Pulmonary Metastasectomy: A Multicenter Comparison of Wedge Resection Versus Anatomic Resection for Single Metastases of Colorectal Cancer
Simple Summary
Abstract
1. Introduction
- Greater safety margins: Anatomical resection provides wider margins, which should reduce the risk of local intrapulmonary recurrence at the resection margin [4].
- Lymph node clearance: Removal of intralobar or intrasegmental lymphoid tissue may include undetected lymph node metastases or lymphatic involvement, thereby reducing rates of locoregional recurrence.
- Reduced occult metastases: The larger volume of tissue removed decreases the likelihood of occult metastases in healthy lung tissue, potentially lowering the risk of new intrapulmonary metastases.
Study Rationale and Objectives
2. Materials and Methods
Statistical Evaluation
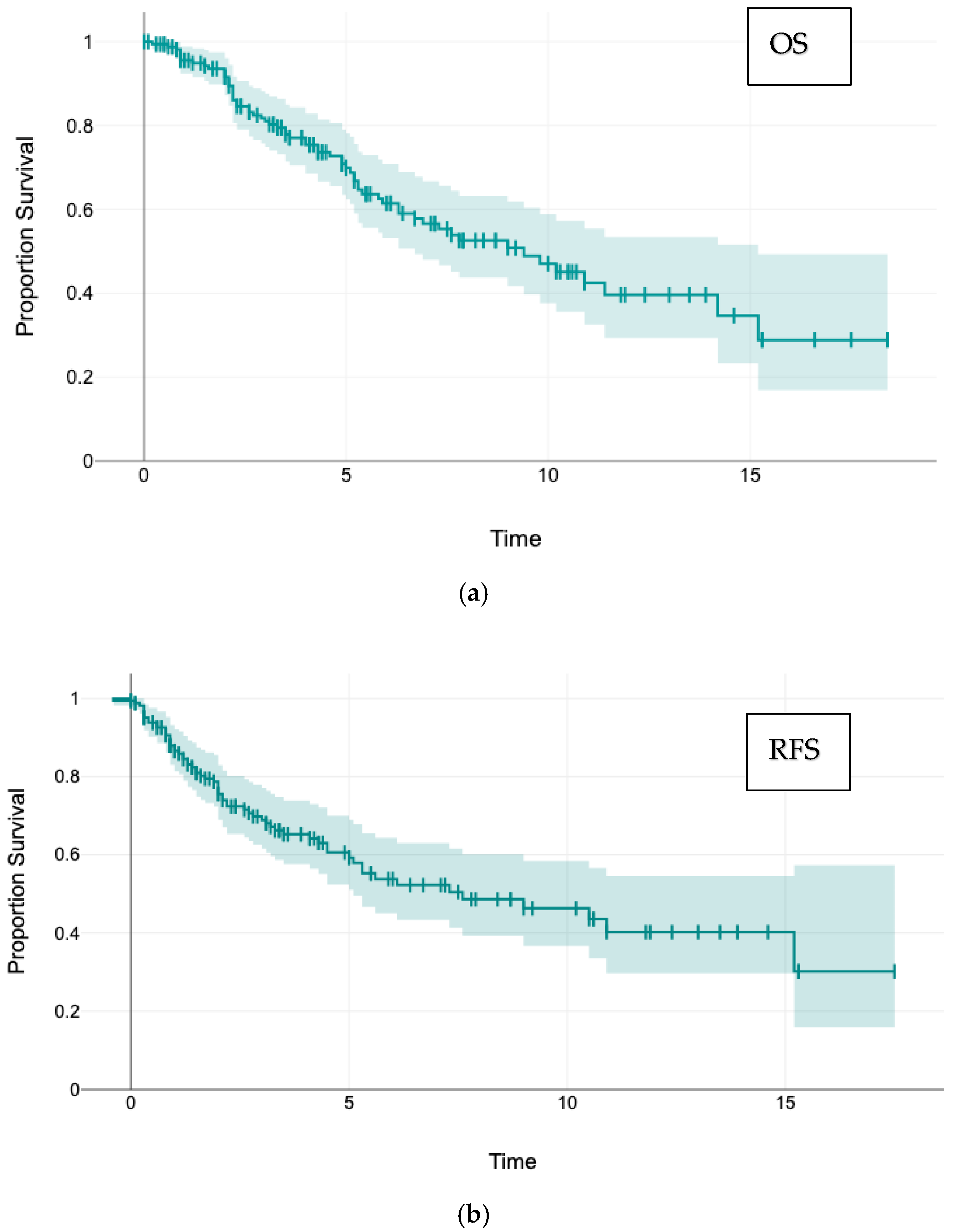
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CRC | colorectal cancer |
| DFI | disease-free interval |
| DFS | disease-free survival |
| HR | hazard ratio |
| IQR | interquartile range |
| LN | lymph node |
| OS | overall survival |
| PSM | propensity score matching |
| RFS | recurrence-free survival |
| SD | standard deviation |
References
- Petrella, F.; Diotti, C.; Rumessa, A.; Spaggiari, L. Pulmonary metastasectomy: An overview. J. Thorac. Dis. 2017, 9, S1291–S1298. [Google Scholar] [CrossRef]
- Arunachalam, P.; Chihara, R.; Nguyen, D.T.; Graviss, E.A.; Kim, M.P. Surgical Resection of a Single Colorectal Lung Metastasis is Associated with Best Survival. J. Surg. Res. 2025, 309, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B., Jr.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef]
- Welter, S.; Essaleh, W.; Dudek, W. Anatomical resection of colorectal lung metastases: A reasonable indication without evidence—Non-systematic and incomplete reporting is a key issue. J. Thorac. Dis. 2022, 14, 4197–4201. [Google Scholar] [CrossRef]
- Renaud, S.; Seitlinger, J.; Al Lawati, Y.; Guerrera, F.; Falcoz, P.-E.; Massard, G.; Ferri, L.M.; Spicer, J.M. Anatomical Resections Improve Survival Following Lung Metastasectomy of Colorectal Cancer Harboring KRAS Mutations. Ann. Surg. 2019, 270, 1170–1177. [Google Scholar] [CrossRef]
