Exploring the Tumor Microenvironment in Osteosarcoma: Driver of Resistance and Progression
Simple Summary
Abstract
1. Introduction
2. TME Components
2.1. Immune Cells
2.1.1. Tumor-Associated Macrophages
2.1.2. Tumor-Associated Neutrophils
2.1.3. Myeloid-Derived Suppressor Cells
2.1.4. Mast Cells
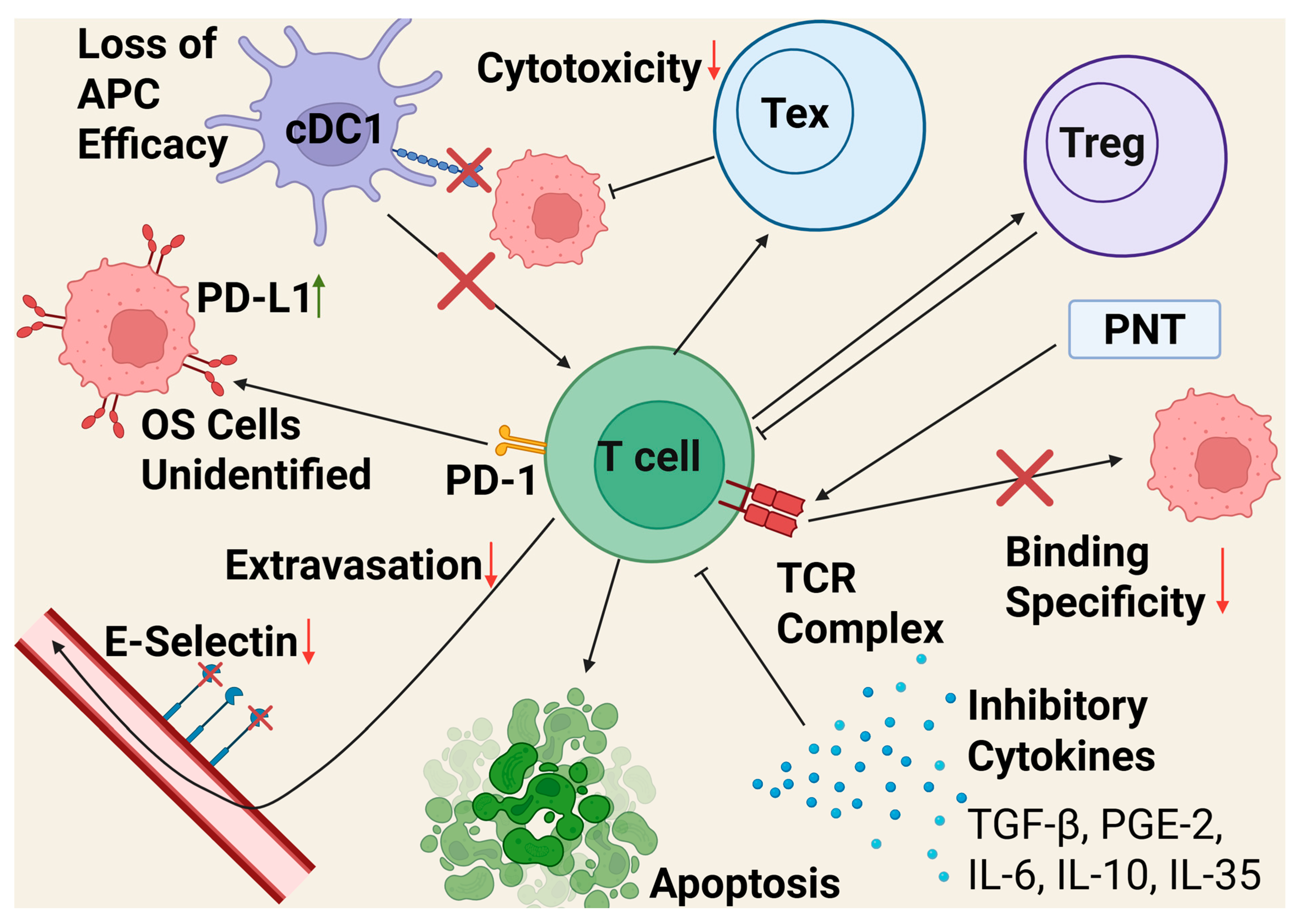
2.1.5. T Cells
2.1.6. B Cells
2.1.7. Natural Killer Cells
2.1.8. Dendritic Cells
2.2. Non-Immune Cells
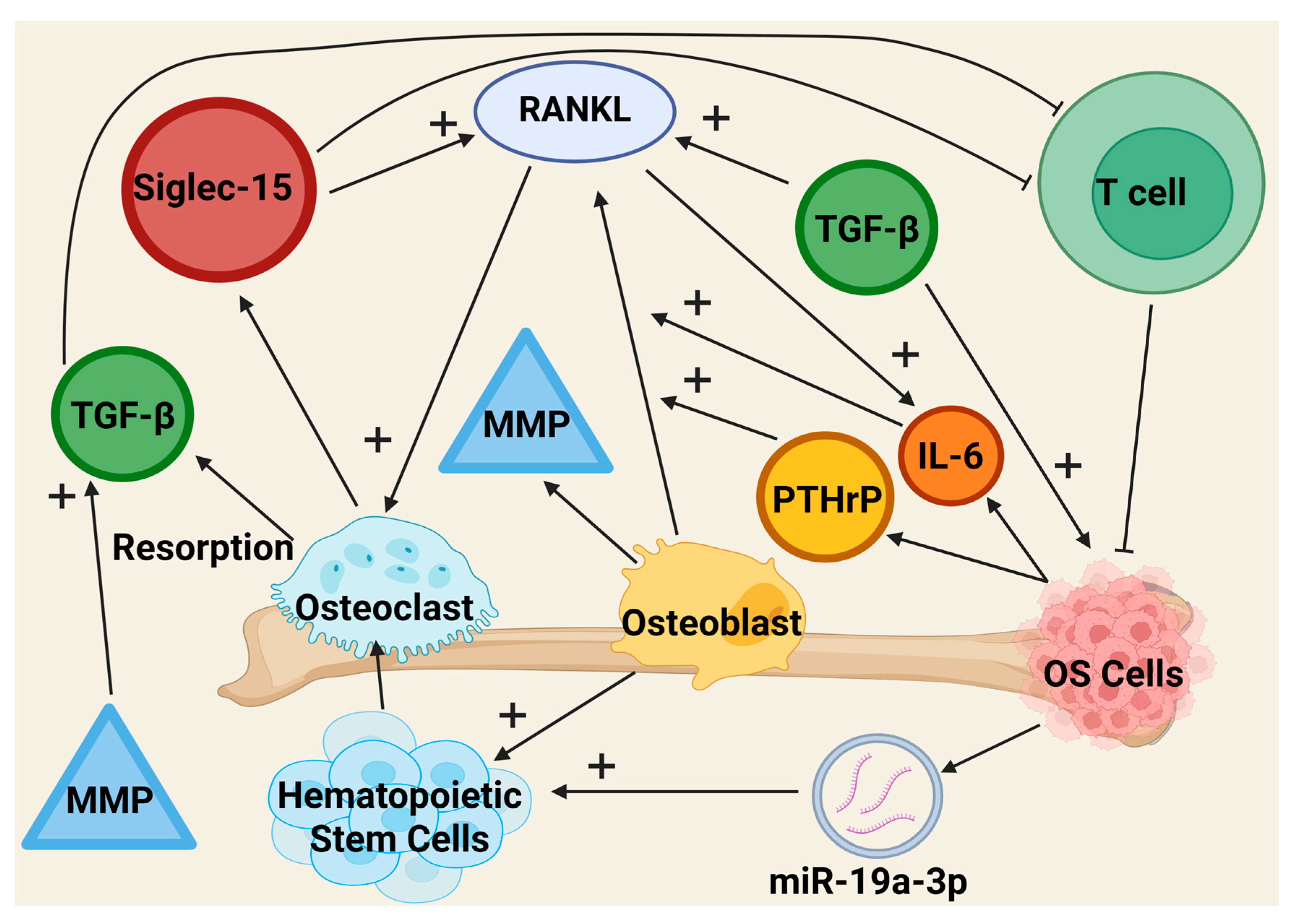
2.2.1. Osteoclasts
2.2.2. Osteoblasts
2.2.3. Fibroblasts
2.3. Extracellular Matrix
2.4. Signaling Pathways
2.4.1. PI3K/AKT/mTOR Signaling Pathway and Osteosarcoma
2.4.2. Notch/Hedgehog/Wnt/120 and Osteosarcoma
2.5. Environment
3. Immunotherapy Treatment
4. TME Evolution with Disease Progression
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Author Correction: Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 82. [Google Scholar] [CrossRef]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer. 2021, 68 (Suppl. S2), e28352. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Voskarides, K.; Giannopoulou, N. The Role of TP53 in Adaptation and Evolution. Cells 2023, 12, 512. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gálvez, C.C.; Ordaz-Favila, J.C.; Villar-Calvo, V.M.; Cancino-Marentes, M.E.; Bosch-Canto, V. Retinoblastoma: Review and new insights. Front. Oncol. 2022, 12, 963780. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef]
- Moukengue, B.; Lallier, M.; Marchandet, L.; Baud’huin, M.; Verrecchia, F.; Ory, B.; Lamoureux, F. Origin and Therapies of Osteosarcoma. Cancers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Fried, S.; Suhrawardy, A.; Rehman, R.; Al-Jamal, M.; Moore, D.; Mehregan, D. Metastatic osteosarcoma involving the skin: A systematic review of patient demographics, clinical characteristics, and treatment outcomes. J. Am. Acad. Dermatol. 2023, 88, 470–471. [Google Scholar] [CrossRef]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.; Pan, F.; Huang, S.; Wang, F.; Zeng, Z.; Chen, H.; Tian, X. MATN4 as a target gene of HIF-1α promotes the proliferation and metastasis of osteosarcoma. Aging 2024, 16, 10462–10476. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, J.; Sun, W.; Zhong, W.; Wu, H. Therapeutic Effects of Zoledronic Acid-Loaded Hyaluronic Acid/Polyethylene Glycol/Nano-Hydroxyapatite Nanoparticles on Osteosarcoma. Front. Bioeng. Biotechnol. 2022, 10, 897641. [Google Scholar] [CrossRef]
- Poudel, B.H.; Koks, S. The whole transcriptome analysis using FFPE and fresh tissue samples identifies the molecular fingerprint of osteosarcoma. Exp. Biol. Med. 2024, 249, 10161. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Ujlaki, G.; Sári, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1225, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Orrapin, S.; Moonmuang, S.; Udomruk, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Unlocking the tumor-immune microenvironment in osteosarcoma: Insights into the immune landscape and mechanisms. Front. Immunol. 2024, 15, 1394284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef]
- Fu, Y.; Bao, Q.; Liu, Z.; He, G.; Wen, J.; Liu, Q.; Xu, Y.; Jin, Z.; Zhang, W. Development and Validation of a Hypoxia-Associated Prognostic Signature Related to Osteosarcoma Metastasis and Immune Infiltration. Front. Cell Dev. Biol. 2021, 9, 633607. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Friedman-DeLuca, M.; Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H.; Entenberg, D. Macrophages in tumor cell migration and metastasis. Front. Immunol. 2024, 15, 1494462. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.L.; Borriello, L.; Karagiannis, G.S.; Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Targeting Tie2 in the Tumor Microenvironment: From Angiogenesis to Dissemination. Cancers 2021, 13, 5730. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Karagiannis, G.S.; Wang, Y.; Xue, E.A.; Lin, Y.; Skobe, M.; Jones, J.G.; Oktay, M.H.; Condeelis, J.S.; Entenberg, D. Hematogenous Dissemination of Breast Cancer Cells From Lymph Nodes Is Mediated by Tumor MicroEnvironment of Metastasis Doorways. Front. Oncol. 2020, 10, 571100. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef]
- Harney, A.S.; Karagiannis, G.S.; Pignatelli, J.; Smith, B.D.; Kadioglu, E.; Wise, S.C.; Hood, M.M.; Kaufman, M.D.; Leary, C.B.; Lu, W.P.; et al. The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2Hi Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors. Mol. Cancer Ther. 2017, 16, 2486–2501. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Komura, N.; Mabuchi, S.; Shimura, K.; Yokoi, E.; Kozasa, K.; Kuroda, H.; Takahashi, R.; Sasano, T.; Kawano, M.; Matsumoto, Y.; et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol. Immunother. 2020, 69, 2477–2499. [Google Scholar] [CrossRef] [PubMed]
- Take, Y.; Koizumi, S.; Nagahisa, A. Prostaglandin E Receptor 4 Antagonist in Cancer Immunotherapy: Mechanisms of Action. Front. Immunol. 2020, 11, 324. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Tatsuno, R.; Ichikawa, J.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Aoki, K.; Hayakawa, K.; Iwata, S.; Jubashi, T.; et al. Pivotal role of IL-8 derived from the interaction between osteosarcoma and tumor-associated macrophages in osteosarcoma growth and metastasis via the FAK pathway. Cell Death Dis. 2024, 15, 108. [Google Scholar] [CrossRef]
