Physical Therapy Utilization and Morbidity Outcomes After Breast Cancer Surgery: A Longitudinal Analysis of Three Combined Cohorts
Simple Summary
Abstract
1. Introduction
2. Objective
3. Methods
3.1. Participants
3.2. Data Collection and Measures
3.3. Arm Morbidity
3.4. Risk Screening for Future Arm Morbidity
3.5. Emotional Well-Being
3.6. Statistical Analysis
3.7. Reporting Guideline
4. Results
5. Discussion
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hauerslev, K.R.; Madsen, A.H.; Overgaard, J.; Damsgaard, T.E.; Christiansen, P. Long-term follow-up on shoulder and arm morbidity in patients treated for early breast cancer. Acta Oncol. 2020, 59, 851–858. [Google Scholar] [CrossRef]
- Tran, T.X.M.; Jung, S.; Lee, E.; Cho, H.; Cho, J.; Lee, E.; Chang, Y.J.; Cho, H. Long-term trajectory of postoperative health-related quality of life in young breast cancer patients: A 15-year follow-up study. J. Cancer Surviv. 2023, 17, 1416–1426. [Google Scholar] [CrossRef]
- Álvarez-Salvago, F.; Gutiérrez-García, P.; Molina-García, C.; Atienzar-Aroca, S.; Jiménez-García, J.D.; Aibar-Almazán, A.; Martínez-Amat, A.; Pujol-Fuentes, C. Is it really over when it is over? physical, mental and emotional health status of long-term breast cancer survivors compared to healthy matched controls. Support. Care Cancer 2024, 32, 641. [Google Scholar] [CrossRef]
- Johnson, M.; McGregor, A.H.; A’Hern, R.; Leff, D.R.; Wells, M. ‘I presumed the pain would eventually get better by itself’; challenges with access to rehabilitation for upper limb dysfunction after breast cancer treatment—Descriptive and qualitative findings from a cross-sectional survey. Eur. J. Surg. Oncol. 2024, 50, 108591. [Google Scholar] [CrossRef]
- Che Bakri, N.A.; Kwasnicki, R.M.; Khan, N.; Ghandour, O.; Lee, A.; Grant, Y.; Dawidziuk, A.; Darzi, A.; Ashrafian, H.; Leff, D.R. Impact of Axillary Lymph Node Dissection and Sentinel Lymph Node Biopsy on Upper Limb Morbidity in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Ann. Surg. 2023, 277, 572–580. [Google Scholar] [CrossRef]
- Manfuku, M.; Nishigami, T.; Mibu, A.; Yamashita, H.; Imai, R.; Kanamori, H.; Sumiyoshi, K. Predictors of persistent post-surgical pain intensity and interference at 1 year after breast cancer surgery: Assessing central sensitization, central sensitivity symptoms, and psychological factors. Breast Cancer 2023, 30, 271–281. [Google Scholar] [CrossRef]
- Lang, A.E.; Murphy, M.; Dickerson, C.R.; Stavness, I.; Kim, S.Y. Shoulder Dysfunction in Breast Cancer Survivors: Can Treatment Type or Musculoskeletal Factors Identify Those at Higher Risk? Rehabil. Oncol. 2021, 39, 143–151. [Google Scholar] [CrossRef]
- Ramirez-Parada, K.; Gonzalez-Santos, A.; Riady-Aleuy, L.; Pinto, M.P.; Ibañez, C.; Merino, T.; Acevedo, F.; Walbaum, B.; Fernández-Verdejo, R.; Sanchez, C. Upper-Limb Disability and the Severity of Lymphedema Reduce the Quality of Life of Patients with Breast Cancer-Related Lymphedema. Curr. Oncol. 2023, 30, 8068–8077. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, C.L.; Taghian, A.G. Breast Cancer–Related Lymphedema: The Prospective Surveillance Model, Early Intervention Strategies, and Role of Complete Decongestive Therapy. Curr. Breast Cancer Rep. 2023, 15, 12–23. [Google Scholar] [CrossRef]
- Braudy, R.; Atoms, B.; Coghlan, J.; Staples, M.; Moga, D.; Tollefsrud, R.; Lawrence, R.L.; Ludewig, P.; Koehler, L. Shoulder Kinematics of Axillary Web Syndrome in Women Treated for Breast Cancer. Arch. Phys. Med. Rehabil. 2023, 104, 403–409. [Google Scholar] [CrossRef] [PubMed]
