CDK4/6 Inhibitors in Patients Aged 80 and Older with HR+/HER2− Metastatic Breast Cancer: A Real-World Multicenter Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
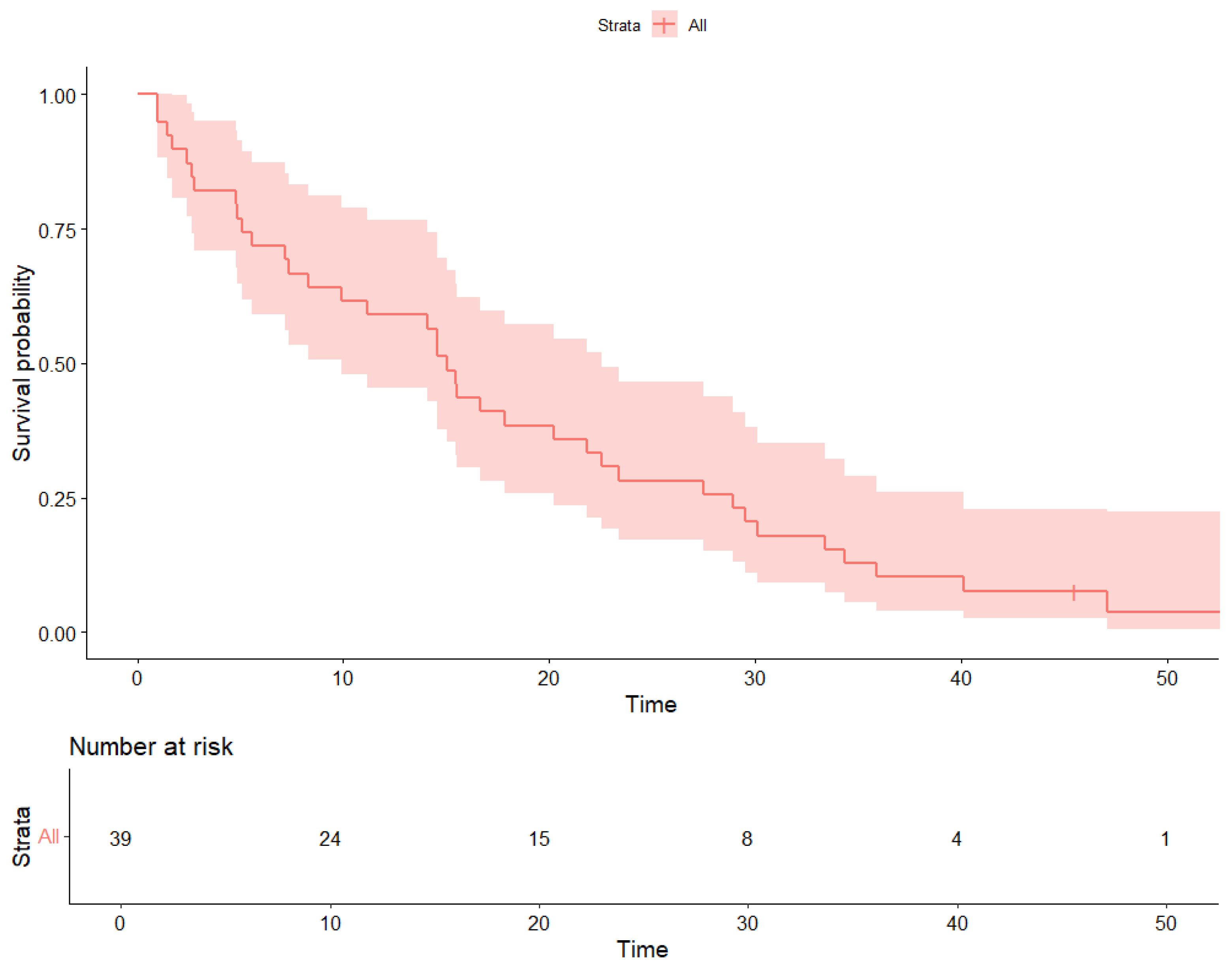
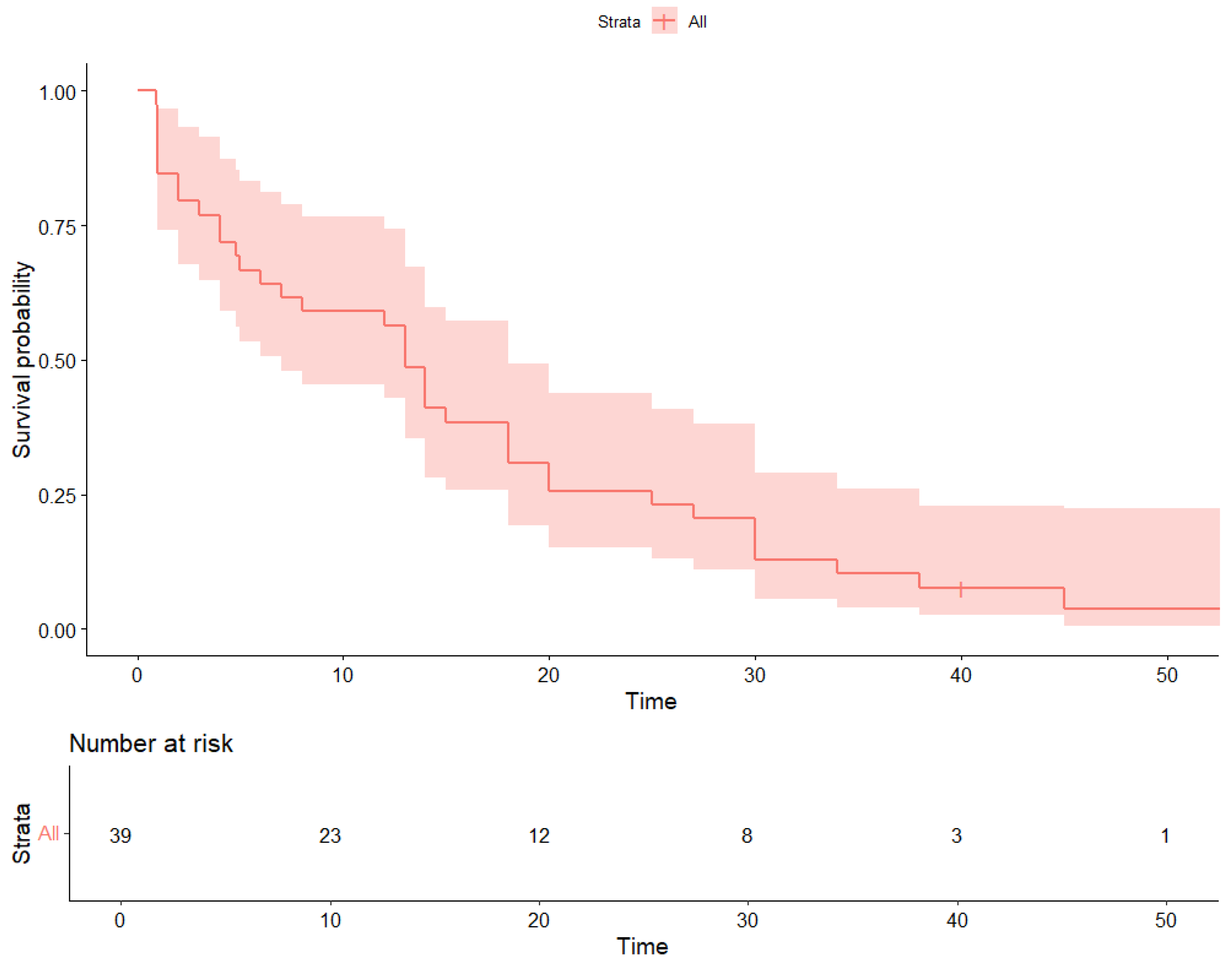
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674–1705. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.; Winer, E.P.; Gauthier, E.; et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Perez, A.T.; et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Takahashi, M.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Battisti, N.M.L.; Mislang, A.R.; Cooper, L.; O’Donovan, A.; Audisio, R.A.; Extermann, M.; Biganzoli, L.; Wildiers, H. Adapting breast cancer management for older patients: Recommendations from the International Society of Geriatric Oncology (SIOG) COVID-19 Working Group. J. Geriatr. Oncol. 2020, 11, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- De Glas, N.A.; Kiderlen, M.; Bastiaannet, E.; de Craen, A.J.M.; van de Water, W.; van de Velde, C.J.H.; Liefers, G.J.; Siesling, S.; Portielje, J.E.A.; van der Velde, S.; et al. Validity of Adjuvant! Online program in older patients with breast cancer: A population-based study. Lancet Oncol. 