- Shiono, S.; Okumura, T.; Boku, N.; Hishida, T.; Ohde, Y.; Sakao, Y.; Yoshiya, K.; Hyodo, I.; Mori, K.; Kondo, H. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur. J. Cardio-Thorac. Surg. 2016, 51, 504–510. [Google Scholar] [CrossRef]
- Hernández, J.; Molins, L.; Fibla, J.J.; Heras, F.; Embún, R.; Rivas, J.J. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann. Oncol. 2016, 27, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, E.; Ceulemans, L.J.; Van Raemdonck, D.E.; Decaluwé, H.; De Leyn, P.; Bertolaccini, L. Impact of the extent of lung resection on postoperative outcomes of pulmonary metastasectomy for colorectal cancer metastases: An exploratory systematic review. J. Thorac. Dis. 2022, 14, 2677–2688. [Google Scholar] [CrossRef]
- Welter, S.; Arfanis, E.; Christoph, D.; Hager, T.; Roesel, C.; Aigner, C.; Weinreich, G.; Theegarten, D. Growth patterns of pulmonary metastases: Should we adjust resection techniques to primary histology and size? Eur. J. Cardio-Thorac. Surg. 2017, 52, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nanji, S.; Karim, S.; Tang, E.; Brennan, K.; McGuire, A.; Pramesh, C.; Booth, C.M. Pulmonary Metastasectomy for Colorectal Cancer: Predictors of Survival in Routine Surgical Practice. Ann. Thorac. Surg. 2018, 105, 1605–1612. [Google Scholar] [CrossRef]
- Rapicetta, C.; Lococo, F.; Davini, F.; Carleo, F.; Kauppi, J.; Di Stefano, T.S.; Ricciardi, S.; Di Martino, M.; Räsänen, J.; Paci, M.; et al. Is Adjuvant Chemotherapy Worthwhile After Radical Resection for Single Lung Metastasis from Colorectal Cancer? A Multicentric Analysis Evaluating the Risk of Recurrence. Front. Oncol. 2019, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Welter, S.; La Raia, R.B.; Gupta, V. Pursuit of an optimal surgical margin in pulmonary metastasectomy. J. Vis. Surg. 2019, 5, 39. [Google Scholar] [CrossRef]
- Chiappetta, M.; Salvatore, L.; Congedo, M.T.; Bensi, M.; De Luca, V.; Ciavarella, L.P.; Camarda, F.; Evangelista, J.; Valentini, V.; Tortora, G.; et al. Management of single pulmonary metastases from colorectal cancer: State of the art. World J. Gastrointest. Oncol. 2022, 14, 820–832. [Google Scholar] [CrossRef]