- González-Rubino, J.B.; Vinolo-Gil, M.J.; Martín-Valero, R. Effectiveness of physical therapy in axillary web syndrome after breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 257. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.L.; Moreira, R.F.C.; Ferrari, A.V.; Alburquerque-Sendín, F.; Camargo, P.R.; Salvini, T.F. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: A systematic review of randomized controlled trials. Clin. Rehabil. 2019, 33, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fernández, M.J.; Medrano-Sánchez, E.M.; Ostos-Díaz, B.; Martín-Valero, R.; Suárez-Serrano, C.; Casuso-Holgado, M.J. Preliminary Study on the Effect of an Early Physical Therapy Intervention after Sentinel Lymph Node Biopsy: A Multicenter Non-Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 1275. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; Galea, M. Optimizing post-acute care in breast cancer survivors: A rehabilitation perspective. J. Multidiscip. Healthc. 2017, 10, 347–357. [Google Scholar] [CrossRef]
- Torres-Lacomba, M.; Prieto-Gómez, V.; Arranz-Martín, B.; Ferrandez, J.C.; Yuste-Sánchez, M.J.; Navarro-Brazález, B.; Romay-Barrero, H. Manual Lymph Drainage with Progressive Arm Exercises for Axillary Web Syndrome After Breast Cancer Surgery: A Randomized Controlled Trial. PTJ Phys. Ther. Rehabil. J. 2022, 102, pzab314. [Google Scholar] [CrossRef]
- Koehler, L.; Day, A.; Hunter, D.; Blaes, A.; Haddad, T.; Shanley, R. Five-Year Cumulative Incidence of Axillary Web Syndrome and Comparison in Upper Extremity Movement, Function, Pain, and Lymphedema in Survivors of Breast Cancer with and Without Axillary Web Syndrome. Arch. Phys. Med. Rehabil. 2022, 103, 1798–1806. [Google Scholar] [CrossRef]
- Klein, I.; Ben David, M.A.; Shahar, D.R.; Rosenberg, I.; Susmallian, S.; Barsuk, D.; Friger, M. Ben David Advancing Breast Cancer Rehabilitation: A Novel Tool for Assessing Physical Morbidity Risk. Oncologist 2025, 30, oyaf060. [Google Scholar] [CrossRef]
- Smith-Forbes, E.; Howell, D.M.; Willoughby, J.; Pitts, G.; Uhl, T.L. Minimal Clinical Important Difference of the Quick Disabilities of the Arm Shoulder and Hand (Quickdash) for Post-surgical Distal Radius Fractures. J. Hand Ther. 2016, 29, 360. [Google Scholar] [CrossRef]
- Modarresi, S.; Lukacs, M.J.; Ghodrati, M.; Salim, S.; MacDermid, J.C.; Walton, D.M. A systematic review and synthesis of psychometric properties of the numeric pain rating scale and the visual analog scale for use in people with neck pain. Clin. J. Pain 2022, 38, 132–148. [Google Scholar] [CrossRef]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and Its Shortened Version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Mordin, M.; Castro, C.; Islam, Z.; Tu, N.; Hackshaw, M.D. Health-related quality of life in women with breast cancer: A review of measures. BMC Cancer 2022, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: A systematic review. BMC Med. Res. Methodol. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Devoogdt, N.; De Groef, A. Physiotherapy management of breast cancer treatment-related sequelae. J. Physiother. 2024, 70, 90–105. [Google Scholar] [CrossRef]
- Metcalfe, J.W.; Orange, S.T.; Madden, L.A.; Marshall, P.; Vince, R.V. Effects of resistance training on quality of life, fatigue, physical function, and muscular strength during chemotherapy treatment: A systematic review and meta-analysis. Support. Care Cancer 2024, 32, 593. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Sheill, G.; O’Neill, L.; O’Connor, L.; Smyth, E.; Guinan, E. Physical Therapists in Oncology Settings: Experiences in Delivering Cancer Rehabilitation Services, Barriers to Care, and Service Development Needs. PTJ Phys. Ther. Rehabil. J. 2022, 102, pzab287. [Google Scholar] [CrossRef]
- Rett, M.T.; Moura, D.P.; Oliveira, F.B.d.; Domingos, H.Y.B.; Oliveira, M.M.F.d.; Gallo, R.B.S.; Silva Junior, W.M.d. Physical therapy after breast cancer surgery improves range of motion and pain over time. Fisioter. Pesqui. 2022, 29, 46–52. [Google Scholar] [CrossRef]
- Nareeba, D.; Ainembabazi, B.; Asiimwe, D.; Atuhaire, G.; Ochom, K.P.; Whitehouse, Z.; Arubaku, W.; Nuwahereza, A.; Kazibwe, H.; Niyonsenga, J.D. Barriers and Facilitators to the Referral of Patients with Cancer for Physiotherapy Rehabilitation at Uganda Cancer Institute (UCI). Research Square. 2023. Preprint (Version 1). Available online: https://www.researchsquare.com/article/rs-2413367/v1 (accessed on 8 October 2025).