2014, 15, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the Task Force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Wildiers, H.; Mauer, M.; Pallis, A.; Hurria, A.; Extermann, M.; Lichtman, S.; Biganzoli, L.; Trimble, E.L.; Audisio, R.; Senior, J.; et al. End points and trial design in geriatric oncology research: A joint EORTC–Alliance–SIOG position article. J. Clin. Oncol. 2013, 31, 3711–3718. [Google Scholar] [CrossRef]
- Biganzoli, L.; Wildiers, H.; Oakman, C.; Marotti, L.; Loibl, S.; Kunkler, I.; Reed, M.; Brain, E.; Cardoso, M.J.; Dilhuydy, J.M.; et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012, 13, e148–e160. [Google Scholar] [CrossRef]
- Bland, K.A.; Mustafa, R.; McTaggart-Cowan, H. Patient Preferences in Metastatic Breast Cancer Care: A Scoping Review. Cancers 2023, 15, 4331. [Google Scholar] [CrossRef]
- Fedele, P.; Landriscina, M.; Moraca, L.; Cusmai, A.; Gnoni, A.; Licchetta, A.; Guarini, C.; Lanotte, L.; Pappagallo, M.N.; Melaccio, A.; et al. Evaluating CDK4/6 inhibitor therapy in elderly patients with metastatic hormone receptor-positive, HER2-negative breast cancer: A retrospective real-world multicenter study. Cancers 2024, 16, 3442. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation). Off. J. Eur. Union. 2016, L119, 1–88.