- Cho, J.H.; Hamaji, M.; Allen, M.S.; Cassivi, S.D.; Nichols, F.C.; Wigle, D.A.; Shen, K.R.; Deschamps, C. The Prognosis of Pulmonary Metastasectomy Depends on the Location of the Primary Colorectal Cancer. Ann. Thorac. Surg. 2014, 98, 1231–1237. [Google Scholar] [CrossRef]
- Suzuki, H.; Kiyoshima, M.; Kitahara, M.; Asato, Y.; Amemiya, R. Long-Term Outcomes After Surgical Resection of Pulmonary Metastases from Colorectal Cancer. Ann. Thorac. Surg. 2015, 99, 435–440. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, S.; Namgung, M.; Choi, Y.S.; Kim, H.K.; Zo, J.I.; Shim, Y.M.; Kim, J. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer. World J. Surg. Oncol. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Forster, C.; Ojanguren, A.; Perentes, J.Y.; Zellweger, M.; Krueger, T.; Abdelnour-Berchtold, E.; Gonzalez, M. Survival prognostic and recurrence risk factors after single pulmonary metastasectomy. J. Cardiothorac. Surg. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Shiono, S.; Ishii, G.; Nagai, K.; Yoshida, J.; Nishimura, M.; Murata, Y.; Tsuta, K.; Nishiwaki, Y.; Kodama, T.; Ochiai, A. Histopathologic Prognostic Factors in Resected Colorectal Lung Metastases. Ann. Thorac. Surg. 2005, 79, 278–282. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Ishida, D.; Tanaka, R. Prognosis of patients with recurrence after pulmonary metastasectomy for colorectal cancer. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1172–1178. [Google Scholar] [CrossRef]
- Blackmon, S.H.; Stephens, E.H.; Correa, A.M.; Hofstetter, W.; Kim, M.P.; Mehran, R.J.; Rice, D.C.; Roth, J.A.; Swisher, S.G.; Walsh, G.L.; et al. Predictors of Recurrent Pulmonary Metastases and Survival After Pulmonary Metastasectomy for Colorectal Cancer. Ann. Thorac. Surg. 2012, 94, 1802–1809. [Google Scholar] [CrossRef]
- Shalabi, A.; Shalabi, S.F.; Graeter, T.; Welter, S.; Ehab, A.; Kuon, J. Low Rates of Intrapulmonary Local Recurrence After Laser Metastasectomy: A Single-Center Retrospective Cohort Study of Colorectal Cancer Metastases. Cancers 2025, 17, 683. [Google Scholar] [CrossRef]
- Renaud, S.; Romain, B.; Falcoz, P.-E.; Olland, A.; Santelmo, N.; Brigand, C.; Rohr, S.; Guenot, D.; Massard, G. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br. J. Cancer 2015, 112, 720–728. [Google Scholar] [CrossRef]