- Liu, T.; Ma, Q.; Zhang, Y.; Wang, X.; Xu, K.; Yan, K.; Dong, W.; Fan, Q.; Zhang, Y.; Qiu, X. Self-seeding circulating tumor cells promote the proliferation and metastasis of human osteosarcoma by upregulating interleukin-8. Cell Death Dis. 2019, 10, 575. [Google Scholar] [CrossRef]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Thind, M.K.; Uhlig, H.H.; Glogauer, M.; Palaniyar, N.; Bourdon, C.; Gwela, A.; Lancioni, C.L.; Berkley, J.A.; Bandsma, R.H.J.; Farooqui, A. A metabolic perspective of the neutrophil life cycle: New avenues in immunometabolism. Front. Immunol. 2023, 14, 1334205. [Google Scholar] [CrossRef]
- Li, X.; Hu, L.; Naeem, A.; Xiao, S.; Yang, M.; Shang, H.; Zhang, J. Neutrophil Extracellular Traps in Tumors and Potential Use of Traditional Herbal Medicine Formulations for Its Regulation. Int. J. Nanomed. 2024, 19, 2851–2877. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Aloe, C.; Wang, H.; Vlahos, R.; Irving, L.; Steinfort, D.; Bozinovski, S. Emerging and multifaceted role of neutrophils in lung cancer. Transl. Lung Cancer Res. 2021, 10, 2806–2818. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; McDowell, S.A.C.; He, X.Y.; Quail, D.F.; Egeblad, M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 2023, 41, 505–526. [Google Scholar] [CrossRef]
- Liu, L.; Mao, Y.; Xu, B.; Zhang, X.; Fang, C.; Ma, Y.; Men, K.; Qi, X.; Yi, T.; Wei, Y.; et al. Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways. Cell Prolif. 2019, 52, e12579. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Gu, J.; Kim, J.E.; Nam, Y.; Song, J.W.; Kim, H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE 2019, 14, e0216055. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e7. [Google Scholar] [CrossRef]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- Deryugina, E.; Carré, A.; Ardi, V.; Muramatsu, T.; Schmidt, J.; Pham, C.; Quigley, J.P. Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events. iScience 2020, 23, 101799. [Google Scholar] [CrossRef]
- Guan, X.; Lu, Y.; Zhu, H.; Yu, S.; Zhao, W.; Chi, X.; Xie, C.; Yin, Z. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J. Hepatocell. Carcinoma 2021, 8, 451–465. [Google Scholar] [CrossRef]
- Jin, W.; Yin, H.; Li, H.; Yu, X.J.; Xu, H.X.; Liu, L. Neutrophil extracellular DNA traps promote pancreatic cancer cells migration and invasion by activating EGFR/ERK pathway. J. Cell Mol. Med. 2021, 25, 5443–5456. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tang, H.; Teng, H.; Feng, W.; Li, F.; Liu, S.; Liu, Y.; Wei, Q. Development and validation of neutrophil extracellular traps-derived signature to predict the prognosis for osteosarcoma patients. Int. Immunopharmacol. 2024, 127, 111364. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Binenbaum, Y.; Maman, R.; Fidel, V.; Shusterman, A.; Vaisman, D.; Sher, O.; Manisterski, M.; Shukrun, R.; Rössig, C.; et al. Neutrophil extracellular traps are associated with poor response to neoadjuvant therapy and poor survival in pediatric osteosarcoma. Front. Oncol. 2025, 15, 1472716. [Google Scholar] [CrossRef]
- Wculek, S.K.; Bridgeman, V.L.; Peakman, F.; Malanchi, I. Early Neutrophil Responses to Chemical Carcinogenesis Shape Long-Term Lung Cancer Susceptibility. iScience 2020, 23, 101277. [Google Scholar] [CrossRef]
- Bui, T.M.; Butin-Israeli, V.; Wiesolek, H.L.; Zhou, M.; Rehring, J.F.; Wiesmüller, L.; Wu, J.D.; Yang, G.Y.; Hanauer, S.B.; Sebag, J.A.; et al. Neutrophils Alter DNA Repair Landscape to Impact Survival and Shape Distinct Therapeutic Phenotypes of Colorectal Cancer. Gastroenterology 2021, 161, 225–238.e15. [Google Scholar] [CrossRef]
- Xia, W.K.; Liu, Z.L.; Shen, D.; Lin, Q.F.; Su, J.; Mao, W.D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Lu, J.; He, J.; Ji, S.; Liao, S.; Wei, Q.; Lu, S.; Liu, Y. Significance of the neutrophil-to-lymphocyte ratio in predicting the response to neoadjuvant chemotherapy in extremity osteosarcoma: A multicentre retrospective study. BMC Cancer 2022, 22, 33. [Google Scholar] [CrossRef]
- Tang, F.; Tie, Y.; Lan, T.; Yang, J.; Hong, W.; Chen, S.; Shi, H.; Li, L.; Zeng, H.; Min, L.; et al. Surgical Treatment of Osteosarcoma Induced Distant Pre-Metastatic Niche in Lung to Facilitate the Colonization of Circulating Tumor Cells. Adv. Sci. 2023, 10, 2207518. [Google Scholar] [CrossRef]
- Arefpour, A.M.; Garousi, M.; Foroughi, A.; Hosseini, S.; Shahin, M.; Javadinia, S.A. Significance of the Pretreatment Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Predicting the Response to Neoadjuvant Chemotherapy and Survival Rates in Extremity Osteosarcoma: A Multicentre Prospective Study. Cancer Investig. 2023, 41, 816–820. [Google Scholar] [CrossRef]
- Tan, S.; Chao, R. An Exploration of Osteosarcoma Metastasis Diagnostic Markers Based on Tumor-Associated Neutrophils. Discov. Med. 2023, 35, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Yang, C.; Tan, J.; Dou, C.; Chen, Y. Beyond immunosuppressive effects: Dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell Mol. Life Sci. 2021, 78, 7161–7183. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Banerjee, S.; White, S.V.; Kortylewski, M. STAT3 in Tumor-Associated Myeloid Cells: Multitasking to Disrupt Immunity. Int. J. Mol. Sci. 2018, 19, 1803. [Google Scholar] [CrossRef]
- Arellano, D.L.; Juárez, P.; Verdugo-Meza, A.; Almeida-Luna, P.S.; Corral-Avila, J.A.; Drescher, F.; Olvera, F.; Jiménez, S.; Elzey, B.D.; Guise, T.A.; et al. Bone Microenvironment-Suppressed T Cells Increase Osteoclast Formation and Osteolytic Bone Metastases in Mice. J. Bone Miner. Res. 2022, 37, 1446–1463. [Google Scholar] [CrossRef]
- Ugolini, A.; Tyurin, V.A.; Tyurina, Y.Y.; Tcyganov, E.N.; Donthireddy, L.; Kagan, V.E.; Gabrilovich, D.I.; Veglia, F. Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI Insight 2020, 5, e138581. [Google Scholar] [CrossRef]