- Yildiz Kabak, V.; Gursen, C.; Aytar, A.; Akbayrak, T.; Duger, T. Physical activity level, exercise behavior, barriers, and preferences of patients with breast cancer–related lymphedema. Support. Care Cancer 2021, 29, 3593–3602. [Google Scholar] [CrossRef]
- Ng, A.H.; Ngo-Huang, A.; Vidal, M.; Reyes-Garcia, A.; Liu, D.D.; Williams, J.L.; Fu, J.B.; Yadav, R.; Bruera, E. Exercise Barriers and Adherence to Recommendations in Patients with Cancer. JCO Oncol. Pract. 2021, 17, e972–e981. [Google Scholar] [CrossRef]
- De Groef, A.; Van der Gucht, E.; Dams, L.; Evenepoel, M.; Teppers, L.; Toppet-Hoegars, J.; De Baets, L. The association between upper limb function and variables at the different domains of the international classification of functioning, disability and health in women after breast cancer surgery: A systematic review. Disabil. Rehabil. 2022, 44, 1176–1189. [Google Scholar] [CrossRef]
- Thalji, S.Z.; Cortina, C.S.; Guo, M.S.; Kong, A.L. Postoperative Complications from Breast and Axillary Surgery. Surg. Clin. N. Am. 2023, 103, 121–139. [Google Scholar] [CrossRef]
- Abbad-Gomez, D.; Domingo, L.; Comas, M.; Santiá, P.; Jansana, A.; Poblador, B.; Sanz, T.; del Cura, I.; Ibañez, B.; Padilla, M.; et al. Effect of comorbidity and multimorbidity on adherence to follow-up recommendations among long-term breast cancer survivors. Maturitas 2024, 182, 107918. [Google Scholar] [CrossRef]
