Cost-Effectiveness of Sacituzumab Govitecan Versus Chemotherapy in Metastatic Triple—Negative Breast Cancer in Taiwan
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Model Structure
2.2. Clinical Data
2.3. Direct Medical Costs and Utility
2.4. Base-Case Analysis
2.5. Sensitivity Analyses
2.6. Scenario Analyses
3. Results
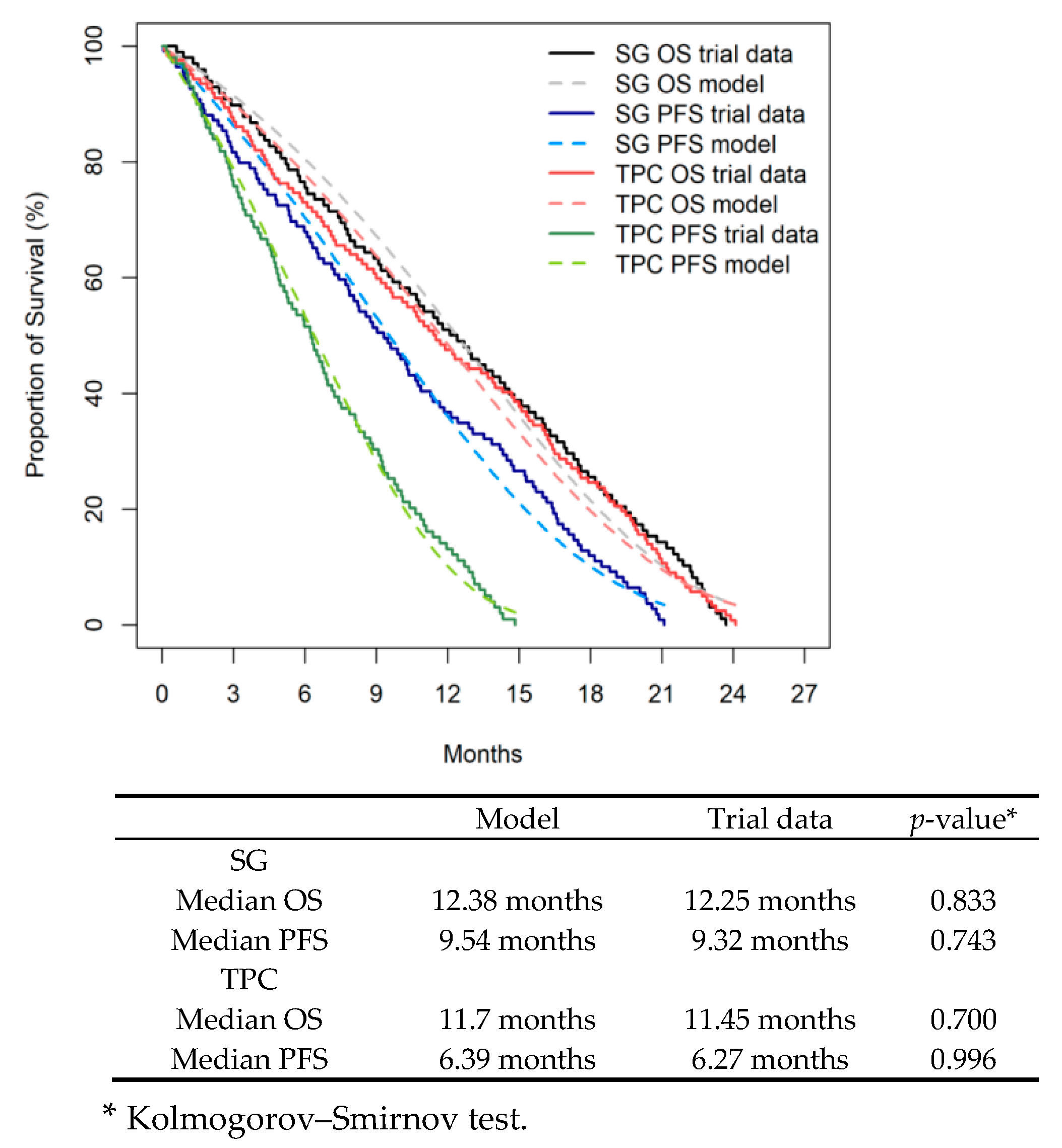
3.1. Internal Validity
3.2. Base-Case Analysis
3.3. One-Way Sensitivity Analyses
3.4. Probabilistic Sensitivity Analyses
3.5. Scenario Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
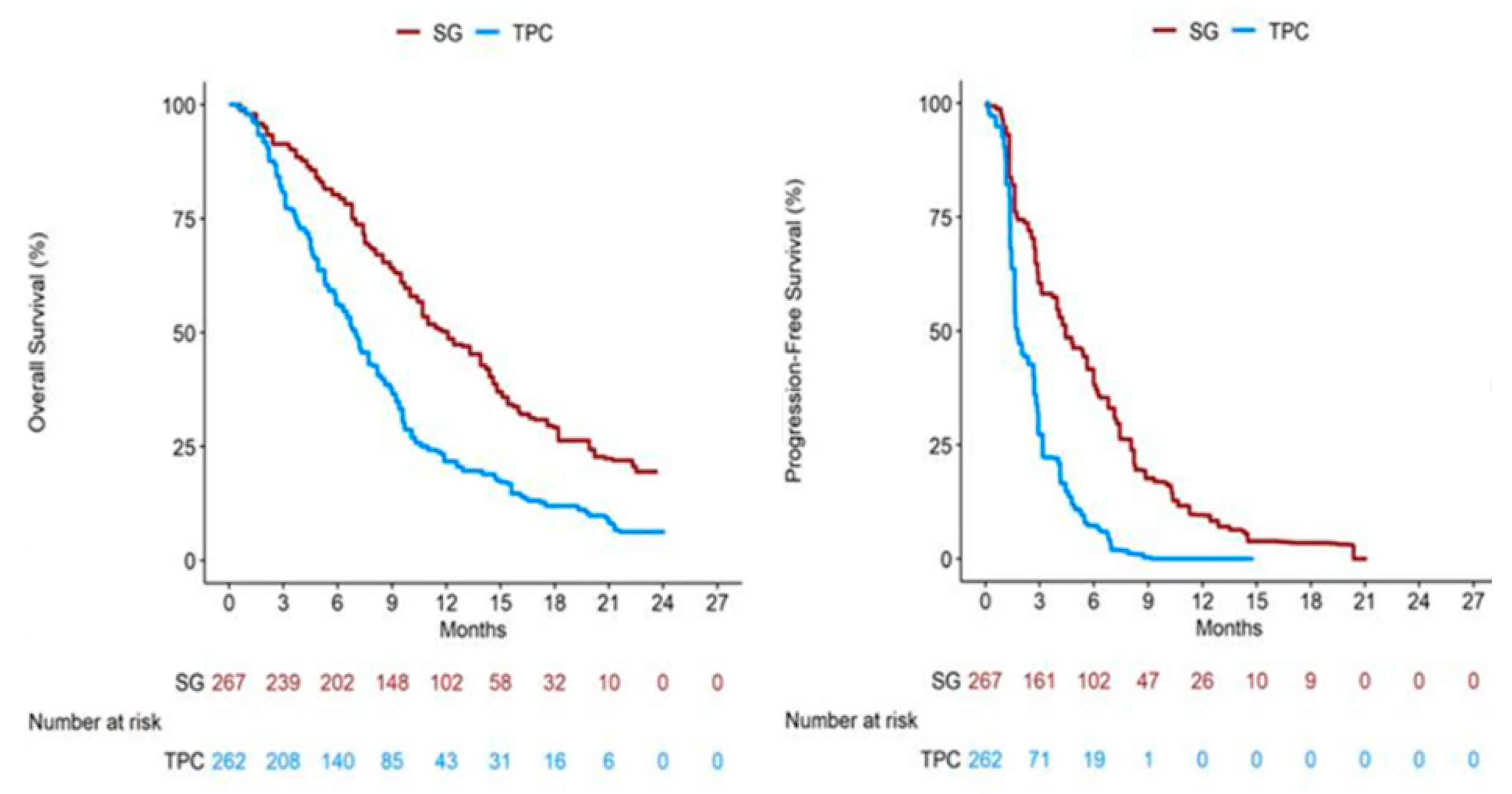
Appendix B

Appendix C
| Strategies | Distributions | Parameters | OS | PFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est | L95% | U95% | SE | AIC | BIC | Est | L95% | U95% | SE | AIC | BIC | |||
| SG | Weibull | shape | 1.792 | 1.518 | 2.116 | 0.152 | 654.085 | 659.255 | 1.474 | 1.258 | 1.726 | 0.119 | 700.750 | 706.133 |
| scale | 13.749 | 12.247 | 15.434 | 0.811 | 10.775 | 9.433 | 12.308 | 0.731 | ||||||
| Gompertz | shape | 2.231 | 1.717 | 2.899 | 0.298 | 662.529 | 667.699 | 1.592 | 1.249 | 2.030 | 0.197 | 708.072 | 713.455 | |
| rate | 0.182 | 0.135 | 0.243 | 0.027 | 0.162 | 0.122 | 0.215 | 0.023 | ||||||
| Gamma | shape | 0.117 | 0.084 | 0.149 | 0.017 | 642.074 | 647.244 | 0.107 | 0.073 | 0.142 | 0.018 | 684.584 | 689.967 | |
| rate | 0.025 | 0.016 | 0.039 | 0.006 | 0.042 | 0.028 | 0.061 | 0.008 | ||||||
| Log-logistic | shape | 2.263 | 1.917 | 2.671 | 0.191 | 681.175 | 686.345 | 1.850 | 1.578 | 2.168 | 0.150 | 733.250 | 738.633 | |
| scale | 10.734 | 9.217 | 12.502 | 0.835 | 8.091 | 6.793 | 9.637 | 0.722 | ||||||
| Log-normal | meanlog | 2.268 | 2.109 | 2.428 | 0.081 | 684.600 | 689.770 | 1.941 | 1.745 | 2.137 | 0.100 | 745.931 | 751.314 | |
| sdlog | 0.807 | 0.701 | 0.928 | 0.058 | 1.044 | 0.915 | 1.193 | 0.071 | ||||||
| TPC | Weibull | shape | 1.588 | 1.367 | 1.845 | 0.122 | 819.397 | 825.005 | 1.628 | 1.385 | 1.913 | 0.134 | 547.543 | 552.733 |
