CHEcking Diagnostic Differential Ability of Real Baseline Variables and Frailty Scores in Tolerance of Anti-Cancer Systemic Therapy in OldEr Patients (CHEDDAR-TOASTIE)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Collection and Items
2.3. Data Analysis
2.3.1. Inclusion/Exclusion Criteria
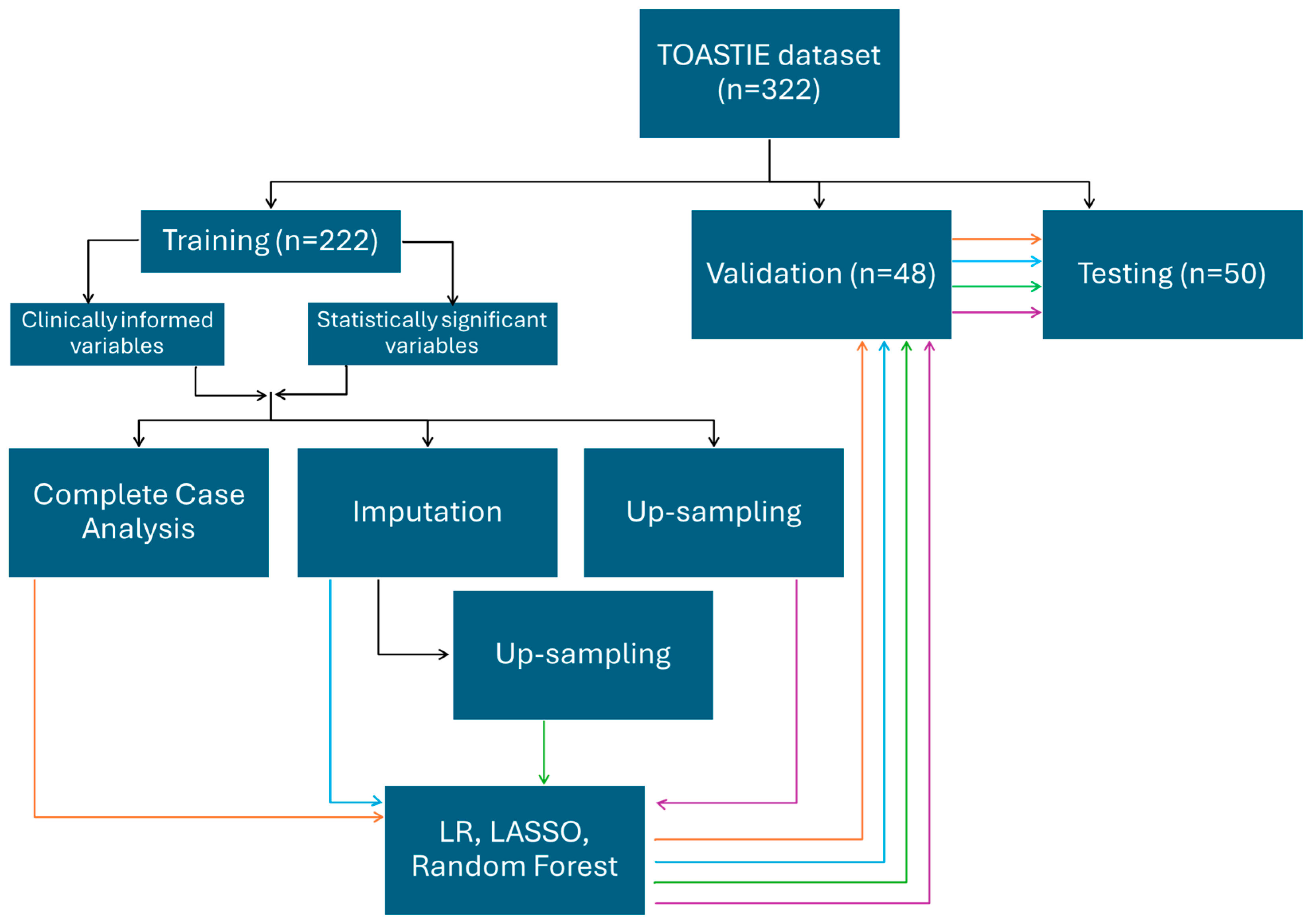
2.3.2. Internal Validation of Models
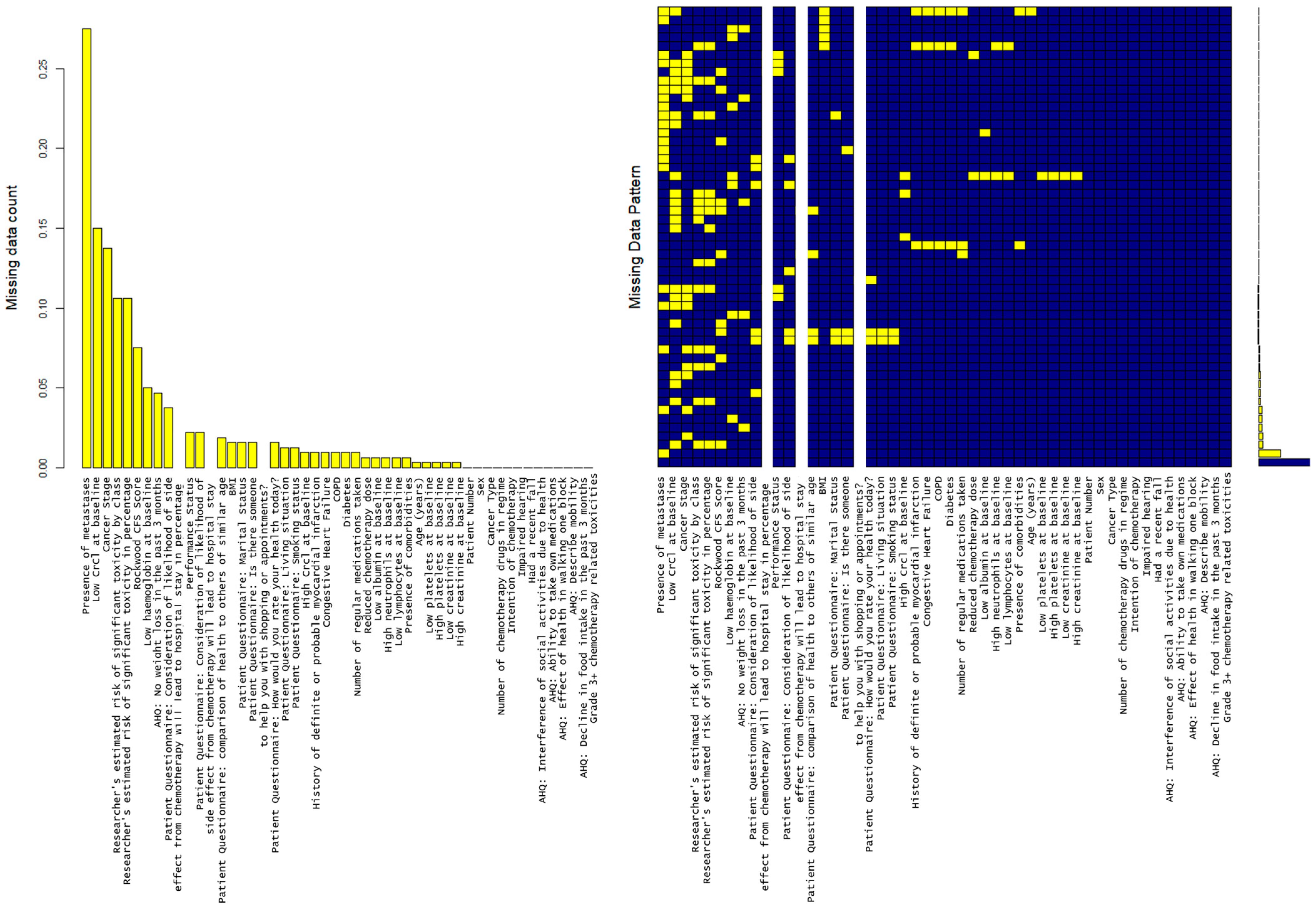
2.3.3. Handling Missing Data
2.3.4. Selecting Variables
Candidate Data Items
Variable Selection
2.3.5. Building Predictive Models
Logistic Regression
LASSO
Random Forest
- Mtry: This parameter determines the number of variables randomly sampled at each split in the decision trees of the random forest. Values of 2, 4, and 6 were tested to evaluate different feature subset sizes.
- splitrule: Two splitting rules, “gini” and “extratrees”, were evaluated to determine the optimal method for partitioning nodes in the decision trees.
- min.node.size: This parameter specifies the minimum number of observations required to create a terminal node in the tree. Values of 1, 3, and 5 were selected to assess model sensitivity to node size.
2.3.6. Model Performance Evaluation
3. Results
3.1. Demographic Results
3.2. Statistical Associations of Variables
3.3. Performance of Predictive Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHQ | Additional Health Questionnaire |
| AUC | Area Under Curve |
| BMI | Body Mass Index |
| CARG | Cancer and Aging Research Group |
| CFS | Clinical Frailty Score |
| CRAN | The Comprehensive R Archive Network |
| CRASH | Chemotherapy Risk Assessment Scale for High-Age Patients |
| MICE | Multivariate Imputation by Chained Equations |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| ROC | Receiver Operator Characteristic |
| TOASTIE | Tolerance of Anti-Systemic Therapies in Older population Study |
| WHO | World Health Organization |
References
- Centre for Ageing Better. Our Ageing Population: The State of Ageing 2023–2024. 2023. Available online: https://ageing-better.org.uk/our-ageing-population-state-ageing-2023-4 (accessed on 1 October 2025).
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef]
- Kalsi, T.; Babic-Illman, G.; Ross, P.J.; Maisey, N.R.; Hughes, S.; Fields, P.; Martin, F.C.; Wang, Y.; Harari, D. The Impact of Comprehensive Geriatric Assessment Interventions on Tolerance to Chemotherapy in Older People. Br. J. Cancer 2015, 112, 1435–1444. [Google Scholar] [CrossRef]
- Bhatt, V.R. Cancer in Older Adults: Understanding Cause and Effects of Chemotherapy-Related Toxicities. Future Oncol. 2019, 15, 2557–2560. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef]
- Hurria, A.; Levit, L.A.; Dale, W.; Mohile, S.G.; Muss, H.B.; Fehrenbacher, L.; Magnuson, A.; Lichtman, S.M.; Bruinooge, S.S.; Soto-Perez-de-Celis, E.; et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J. Clin. Oncol. 2015, 33, 3826–3833. [Google Scholar] [CrossRef] [PubMed]
- Scher, K.S.; Hurria, A. Under-Representation of Older Adults in Cancer Registration Trials: Known Problem, Little Progress. J. Clin. Oncol. 2012, 30, 2036–2038. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.; Lund, C.; te Molder, M.; Soubeyran, P.; Wildiers, H.; van Huis, L.; Rostoft, S. Geriatric Assessment in the Management of Older Patients with Cancer—A Systematic Review (Update). J. Geriatr. Oncol. 2022, 13, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef]
- LICHTMAN, S. Chemotherapy in the Elderly. Semin. Oncol. 2004, 31, 160–174. [Google Scholar] [CrossRef]