- Won, W.J.; Deshane, J.S.; Leavenworth, J.W.; Oliva, C.R.; Griguer, C.E. Metabolic and functional reprogramming of myeloid-derived suppressor cells and their therapeutic control in glioblastoma. Cell Stress 2019, 3, 47–65. [Google Scholar] [CrossRef]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431. [Google Scholar] [CrossRef]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef] [PubMed]
- Stiff, A.; Trikha, P.; Mundy-Bosse, B.; McMichael, E.; Mace, T.A.; Benner, B.; Kendra, K.; Campbell, A.; Gautam, S.; Abood, D.; et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018, 24, 1891–1904. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Hu, X.; Jin, F.; Gao, Z.; Yin, L.; Qin, J.; Yin, F.; Li, C.; Wang, Y. IDO1 impairs NK cell cytotoxicity by decreasing NKG2D/NKG2DLs via promoting miR-18a. Mol. Immunol. 2018, 103, 144–155. [Google Scholar] [CrossRef]
- Wang, Y.; Schafer, C.C.; Hough, K.P.; Tousif, S.; Duncan, S.R.; Kearney, J.F.; Ponnazhagan, S.; Hsu, H.C.; Deshane, J.S. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J. Immunol. 2018, 201, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.W.; Muhitch, J.B.; Powers, C.A.; Diehl, M.; Kim, M.; Fisher, D.T.; Sharda, A.P.; Clements, V.K.; O’Loughlin, K.; Minderman, H.; et al. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. eLife 2016, 5, e17375. [Google Scholar] [CrossRef]
- Shen, M.; Wang, J.; Yu, W.; Zhang, C.; Liu, M.; Wang, K.; Yang, L.; Wei, F.; Wang, S.E.; Sun, Q.; et al. A novel MDSC-induced PD-1-PD-L1+ B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology 2018, 7, e1413520. [Google Scholar] [CrossRef]
- Siret, C.; Collignon, A.; Silvy, F.; Robert, S.; Cheyrol, T.; André, P.; Rigot, V.; Iovanna, J.; van de Pavert, S.; Lombardo, D.; et al. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2019, 10, 3070. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Sheng, J.; Ng, P.K.S.; Harder, J.M.; Kumar, P.; Ahn, J.Y.; Cao, Y.; Dzis, A.M.; Jillette, N.L.; Goodspeed, A.; et al. Immunosuppressive Tumor Microenvironment of Osteosarcoma. Cancers 2025, 17, 2117. [Google Scholar] [CrossRef]
- Nirala, B.K.; Yamamichi, T.; Petrescu, D.I.; Shafin, T.N.; Yustein, J.T. Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers 2023, 15, 5108. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, R.; Peng, Z.; Dong, D.; Wei, G.; Wang, Y. Inhibition of IL-18-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy against osteosarcoma cancer. J. Bone Oncol. 2017, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, J.; Zhang, J.; Wang, L.; Zhang, Q.; Ge, J.; Guo, Y.; Wang, B.; Huang, Y.; Yang, T.; et al. SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy. Int. Immunopharmacol. 2019, 75, 105818. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, X.; Wang, H.; Yu, Z.; Zhu, Y.; Gao, Y. Specific inhibition of PI3Kδ/γ enhances the efficacy of anti-PD1 against osteosarcoma cancer. J. Bone Oncol. 2018, 16, 100206. [Google Scholar] [CrossRef]
- Solimando, A.G.; Desantis, V.; Ribatti, D. Mast Cells and Interleukins. Int. J. Mol. Sci. 2022, 23, 14004. [Google Scholar] [CrossRef]
- Le, T.; Su, S.; Shahriyari, L. Immune classification of osteosarcoma. Math. Biosci. Eng. 2021, 18, 1879–1897. [Google Scholar] [CrossRef]
- Heymann, M.F.; Lézot, F.; Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell Immunol. 2019, 343, 103711. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Sammarco, G.; Varricchi, G.; Ferraro, V.; Ammendola, M.; De Fazio, M.; Altomare, D.F.; Luposella, M.; Maltese, L.; Currò, G.; Marone, G.; et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 2106. [Google Scholar] [CrossRef]
- Shi, S.; Ye, L.; Yu, X.; Jin, K.; Wu, W. Focus on mast cells in the tumor microenvironment: Current knowledge and future directions. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188845. [Google Scholar] [CrossRef]
- Lv, Y.P.; Peng, L.S.; Wang, Q.H.; Chen, N.; Teng, Y.S.; Wang, T.T.; Mao, F.Y.; Zhang, J.Y.; Cheng, P.; Liu, Y.G.; et al. Degranulation of mast cells induced by gastric cancer-derived adrenomedullin prompts gastric cancer progression. Cell Death Dis. 2018, 9, 1034. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.; Wang, F.; Wang, X.; Yang, F.; Zhao, C.; Feng, C.; Li, T. Role of MMP-9 in epithelial-mesenchymal transition of thyroid cancer. World J. Surg. Oncol. 2020, 18, 181. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Furuhata, A.; Suzuki, E.; Toi, M.; Tsuruyama, T.; Okayama, Y.; Haga, H. The Killer Cell Ig-like Receptor 2DL4 Expression in Human Mast Cells and Its Potential Role in Breast Cancer Invasion. Cancer Immunol. Res. 2015, 3, 871–880. [Google Scholar] [CrossRef]
- Yin, Y.; Shelke, G.V.; Lässer, C.; Brismar, H.; Lötvall, J. Extracellular vesicles from mast cells induce mesenchymal transition in airway epithelial cells. Respir. Res. 2020, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Kim, H.J.; Song, I.S.; Park, C.; Jung, J.W.; Park, D.S.; Oh, S.H.; Kim, Y.S.; Kim, H.R. Salinomycin suppresses TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the AMPK/SIRT1 pathway in non-small cell lung cancer. Int. J. Med. Sci. 2021, 18, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Chen, Y.; Yeh, C.R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842–7855. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hookway, E.; Williams, K.A.; Hassan, A.B.; Oppermann, U.; Tanaka, Y.; Soilleux, E.; Athanasou, N.A. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016, 6, 13. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef]