| scale | 13.002 | 11.570 | 14.610 | 0.774 | 7.351 | 6.475 | 8.346 | 0.476 | ||||||
| Gamma | shape | 1.770 | 1.405 | 2.231 | 0.209 | 829.684 | 835.292 | 1.989 | 1.536 | 2.576 | 0.262 | 553.310 | 558.500 | |
| rate | 0.150 | 0.115 | 0.196 | 0.020 | 0.301 | 0.224 | 0.404 | 0.045 | ||||||
| Log-logistic | shape | 0.107 | 0.078 | 0.136 | 0.015 | 798.914 | 804.522 | 0.161 | 0.109 | 0.214 | 0.027 | 541.211 | 546.401 | |
| rate | 0.030 | 0.020 | 0.044 | 0.006 | 0.062 | 0.042 | 0.092 | 0.013 | ||||||
| Gompertz | shape | 1.978 | 1.703 | 2.296 | 0.151 | 856.600 | 862.208 | 2.137 | 1.813 | 2.520 | 0.180 | 570.547 | 575.737 | |
| scale | 9.951 | 8.523 | 11.618 | 0.787 | 5.569 | 4.745 | 6.537 | 0.455 | ||||||
| Log-normal | meanlog | 2.158 | 1.984 | 2.332 | 0.089 | 872.286 | 877.894 | 1.617 | 1.446 | 1.787 | 0.087 | 576.399 | 581.589 | |
| sdlog | 0.982 | 0.866 | 1.113 | 0.063 | 0.865 | 0.753 | 0.995 | 0.061 | ||||||
Appendix D
| Parameters | Unit Cost (USD) | Total Cost (USD) | Source | ||
|---|---|---|---|---|---|
| Sacituzumab | Chemotherapy | Sacituzumab | Chemo therapy | ||
| Progression free survival state, total cost | 66,340 | 9600 | |||
| Drug Cost | 180 mg/vial | 55,274 | 6859 | TNHIA reimbursement price list [45] | |
| Eribulin, 1.0 mg/2 mL/vial | 297/vial | 4336 | |||
| Vinorelbine 50 mg/5 mL/vial | 208/vial | 1248 | |||
| Capecitabine 500 mg/capsule | 2.5/tablet | 599 | |||
| Gemcitabine 1000 mg/vial | 61.5/vial | 676 | |||
| Administration cost, total | 263 | 92 | TNHIA reimbursement price list [46] | ||
| Physician OPD per visit | 15.9 | 15.9 | 229 | 81 | |
| Dispensing fee, per patient | 2.38 | 2.38 | 34 | 11 | |
| Laboratory test, total cost | 115.8 | 35.2 | |||
| Complete blood count | 6.897 | 6.897 | 38.6 | 11.7 | |
| CA-125 | 13.79 | 13.79 | 77.2 | 23.5 | TNHIA reimbursement price list [46] |
| Assessment cost, total | 5796 | 1165 | TNHIA reimbursement price list [46] | ||
| MRI with contrast | 1554 | 1554 | 5796 | 1165 | |