- Gorzelitz, J.S.; Brick, R.; Deng, L.; Mollica, M.; Stout, N.; Stoller, S.; Williams, C.P. Sociodemographic and clinical characteristics associated with rehabilitation services utilization in older women with early-stage breast cancer from SEER-Medicare 2009–2018. J. Cancer Surviv. 2024, 18, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Donahue, P.M.C.; MacKenzie, A.; Filipovic, A.; Koelmeyer, L. Advances in the prevention and treatment of breast cancer-related lymphedema. Breast Cancer Res. Treat. 2023, 200, 1–14. [Google Scholar] [CrossRef]
- Cheville, A.L.; McLaughlin, S.A.; Haddad, T.C.; Lyons, K.D.; Newman, R.; Ruddy, K.J. Integrated Rehabilitation for Breast Cancer Survivors. Am. J. Phys. Med. Rehabil. 2019, 98, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Keesing, S.; Rosenwax, L.; McNamara, B. A call to action: The need for improved service coordination during early survivorship for women with breast cancer and partners. Women Health 2019, 59, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Pan, P. The use of rehabilitation among patients with breast cancer: A retrospective longitudinal cohort study. BMC Health Serv. Res. 2012, 12, 282. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Speck, R.M.; Rye, S.A.; DiSipio, T.; Hayes, S.C. Prevalence of breast cancer treatment sequelae over 6 years of follow-up. Cancer 2012, 118, 2217–2225. [Google Scholar] [CrossRef]
- Gerber, L.H.; Stout, N.L.; Schmitz, K.H.; Stricker, C.T. Integrating a prospective surveillance model for rehabilitation into breast cancer survivorship care. Cancer 2012, 118, 2201–2206. [Google Scholar] [CrossRef]
