0.75–2.02 | 0.416 | - | ||
| Smoking history | ||||||
| No | Ref | - | ||||
| Yes | 1.11 | 0.73–1.67 | 0.633 | - | ||
| Histological type | ||||||
| Non-squamous | Ref | - | ||||
| Squamous | 1.28 | 0.85–1.94 | 0.235 | - | ||
| PD-L1 TPS | ||||||
| <1% | Ref | Ref | ||||
| 1–49% | 0.09 | 0.03–0.26 | <0.001 | 0.29 | 0.10–0.84 | 0.022 |
| ≥50% | 0.11 | 0.04–0.29 | <0.001 | 0.26 | 0.10–0.69 | 0.007 |
| Unknown | 0.15 | 0.06–0.39 | <0.001 | 0.33 | 0.13–0.85 | 0.021 |
| Immunotherapy drugs | ||||||
| Camrelizumab | Ref | - | ||||
| Pembrolizumab | 1.62 | 0.92–2.87 | 0.095 | - | ||
| Tislelizumab | 1.81 | 0.91–3.60 | 0.091 | - | ||
| Sintilimab | 1.36 | 0.70–2.64 | 0.367 | - | ||
| Others | 1.37 | 0.53–3.51 | 0.514 | - | ||
| Immunotherapy monotherapy | ||||||
| No | Ref | Ref | ||||
| Yes | 0.22 | 0.13–0.35 | <0.001 | 0.24 | 0.14–0.40 | <0.001 |
| Response assessment | ||||||
| 2-month | Ref | - | ||||
| 3-month | 0.78 | 0.53–1.14 | 0.200 | - | ||
| PFS stratification | ||||||
| ≤median PFS | Ref | Ref | ||||
| >median PFS | 0.11 | 0.07–0.17 | <0.001 | 0.13 | 0.08–0.21 | <0.001 |
| Radiotherapy history | ||||||
| No | Ref | - | ||||
| Yes | 1.25 | 0.83–1.87 | 0.292 | - | ||
| Top Cancer Hospitals in the US * | CT Scan of Chest with and Without Contrast | CT Abdomen with and Without Contrast | CBC | CMP | TSH | FT4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min Charge | Max Charge | Min Charge | Max Charge | Min Charge | Max Charge | Min Charge | Max Charge | Min Charge | Max Charge | Min Charge | Max Charge | |
| University of Texas MD Anderson Cancer Center | 180.34 | 3605.98 | 180.34 | 3817.54 | 5.43 | 86.86 | 8.87 | 649.30 | 14.11 | 254.56 | 7.58 | 138.46 |
| Memorial Sloan Kettering Cancer Center | 383.97 | 2604.60 | 383.97 | 2168.00 | 13.65 | 134.10 | 31.86 | 323.10 | 31.13 | 342.90 | 31.13 | 263.70 |
| Massachusetts General Hospital | 167.95 | 1305.68 | 201.74 | 1305.68 | 6.34 | 48.56 | 10.35 | 79.34 | 16.46 | 126.10 | 8.84 | 67.67 |
| Mount Sinai Hospital | 164.11 | 775.89 | 191.23 | 1042.28 | 65.76 | 78.72 | 137.00 | 164.00 | 109.60 | 131.20 | NA | NA |
| The Hospital of the University of Pennsylvania | 93.36 | 7221.00 | 93.36 | 7370.00 | 3.89 | 260.00 | 5.28 | 350.00 | 6.00 | 75.00 | 4.51 | 369.00 |
| Mean | 197.95 | 3102.63 | 210.13 | 3140.70 | 19.01 | 121.65 | 38.67 | 313.15 | 35.46 | 185.95 | 13.02 | 209.71 |
| Min mean standard charge | USD 514.24 | |||||||||||
| Max mean standard charge | USD 7073.79 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Soth, V.; Liu, X.; Li, Y.; Chen, X.; Xue, J.; Gong, Y. Impact of Response Assessment Intervals on Survival and Economic Burden in Long-Term Responders to Immunotherapy for Advanced Non-Small-Cell Lung Cancer. Cancers 2025, 17, 3312. https://doi.org/10.3390/cancers17203312
Wang M, Soth V, Liu X, Li Y, Chen X, Xue J, Gong Y. Impact of Response Assessment Intervals on Survival and Economic Burden in Long-Term Responders to Immunotherapy for Advanced Non-Small-Cell Lung Cancer. Cancers. 2025; 17(20):3312. https://doi.org/10.3390/cancers17203312
Chicago/Turabian StyleWang, Min, Vannhong Soth, Xingzhu Liu, Yuxi Li, Xianyan Chen, Jianxin Xue, and Youling Gong. 2025. "Impact of Response Assessment Intervals on Survival and Economic Burden in Long-Term Responders to Immunotherapy for Advanced Non-Small-Cell Lung Cancer" Cancers 17, no. 20: 3312. https://doi.org/10.3390/cancers17203312
APA StyleWang, M., Soth, V., Liu, X., Li, Y., Chen, X., Xue, J., & Gong, Y. (2025). Impact of Response Assessment Intervals on Survival and Economic Burden in Long-Term Responders to Immunotherapy for Advanced Non-Small-Cell Lung Cancer. Cancers, 17(20), 3312. https://doi.org/10.3390/cancers17203312