| Managing grade 3/4 adverse events cost, total | 4891 | 1448 | TNHIA reimbursement price list [47] | ||
| Leukopenia | 382 | 382 | 2138 | 649 | |
| Anemia | 205 | 205 | 1153 | 349 | |
| Neutropenia | 288 | 288 | 1600 | 450 | |
| Progression disease health state, total cost | 18,337 | 7497 | |||
| MRI with contrast | 1554 | TNHIA reimbursement price list [46] | |||
| Palliative care | 1206 | [47] | |||
| Hospice per event. | 1650 | [48] | |||
Appendix E
| Variables | Baseline | Lower Range | Upper Range | Distribution | Reference |
|---|---|---|---|---|---|
| cPFS state | 52,263 | 36,586 | 67,945 | Gamma | TNHIA reference price list |
| cPFS1 state | 9600 | 6720 | 12,480 | Gamma | TNHIA reference price list |
| cPostProgression state | 18,337 | 12,836 | 23,838 | Gamma | TNHIA reference price list |
| cPostProgression state1 | 7497 | 5248 | 9746 | Gamma | TNHIA reference price list |
| Rate OS | 0.502 | 0.351 | 0.653 | Beta | Restructed KM data |
| Rate PFS | 0.473 | 0.331 | 0.615 | Beta | Restructed KM data |
| Shape OS | 0.117 | 0.082 | 0.152 | Beta | Restructed KMdata |
| Shape PFS | 0.107 | 0.075 | 0.139 | Beta | Restructed KMdata |
| Utility PFS | 0.907 | 0.635 | 0.635 | Beta | [26] |
| Utility PP | 0.730 | 0.511 | 0.949 | Beta | [26] |
| Rate OS1 | 0.475 | 0.333 | 0.618 | Beta | Restructed KMdata |
| Rate PFS1 | 0.505 | 0.353 | 0.657 | Beta | Restructed KMdata |
| Shape OS1 | 0.107 | 0.075 | 0.139 | Beta | Restructed KMdata |
| Shape PFS1 | 0.161 | 0.113 | 0.209 | Beta | Restructed KMdata |
| Utility PFS1 | 0.860 | 0.602 | 1.118 | Beta | [26] |
| Utility PP1 | 0.730 | 0.511 | 0.949 | Beta | [26] |
| Time Horizon | 10 | 3 | 13 | Beta |
References
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2025. Atlanta: American Cancer Society. 2025. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html (accessed on 5 September 2025).