- National Cancer Institute. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; NIH: Bethesda, MD, USA, 2017. [Google Scholar]
- Wildiers, H.; Vidal, M.; Andre, F.; Jacot, W.; Bachelot, T.; Diéras, V.; Cortes, J.; Tredan, O.; Ferrero, J.M.; Jerusalem, G.; et al. PALOMAGE: A real-world study of palbociclib plus endocrine therapy in women aged ≥70 years. Oncologist 2021, 26, e1513–e1520. [Google Scholar] [CrossRef]
- Petrelli, F.; Dottorini, L.; Di Menna, G.; Borgonovo, K.; Parati, M.C.; Rea, C.G.; Ghilardi, M.; Ghidini, A.; Luciani, A. The role of CDK4/6 inhibitors in older and younger patients with breast cancer: A systematic review and meta-analysis. Breast 2023, 71, 138–142. [Google Scholar] [CrossRef]
- Vigneri, P.; Pizzuti, L.; Moscetti, L.; Schettini, F.; Michelotti, A.; Giuliano, M.; Santini, D.; Arpino, G.; Monterisi, S.; Masiello, V.; et al. Real-world comparison of palbociclib, ribociclib, and abemaciclib: The PALMARES study. Breast 2023, 69, 102–110. [Google Scholar] [CrossRef]
- Rugo, H.S.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Brufsky, A. Real-world treatment patterns for palbociclib plus an aromatase inhibitor, or an aromatase inhibitor alone, for patients with metastatic breast cancer in the Flatiron Database. Int. J. Cancer 2023, 154, 701–711. [Google Scholar] [CrossRef]
- Karuturi, M.S.; Cappelleri, J.C.; Blum, J.L.; Patel, K.; Telivala, B.; McCune, S.; Cuevas, J.D.; Lakhanpal, S.; Montelongo, M.Z.; Wang, Y.; et al. Measures of functional status in older patients treated with palbociclib for advanced breast cancer. J. Geriatr. Oncol. 2023, 15, 101670. [Google Scholar] [CrossRef]
- Avcı, O.; İrIağaç, Y.; Çavdar, E.; Karaboyun, K.; Araz, M.; Şakala, T.; Değerli, E.; Özdemir, Ö.; İnAl, A.; Ocak, B.; et al. PROPSEA, safety evaluation of palbociclib and ribociclib in older patients with breast cancer: A prospective real-world TOG study. J. Geriatr. Oncol. 2023, 14, 101604. [Google Scholar] [CrossRef]
- Brain, E.; Chen, C.; Simon, S.; Pasupuleti, V.; Pfitzer, K.V.; Gelmon, K.A. Palbociclib in Older Patients with Advanced/Metastatic Breast Cancer: A Systematic Review. Target. Oncol. 2024, 19, 303–320. [Google Scholar] [CrossRef]
- Fedele, P.; Landriscina, M.; Moraca, L.; Gadaleta-Caldarola, A.; Cusmai, A.; Giuliani, F.; Chiuri, V.; Giotta, F.; Pinto, A.; Mirisola, V.; et al. Standard versus reduced CDK4/6 inhibitor therapy in elderly patients with metastatic HR+/HER2− breast cancer: An observational multicenter study. J. Clin. Med. 2024, 13, 7441. [Google Scholar]
| n (%) | ||
|---|---|---|
| Stage at diagnosis | I–III | 25 (31.3%) |
| IV | 55 (68.7%) | |
| No. metastasis site | Soft Tissue/Bone | 37 (46.2%) |
| Visceral | 43 (53.8%) | |
| BC subtype | Luminal A | 48 (60.0%) |
| Luminal B | 32 (40.0%) | |
| Comorbidities | 0 | 8 (10.0%) |
| 1 | 55 (68.7%) | |
| 2 or more | 17 (21.3%) | |
| ECOG PS | 0 | 2 (2.5%) |