- Talarico, L.; Chen, G.; Pazdur, R. Enrollment of Elderly Patients in Clinical Trials for Cancer Drug Registration: A 7-Year Experience by the US Food and Drug Administration. J. Clin. Oncol. 2004, 22, 4626–4631. [Google Scholar] [CrossRef]
- Ortland, I.; Mendel Ott, M.; Kowar, M.; Sippel, C.; Jaehde, U.; Jacobs, A.H.; Ko, Y.-D. Comparing the Performance of the CARG and the CRASH Score for Predicting Toxicity in Older Patients with Cancer. J. Geriatr. Oncol. 2020, 11, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Pinker, I.; Lafont, C.; Liposits, G.; Vidra, R.; Cunquero-Tomás, A.J.; Korobeinikova, E.; Neuendorff, N.R.; Slavova-Boneva, V.; Baltussen, J.; Chovanec, M.; et al. Representation of Geriatric Oncology in Cancer Care Guidelines in Europe: A Scoping Review by the International Society of Geriatric Oncology (SIOG). ESMO Open 2025, 10, 105052. [Google Scholar] [CrossRef]
- Joint Collegiate Council for Oncology (JCCO) (made up of the Royal College of Physicians and The Royal College of Radiologists). Implementing Frailty Assessment and Management in Oncology Services; Joint Collegiate Council for Oncology (JCCO): London, UK, 2023. [Google Scholar]
- Soto-Perez-De-Celis, E.; Lichtman, S.M. Considerations for Clinical Trial Design in Older Adults with Cancer. Expert Opin. Investig. Drugs 2017, 26, 1099–1102. [Google Scholar] [CrossRef]
- Kingston, A.; Comas-Herrera, A.; Jagger, C. Forecasting the Care Needs of the Older Population in England over the next 20 Years: Estimates from the Population Ageing and Care Simulation (PACSim) Modelling Study. Lancet Public Health 2018, 3, e447–e455. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J.; et al. Predicting the Risk of Chemotherapy Toxicity in Older Patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) Score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef]
- Dearden, H.C.; Rowe, M.; Peters, A.; Rohan, M.; Marsh, A.; Gee, A.L.; Zucker, K.; Quesne, G.; Heseltine, J.; Prichard, R.; et al. A United Kingdom Prospective, Multicentre, Observational Cohort Study Investigating Tolerance of Anti-Cancer Systemic Therapy in the Elderly: The TOASTIE Study. J. Clin. Oncol. 2024, 42, 1521. [Google Scholar] [CrossRef]
- Dearden, H.; Baxter, M.A.; Martin, S.; Rowe, M.; Zucker, K.; Jones, C.M.; Olsson-Brown, A.C.; Petty, R.D.; Swinson, D. Observational Study Investigating Tolerance Of Anticancer Systemic Therapy In the Elderly (TOASTIE): A Protocol. BMJ Open 2021, 11, e051104. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Rowe, M.; Zucker, K.; Peters, A.L.; Rohan, M.; Marsh, A.; Gee, A.L.; Quesne, G.; Heseltine, J.; Prichard, R.; et al. UK National Observational Cohort Study Investigating Tolerance of Anti-Cancer Systemic Therapy in the Elderly: The TOASTIE Study. BMJ Oncol. 2024, 3, e000459. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A Global Clinical Measure of Fitness and Frailty in Elderly People. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). National Cancer Institute Division of Cancer Treatment and Diagnosis, USA. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 1 October 2025).
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728–736. [Google Scholar] [CrossRef]
- Moth, E.B.; Kiely, B.E.; Stefanic, N.; Naganathan, V.; Martin, A.; Grimison, P.; Stockler, M.R.; Beale, P.; Blinman, P. Predicting Chemotherapy Toxicity in Older Adults: Comparing the Predictive Value of the CARG Toxicity Score with Oncologists’ Estimates of Toxicity Based on Clinical Judgement. J. Geriatr. Oncol. 2019, 10, 202–209. [Google Scholar] [CrossRef]