- Sung, H.; Jiang, C.; Bandi, P.; Minihan, A.; Fidler-Benaoudia, M.; Islami, F.; Siegel, R.L.; Jemal, A. Differences in cancer rates among adults born between 1920 and 1990 in the USA: An analysis of population-based cancer registry data. Lancet Public Health 2024, 9, e583–e593. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Chang, C.-J.; Cheng, J.-S. Survival, treatment regimens and medical costs of women newly diagnosed with metastatic triple-negative breast cancer. Sci. Rep. 2022, 12, 729. [Google Scholar] [CrossRef]
- Gao, H.-F.; Lin, Y.-Y.; Zhu, T.; Ji, F.; Zhang, L.-L.; Yang, C.-Q.; Yang, M.; Li, J.-Q.; Cheng, M.-Y.; Wang, K. Adjuvant CDK4/6 inhibitors combined with endocrine therapy in HR-positive, HER2-negative early breast cancer: A meta-analysis of randomized clinical trials. Breast 2021, 59, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhao, M.; Guan, A.-J.; Shao, T.; Zhou, D.; Yu, G.; Tang, W. A network meta-analysis of efficacy and safety for first-line and second/further-line therapies in postmenopausal women with hormone receptor-positive, HER2-negative, advanced breast cancer. BMC Med. 2024, 22, 13. [Google Scholar] [CrossRef]
- Elazzazy, S.; Al-Ziftawi, N.H.; Mohamed Ibrahim, M.I.; Bujassoum, S.; Hamad, A. Comparative cost-effectiveness analysis of CDK4/6 inhibitors in the first-line treatment of HR-positive and HER2-negative advanced breast cancer: A Markov’s model-based evaluation. Front. Oncol. 2024, 14, 1413676. [Google Scholar] [CrossRef]
- Masurkar, P.P.; Damgacioglu, H.; Deshmukh, A.A.; Trivedi, M.V. Cost Effectiveness of CDK4/6 Inhibitors in the First-Line Treatment of HR+/HER2- Metastatic Breast Cancer in Postmenopausal Women in the USA. PharmacoEconomics 2023, 41, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Look, K.A. Health Care Expenditure Burden of Cancer Care in the United States. Inq. J. Health Care Organ. Provis. Financ. 2019, 56, 0046958019880696. [Google Scholar] [CrossRef]
- Taiwan Cancer Registry Annual Report in 2021. Taiwan Cancer Registry Annual Report in 2022. Available online: https://twcr.tw/wp-content/uploads/2024/02/Top-10-cancers-in-Taiwan-2021.pdf (accessed on 11 September 2025).