- Kroner, J.; Kovtun, A.; Kemmler, J.; Messmann, J.J.; Strauss, G.; Seitz, S.; Schinke, T.; Amling, M.; Kotrba, J.; Froebel, J.; et al. Mast Cells Are Critical Regulators of Bone Fracture-Induced Inflammation and Osteoclast Formation and Activity. J. Bone Miner. Res. 2017, 32, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Brunell, A.E.; Lahesmaa, R.; Autio, A.; Thotakura, A.K. Exhausted T cells hijacking the cancer-immunity cycle: Assets and liabilities. Front. Immunol. 2023, 14, 1151632. [Google Scholar] [CrossRef]
- Khan, O.; Giles, J.R.; McDonald, S.; Manne, S.; Ngiow, S.F.; Patel, K.P.; Werner, M.T.; Huang, A.C.; Alexander, K.A.; Wu, J.E.; et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 2019, 571, 211–218. [Google Scholar] [CrossRef]
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016, 354, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Kersten, K.; Hu, K.H.; Combes, A.J.; Samad, B.; Harwin, T.; Ray, A.; Rao, A.A.; Cai, E.; Marchuk, K.; Artichoker, J.; et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 2022, 40, 624–638.e9. [Google Scholar] [CrossRef]
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef]
- Vignali, P.D.A.; DePeaux, K.; Watson, M.J.; Ye, C.; Ford, B.R.; Lontos, K.; McGaa, N.K.; Scharping, N.E.; Menk, A.V.; Robson, S.C.; et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat. Immunol. 2023, 24, 267–279. [Google Scholar] [CrossRef]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef]
- van de Veen, W.; Globinska, A.; Jansen, K.; Straumann, A.; Kubo, T.; Verschoor, D.; Wirz, O.F.; Castro-Giner, F.; Tan, G.; Rückert, B.; et al. A novel proangiogenic B cell subset is increased in cancer and chronic inflammation. Sci. Adv. 2020, 6, eaaz3559. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, F.; Meiser, P.; Donakonda, S.; Hirschberger, A.; Lacher, S.B.; Pedde, A.M.; Hermann, C.D.; Elewaut, A.; Knolle, M.; Ramsauer, L.; et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 2023, 56, 1341–1358.e11. [Google Scholar] [CrossRef]
- Daley, D.; Zambirinis, C.P.; Seifert, L.; Akkad, N.; Mohan, N.; Werba, G.; Barilla, R.; Torres-Hernandez, A.; Hundeyin, M.; Kumar Mani, V.R.; et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell 2020, 183, 1134–1136. [Google Scholar] [CrossRef]
- Morath, A.; Schamel, W.W. αβ and γδ T cell receptors: Similar but different. J. Leukoc. Biol. 2020, 107, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yin, H. Gamma delta (γδ) T cells in cancer immunotherapy; where it comes from, where it will go? Eur. J. Pharmacol. 2022, 919, 174803. [Google Scholar] [CrossRef]
- Kõks, G.; Uudelepp, M.L.; Limbach, M.; Peterson, P.; Reimann, E.; Kõks, S. Smoking-Induced Expression of the GPR15 Gene Indicates Its Potential Role in Chronic Inflammatory Pathologies. Am. J. Pathol. 2015, 185, 2898–2906. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Li, J.; Wang, K.; Zhu, W.; Zeng, Y. The Dual Role of B Cells in the Tumor Microenvironment: Implications for Cancer Immunology and Therapy. Int. J. Mol. Sci. 2024, 25, 11825. [Google Scholar] [CrossRef]
- Esteve-Solé, A.; Luo, Y.; Vlagea, A.; Deyà-Martínez, Á.; Yagüe, J.; Plaza-Martín, A.M.; Juan, M.; Alsina, L. B Regulatory Cells: Players in Pregnancy and Early Life. Int. J. Mol. Sci. 2018, 19, 2099. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Song, C.; Tong, T.; Dai, B.; Zhu, Y.; Chen, E.; Zhang, M.; Zhang, W. Osteoimmunology in bone malignancies: A symphony with evil. J. Natl. Cancer Cent. 2024, 4, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, X.; Qi, Y.; Wu, J. Exploration of Immune-Related Gene Expression in Osteosarcoma and Association With Outcomes. JAMA Netw. Open 2021, 4, e2119132. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Duan, Y.; Li, X.; Pan, J.; Wang, G.; Shen, B. Identification of B cell marker genes based on single-cell sequencing to establish a prognostic model and identify immune infiltration in osteosarcoma. Front. Immunol. 2022, 13, 1026701. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, F.M.; Wilmott, J.S.; Volders, N.; Mercier, M.; Wouters, J.; Stas, M.; Blokx, W.A.; Massi, D.; Thompson, J.F.; Scolyer, R.A.; et al. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod. Pathol. 2016, 29, 347–358. [Google Scholar] [CrossRef]
- Isaeva, O.I.; Sharonov, G.V.; Serebrovskaya, E.O.; Turchaninova, M.A.; Zaretsky, A.R.; Shugay, M.; Chudakov, D.M. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J. Immunother. Cancer 2019, 7, 279. [Google Scholar] [CrossRef]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef]
- Welinder, C.; Jirström, K.; Lehn, S.; Nodin, B.; Marko-Varga, G.; Blixt, O.; Danielsson, L.; Jansson, B. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon 2016, 2, e00143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, J.; Ni, Y. Screening of candidate key genes associated with human osteosarcoma using bioinformatics analysis. Oncol. Lett. 2017, 14, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- N, J.; J, T.; Sl, N.; Gt, B. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10, 1900508. [Google Scholar] [CrossRef] [PubMed]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 2689. [Google Scholar] [CrossRef]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef]
- Casu, B.; Dondero, A.; Regis, S.; Caliendo, F.; Petretto, A.; Bartolucci, M.; Bellora, F.; Bottino, C.; Castriconi, R. Novel Immunoregulatory Functions of IL-18, an Accomplice of TGF-β1. Cancers 2019, 11, 75. [Google Scholar] [CrossRef]
- Bruno, A.; Bassani, B.; D’Urso, D.G.; Pitaku, I.; Cassinotti, E.; Pelosi, G.; Boni, L.; Dominioni, L.; Noonan, D.M.; Mortara, L.; et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. 2018, 32, 5365–5377. [Google Scholar] [CrossRef]