- Kotzerke, D.; Moritz, F.; Mantovani, L.; Hambsch, P.; Hering, K.; Kuhnt, T.; Yahiaoui-Doktor, M.; Forstmeyer, D.; Lordick, F.; Knödler, M. The Performance of Three Oncogeriatric Screening Tools—G8, Optimised G8 and CARG—in Predicting Chemotherapy-Related Toxicity in Older Patients with Cancer. A Prospective Clinical Study. J. Geriatr. Oncol. 2019, 10, 937–943. [Google Scholar] [CrossRef]
- Magnuson, A.; Sedrak, M.S.; Gross, C.P.; Tew, W.P.; Klepin, H.D.; Wildes, T.M.; Muss, H.B.; Dotan, E.; Freedman, R.A.; O’Connor, T.; et al. Development and Validation of a Risk Tool for Predicting Severe Toxicity in Older Adults Receiving Chemotherapy for Early-Stage Breast Cancer. J. Clin. Oncol. 2021, 39, 608–618. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, I.M.G.; Marcadenti, A.; de Medeiros, G.O.C.; Bezerra, R.A.; de Rego, J.F.M.; Gonzalez, M.C.; Fayh, A.P.T. Is Cachexia Associated with Chemotherapy Toxicities in Gastrointestinal Cancer Patients? A Prospective Study. J. Cachexia Sarcopenia Muscle 2019, 10, 445–454. [Google Scholar] [CrossRef]
- Gendarme, S.; Zebachi, S.; Corre, R.; Greillier, L.; Justeau, G.; Bylicki, O.; Decroisette, C.; Auliac, J.-B.; Guisier, F.; Geier, M.; et al. Predictors of Three-Month Mortality and Severe Chemotherapy-Related Adverse Events in Patients Aged 70 Years and Older with Metastatic Non-Small-Cell Lung Cancer: A Secondary Analysis of ESOGIA-GFPC-GECP 08–02 Study. J. Geriatr. Oncol. 2024, 15, 101506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, W.; Wang, J.; Zhu, M.; Zhuang, Z. Pretreated Baseline Neutrophil Count and Chemotherapy-induced Neutropenia May Be Conveniently Available as Prognostic Biomarkers in Advanced Gastric Cancer. Intern. Med. J. 2015, 45, 854–859. [Google Scholar] [CrossRef]
- Oh, G.H.; Yeom, C.-W.; Shim, E.-J.; Jung, D.; Lee, K.-M.; Son, K.-L.; Kim, W.-H.; Moon, J.Y.; Jung, S.; Kim, T.-Y.; et al. The Effect of Perceived Social Support on Chemotherapy-Related Symptoms in Patients with Breast Cancer: A Prospective Observational Study. J. Psychosom. Res. 2020, 130, 109911. [Google Scholar] [CrossRef]
| Dependent: Severe Chemotherapy-Related Toxicities | Grade ≤ 2 Toxicity | GradeRADE 3+ Toxicity | Total | x2 (p) | |
|---|---|---|---|---|---|
| Total N (%) | 251 (78.0) | 71 (22.0) | 322 | ||
| WHO/ECOG Performance Status (0–4) | Mean (SD) | 0.7 (0.7) | 1.0 (0.7) | 0.8 (0.7) | 0.004 |
| High baseline neutrophils | FALSE | 208 (83.9) | 50 (71.4) | 258 (81.1) | 0.03 |
| TRUE | 40 (16.1) | 20 (28.6) | 60 (18.9) | ||
| AHQ: interference of social activities due to health | All the time | 10 (4.0) | 10 (14.1) | 20 (6.2) | 0.004 |
| Most of the time | 28 (11.2) | 9 (12.7) | 37 (11.5) | ||
| Some of the time | 38 (15.1) | 15 (21.1) | 53 (16.5) | ||
| A little of the time | 21 (8.4) | 8 (11.3) | 29 (9.0) | ||
| None of the time | 153 (61.0) | 28 (39.4) | 181 (56.2) | ||
| (Missing) | 1 (0.4) | 1 (1.4) | 2 (0.6) | ||
| AHQ: ability to take own medications | Without help | 240 (95.6) | 62 (87.3) | 302 (93.8) | 0.035 |
| With some help or reminders | 9 (3.6) | 6 (8.5) | 15 (4.7) | ||
| Unable | 1 (0.4) | 2 (2.8) | 3 (0.9) | ||
| (Missing) | 1 (0.4) | 1 (1.4) | 2 (0.6) | ||
| AHQ: effect of health on walking one block | Limited a lot | 10 (4.0) | 6 (8.5) | 16 (5.0) | 0.001 |
| Limited a little | 21 (8.4) | 16 (22.5) | 37 (11.5) | ||
| No limitations | 219 (87.3) | 48 (67.6) | 267 (82.9) | ||
| (Missing) | 1 (0.4) | 1 (1.4) | 2 (0.6) | ||
| AHQ: weight loss in the past 3 months | Yes | 130 (51.8) | 48 (67.6) | 178 (55.3) | 0.004 |
| No | 111 (44.2) | 16 (22.5) | 127 (39.4) | ||