- Spring, L.M.; Nakajima, E.; Hutchinson, J.; Viscosi, E.; Blouin, G.; Weekes, C.; Rugo, H.; Moy, B.; Bardia, A. Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer: Clinical Overview and Management of Potential Toxicities. Oncologist 2021, 26, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Bednova, O.; Leyton, J.V. Targeted Molecular Therapeutics for Bladder Cancer—A New Option beyond the Mixed Fortunes of Immune Checkpoint Inhibitors? Int. J. Mol. Sci. 2020, 21, 7268. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Chai, Q.; Tao, W.; Liao, Y.; Liu, X.; Wu, B. Cost-effectiveness of sacituzumab govitecan versus chemotherapy in advanced or metastatic triple-negative breast cancer. Breast 2023, 68, 173–180. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Li, Y.; Li, J. Cost-effectiveness of sacituzumab govitecan versus chemotherapy in patients with relapsed or refractory metastatic triple-negative breast cancer. BMC Health Serv. Res. 2023, 23, 706. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Zhang, Z.; Li, F.; Ruan, Y.; He, Y.; Huang, J.; Zheng, X. Cost-effectiveness of Sacituzumab Govitecan versus Single-agent Chemotherapy for Patients with Metastatic Triple-Negative Breast Cancer in China. Clin. Breast Cancer 2024, 24, e545–e553.e6. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Chen, H.; Wan, Z.; Dou, L.; Li, S. Cost-effectiveness of sacituzumab govitecan for hormone receptor-positive human epidermal growth factor receptor 2-negative metastatic breast cancer based on the EVER-132-002 trial in China. Cost Eff. Resour. Alloc. 2025, 23, 8. [Google Scholar] [CrossRef]
- Lai, S.P.; Wang, S.-Y.; Chan, A.L.; Leung, J.H.; Yip, H.-T. Cost-effectiveness and drug wastage of bevacizumab biosimilar with or without chemotherapy for platinum-resistant recurrent ovarian cancer. Expert Rev. Pharmacoeconomics Outcomes Res. 2024, 24, 541–549. [Google Scholar] [CrossRef]
- NIEHS (Natl. Inst. Environ. Health Sci.), Translational Research Framework. 2019. Available online: https://www.niehs.nih.gov/research/programs/translational/framework-details/index.cfm (accessed on 10 September 2025).
- Shafrin, J.; May, S.; Skornicki, M.; Hathway, J.; Macaulay, R.; Villeneuve, J.; Lees, M.; Hertel, N.; Penrod, J.; Jansen, J. Use of Net Monetary Benefit Analysis to Comprehensively Understand the Value of Innovative Treatments. Value Health 2016, 19, A731. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Leung, J.H.; Tai, Y.-S.; Wang, S.-Y.; Fion, H.-T.Y.; Tsung-Chin, H.; Chan, A.L. Cost-effectiveness of trastuzumab biosimilar combination therapy and drug wastage as first-line treatment for HER2-positive metastatic breast cancer. Breast 2022, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Smare, C.; Lakhdari, K.; Doan, J.; Posnett, J.; Johal, S. Evaluating Partitioned Survival and Markov Decision-Analytic Modeling Approaches for Use in Cost-Effectiveness Analysis: Estimating and Comparing Survival Outcomes. PharmacoEconomics 2019, 38, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Danesi, V.; Andalò, A.; Cavallucci, M.; Balzi, W.; Gentili, N.; Altini, M.; Maltoni, R.; Massa, I.; Vallicelli, G.; Montella, M.T.; et al. Body weight and body surface area of adult patients with selected cancers: An Italian multicenter study. PLoS ONE 2024, 19, e0314452. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, Department of Statistics, Taiwan. Available online: https://www.mohw.gov.tw/lp-7324-2.html (accessed on 9 October 2025).