- Welter, S.; Theegarten, D.; Trarbach, T.; Maletzki, F.; Stamatis, G.; Tötsch, M. Safety distance in the resection of colorectal lung metastases: A prospective evaluation of satellite tumor cells with immunohistochemistry. J. Thorac. Cardiovasc. Surg. 2011, 141, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Tayob, N.; Mitchell, K.G.; Correa, A.M.; Hofstetter, W.L.; Sepesi, B.; Walsh, G.L.; Vaporciyan, A.A.; Swisher, S.G.; Antonoff, M.B.; et al. Surgical margins and risk of local recurrence after wedge resection of colorectal pulmonary metastases. J. Thorac. Cardiovasc. Surg. 2019, 157, 1648–1655. [Google Scholar] [CrossRef]
- Metastatic Lung Tumor Study Group of Japan; Shiono, S.; Matsutani, N.; Hashimoto, H.; Yamauchi, Y.; Matsuguma, H.; Mun, M.; Kuroda, H.; Funai, K.; Nakajima, J.; et al. Prospective study of recurrence at the surgical margin after wedge resection of pulmonary metastases. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 950–959. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, M.; Bousema, J.E.; Torensma, B.; Dickhoff, C.; Broek, F.J.C.v.D.; Schreurs, W.H.; Gonzalez, M.; Kazemier, G.; Heineman, D.J. Pulmonary metastasectomy with lymphadenectomy for colorectal pulmonary metastases: A systematic review. Eur. J. Surg. Oncol. (EJSO) 2022, 48, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lan, H.; Yang, D.; Wang, L.; Hu, L. Prognostic value of circulating tumor cells in patients with recurrent and metastatic colorectal cancer: A systematic review and meta-analysis. Medicine 2024, 103, e36819. [Google Scholar] [CrossRef]
- Le, U.-T.; Bronsert, P.; Picardo, F.; Riethdorf, S.; Haager, B.; Rylski, B.; Czerny, M.; Beyersdorf, F.; Wiesemann, S.; Pantel, K.; et al. Intraoperative detection of circulating tumor cells in pulmonary venous blood during metastasectomy for colorectal lung metastases. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Horie, T.; Kanemitsu, Y.; Takamizawa, Y.; Moritani, K.; Tsukamoto, S.; Shida, D. Prognostic differences between oligometastatic and polymetastatic disease after resection in patients with colorectal cancer and hepatic or lung metastases: Retrospective analysis of a large cohort at a single institution. Surgery 2022, 173, 328–334. [Google Scholar] [CrossRef]