| 1–2 | 78 (97.5%) | |
| G8 score | >14 | 47 (58.8%) |
| ≤14 | 33 (41.2%) | |
| Endocrine therapy | Letrozole/Anastrozole | 41 (51.3%) |
| Fulvestrant | 39 (48.7%) | |
| CDK4/6i | Abemaciclib | 7 (8.8%) |
| Palbociclib | 62 (77.5%) | |
| Ribociclib | 11 (13.7%) | |
| Starting Dose | Standard | 45 (56.4%) |
| Reduced | 35 (43.6%) | |
| BMI | <25 | 33 (41.3%) |
| ≥25 | 47 (58.7%) |
| AEs | Abemaciclib (N = 7) Any Grade | Ribociclib (N = 11) Any Grade | Palbociclib (N = 62) Any Grade | Abemaciclib (N = 7) Grade ≥ 3 | Ribociclib (N = 11) Grade ≥ 3 | Palbociclib (N = 62) Grade ≥ 3 | All Patients (N = 80) Any Grade | All Patients (N = 80) Grade ≥ 3 |
|---|---|---|---|---|---|---|---|---|
| Anemia | 1 (14%) | 1 (9%) | 8 (13%) | 1 (14%) | 0 (0%) | 1 (2%) | 10 (13%) | 2 (3%) |
| Neutropenia | 2 (29%) | 7 (64%) | 38 (61%) | 1 (14%) | 2 (18%) | 17 (27%) | 47 (59%) | 20 (25%) |
| Thrombocytopenia | 0 (0%) | 0 (0%) | 3 (5%) | 0 (0%) | 0 (0%) | 1 (2%) | 3 (4%) | 1 (1%) |
| Asthenia | 1 (14%) | 2 (18%) | 10 (16%) | 1 (14%) | 1 (9%) | 2 (3%) | 13 (16%) | 4 (5%) |
| Diarrhea | 3 (43%) | 0 (0%) | 0 (0%) | 1 (14%) | 0 (0%) | 0 (0%) | 3 (4%) | 1 (1%) |
| ALT/AST increased | 1 (14%) | 1 (9%) | 5 (8%) | 0 (0%) | 0 (0%) | 1 (2%) | 7 (9%) | 1 (1%) |
| QTc prolongation | 0 (0%) | 2 (18%) | 0 (0%) | 0 (0%) | 1 (9%) | 0 (0%) | 2 (3%) | 1 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedele, P.; Landriscina, M.; Moraca, L.; Cusmai, A.; Gnoni, A.; Licchetta, A.; Lanotte, L.; Pappagallo, M.N.; Melaccio, A.; Giordano, G.; et al. CDK4/6 Inhibitors in Patients Aged 80 and Older with HR+/HER2− Metastatic Breast Cancer: A Real-World Multicenter Study. Cancers 2025, 17, 3302. https://doi.org/10.3390/cancers17203302
Fedele P, Landriscina M, Moraca L, Cusmai A, Gnoni A, Licchetta A, Lanotte L, Pappagallo MN, Melaccio A, Giordano G, et al. CDK4/6 Inhibitors in Patients Aged 80 and Older with HR+/HER2− Metastatic Breast Cancer: A Real-World Multicenter Study. Cancers. 2025; 17(20):3302. https://doi.org/10.3390/cancers17203302
Chicago/Turabian StyleFedele, Palma, Matteo Landriscina, Lucia Moraca, Antonio Cusmai, Antonio Gnoni, Antonella Licchetta, Laura Lanotte, Maria Nicla Pappagallo, Assunta Melaccio, Guido Giordano, and et al. 2025. "CDK4/6 Inhibitors in Patients Aged 80 and Older with HR+/HER2− Metastatic Breast Cancer: A Real-World Multicenter Study" Cancers 17, no. 20: 3302. https://doi.org/10.3390/cancers17203302
APA StyleFedele, P., Landriscina, M., Moraca, L., Cusmai, A., Gnoni, A., Licchetta, A., Lanotte, L., Pappagallo, M. N., Melaccio, A., Giordano, G., Maselli, F. M., Giuliani, F., Chiuri, V., Giotta, F., Fumai, F., & Gadaleta-Caldarola, G. (2025). CDK4/6 Inhibitors in Patients Aged 80 and Older with HR+/HER2− Metastatic Breast Cancer: A Real-World Multicenter Study. Cancers, 17(20), 3302. https://doi.org/10.3390/cancers17203302