- Koelmeyer, L.; Gaitatzis, K.; Ridner, S.H.; Boyages, J.; Nelms, J.; Hughes, T.M.; Elder, E.; French, J.; Ngui, N.; Hsu, J.; et al. Implementing a prospective surveillance and early intervention model of care for breast cancer–related lymphedema into clinical practice: Application of the RE-AIM framework. Support. Care Cancer 2021, 29, 1081–1089. [Google Scholar] [CrossRef]
- Stout, N.L.; Utzman, R.; Jenkins, H.H.; Burkart, M.; Swisher, A.K. Implementing and sustaining a breast cancer prospective surveillance rehabilitation program: An institutional perspective. J. Cancer Surviv. 2023, 17, 509–517. [Google Scholar] [CrossRef]
- Fu, M.R.; Aouizerat, B.E.; Yu, G.; Conley, Y.; Axelrod, D.; Guth, A.A.; Gagner, J.; Qiu, J.M.; Zagzag, D. Model-Based Patterns of Lymphedema Symptomatology: Phenotypic and Biomarker Characterization. Curr. Breast Cancer Rep. 2021, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Laza-Cagigas, R.; Pagarkar, A.; Olaoke, A.; El Gammal, M.; Rampal, T. The Feasibility of Prehabilitation as Part of the Breast Cancer Treatment Pathway. PM&R 2021, 13, 1237–1246. [Google Scholar]
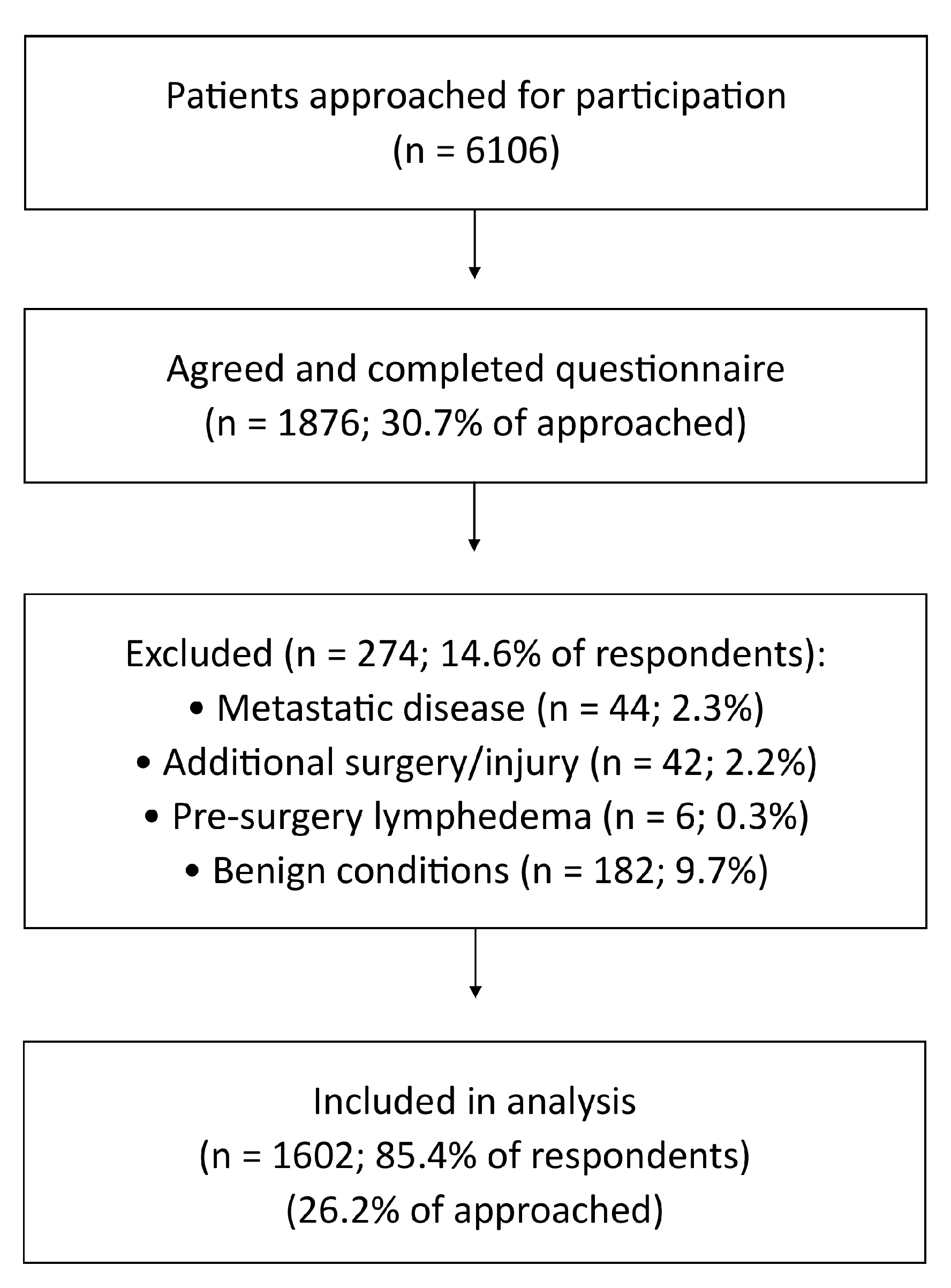
| Sociodemographic Characteristics | |||
| Age (n = 1602) | median 58 IQR [50;67] | ||
| BMI (n = 1568) | median 25 [22;29] | ||
| Family status (n, %) | Single | 358 | 23.4 |
| Married | 897 | 58.7 | |
| Divorced | 168 | 11.0 | |
| Widowed | 106 | 6.9 | |
| Years of education (n, %) | 8–12 | 295 | 20.1 |
| 13–16 | 551 | 37.6 | |
| 17–18 | 363 | 24.7 | |
| 19< | 258 | 17.6 | |
| BRCA gene, positive (n, %) | 254 | 19.9 | |
| Smoking, yes (n, %) | 229 | 15.7 | |
| Clinical Characteristics | |||
| Surgery type (n, %) | Lumpectomy | 1091 | 76.9 |
| Mastectomy | 279 | 19.6 | |