- Guan, Y.; Chambers, C.B.; Tabatabai, T.; Hatley, H.; Delfino, K.R.; Robinson, K.; Alanee, S.R.; Ran, S.; Torry, D.S.; Wilber, A. Renal cell tumors convert natural killer cells to a proangiogenic phenotype. Oncotarget 2020, 11, 2571–2585. [Google Scholar] [CrossRef]
- Palano, M.T.; Gallazzi, M.; Cucchiara, M.; De Lerma Barbaro, A.; Gallo, D.; Bassani, B.; Bruno, A.; Mortara, L. Neutrophil and Natural Killer Cell Interactions in Cancers: Dangerous Liaisons Instructing Immunosuppression and Angiogenesis. Vaccines 2021, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Gallazzi, M.; Baci, D.; Mortara, L.; Bosi, A.; Buono, G.; Naselli, A.; Guarneri, A.; Dehò, F.; Capogrosso, P.; Albini, A.; et al. Prostate Cancer Peripheral Blood NK Cells Show Enhanced CD9, CD49a, CXCR4, CXCL8, MMP-9 Production and Secrete Monocyte-Recruiting and Polarizing Factors. Front. Immunol. 2020, 11, 586126. [Google Scholar] [CrossRef]
- Liu, W.; Hu, H.; Shao, Z.; Lv, X.; Zhang, Z.; Deng, X.; Song, Q.; Han, Y.; Guo, T.; Xiong, L.; et al. Characterizing the tumor microenvironment at the single-cell level reveals a novel immune evasion mechanism in osteosarcoma. Bone Res. 2023, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef]
- Kansara, M.; Thomson, K.; Pang, P.; Dutour, A.; Mirabello, L.; Acher, F.; Pin, J.P.; Demicco, E.G.; Yan, J.; Teng, M.W.L.; et al. Infiltrating Myeloid Cells Drive Osteosarcoma Progression via GRM4 Regulation of IL23. Cancer Discov. 2019, 9, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Zhang, J.; Ai, J.; Hao, X.; Luan, R. Aberrant expression of IL-23/IL-23R in patients with breast cancer and its clinical significance. Mol. Med. Rep. 2018, 17, 4639–4644. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Nie, W.; Yu, T.; Sang, Y.; Gao, X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macrophages and neutrophils in tumor microenvironment. Biochem. Biophys. Res. Commun. 2017, 482, 1400–1406. [Google Scholar] [CrossRef]
- Gao, Y.M.; Pei, Y.; Zhao, F.F.; Wang, L. Osteoclasts in Osteosarcoma: Mechanisms, Interactions, and Therapeutic Prospects. Cancer Manag. Res. 2023, 15, 1323–1337. [Google Scholar] [CrossRef]
- Luo, T.; Zhou, X.; Jiang, E.; Wang, L.; Ji, Y.; Shang, Z. Osteosarcoma Cell-Derived Small Extracellular Vesicles Enhance Osteoclastogenesis and Bone Resorption Through Transferring MicroRNA-19a-3p. Front. Oncol. 2021, 11, 618662. [Google Scholar] [CrossRef]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398. [Google Scholar] [CrossRef]
- Yu, Y.; Li, K.; Peng, Y.; Zhang, Z.; Pu, F.; Shao, Z.; Wu, W. Tumor microenvironment in osteosarcoma: From cellular mechanism to clinical therapy. Genes. Dis. 2025, 12, 101569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Wu, K.L.; Yu, L.; Wang, C.; Ding, H.; Gao, Y.; Sun, H.; Wu, Y.H.; Xia, M.; et al. Siglec-15/sialic acid axis as a central glyco-immune checkpoint in breast cancer bone metastasis. Proc. Natl. Acad. Sci. USA 2024, 121, e2312929121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Chen, T.; Qiu, G.; Han, Y. The emerging role of osteoclasts in the treatment of bone metastases: Rationale and recent clinical evidence. Front. Oncol. 2024, 14, 1445025. [Google Scholar] [CrossRef]
- Huang, F.; Wong, P.; Li, J.; Lv, Z.; Xu, L.; Zhu, G.; He, M.; Luo, Y. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J. Cell. Mol. Med. 2022, 26, 3591–3597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, W.; Li, J.; Su, T.; Wang, C.; Fu, Y.; Li, C.; Hua, P.; Liang, X.; Zhu, Y.; Cui, H. Osteoclast Activation and Inflammatory Bone Diseases: Focusing on Receptors in Osteoclasts. J. Inflamm. Res. 2025, 18, 3201–3213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- Vimalraj, S.; Sekaran, S. RUNX Family as a Promising Biomarker and a Therapeutic Target in Bone Cancers: A Review on Its Molecular Mechanism(s) behind Tumorigenesis. Cancers 2023, 15, 3247. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, R.; Roth, M.; Piperdi, S.; Zhang, W.; Dorfman, H.; Rao, P.; Park, A.; Tripathi, S.; Freeman, C.; et al. Genetically transforming human osteoblasts to sarcoma: Development of an osteosarcoma model. Genes Cancer 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chow, S.O.; Boernert, K.; Basel, D.; Mikuscheva, A.; Kim, S.; Fong-Yee, C.; Trivedi, T.; Buttgereit, F.; Sutherland, R.L.; et al. Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J. Bone Miner. Res. 2014, 29, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating osteoimmunology: Regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacifici, R. T cells, osteoblasts, and osteocytes: Interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 2016, 1364, 11–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Galbo, P.M.; Zang, X.; Zheng, D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.P.; Cronise, K.E.; Haines, L.A.; Das, S.; Offermann, A.; Easton, C.P.; Regan, D.P. Osteosarcoma Exosome Priming of Primary Human Lung Fibroblasts Induces an Immune Modulatory and Protumorigenic Phenotype. Cancer Res. Commun. 2025, 5, 594–608. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, X.F.; Gu, X.J.; Jiang, X.H. Exosomal miR-1228 From Cancer-Associated Fibroblasts Promotes Cell Migration and Invasion of Osteosarcoma by Directly Targeting SCAI. Oncol. Res. 2019, 27, 979–986. [Google Scholar] [CrossRef]
- Lin, L.; Liu, D.; Liang, H.; Xue, L.; Su, C.; Liu, M. MiR-1228 promotes breast cancer cell growth and metastasis through targeting SCAI protein. Int. J. Clin. Exp. Pathol. 2015, 8, 6646–6655. [Google Scholar]