| (Missing) | 10 (4.0) | 7 (9.9) | 17 (5.3) | ||
| AHQ: describe mobility | Bed- or chair-bound | 1 (0.4) | 0 (0.0) | 1 (0.3) | <0.001 |
| Can get out, but does not | 7 (2.8) | 11 (15.5) | 18 (5.6) | ||
| Gets out | 242 (96.4) | 59 (83.1) | 301 (93.5) | ||
| (Missing) | 1 (0.4) | 1 (1.4) | 2 (0.6) | ||
| AHQ: decline in food intake in the past 3 months | Severe decrease | 36 (14.3) | 15 (21.1) | 51 (15.8) | 0.004 |
| Moderate decrease | 76 (30.3) | 32 (45.1) | 108 (33.5) | ||
| No decrease | 138 (55.0) | 23 (32.4) | 161 (50.0) | ||
| (Missing) | 1 (0.4) | 1 (1.4) | 2 (0.6) | ||
| Rockwood CFS | Mean (SD) | 2.5 (1.2) | 3.0 (1.3) | 2.6 (1.2) | 0.004 |
| Patient questionnaire: comparison of health to others of similar age | Not as good | 24 (9.6) | 14 (19.7) | 38 (11.8) | 0.007 |
| As good | 102 (40.6) | 19 (26.8) | 121 (37.6) | ||
| Better | 111 (44.2) | 31 (43.7) | 142 (44.1) | ||
| Does not know | 7 (2.8) | 6 (8.5) | 13 (4.0) | ||
| (Missing) | 7 (2.8) | 1 (1.4) | 8 (2.5) |
| Dependent: Grade 3+ Toxicities | FALSE | TRUE | OR (Univariable) | OR (Multivariable) | OR (Multiple Imputation) | |
|---|---|---|---|---|---|---|
| Age | Mean (SD) | 72.6 (4.9) | 72.7 (4.8) | 1.00 (0.94–1.07, p = 0.899) | 1.00 (0.92–1.08, p = 0.996) | 0.80 (0.63–1.03, p = 0.078) |
| Sex | Female | 92 (52.3) | 23 (46.9) | - | - | - |
| Male | 84 (47.7) | 26 (53.1) | 1.24 (0.66–2.35, p = 0.509) | 1.28 (0.57–2.96, p = 0.551) | 0.89 (0.14–5.72, p = 0.901) | |
| BMI | Mean (SD) | 27.1 (5.4) | 26.8 (5.5) | 0.99 (0.93–1.05, p = 0.703) | 0.98 (0.92–1.05, p = 0.595) | 1.06 (0.88–1.27, p = 0.540) |
| ECOG Performance Status (0–4) | Mean (SD) | 0.8 (0.7) | 0.9 (0.6) | 1.49 (0.93–2.39, p = 0.095) | 1.94 (1.09–3.55, p = 0.027) | 2.27 (0.40–12.97, p = 0.346) |
| Cancer Type | Upper GI | 45 (25.6) | 12 (24.5) | - | - | - |
| Gynecological | 28 (15.9) | 5 (10.2) | 0.67 (0.20–2.02, p = 0.492) | 0.69 (0.15–2.96, p = 0.628) | 0.13 (0.00–3.75, p = 0.227) | |
| Lung | 12 (6.8) | 2 (4.1) | 0.62 (0.09–2.72, p = 0.571) | 0.57 (0.08–2.74, p = 0.521) | 0.04 (0.00–2.86, p = 0.139) | |
| Breast | 15 (8.5) | 6 (12.2) | 1.50 (0.46–4.61, p = 0.486) | 2.28 (0.57–8.91, p = 0.233) | 3.89 (0.24–62.61, p = 0.335) | |
| Lower GI | 36 (20.5) | 10 (20.4) | 1.04 (0.40–2.69, p = 0.933) | 1.62 (0.54–4.94, p = 0.391) | 0.67 (0.07–6.70, p = 0.730) | |
| HPB | 9 (5.1) | 6 (12.2) | 2.50 (0.72–8.42, p = 0.139) | 1.97 (0.52–7.12, p = 0.303) | 0.99 (0.04–24.60, p = 0.996) | |
| Urological | 20 (11.4) | 6 (12.2) | 1.13 (0.35–3.34, p = 0.836) | 1.99 (0.53–7.13, p = 0.294) | 0.26 (0.01–5.52, p = 0.385) | |
| Other | 11 (6.2) | 2 (4.1) | 0.68 (0.10–3.01, p = 0.646) | 1.00 (0.13–5.01, p = 0.996) | 0.13 (0.00–12.95, p = 0.372) | |
| Cancer Stage | 1 | 9 (5.8) | 1 (2.2) | - | - | - |
| 2 | 25 (16.2) | 6 (13.0) | 2.16 (0.31–43.74, p = 0.502) | - | 39.54 (0.14–11,309.76, p = 0.195) | |
| 3 | 52 (33.8) | 19 (41.3) | 3.29 (0.56–62.70, p = 0.274) | - | 125.65 (0.37–42,966.83, p = 0.101) | |
| 4 | 68 (44.2) | 20 (43.5) | 2.65 (0.46–50.30, p = 0.369) | - | 18.25 (0.02–14,521.60, p = 0.374) | |
| Presence of metastases | FALSE | 44 (35.2) | 16 (39.0) | - | - | - |
| TRUE | 81 (64.8) | 25 (61.0) | 0.85 (0.41–1.78, p = 0.658) | - | 1.38 (0.10–18.85, p = 0.806) | |
| Number of chemotherapy drugs | Mean (SD) | 2.1 (0.6) | 2.2 (0.6) | 1.64 (0.98–2.77, p = 0.059) | 2.01 (1.04–4.09, p = 0.043) | 2.50 (0.52–12.05, p = 0.250) |
| Intention of chemotherapy | Neo-adjuvant | 52 (29.5) | 14 (28.6) | - | - | - |
| Adjuvant | 42 (23.9) | 11 (22.4) | 0.97 (0.39–2.36, p = 0.951) | - | 0.35 (0.04–2.93, p = 0.328) | |