| Prophylactic mastectomy | 50 | 3.5 | |
| Lymph node dissection (n, %) | No nodes removed | 102 | 8.4 |
| 1–4 | 931 | 76.8 | |
| ≥5 nodes | 180 | 14.8 | |
| Breast reconstruction, yes (n, %) | 211 | 15.3 | |
| Stage (n, %) | I | 982 | 88.4 |
| II | 85 | 7.7 | |
| III | 42 | 3.8 | |
| Comorbidity, yes (n, %) | 614 | 38.1 | |
| ARM-BCT score (n = 1571) | median 6 IQR [4;8] | ||
| QuickDASH (n = 1184) | median 13 IQR [0;17] | ||
| Physical activity score (n = 1062) | median 2 IQR [2;8] | ||
| Treatment Characteristics | |||
| Chemotherapy, yes (n, %) | 302 | 20.5 | |
| Radiation therapy, yes (n, %) | 525 | 35.7 | |
| Time Range (Months) | p Value | ||||
|---|---|---|---|---|---|
| 0–6 N = 419 | 7–12 N = 80 | 13–24 N = 733 | 25–36 N = 370 | ||
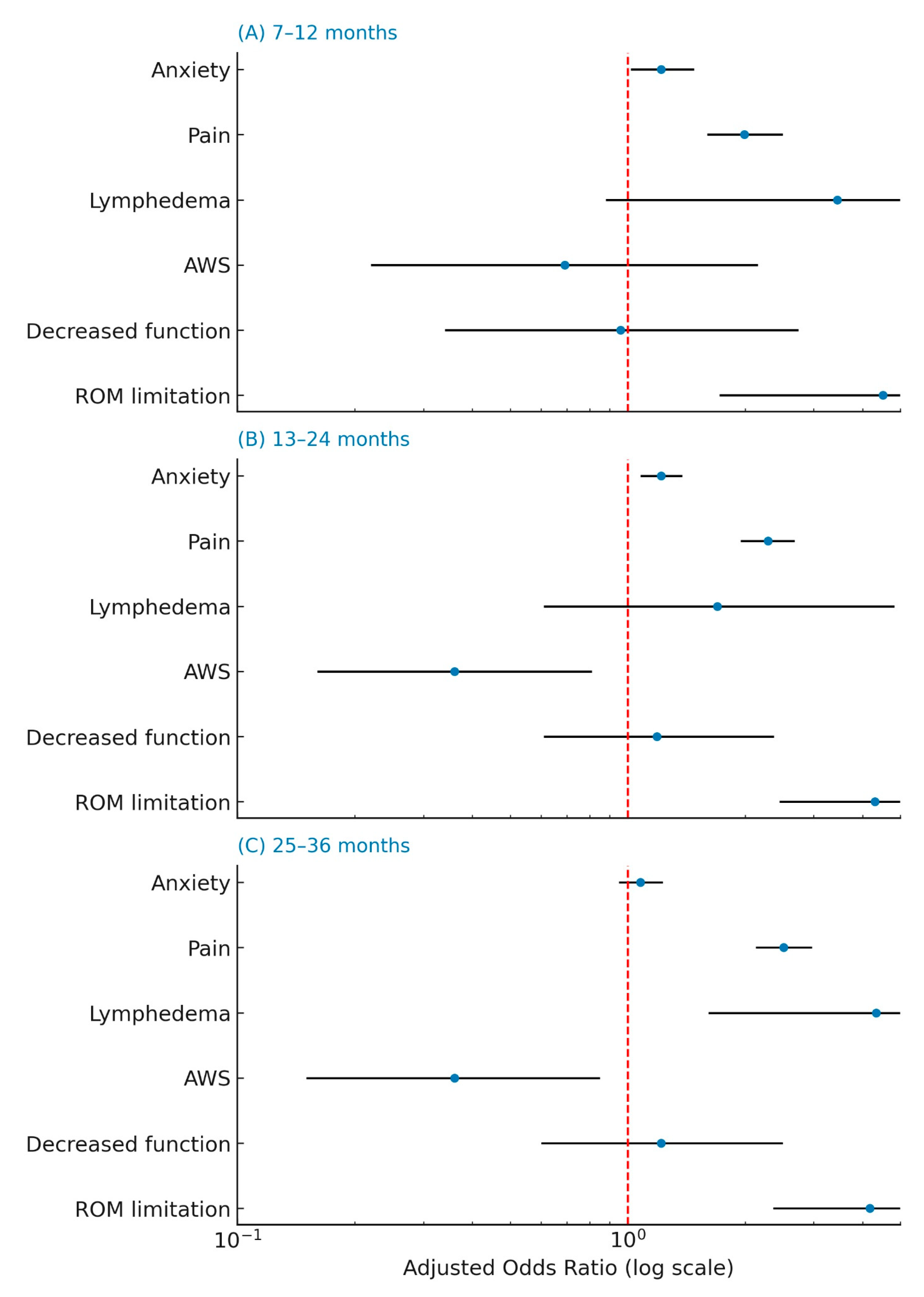
| ROM limitation, yes n (%) | 101 (31.7) | 24 (45.3) | 116 (40.3) | 87 (35.7) | 0.145 |
| Decreased function, yes n (%) | 124 (38.8) | 26 (49.1) | 120 (41.7) | 84 (34.6) | 0.273 |
| Lymphedema, yes n (%) | 25 (7.8) | 3 (5.7) | 21 (7.3) | 23 (9.9) | 0.665 |
| AWS, yes n (%) | 62 (19.4) | 4 (7.5) | 21 (7.3) | 13 (5.3) | <0.001 |
| Pain level median [IQR] | 1 [0;2] | 2 [1;4] | 1 [1;5] | 1 [1;4] | <0.001 |
| Anxiety level median [IQR] | 3 [2;5] | 3 [0;5] | 1 [0;5] | 0 [0;4] | <0.001 |
| Did Not Receive PT N = 820 (51.6%) | Received PT N = 769 (48.4%) | p Value | |
|---|---|---|---|
| Age, median [IQR] | 59 [50;67] | 58 [50;67] | 0.647 |
| BMI, median [IQR] | 25 [22;29] | 25 [22;28] | 0.165 |
| Surgery type | |||
| Lumpectomy | 609 (71.6) | 456 (81.6) | 0.008 |
| Mastectomy | 194 (22.8) | 85 (15.2) | |