- Bertram, M.Y.; Lauer, J.A.; De Joncheere, K.; Edejer, T.; Hutubessy, R.; Kieny, M.P.; Hill, S.R. Cost-effectiveness thresholds: Pros and cons. Bull. World Health Organ. 2016, 94, 925–930. [Google Scholar] [CrossRef]
- Chen, J.; Han, M.; Liu, A.; Shi, B. Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US. Front. Oncol. 2021, 11, 734594. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S.; Liu, X.; Chen, Y.; Luan, J.; Wang, S. Cost-effectiveness of sacituzumab govitecan versus single-agent chemotherapy for metastatic triple-negative breast cancer: A trial-based analysis. Cost Eff. Resour. Alloc. 2024, 22, 32. [Google Scholar] [CrossRef]
- Chen, G.-T.; Chang, S.-C.; Chang, C.-J. New Drug Reimbursement and Pricing Policy in Taiwan. Value Health Reg. Issues 2018, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.H.; Chang, C.W.; Chan, A.L.; Lang, H.C. Cost-effectiveness of immune checkpoint inhibitors in the treatment of non-small-cell lung cancer as a second line in Taiwan. Future Oncol. 2022, 18, 859–870. [Google Scholar] [CrossRef]
- Reckers-Droog, V.; van Exel, J.; Brouwer, W. Willingness to Pay for Health-Related Quality of Life Gains in Relation to Disease Severity and the Age of Patients. Value Health 2021, 24, 1182–1192. [Google Scholar] [CrossRef]
- Chen, P.; Lee, Y. Willingness to Pay Per Quality-Adjusted Life in Taiwan. Value Health 2018, 21, S92. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, S.; Yan, M.; Sohn, J.; Li, W.; Tang, J.; Wang, X.; Wang, Y.; Im, S.-A.; Jiang, D.; et al. Sacituzumab govitecan in HR+HER2− metastatic breast cancer: The randomized phase 3 EVER-132-002 trial. Nat. Med. 2024, 30, 3709–3716. [Google Scholar] [CrossRef] [PubMed]
- Cher, B.P.; Goh, S.; Aziz, M.I.A.; Wong, G.; Ng, H.R.; Ong, B.S.; Ng, K.H. Cost-utility analysis of sacituzumab govitecan versus chemotherapy for the treatment of metastatic triple-negative breast cancer in Singapore. Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 217–225. [Google Scholar] [CrossRef]
- Shi, D.; Li, Y.; Liang, X.; Chen, L. Cost-effectiveness of sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Front. Oncol. 2023, 13, 1162360. [Google Scholar] [CrossRef]
- Metreau, E.; Young, K.E.; Eapen, S.G. Understanding Country Income: World Bank Group Income Classifications for FY26 (1 July 2025–June 2026). Available online: https://blogs.worldbank.org/en/opendata/understanding-country-income--world-bank-group-income-classifica (accessed on 4 October 2025).
- Hong, J.; Bae, E.-Y.; Lee, H.-J.; Lee, T.-J.; Clarke, P. Values, challenges, and responses associated with high-priced potential cures: Perspectives of diverse stakeholders in South Korea. Cost Eff. Resour. Alloc. 2024, 22, 20. [Google Scholar] [CrossRef]
- Kakinuma, H.; Takada, D.; Itoshima, H.; Kunisawa, S.; Moriwaki, K.; Honda, M.; Fushimi, K.; Imanaka, Y. Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus chemotherapy as first line chemotherapy for patients with unresectable advanced esophageal cancer in Japan. Esophagus 2023, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sukmanee, J.; Soon, K.W.; Lim, J.; D’SOuza, J.L.A.; Teerawattananon, Y. Economic Evaluation of the Next Generation Electronic Medical Records in Singapore: Cost-Utility Analysis. J. Med. Internet Res. 2025, 27, e70484. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C. ICER Thresholds Across Asia—What They Are, How Are They Determined and Used? What Are the Implications of This for Patient Access? ISPOR, Asia Pacific Summit. 20–21 September 2022. Available online: https://www.ispor.org/docs/default-source/asia2022/c-taylorce-thresholdsispor-ac.pdf?sfvrsn=6bf9c567_0 (accessed on 8 October 2025).
- Parmar, A.; Jiao, T.; Saluja, R.; Chan, K.K.W. Value-based pricing: Toward achieving a balance between individual and population gains in health benefits. Cancer Med. 2019, 9, 94–103. [Google Scholar] [CrossRef]
- Pros and Cons of the Pay-For-Performance Model. Indeed Editor Team. 9 June 2025. Available online: https://www.indeed.com/career-advice/pay-salary/pros-and-cons-of-pay-for-performance (accessed on 10 September 2025).
- Paying for Value—Thresholds and International Reference Pricing. The Need for Greater Transparency Around Willingness-To-Pay Thresholds. Available online: https://www.costellomedical.com/research/ispor-apac-2022/paying-for-value/#:~:text=The%20Need%20for%20Greater%20Transparn-cy%20Around%20Willingness%2DTo%2DPay,HTA%20decision%2Dmaking%2C%20would%20help%20to%20improve%20transparency (accessed on 4 October 2025).
- National Health Insurance Administration, Ministry of Health and Welfare. Online Search for Health Insurance Drug Items. Available online: https://info.nhi.gov.tw/INAE3000/INAE3000S01 (accessed on 8 October 2025).