| Palliative | 82 (46.6) | 24 (49.0) | 1.09 (0.52–2.33, p = 0.826) | - | 3.23 (0.19–53.57, p = 0.409) | |
| Dose reduction | FALSE | 115 (65.3) | 27 (55.1) | - | - | - |
| TRUE | 61 (34.7) | 22 (44.9) | 1.54 (0.80–2.92, p = 0.191) | - | 1.58 (0.35–7.04, p = 0.544) | |
| Low baseline hemoglobin | FALSE | 112 (66.3) | 23 (52.3) | - | - | - |
| TRUE | 57 (33.7) | 21 (47.7) | 1.79 (0.91–3.52, p = 0.088) | - | 4.54 (0.31–66.74, p = 0.249) | |
| Low platelet count at baseline | FALSE | 169 (96.0) | 48 (98.0) | - | - | - |
| TRUE | 7 (4.0) | 1 (2.0) | 0.50 (0.03–2.92, p = 0.525) | - | 0.02 (0.00–1.71, p = 0.083) | |
| High platelet count at baseline | FALSE | 147 (83.5) | 43 (87.8) | - | - | - |
| TRUE | 29 (16.5) | 6 (12.2) | 0.71 (0.25–1.71, p = 0.471) | - | 0.14 (0.01–1.79, p = 0.131) | |
| Low creatinine at baseline | FALSE | 141 (80.1) | 36 (73.5) | - | - | - |
| TRUE | 35 (19.9) | 13 (26.5) | 1.45 (0.68–2.99, p = 0.317) | - | 2.24 (0.35–14.23, p = 0.386) | |
| High creatinine at baseline | FALSE | 159 (90.3) | 41 (83.7) | - | - | - |
| TRUE | 17 (9.7) | 8 (16.3) | 1.82 (0.70–4.41, p = 0.194) | - | 4.62 (0.37–57.69, p = 0.231) | |
| Low creatinine clearance at baseline | FALSE | 18 (12.2) | 5 (11.1) | - | - | - |
| TRUE | 129 (87.8) | 40 (88.9) | 1.12 (0.41–3.55, p = 0.838) | - | 0.32 (0.01–12.33, p = 0.518) | |
| High bilirubin count at baseline | FALSE | 167 (94.9) | 48 (100.0) | - | - | - |
| TRUE | 9 (5.1) | 0.00 (NA–∞, p = 0.985) | - | 0.00 (0.00–Inf, p = 0.993) | ||
| Low albumin count at baseline | FALSE | 130 (74.3) | 34 (69.4) | - | - | - |
| TRUE | 45 (25.7) | 15 (30.6) | 1.27 (0.62–2.52, p = 0.494) | - | 0.67 (0.10–4.29, p = 0.668) | |
| High neutrophil count at baseline | FALSE | 147 (83.5) | 36 (73.5) | - | - | - |
| TRUE | 29 (16.5) | 13 (26.5) | 1.83 (0.85–3.82, p = 0.114) | - | 8.38 (0.90–77.83, p = 0.061) | |
| Low lymphocyte count at baseline | FALSE | 139 (79.0) | 39 (79.6) | - | - | - |
| TRUE | 37 (21.0) | 10 (20.4) | 0.96 (0.42–2.05, p = 0.925) | - | 1.19 (0.17–8.26, p = 0.856) | |
| Presence of comorbidities | FALSE | 58 (33.3) | 12 (24.5) | - | - | - |
| TRUE | 116 (66.7) | 37 (75.5) | 1.54 (0.77–3.29, p = 0.241) | 1.76 (0.78–4.21, p = 0.186) | 6.96 (0.66–73.31, p = 0.104) | |
| History of definite or probable myocardial infarction | FALSE | 156 (89.7) | 47 (95.9) | - | - | - |
| TRUE | 18 (10.3) | 2 (4.1) | 0.37 (0.06–1.34, p = 0.191) | - | 0.06 (0.00–1.21, p = 0.066) | |
| History of congestive heart failure | FALSE | 165 (94.8) | 44 (89.8) | - | - | - |
| TRUE | 9 (5.2) | 5 (10.2) | 2.08 (0.61–6.35, p = 0.208) | - | 7.03 (0.25–199.13, p = 0.248) | |
| History of COPD | FALSE | 162 (93.1) | 46 (93.9) | - | - | - |
| TRUE | 12 (6.9) | 3 (6.1) | 0.88 (0.19–2.91, p = 0.849) | - | 0.91 (0.06–13.91, p = 0.948) | |
| History of diabetes | FALSE | 154 (88.5) | 42 (85.7) | - | - | - |
| TRUE | 20 (11.5) | 7 (14.3) | 1.28 (0.48–3.12, p = 0.597) | - | 1.69 (0.13–21.57, p = 0.683) | |
| Number of regular medications taken | Mean (SD) | 3.8 (2.8) | 4.1 (2.6) | 1.04 (0.92–1.16, p = 0.524) | 0.97 (0.83–1.13, p = 0.726) | 0.83 (0.55–1.24, p = 0.353) |
| Researcher’s estimated risk of significant toxicity (CARG class) | Low | 26 (16.6) | 8 (17.4) | - | - | - |
| Low–medium | 82 (52.2) | 19 (41.3) | 0.75 (0.30–2.01, p = 0.553) | - | 0.41 (0.04–4.66, p = 0.458) | |
| Medium–high | 44 (28.0) | 18 (39.1) | 1.33 (0.52–3.63, p = 0.562) | - | 1.71 (0.05–55.17, p = 0.760) | |
| High | 5 (3.2) | 1 (2.2) | 0.65 (0.03–4.88, p = 0.712) | - | 7.44 (0.01–9018.15, p = 0.564) | |