| Prophylactic mastectomy | 39 (4.6) | 11 (2.0) | |
| Lymph node dissection | |||
| No nodes removed | 66 (8.1) | 34 (8.8) | 0.140 |
| 1–4 | 626 (75.5) | 299 (77.3) | |
| ≥5 nodes | 126 (15.4) | 54 (14.0) | |
| Breast reconstruction | 100 (12.1) | 111 (14.7) | 0.254 |
| Comorbidity | 259 (31.6) | 351 (45.6) | <0.001 |
| Chemotherapy | 138 (19.4) | 162 (21.7) | 0.147 |
| Radiation therapy | 247 (34.7) | 276 (37.0) | 0.194 |
| ARM-BCT score, median [IQR] | 5 [3;8] | 6 [4;8] | <0.001 |
| QuickDASH score, median [IQR] | 11 [0;16] | 15 [12;18] | <0.001 |
| ROM limitation | 141 (36.1) | 271 (36.4) | 0.478 |
| Decreased function | 173 (44.4) | 274 (36.8) | 0.015 |
| Lymphedema | 38 (9.7) | 53 (7.1) | 0.136 |
| AWS | 56 (14.2) | 61 (8.2) | 0.002 |
| Pain level, median [IQR] | 1 [0;4] | 1 [1;3] | 0.820 |
| Anxiety level, median [IQR] | 2 [1;5] | 1 [1;3] | <0.001 |
| PT during hospital stay | 242 (50.9) | 350 (45.5) | 0.086 |
| Verbal recommendation from medical staff | 41 (8.3) | 190 (28.2) | <0.001 |
| Lack of family support | 57 (7.4) | 69 (9.2) | 0.212 |
| Factor | No PT vs. Early PT | Late PT vs. Early PT | ||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value | |
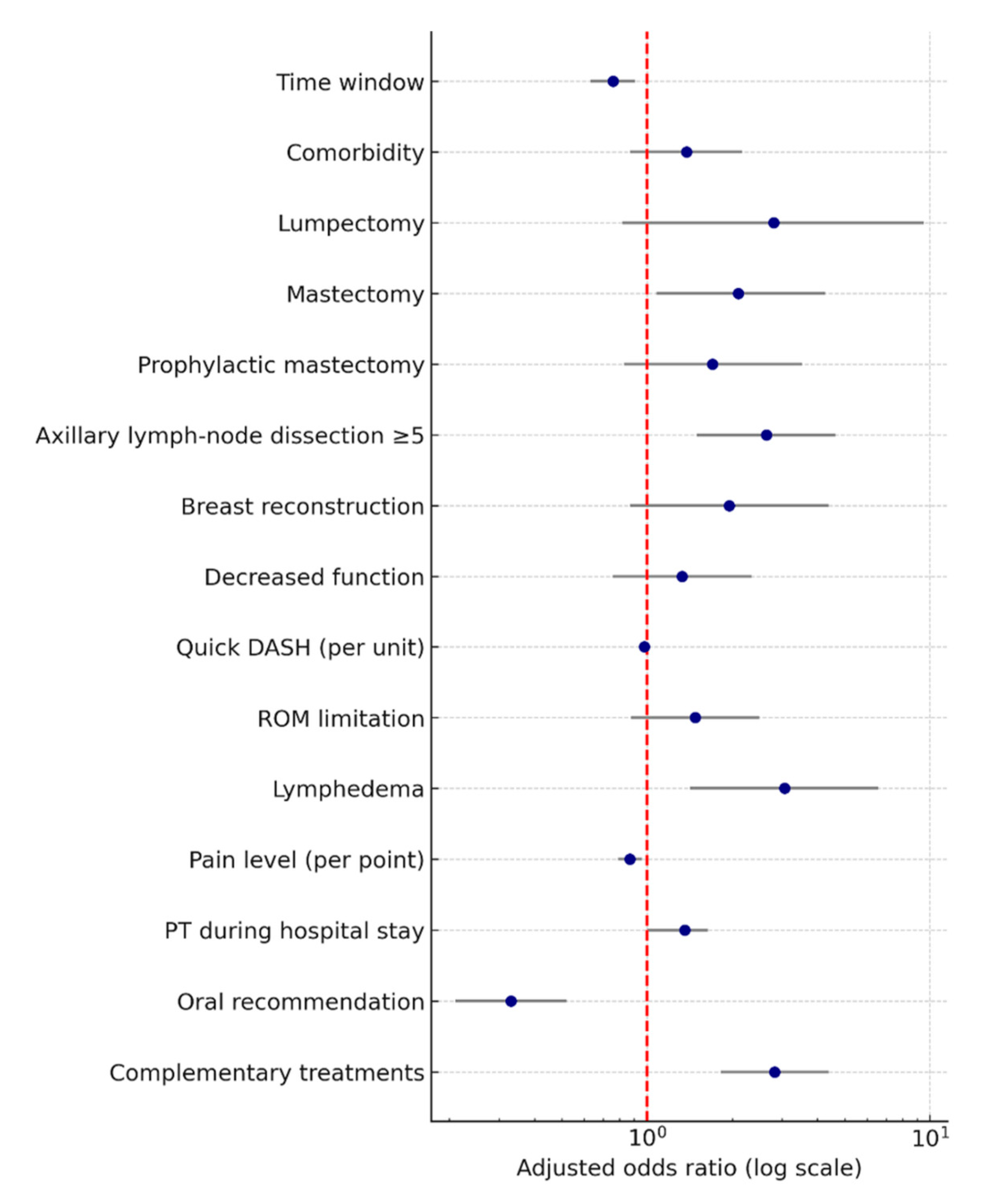
| Pain (higher level) | 1.11 | 1.01–1.22 | 0.037 | 1.21 | 1.11–1.33 | <0.001 |
| Lymphedema | 2.59 | 1.10–6.07 | 0.029 | 3.04 | 1.31–7.04 | 0.010 |
| ROM limitation | 0.80 | 0.52–1.25 | 0.323 | 0.79 | 0.52–1.20 | 0.267 |
| AWS | 0.87 | 0.52–1.46 | 0.587 | 2.48 | 1.42–4.34 | 0.001 |
| Decreased function | 1.33 | 0.84–2.10 | 0.226 | 1.00 | 0.64–1.56 | 0.995 |