- National Health Insurance Administration, Ministry of Health and Welfare. Available online: http://sc-dr.tw/health_form/files/form4-001.pdf (accessed on 8 October 2025).
- Tseng, T.H.; Chiang, S.C.; Hsu, J.C.; Ko, Y. Cost-effectiveness analysis of granulocyte colony-stimulating factors for the prophylaxis of chemotherapy-induced febrile neutropenia in patients with breast cancer in Taiwan. PLoS ONE 2024, 19, e0303294. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Simon, M.S.; Bylsma, L.C.; Ruterbusch, J.J.; Beebe-Dimmer, J.L.; Schultz, N.M.; Flanders, S.C.; Barlev, A.; Fryzek, J.P.; Quek, R.G.W. Clinical and economic burden associated with stage III to IV triple-negative breast cancer: A SEER-Medicare historical cohort study in elderly women in the United States. Cancer 2018, 124, 2104–2114. [Google Scholar] [CrossRef]

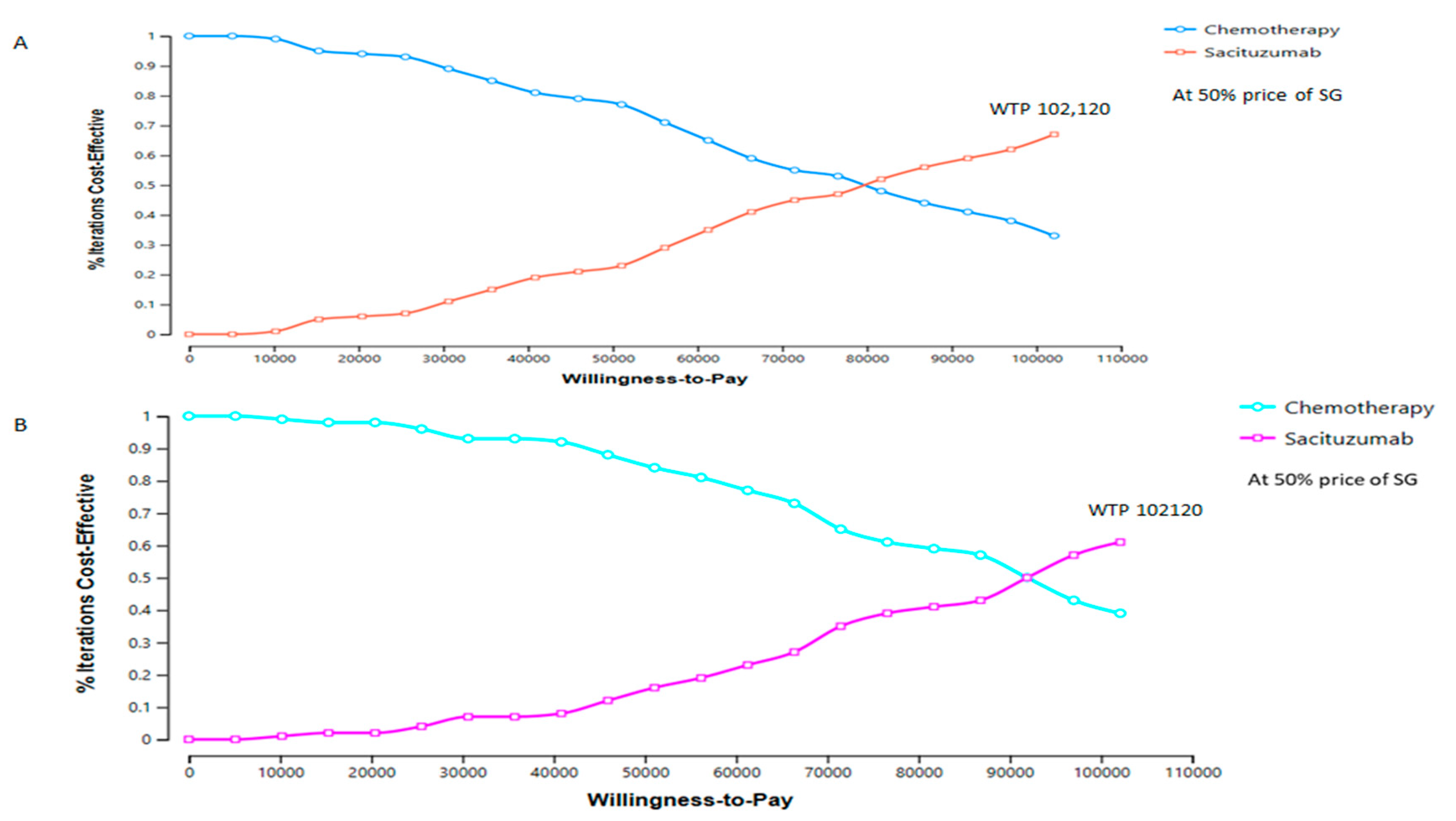
| Strategy | Cost | Increment Cost | Effectiveness | Incremental Effectiveness | ICER | NMB | |
|---|---|---|---|---|---|---|---|
| Overall Population | Chemotherapy | 75,273 | 4.94 | 171,498 | |||
| Sacituzumab | 581,126 | 505,854 | 9.40 | 4.47 | 113,277 | −111,074 | |
| BMN subgroup | Chemotherapy | 75,273 | 4.94 | 171,498 | |||
| Sacituzumab | 641,680 | 566,407 | 9.40 | 4.47 | 126,837 | −171,627 |
| Scenario 1 | Strategy | Cost | Incr Cost | Effectiveness | Incr Eff | ICER | NMB |
|---|---|---|---|---|---|---|---|
| At 30% price of SG | Chemotherapy | 53,738 | 3.5 | 120,873 | |||
| Overall population | Sacituzumab | 324,059 | 270,321 | 6.1 | 2.6 | 105,434 | −21,254 |
| BMN subgroup | Chemotherapy | 53,738 | 3.5 | 120,873 | |||
| Sacituzumab | 356,845 | 303,107 | 6.1 | 2.6 | 118,222 | −54,039 | |
| Scenario 2 | Strategy | Cost | Incr Cost | Effectiveness | Incr Eff | ICER | NMB |
| At 50% price of SG | Chemotherapy | 53,738 | 3.5 | 120,873 | |||
| Overall population | Sacituzumab | 262,314 | 208,576 | 6.1 | 2.6 | 81,352 | 40,492 |
| BMN subgroup | Chemotherapy | 53,738 | 3.5 | 120,873 | |||