| Researcher’s estimated risk of significant toxicity in percentage | Mean (SD) | 34.2 (20.2) | 35.9 (19.4) | 1.00 (0.99–1.02, p = 0.624) | - | 0.92 (0.85–0.99, p = 0.036) |
| AHQ: Presence of impaired hearing | FALSE | 136 (77.3) | 37 (75.5) | - | - | - |
| TRUE | 40 (22.7) | 12 (24.5) | 1.10 (0.51–2.27, p = 0.796) | - | 3.19 (0.46–22.19, p = 0.235) | |
| AHQ: Fall(s) in last 6 months | FALSE | 147 (83.5) | 44 (89.8) | - | - | - |
| TRUE | 29 (16.5) | 5 (10.2) | 0.58 (0.19–1.46, p = 0.283) | - | 0.46 (0.05–4.51, p = 0.499) | |
| AHQ: Interference of social activities due to health | All the time | 6 (3.4) | 4 (8.2) | - | - | - |
| Most of the time | 21 (11.9) | 8 (16.3) | 0.57 (0.13–2.73, p = 0.466) | - | 0.51 (0.01–19.88, p = 0.718) | |
| Some of the time | 25 (14.2) | 9 (18.4) | 0.54 (0.12–2.52, p = 0.413) | - | 0.11 (0.00–7.74, p = 0.303) | |
| A little of the time | 12 (6.8) | 7 (14.3) | 0.88 (0.18–4.44, p = 0.868) | - | 0.32 (0.00–44.30, p = 0.636) | |
| None of the time | 112 (63.6) | 21 (42.9) | 0.28 (0.07–1.18, p = 0.065) | - | 0.13 (0.00–4.51, p = 0.257) | |
| AHQ: Ability to take own medications | Without help | 169 (96.0) | 45 (91.8) | - | - | - |
| With some help or reminders | 7 (4.0) | 3 (6.1) | 1.61 (0.34–6.04, p = 0.503) | - | 0.20 (0.00–13.74, p = 0.438) | |
| Unable | 0 (0.0) | 1 (2.0) | 7,954,942.58 (0.00–NA, p = 0.986) | - | 3257.64 (0.00–Inf, p = 0.999) | |
| AHQ: Effect of health on walking one block | Limited a lot | 8 (4.5) | 4 (8.2) | - | - | - |
| Limited a little | 15 (8.5) | 11 (22.4) | 1.47 (0.36–6.66, p = 0.600) | - | 5.19 (0.08–338.32, p = 0.436) | |
| No limitations | 153 (86.9) | 34 (69.4) | 0.44 (0.13–1.74, p = 0.206) | - | 2.67 (0.05–152.63, p = 0.632) | |
| AHQ: Weight loss in the past 3 months | Yes | 89 (52.4) | 31 (67.4) | - | - | - |
| No | 81 (47.6) | 15 (32.6) | 0.53 (0.26–1.04, p = 0.071) | - | 0.50 (0.07–3.62, p = 0.490) | |
| AHQ: Describe mobility | Bed- or chair-bound | 1 (0.6) | 0 (0.0) | - | - | - |
| Can get out, but does not | 4 (2.3) | 5 (10.2) | 2,647,724.96 (0.00–NA, p = 0.987) | - | 415,273,785,083.42 (0.00–Inf, p = 0.998) | |
| Gets out | 171 (97.2) | 44 (89.8) | 545,028.76 (0.00–NA, p = 0.988) | - | 1,644,914,644.46 (0.00–Inf, p = 0.998) | |
| AHQ: Decline in food intake in the past 3 months | Severe decrease | 29 (16.5) | 8 (16.3) | - | - | - |
| Moderate decrease | 51 (29.0) | 22 (44.9) | 1.56 (0.64–4.15, p = 0.345) | - | 6.76 (0.32–142.17, p = 0.207) | |
| No decrease | 96 (54.5) | 19 (38.8) | 0.72 (0.29–1.89, p = 0.481) | - | 2.22 (0.17–29.80, p = 0.540) | |
| Rockwood CFS | Mean (SD) | 2.4 (1.2) | 3.0 (1.4) | 1.38 (1.07–1.80, p = 0.012) | - | 1.32 (0.42–4.14, p = 0.618) |
| Patient Questionnaire: Marital Status | Single | 29 (16.7) | 7 (14.3) | - | - | - |
| Married | 116 (66.7) | 34 (69.4) | 1.21 (0.51–3.23, p = 0.676) | - | 2.94 (0.07–119.76, p = 0.556) | |
| Widowed | 18 (10.3) | 8 (16.3) | 1.84 (0.57–6.11, p = 0.308) | - | 5.46 (0.44–67.40, p = 0.183) | |
| Other | 11 (6.3) | 0 (0.0) | 0.00 (0.00–∞, p = 0.989) | - | 0.00 (0.00–Inf, p = 0.994) | |
| Patient Questionnaire: Living situation | Alone | 46 (26.4) | 13 (26.5) | - | - | - |
| With partner/family | 124 (71.3) | 34 (69.4) | 0.97 (0.48–2.05, p = 0.935) | - | 0.24 (0.01–10.12, p = 0.450) | |
| Carer for partner/family | 4 (2.3) | 2 (4.1) | 1.77 (0.23–10.18, p = 0.536) | - | 1.53 (0.00–479.14, p = 0.882) | |
| Other | 0 (0.0) | 0 (0.0) | - | - | - | |
| Patient Questionnaire: Is there someone to help you with shopping or appointments? | Always | 119 (68.8) | 34 (69.4) | - | - | - |