- Sun, F.; Chen, L.; Shi, M.; Yang, X.; Li, M.; Yang, X.; Dai, X.; Zhan, C.; Shi, Y.; Wang, Q. Prognosis of video-assisted thoracoscopic pulmonary metastasectomy in patients with colorectal cancer lung metastases: An analysis of 154 cases. Int. J. Color. Dis. 2017, 32, 897–905. [Google Scholar] [CrossRef]
- Kamigaichi, A.; Hamada, A.; Tsutani, Y. Segmentectomy for patients with early-stage pure-solid non-small cell lung cancer. Front. Oncol. 2023, 13, 1287088. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.; Huang, Q.; Li, R.Z.; Chandran, U.; Zhang, Y.; Amos, T.B.; Wright, G.W.; Ferko, N.C.; Kalsekar, I. Recurrence-Free Survival in Patients with Surgically Resected Non-Small Cell Lung Cancer. Chest 2023, 165, 1260–1270. [Google Scholar] [CrossRef] [PubMed]

| Variable | N (Valid Values) | (% Valid Values) | |
|---|---|---|---|
| Gender | Male | 104 | 62.6 |
| Female | 62 | 37.7 | |
| Age (y) | 166 | 100 | |
| median 69, mean 67 (39.2–92.6) | |||
| Primary tumor | Rectum | 98 | 59 |
| Colon | 68 | 41 | |
| Lymph node involvement primary tumor | No | 80 | 53 |
| Yes | 70 | 47 | |
| Resected liver metastasis | No | 129 | 77.7 |
| Yes | 37 | 22.3 | |
| First pulmonary metastasis | Metachronous | 144 | 86.7 |
| Synchronous | 22 | 13.3 | |
| Operative access | Open thoracotomy | 126 | 75.9 |
| VATS | 40 | 24.1 | |
| Extent of resection | Enucleation | 41 | 24.7 |
| Atypical Resection | 32 | 19.3 | |
| Segmentectomy | 36 | 21.7 | |
| >1 Segments | 8 | 4.8 | |
| Lobectomy | 42 | 25.3 | |
| Bilobectomy | 7 | 4.2 | |
| Amount of lung tissue removed | Anatomic Resection | 93 | 56.0 |
| Non-anatomic Res. | 73 | 44.0 | |
| Resection method | Staplers | 115 | 71.0 |
| Laser resection | 45 | 27.8 | |
| Cautery | 2 | 1.2 | |
| Number of specimens removed | 1 specimen | 123 | 74.1 |
| 2 specimens | 25 | 15.1 | |
| 3 specimens | 15 | 9.0 | |
| >3 specimens | 3 | 1,8 | |
| Maximum size of the metastasis (mm) | 158 | 95.2 | |
| median 21.0; mean 27.3 (4–115) | |||
| Minimum safety margin (mm) | 106 | 63.9 | |
| median 5.0; mean 9.9 (0–50) | |||
| Disease-free interval (years) | 166 | 100 | |
| median 2.7; mean 3.2 (0–12.9) | |||
| Follow-up (years) | 166 | 100 | |
| median 4.3; mean 5.3 (0–18.4) | |||