- Cortini, M.; Macchi, F.; Reggiani, F.; Vitale, E.; Lipreri, M.V.; Perut, F.; Ciarrocchi, A.; Baldini, N.; Avnet, S. Endogenous Extracellular Matrix Regulates the Response of Osteosarcoma 3D Spheroids to Doxorubicin. Cancers 2023, 15, 1221. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular matrix stiffness: Mechanisms in tumor progression and therapeutic potential in cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Tian, H.; Shi, H.; Yu, J.; Ge, S.; Ruan, J. Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT. Glob. Chall. 2022, 6, 2100094. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.K.; Viloria-Petit, A.M. Targeting Mechanotransduction in Osteosarcoma: A Comparative Oncology Perspective. Int. J. Mol. Sci. 2020, 21, 7595. [Google Scholar] [CrossRef]
- Kalli, M.; Poskus, M.D.; Stylianopoulos, T.; Zervantonakis, I.K. Beyond matrix stiffness: Targeting force-induced cancer drug resistance. Trends Cancer 2023, 9, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, J.; Lan, Y.; Yi, Q.; Liu, H. Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies. MedComm 2023, 4, e308. [Google Scholar] [CrossRef]
- Shao, W.; Feng, Y.; Huang, J.; Li, T.; Gao, S.; Yang, Y.; Li, D.; Yang, Z.; Yao, Z. Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma. Open Life Sci. 2024, 19, 20220936. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Yin, C.; Feng, C.; Liu, B.; Xu, H.; Jin, X.; Tu, C.; Li, Z. PRKDC Induces Chemoresistance in Osteosarcoma by Recruiting GDE2 to Stabilize GNAS and Activate AKT. Cancer Res. 2024, 84, 2873–2887. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yin, L.; Yuan, Q.; Su, X.; Shen, Y.; Deng, Z. DCC-2036 inhibits osteosarcoma via targeting HCK and the PI3K/AKT-mTORC1 axis to promote autophagy. World J. Surg. Oncol. 2025, 23, 115. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Self-Renewal and Pluripotency in Osteosarcoma Stem Cells’ Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers. Int. J. Mol. Sci. 2023, 24, 8401. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Q. Wnt/β-catenin signaling pathway: An attractive potential therapeutic target in osteosarcoma. Front. Oncol. 2024, 14, 1456959. [Google Scholar] [CrossRef]
- Jiang, S.; Kong, P.; Liu, X.; Yuan, C.; Peng, K.; Liang, Y. LncRNA FLVCR1-AS1 accelerates osteosarcoma cells to proliferate, migrate and invade via activating wnt/β-catenin pathway. J. BUON 2020, 25, 2078–2085. [Google Scholar]
- Matsuoka, K.; Bakiri, L.; Wolff, L.I.; Linder, M.; Mikels-Vigdal, A.; Patiño-García, A.; Lecanda, F.; Hartmann, C.; Sibilia, M.; Wagner, E.F. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020, 30, 885–901. [Google Scholar] [CrossRef]
- Zhang, W.; Lyu, P.; Andreev, D.; Jia, Y.; Zhang, F.; Bozec, A. Hypoxia-immune-related microenvironment prognostic signature for osteosarcoma. Front. Cell Dev. Biol. 2022, 10, 974851. [Google Scholar] [CrossRef] [PubMed]
- Di Giovannantonio, M.; Hartley, F.; Elshenawy, B.; Barberis, A.; Hudson, D.; Shafique, H.S.; Allott, V.E.S.; Harris, D.A.; Lord, S.R.; Haider, S.; et al. Defining hypoxia in cancer: A landmark evaluation of hypoxia gene expression signatures. Cell Genom. 2025, 5, 100764. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, D.; Li, N. MicroRNA-20b Downregulates HIF-1α and Inhibits the Proliferation and Invasion of Osteosarcoma Cells. Oncol. Res. 2016, 23, 257–266. [Google Scholar] [CrossRef]
- Pierrevelcin, M.; Fuchs, Q.; Lhermitte, B.; Messé, M.; Guérin, E.; Weingertner, N.; Martin, S.; Lelong-Rebel, I.; Nazon, C.; Dontenwill, M.; et al. Focus on Hypoxia-Related Pathways in Pediatric Osteosarcomas and Their Druggability. Cells 2020, 9, 1998. [Google Scholar] [CrossRef] [PubMed]
- Tafech, A.; Stéphanou, A. On the Importance of Acidity in Cancer Cells and Therapy. Biology 2024, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Meftahpour, V.; Aghebati-Maleki, A.; Fotouhi, A.; Safarzadeh, E.; Aghebati-Maleki, L. Prognostic significance and therapeutic potentials of immune checkpoints in osteosarcoma. EXCLI J. 2022, 21, 250–268. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, X.; Jiang, Z.; Wang, H.; Yuan, H. Immune checkpoint inhibitors in osteosarcoma: A hopeful and challenging future. Front. Pharmacol. 2022, 13, 1031527. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Geoerger, B.; Zwaan, C.M.; Marshall, L.V.; Michon, J.; Bourdeaut, F.; Casanova, M.; Corradini, N.; Rossato, G.; Farid-Kapadia, M.; Shemesh, C.S.; et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): A multicentre phase 1-2 study. Lancet Oncol. 2020, 21, 134–144. [Google Scholar] [CrossRef]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: Results from the PEMBROSARC study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef]
- Davar, D.; Boasberg, P.; Eroglu, Z.; Falchook, G.; Gainor, J.; Hamilton, E. A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in combination with TSR-042 (anti-PD-1) in patients with colorectal cancer and post-PD-1 NSCLC and melanoma. J. Immunother. Cancer 2018, 6 (Suppl. S1), 115O21.30400822. [Google Scholar]
- Davis, K.L.; Fox, E.; Isikwei, E.; Reid, J.M.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; Mackall, C.L. A Phase I/II Trial of Nivolumab plus Ipilimumab in Children and Young Adults with Relapsed/Refractory Solid Tumors: A Children’s Oncology Group Study ADVL1412. Clin. Cancer Res. 2022, 28, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Comito, F.; Massari, F.; Gelsomino, F. Relatlimab and nivolumab in untreated advanced melanoma: Insight into RELATIVITY. Immunotherapy 2023, 15, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chen, G.; Wang, D. Emerging immunotherapies in osteosarcoma: From checkpoint blockade to cellular therapies. Front. Immunol. 2025, 16, 1579822. [Google Scholar] [CrossRef]