| Sometimes | 17 (9.8) | 7 (14.3) | 1.44 (0.52–3.64, p = 0.455) | - | 1.64 (0.18–15.14, p = 0.659) | |
| Never | 17 (9.8) | 2 (4.1) | 0.41 (0.06–1.53, p = 0.251) | - | 0.16 (0.01–4.44, p = 0.268) | |
| I do not need help | 20 (11.6) | 6 (12.2) | 1.05 (0.36–2.69, p = 0.923) | - | 1.11 (0.09–13.02, p = 0.934) | |
| Patient Questionnaire: Smoking status | Current | 15 (8.6) | 3 (6.1) | - | - | - |
| Ex-smoker | 84 (48.3) | 21 (42.9) | 1.25 (0.37–5.75, p = 0.742) | - | 7.67 (0.25–236.82, p = 0.233) | |
| Never | 75 (43.1) | 25 (51.0) | 1.67 (0.50–7.62, p = 0.448) | - | 24.74 (0.55–1108.66, p = 0.094) | |
| Patient Questionnaire: comparison of health to others of similar age | Not as good | 17 (9.9) | 8 (16.3) | - | - | - |
| As good | 74 (43.0) | 13 (26.5) | 0.37 (0.13–1.07, p = 0.060) | - | 0.92 (0.09–9.44, p = 0.945) | |
| Better | 77 (44.8) | 24 (49.0) | 0.66 (0.26–1.80, p = 0.399) | - | 8.80 (0.48–161.63, p = 0.139) | |
| Does not know | 4 (2.3) | 4 (8.2) | 2.12 (0.41–11.28, p = 0.362) | - | 1.65 (0.01–523.73, p = 0.855) | |
| Patient Questionnaire: Consideration of likelihood of side effect from chemotherapy will lead to hospital stay | Unlikely | 84 (48.6) | 19 (39.6) | - | - | - |
| Not very likely | 74 (42.8) | 22 (45.8) | 1.31 (0.66–2.64, p = 0.437) | - | 6.32 (0.72–55.11, p = 0.091) | |
| Quite likely | 13 (7.5) | 7 (14.6) | 2.38 (0.80–6.67, p = 0.104) | - | 13.70 (0.47–402.77, p = 0.127) | |
| Very likely | 2 (1.2) | 0 (0.0) | 0.00 (NA–∞, p = 0.989) | - | 0.00 (0.00–Inf, p = 0.998) | |
| Patient Questionnaire: Consideration of likelihood of side effect from chemotherapy will lead to hospital stay in percentage | Mean (SD) | 24.2 (23.2) | 28.0 (23.2) | 1.01 (0.99–1.02, p = 0.323) | - | 1.01 (0.98–1.05, p = 0.557) |
| Patient Questionnaire: How does the patient rate their health today in percentage | Mean (SD) | 67.6 (25.9) | 65.2 (26.2) | 1.00 (0.98–1.01, p = 0.563) | - | 0.98 (0.95–1.02, p = 0.272) |
| (a) Logistic Regression | ||||||||
| Selected Clinical Variables | Significant Variables | |||||||
| CCA | Imp | Up-Sample | Imp with Up-Sample | CCA | Imp | Up-Sample | Imp with Up-Sample | |
| Accuracy | 0.6000 | 0.6600 | 0.5600 | 0.6200 | 0.5800 | 0.6600 | 0.6400 | 0.6600 |
| 95% CI | 0.4518–0.7359 | 0.5123–0.7879 | 0.4125–0.7001 | 0.4717–0.7535 | 0.4321–0.7181 | 0.5123–0.7879 | 0.4919–0.7708 | 0.5123–0.7879 |
| p-value | 0.9962 | 0.9616 | 0.9995 | 0.9912 | 0.9985 | 0.9616 | 0.9809 | 0.9616 |
| Balanced accuracy | 0.5658 | 0.5197 | 0.5395 | 0.6075 | 0.6382 | 0.5768 | 0.5921 | 0.5768 |
| Sensitivity (recall) | 0.5000 | 0.2500 | 0.5000 | 0.5833 | 0.7500 | 0.4167 | 0.5000 | 0.4167 |
| Specificity | 0.6316 | 0.7895 | 0.5789 | 0.6316 | 0.5263 | 0.7368 | 0.6842 | 0.7368 |
| PPV (precision) | 0.3000 | 0.2727 | 0.2727 | 0.3333 | 0.3333 | 0.3333 | 0.3333 | 0.3333 |
| NPV | 0.8000 | 0.7692 | 0.7857 | 0.8276 | 0.8696 | 0.8000 | 0.8125 | 0.8000 |
| AUC | 0.5789 | 0.6360 | 0.6075 | 0.6184 | 0.6590 | 0.6524 | 0.6590 | 0.6546 |
| AUC 95% CI | 0.3730–0.7849 | 0.4557–0.8162 | 0.4279–0.7871 | 0.4393–0.7976 | 0.4708–8471 | 0.4682–0.8366 | 0.4691–0.8489 | 0.4666–0.8427 |
| (b) LASSO | ||||||||
| Selected Clinical Variables | Significant Variables | |||||||
| CCA | Imp | Up-Sample | Imp with Up-Sample | CCA | Imp | Up-Sample | Imp with Up-Sample | |
| Accuracy | 0.5800 | 0.2200 | 0.5400 | 0.5200 | 0.7000 | 0.7400 | 0.6800 | 0.7200 |
| 95% CI | 0.4321–0.7187 | 0.1153–0.3596 | 0.3932–0.6819 | 0.3742–0.6634 | 0.5539–0.8214 | 0.5966–0.8537 | 0.5330–0.8048 | 0.5751–0.8377 |
| p-value | 0.9985 | 1.0000 | 0.9999 | 0.9999 | 0.8753 | 0.6977 | 0.9282 | 0.7987 |