| Sacituzumab | 285,247 | 231,509 | 6.1 | 2.6 | 90,296 | 17,559 |
| Scenario Description | Drug Cost (USD) | WTP Threshold | Probability of Cost-Effectiveness (%) | |||
|---|---|---|---|---|---|---|
| 1 | In Taiwan, SG cost maintained at base-case value | SG | TPS | Cost/QALY | SG | TPC |
| WTP threshold increased by 10% | 55,274 | 6859 | 102,120 | 56 | 44 | |
| WTP threshold increased by 30% | 55,274 | 6859 | 112,330 | 71 | 29 | |
| 2 | In Netherlands, WTP threshold is EUR 20,000 to EUR 80,000 per QALY, assuming same drug cost as in Taiwan. | |||||
| WTP threshold increased by 10% of USD 94,118 | 55,274 | 6859 | 103,530 | 52 | 48 | |
| 3 | In England, WTP threshold is GBP 20,000–GBP 30,000 per QALY, assuming the same drug cost as in Taiwan. | |||||
| WTP threshold was raised to 2.9 times the current upper limit | 55,274 | 6859 | 118,919 (GBP 88,000) | 57 | 43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Tai, Y.-S.; Leung, H.W.C.; Leung, S.H.; Chan, A.L.F. Cost-Effectiveness of Sacituzumab Govitecan Versus Chemotherapy in Metastatic Triple—Negative Breast Cancer in Taiwan. Cancers 2025, 17, 3305. https://doi.org/10.3390/cancers17203305
Wang S-Y, Tai Y-S, Leung HWC, Leung SH, Chan ALF. Cost-Effectiveness of Sacituzumab Govitecan Versus Chemotherapy in Metastatic Triple—Negative Breast Cancer in Taiwan. Cancers. 2025; 17(20):3305. https://doi.org/10.3390/cancers17203305
Chicago/Turabian StyleWang, Shyh-Yau, Yun-Sheng Tai, Henry W. C. Leung, Shin Hang Leung, and Agnes L. F. Chan. 2025. "Cost-Effectiveness of Sacituzumab Govitecan Versus Chemotherapy in Metastatic Triple—Negative Breast Cancer in Taiwan" Cancers 17, no. 20: 3305. https://doi.org/10.3390/cancers17203305
APA StyleWang, S.-Y., Tai, Y.-S., Leung, H. W. C., Leung, S. H., & Chan, A. L. F. (2025). Cost-Effectiveness of Sacituzumab Govitecan Versus Chemotherapy in Metastatic Triple—Negative Breast Cancer in Taiwan. Cancers, 17(20), 3305. https://doi.org/10.3390/cancers17203305