| Anxiety (higher score) | 0.98 | 0.91–1.06 | 0.623 | 0.94 | 0.87–1.01 | 0.099 |
| Physical activity score (higher) | 0.99 | 0.97–1.02 | 0.624 | 1.00 | 0.98–1.02 | 0.777 |
| QuickDASH score (higher) | 1.00 | 0.97–1.03 | 0.774 | 0.98 | 0.95–1.01 | 0.135 |
| ARM-BCT score (higher) | 1.08 | 1.00–1.18 | 0.062 | 0.99 | 0.91–1.07 | 0.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, I.; Shahar, D.R.; Friger, M.; Rosenberg, I.; Barsuk, D.; Ben-David, M.A.; Susmallian, S. Physical Therapy Utilization and Morbidity Outcomes After Breast Cancer Surgery: A Longitudinal Analysis of Three Combined Cohorts. Cancers 2025, 17, 3296. https://doi.org/10.3390/cancers17203296
Klein I, Shahar DR, Friger M, Rosenberg I, Barsuk D, Ben-David MA, Susmallian S. Physical Therapy Utilization and Morbidity Outcomes After Breast Cancer Surgery: A Longitudinal Analysis of Three Combined Cohorts. Cancers. 2025; 17(20):3296. https://doi.org/10.3390/cancers17203296
Chicago/Turabian StyleKlein, Ifat, Danit R. Shahar, Michael Friger, Irena Rosenberg, Daphna Barsuk, Merav A. Ben-David, and Sergio Susmallian. 2025. "Physical Therapy Utilization and Morbidity Outcomes After Breast Cancer Surgery: A Longitudinal Analysis of Three Combined Cohorts" Cancers 17, no. 20: 3296. https://doi.org/10.3390/cancers17203296
APA StyleKlein, I., Shahar, D. R., Friger, M., Rosenberg, I., Barsuk, D., Ben-David, M. A., & Susmallian, S. (2025). Physical Therapy Utilization and Morbidity Outcomes After Breast Cancer Surgery: A Longitudinal Analysis of Three Combined Cohorts. Cancers, 17(20), 3296. https://doi.org/10.3390/cancers17203296