| Balanced accuracy | 0.5526 | 0.4298 | 0.5548 | 0.5132 | 0.6031 | 0.5724 | 0.6469 | 0.6447 |
| Sensitivity (recall) | 0.6053 | 0.0263 | 0.5263 | 0.5263 | 0.7895 | 0.8947 | 0.7105 | 0.7895 |
| Specificity | 0.5000 | 0.8333 | 0.5833 | 0.5000 | 0.4167 | 0.2500 | 0.5833 | 0.5000 |
| PPV (precision) | 0.7931 | 0.3333 | 0.8000 | 0.7692 | 0.8108 | 0.7907 | 0.8438 | 0.8333 |
| NPV | 0.2857 | 0.2128 | 0.2800 | 0.2500 | 0.3846 | 0.4286 | 0.3889 | 0.4286 |
| AUC | 0.5526 | 0.4298 | 0.5548 | 0.5132 | 0.6031 | 0.5724 | 0.6469 | 0.6447 |
| AUC 95% CI | 0.3852–0.7200 | 0.3167–0.5429 | 0.3884–0.7212 | 0.3449–0.6814 | 0.4433–0.7629 | 0.4352–0.7095 | 0.4840–0.8099 | 0.4831–0.8064 |
| (c) Random Forest | ||||||||
| Selected clinical Variables | Significant Variables | |||||||
| CCA | Imp | Up-Sample | Imp with Up-Sample | CCA | Imp | Up-Sample | Imp with Up-Sample | |
| Accuracy | 0.7200 | 0.7000 | 0.6400 | 0.7000 | 0.7000 | 0.7600 | 0.7000 | 0.7400 |
| 95% CI | 0.5751–0.8377 | 0.5539–0.8214 | 0.4919–0.7708 | 0.5539–0.8214 | 0.5539–0.8214 | 0.6183–0.8694 | 0.5539–0.8214 | 0.5966- 0.8537 |
| p-value | 0.79873 | 0.87529 | 0.9809 | 0.8753 | 0.8753 | 0.57668 | 0.8753 | 1 |
| Balanced accuracy | 0.4737 | 0.4605 | 0.4781 | 0.5175 | 0.4890 | 0.500 | 0.6031 | 0.6294 |
| Sensitivity (recall) | 0.0000 | 0.0000 | 0.1667 | 0.1667 | 0.08333 | 0.0000 | 0.4167 | 0.4167 |
| Specificity | 0.9474 | 0.9211 | 0.7895 | 0.8684 | 0.89474 | 1.0000 | 0.7895 | 0.8421 |
| PPV (precision) | 0.0000 | 0.0000 | 0.2000 | 0.2857 | 0.2000 | - | 0.3846 | 0.4545 |
| NPV | 0.7500 | 0.7447 | 0.7500 | 0.7674 | 0.75556 | 0.7600 | 0.8108 | 0.8205 |
| AUC | 0.4737 | 0.5746 | 0.4781 | 0.5033 | 0.4890 | 0.5000 | 0.6031 | 0.6557 |
| AUC 95% CI | 0.4377–0.5097 | 0.368–0.781 | 0.3499–0.6063 | 0.3789–0.6276 | 0.3936–0.5845 | 0.500–0.500 | 0.4433–0.7629 | 0.4819–0.8295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, H.H.L.; Mahmood, I.; Aggrey, F.; Dearden, H.; Baxter, M.; Zucker, K., on behalf of the National Oncology Trainees Collaborative for Healthcare Research (NOTCH). CHEcking Diagnostic Differential Ability of Real Baseline Variables and Frailty Scores in Tolerance of Anti-Cancer Systemic Therapy in OldEr Patients (CHEDDAR-TOASTIE). Cancers 2025, 17, 3303. https://doi.org/10.3390/cancers17203303
Ng HHL, Mahmood I, Aggrey F, Dearden H, Baxter M, Zucker K on behalf of the National Oncology Trainees Collaborative for Healthcare Research (NOTCH). CHEcking Diagnostic Differential Ability of Real Baseline Variables and Frailty Scores in Tolerance of Anti-Cancer Systemic Therapy in OldEr Patients (CHEDDAR-TOASTIE). Cancers. 2025; 17(20):3303. https://doi.org/10.3390/cancers17203303
Chicago/Turabian StyleNg, Helen H. L., Isa Mahmood, Francis Aggrey, Helen Dearden, Mark Baxter, and Kieran Zucker on behalf of the National Oncology Trainees Collaborative for Healthcare Research (NOTCH). 2025. "CHEcking Diagnostic Differential Ability of Real Baseline Variables and Frailty Scores in Tolerance of Anti-Cancer Systemic Therapy in OldEr Patients (CHEDDAR-TOASTIE)" Cancers 17, no. 20: 3303. https://doi.org/10.3390/cancers17203303
APA StyleNg, H. H. L., Mahmood, I., Aggrey, F., Dearden, H., Baxter, M., & Zucker, K., on behalf of the National Oncology Trainees Collaborative for Healthcare Research (NOTCH). (2025). CHEcking Diagnostic Differential Ability of Real Baseline Variables and Frailty Scores in Tolerance of Anti-Cancer Systemic Therapy in OldEr Patients (CHEDDAR-TOASTIE). Cancers, 17(20), 3303. https://doi.org/10.3390/cancers17203303