| Postoperative complications (Clavien–Dindo) | 165 | 99.4 | |
| No complication | 80 | 48.5 | |
| Grade I | 56 | 33.9 | |
| Grade II | 22 | 13.3 | |
| Grade IIIa | 4 | 2.4 | |
| Grade IIIb | 3 | 1.8 | |
| Laterality | Left | 85 | 51.2 |
| Right | 81 | 48.8 | |
| Affected lobe | Lower lobe | 86 | 51.8 |
| Upper lobe | 62 | 37.4 | |
| Middle lobe | 18 | 10.8 | |
| Resection margin | R0 | 159 | 95.8 |
| R1 | 7 | 4.2 | |
| Lymphadenectomy | None | 29 | 17.6 |
| Sampling | 79 | 47.9 | |
| Radical | 57 | 34.5 | |
| Number of removed lymph nodes | 162 | 97.6 | |
| median 4; mean 8.2 (0–42) | |||
| Lymph node involvement | N0 | 147 | 90.7 |
| N1 | 9 | 5.6 | |
| N2 | 6 | 3.7 | |
| Position of the metastasis | Peripherical | 77 | 47.0 |
| Intermediate | 35 | 21.3 | |
| Central | 52 | 31.7 | |
| Last status | Alive | 102 | 61.4 |
| Deceased | 64 | 38.6 | |
| Any tumor recurrence | Yes | 87 | 54.0 |
| No | 74 | 46.0 | |
| Tumor recurrence within the lung | Yes | 62 | 38.5 |
| No | 99 | 61. | |
| Intrapulmonary local margin recurrence | Yes | 25 | 17.2 |
| No | 120 | 82.8 | |
| Intrathoracic lymph node recurrence | Yes | 14 | 10.1 |
| No | 124 | 89.9 | |
| Location of distant recurrence | None | 81 | 48.8 |
| Unknown | 44 | 26.5 | |
| Primary CRC | 13 | 7.8 | |
| Brain | 9 | 5.4 | |
| Liver | 7 | 4.2 | |
| Multiple sites | 7 | 4.2 | |
| Bone | 3 | 1.8 | |
| Others | 2 | 1.2 | |
| Variable | n | Intrapulmonary Local Recurrence (%) | Chi-Squared | |
|---|---|---|---|---|
| Gender | Male | 89 | 19 (21.4) | |
| Female | 56 | 6 (10.7) | 0.154 | |
| Primary | Rectum | 86 | 18 (20.9) | |
| Colon | 59 | 7 (13.5) | 0.232 | |
| Resected liver metastasis | no | 110 | 18 (16.4) | |
| yes | 35 | 7 (38.9) | 0.811 | |
| Appearance of metastases | Metachronous | 126 | 21 (16.7) | |
| Synchronous | 19 | 19 (26.7) | 0.884 | |
| Operative access | Thoracotomy | 107 | 19 (17.8) | |
| VATS | 38 | 6 (15.8) | 0.979 | |
| Mode of resection | Non-anatomic | 59 | 15 (25.4) | |
| Anatomic | 86 | 10 (11.6) | 0.052 | |
| Technique of resection | Stapler resection | 106 | 17 (16.0) | |
| Laser resection | 33 | 8 (24.2) | ||
| Cautery | 2 | 0 (0) | 0.450 | |
| Laterality | Right side | 74 | 14 (18.9) | |
| Left side | 71 | 11 (15.5) | 0.744 | |
| Affected lobe | Upper lobe | 56 | 9 (16.1) | |