- Mao, C.; Xiong, A.; Qian, J.; Wang, W.; Liu, Y.; Zhang, T.; Wu, Z.; Ni, H.; Lu, J.; Long, S.; et al. Dual inhibition of LAG-3 and PD-1 with IBI110 and sintilimab in advanced solid tumors: The first-in-human phase Ia/Ib study. J. Hematol. Oncol. 2024, 17, 132. [Google Scholar] [CrossRef]
- Gunassekaran, G.R.; Poongkavithai Vadevoo, S.M.; Baek, M.C.; Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef]
- Lv, J.; Chen, F.K.; Liu, C.; Liu, P.J.; Feng, Z.P.; Jia, L.; Yang, Z.X.; Hou, F.; Deng, Z.Y. Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway. Life Sci. 2020, 256, 117925. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, D.X.; Yu, X.J.; Sun, H.W.; Xu, Y.T.; Zhang, Y.J.; Xu, J. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology 2019, 8, e1652540. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, Y.; Weng, L.; Wu, Q.; Zhang, J.; Zhao, P.; Fang, L.; Shi, Y.; Wang, P. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Wang, J.; Zhou, Y.; Watowich, S.S.; Kleinerman, E.S. CD103+ cDC1 Dendritic Cell Vaccine Therapy for Osteosarcoma Lung Metastases. Cancers 2024, 16, 3251. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Zhang, B.; Yang, C.; Wang, Y.; Zheng, S.; Tang, L.; Zhou, C.; Qian, G.; Huang, Y.; et al. A pilot study of multi-antigen stimulated cell therapy-I plus camrelizumab and apatinib in patients with advanced bone and soft-tissue sarcomas. BMC Med. 2023, 21, 470. [Google Scholar] [CrossRef]
- Krishnadas, D.K.; Shusterman, S.; Bai, F.; Diller, L.; Sullivan, J.E.; Cheerva, A.C.; George, R.E.; Lucas, K.G. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015, 64, 1251–1260. [Google Scholar] [CrossRef]
- Deng, C.; Xu, Y.; Fu, J.; Zhu, X.; Chen, H.; Xu, H.; Wang, G.; Song, Y.; Song, G.; Lu, J.; et al. Reprograming the tumor immunologic microenvironment using neoadjuvant chemotherapy in osteosarcoma. Cancer Sci. 2020, 111, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Santos, I.M.; Taverna, F.; Kuhlencord, M.; Gensch, V.; Päsler, S.; Vinckier, S.; et al. Blockade of Myeloid-Derived Suppressor Cell Expansion with All-Trans Retinoic Acid Increases the Efficacy of Antiangiogenic Therapy. Cancer Res. 2018, 78, 3220–3232. [Google Scholar] [CrossRef]
- Fujiwara, T.; Yakoub, M.A.; Chandler, A.; Christ, A.B.; Yang, G.; Ouerfelli, O.; Rajasekhar, V.K.; Yoshida, A.; Kondo, H.; Hata, T.; et al. CSF1/CSF1R Signaling Inhibitor Pexidartinib (PLX3397) Reprograms Tumor-Associated Macrophages and Stimulates T-cell Infiltration in the Sarcoma Microenvironment. Mol. Cancer Ther. 2021, 20, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Benner, B.; Good, L.; Quiroga, D.; Schultz, T.E.; Kassem, M.; Carson, W.E.; Cherian, M.A.; Sardesai, S.; Wesolowski, R. Pexidartinib, a Novel Small Molecule CSF-1R Inhibitor in Use for Tenosynovial Giant Cell Tumor: A Systematic Review of Pre-Clinical and Clinical Development. Drug Des. Devel Ther. 2020, 14, 1693–1704. [Google Scholar] [CrossRef]
- Monestime, S.; Lazaridis, D. Pexidartinib (TURALIOTM): The First FDA-Indicated Systemic Treatment for Tenosynovial Giant Cell Tumor. Drugs R D 2020, 20, 189–195. [Google Scholar] [CrossRef]
- Tap, W.D.; Gelderblom, H.; Palmerini, E.; Desai, J.; Bauer, S.; Blay, J.Y.; Alcindor, T.; Ganjoo, K.; Martín-Broto, J.; Ryan, C.W.; et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet 2019, 394, 478–487. [Google Scholar] [CrossRef]
- Dong, J.; Chai, X.; Xue, Y.; Shen, S.; Chen, Z.; Wang, Z.; Yinwang, E.; Wang, S.; Chen, L.; Wu, F.; et al. ZIF-8-Encapsulated Pexidartinib Delivery via Targeted Peptide-Modified M1 Macrophages Attenuates MDSC-Mediated Immunosuppression in Osteosarcoma. Small 2024, 20, e2309038. [Google Scholar] [CrossRef]
- Sholevar, C.J.; Liu, N.M.; Mukarrama, T.; Kim, J.; Lawrence, J.; Canter, R.J. Myeloid Cells in the Immunosuppressive Microenvironment as Immunotargets in Osteosarcoma. Immunotargets Ther. 2025, 14, 247–258. [Google Scholar] [CrossRef]
- Fan, Q.; Zuo, J.; Tian, H.; Huang, C.; Nice, E.C.; Shi, Z.; Kong, Q. Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells. J. Exp. Clin. Cancer Res. 2022, 41, 162. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Shang, G. Current status and future challenges of CAR-T cell therapy for osteosarcoma. Front. Immunol. 2023, 14, 1290762. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer 2022, 21, 194. [Google Scholar] [CrossRef]
- Mensali, N.; Köksal, H.; Joaquina, S.; Wernhoff, P.; Casey, N.P.; Romecin, P.; Panisello, C.; Rodriguez, R.; Vimeux, L.; Juzeniene, A.; et al. ALPL-1 is a target for chimeric antigen receptor therapy in osteosarcoma. Nat. Commun. 2023, 14, 3375. [Google Scholar] [CrossRef]
- Hui, X.; Farooq, M.A.; Chen, Y.; Ajmal, I.; Ren, Y.; Xue, M.; Ji, Y.; Du, B.; Wu, S.; Jiang, W. A novel strategy of co-expressing CXCR5 and IL-7 enhances CAR-T cell effectiveness in osteosarcoma. Front. Immunol. 2024, 15, 1462076. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.K.V. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef]
- Yang, B.; Su, Z.; Chen, G.; Zeng, Z.; Tan, J.; Wu, G.; Zhu, S.; Lin, L. Identification of prognostic biomarkers associated with metastasis and immune infiltration in osteosarcoma. Oncol. Lett. 2021, 21, 180. [Google Scholar] [CrossRef]