| Middle lobe | 16 | 2 (14.3) | ||
| Lower lobe | 73 | 14 (19.2) | 0.780 | |
| Resection margin | R0 | 138 | 23 (16.7) | |
| R1 | 7 | 2 (28.6) | 0.764 | |
| Lymphadenectomy | None | 25 | 6 (24.0) | |
| Sampling | 67 | 15 (22.4) | ||
| Radical | 53 | 4 (7.6) | 0.063 | |
| Lymph node involvement | N0 | 129 | 23 (17.8) | |
| N1 | 8 | 1 (12.5) | ||
| N2 | 6 | 0 (0) | ||
| N3 | 1 | 1 (100) | 0.104 | |
| Position of the metastasis | Peripherical | 65 | 11 (16.9) | |
| Intermediate | 33 | 4 (12.1) | ||
| Central | 46 | 10 (21.7) | 0.534 | |
| Last status | Alive | 92 | 12 (13.0) | |
| Deceased | 53 | 13 (24.5) | 0.125 | |
| Intrathoracic lymph node recurrence | No | 124 | 15 (12.1) | |
| Yes | 14 | 7 (50) | 0.001 | |
| No local margin recurrence Mean/median (mm) | Local margin recurrence Mean/median (mm) | |||
| Maximum size of the metastasis | 27.4/20.0 | 23.2/21.0 | 0.212 | |
| Minimum safety margin | 10.7/5.0 | 4.5/3.0 | <0.001 | |
| Number of removed lymph nodes | 9.3/6.0 | 4.5/3.0 | <0.001 | |
| Age (years) | 66.7/68.8 | 69.5/70.5 | 0.148 | |
| Disease-free interval (years) | 3.1/2.5 | 4.3/3.6 | 0.101 | |
| Parameter | n | Intrapulmonary Lymph Node Recurrence (%) | Chi-Squared | |
|---|---|---|---|---|
| Primary | Rectum | 82 | 12 (14.6) | |
| Colon | 56 | 2 (3.6) | 0.068 | |
| Lymph node involvement | N0 | 122 | 10 (8.2) | |
| N1 | 8 | 3 (37.5) | ||
| N2 | 6 | 1 (16.7) | ||
| Nx | 1 | 0 (0) | 0.060 | |
| Last status | Alive | 87 | 4 (4.6) | |
| Deceased | 51 | 10 (19.6) | 0.012 | |
| Pulmonary tumor recurrence | No | 84 | 2 (2.4) | |
| Yes | 42 | 12 (28.6) | <0.001 | |
| Intrapulmonary local margin recurrence | No | 116 | 7 (6.0) | |
| Yes | 22 | 7 (31.8) | 0.001 | |
| Parameter | n (%) Anatomic res. | n (%) Non-Anatomic res. | Chi2 | |
|---|---|---|---|---|
| Resection margin | R0 | 93 (100) | 66 (90.4) | |
| R1 | 0 (0) | 7 (9.6) | 0.007 | |
| Lymphadenectomy | None | 12 (12.9) | 17 (23.6) | |
| Sampling | 27 (29.0) | 52 (72.2) | ||
| radical | 54 (58.1) | 3 (4.2) | <0.001 | |
| Lymph node involvement | N0 | 78 (84.8) | 67 (98.5) | |
| N1 | 9 (9.8) | 0 (0) | ||
| N2 | 5 (5.4) | 1 (1,5) | 0.027 | |
| Position of the metastasis | Peripherical | 28 (30.1) | 49 (69.0) | |
| Intermediate | 26 (28.0) | 9 (12.7) | ||