- Marchais, A.; Marques da Costa, M.E.; Job, B.; Abbas, R.; Drubay, D.; Piperno-Neumann, S.; Fromigué, O.; Gomez-Brouchet, A.; Redini, F.; Droit, R.; et al. Immune Infiltrate and Tumor Microenvironment Transcriptional Programs Stratify Pediatric Osteosarcoma into Prognostic Groups at Diagnosis. Cancer Res. 2022, 82, 974–985. [Google Scholar] [CrossRef]
- Zhihao, Z.; Cheng, J.; Xiaoshuang, Z.; Yangguang, M.; Tingyu, W.; Yongyong, Y.; Zhou, Y.; Jie, Z.; Tao, Z.; Xueyu, H.; et al. Cancer-associated fibroblast infiltration in osteosarcoma: The discrepancy in subtypes pathways and immunosuppression. Front. Pharmacol. 2023, 14, 1136960. [Google Scholar] [CrossRef]
- Hu, J.; Lazar, A.J.; Ingram, D.; Wang, W.L.; Zhang, W.; Jia, Z.; Ragoonanan, D.; Wang, J.; Xia, X.; Mahadeo, K.; et al. Cell membrane-anchored and tumor-targeted IL-12 T-cell therapy destroys cancer-associated fibroblasts and disrupts extracellular matrix in heterogenous osteosarcoma xenograft models. J. Immunother. Cancer 2024, 12, e006991. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, X.; Wang, Z.; Song, C.L.; Huang, Z.; Chen, K.N.; Duan, J.; Bai, H.; Xu, J.; Zhao, J.; et al. Multiregion Sequencing Reveals the Genetic Heterogeneity and Evolutionary History of Osteosarcoma and Matched Pulmonary Metastases. Cancer Res. 2019, 79, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, J.B.; Jimenez Garcia, L.; Gross, A.C.; Cam, M.; Cannon, M.V.; Gust, M.J.; Sheridan, J.P.; Gryder, B.E.; Dries, R.; Roberts, R.D. Aberrant Activation of Wound-Healing Programs within the Metastatic Niche Facilitates Lung Colonization by Osteosarcoma Cells. Clin. Cancer Res. 2025, 31, 414–429. [Google Scholar] [CrossRef]
| TME Cell | OS Carcinogenic Function | Therapeutic Implications | Ref. |
|---|---|---|---|
| TAM | Promotes angiogenesis through increased VEGF, immunosuppression via PD-1, increased incidence of metastasis and invasion through promotion of Cox-2 and IL-8 | ICI less effective if used as monotherapy, treatment that reduces function correlated with better prognoses | [22,28,33,35,42,210,211,212] |
| Neutrophil | NET formation, causing T cell suppression with a potential role in metastasis; when elevated causes an increase in drug resistance; aids in formation of pre-metastatic niche | Development of drugs targeting NETs could be massively beneficial, reducing infiltration may increase treatment efficacy | [62,63,66,67,68,69] |
| MDSC | Immunosuppression through secretion of TGF-β, IL-10, and COX2, promotion of tumor growth via VEGF, MMP-9, and FGF, promotes angiogenesis, invasion, and metastasis, progression and migration via NF-kB pathway | Improvement noted when MDSC function inhibited; targeting of secreted factors may improve therapeutic results | [71,88,89,90,91,92] |
| Mast Cell | Infiltration correlated with death, stimulates bone resorption, local and systemic inflammation | Reducing infiltration and activation potentially beneficial | [94,95] |
| T Cells | Immunosuppression through Tex and Treg | Tex are major components of OS TME, drugs targeting them or increasing their anti-tumor properties may be effective | [109,116] |
| B Cells | Infiltration potentially linked to poorer prognoses, secretion of IgE correlated with OS development | IgE blockers may reduce inflammation and OS development | [127,128,129,135,136] |
| NK Cells | Impaired cytotoxic effects allow for further tumor growth, potential weak angiogenic contribution | Further research required, reestablishing NK cytotoxicity potentially advantageous in immunity | [109,137,138,144] |
| DC | Recruit Tregs, potential role in metastasis, tumor growth through loss of GRM4 and elevation of IL-23 | DC vaccines shown to be useful in treated and untreated lesions and beneficial with ICI | [140,146,147,213,214] |
| Osteoclasts | Dysfunction correlated with tumor cell proliferation, TGF-β release supports OS development and RANKL feedback loop, downregulates T cell proliferation via Siglec-15 | RANKL feedback loop disruption may be a valuable target for decreasing bone remodeling | [152,154,156] |
| Osteoblasts | Stimulation of TGF-β production requires RUNX2, which is linked with OS occurrence, upregulate production of RANKL | RUNX2 inhibitors may reduce OS growth | [154,161,162] |
| CAF | Increases proliferative abilities of tumor cells through exosome-mediated intercellular communication | Reducing TME inflammation may decrease CAF formation; targeting exosomes may be helpful | [166,168,169,170] |
| ECM | Upregulation of MMP-2 promotes metastasis, increased mechanical rigidity enhances survival, EMT, and migration, and inhibit ABC transporters | Drugs that utilize ABC transporters may be ineffective; treatments targeting MMP-2 may prove beneficial | [20,172,175,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, A.A.; Prasad, A.; Huisman, A.R.; Wakefield, M.R.; Fang, Y. Exploring the Tumor Microenvironment in Osteosarcoma: Driver of Resistance and Progression. Cancers 2025, 17, 3106. https://doi.org/10.3390/cancers17193106
Schmidt AA, Prasad A, Huisman AR, Wakefield MR, Fang Y. Exploring the Tumor Microenvironment in Osteosarcoma: Driver of Resistance and Progression. Cancers. 2025; 17(19):3106. https://doi.org/10.3390/cancers17193106
Chicago/Turabian StyleSchmidt, Aidan A., Advay Prasad, Alex R. Huisman, Mark R. Wakefield, and Yujiang Fang. 2025. "Exploring the Tumor Microenvironment in Osteosarcoma: Driver of Resistance and Progression" Cancers 17, no. 19: 3106. https://doi.org/10.3390/cancers17193106
APA StyleSchmidt, A. A., Prasad, A., Huisman, A. R., Wakefield, M. R., & Fang, Y. (2025). Exploring the Tumor Microenvironment in Osteosarcoma: Driver of Resistance and Progression. Cancers, 17(19), 3106. https://doi.org/10.3390/cancers17193106