| Central | 39 (41.9) | 13 (18.3) | 0.012 | |
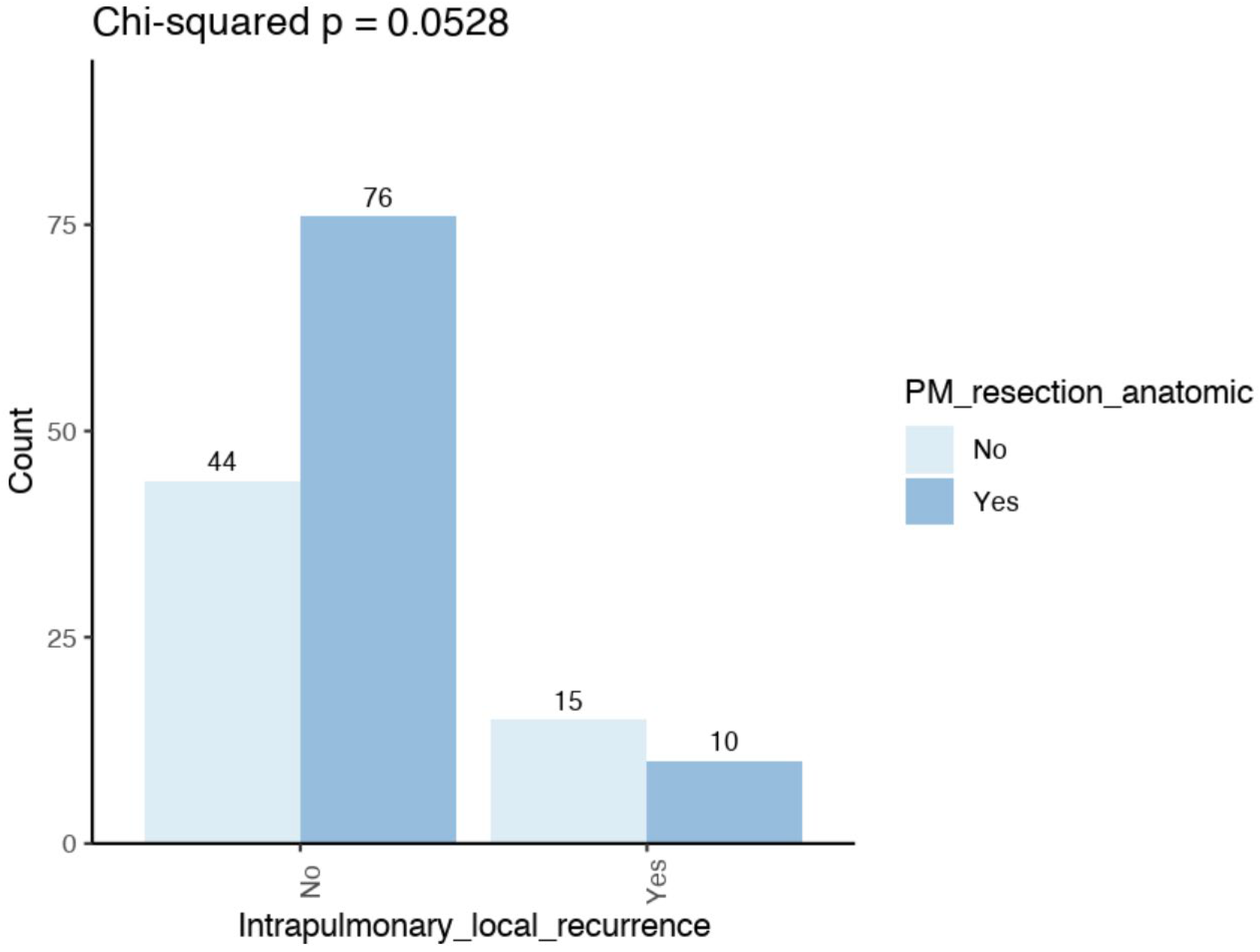
| Intrapulmonary local recurrence | No | 76 (88.4) | 44 (74.6) | |
| yes | 10 (11.6) | 15 (25.4) | 0.052 | |
| Mean/median | Mean/median | |||
| Age (years) | 66.9/69.8 | 67.2/68.0 | 0.841 | |
| Disease-free interval (Years) | 3.5/3.1 | 3.0/2.3 | 0.215 | |
| Maximum size of the metastasis (mm) | 32.9/28.0 | 19.9/18.0 | <0.001 | |
| Minimum safety distance (mm) | 11.8/7.0 | 5.5/4.5 | <0.001 | |
| Number of lymph nodes removed | 12.5/12.0 | 2.7/2.0 | <0.001 | |
| Parameter | No Local Margin Recurrence | Local Margin Recurrence | 95% CI | OR | p | |
|---|---|---|---|---|---|---|
| Mode of resection | Anatomical | 47 | 4 | |||
| Non-anatomical | 42 | 11 | 1.01–7.18 | 2.63 | 0.042 | |
| no intrath. LN recurrence | intrathorac LN recurrence | |||||
| Mode of resection | Anatomical | 48 | 3 | |||
| Non-anatomical | 46 | 5 | 0.47–6.04 | 1.68 | 0.392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welter, S.; Moneke, I.; Wara, R.; Le, A.U.-T.; Shalabi, A.; Graeter, T.; Ploenes, T.; Baum, D. Pulmonary Metastasectomy: A Multicenter Comparison of Wedge Resection Versus Anatomic Resection for Single Metastases of Colorectal Cancer. Cancers 2025, 17, 3299. https://doi.org/10.3390/cancers17203299
Welter S, Moneke I, Wara R, Le AU-T, Shalabi A, Graeter T, Ploenes T, Baum D. Pulmonary Metastasectomy: A Multicenter Comparison of Wedge Resection Versus Anatomic Resection for Single Metastases of Colorectal Cancer. Cancers. 2025; 17(20):3299. https://doi.org/10.3390/cancers17203299
Chicago/Turabian StyleWelter, Stefan, Isabelle Moneke, Ramzi Wara, Antonia Uyen-Thao Le, Ahmad Shalabi, Thomas Graeter, Till Ploenes, and Daniel Baum. 2025. "Pulmonary Metastasectomy: A Multicenter Comparison of Wedge Resection Versus Anatomic Resection for Single Metastases of Colorectal Cancer" Cancers 17, no. 20: 3299. https://doi.org/10.3390/cancers17203299
APA StyleWelter, S., Moneke, I., Wara, R., Le, A. U.-T., Shalabi, A., Graeter, T., Ploenes, T., & Baum, D. (2025). Pulmonary Metastasectomy: A Multicenter Comparison of Wedge Resection Versus Anatomic Resection for Single Metastases of Colorectal Cancer. Cancers, 17(20), 3299. https://doi.org/10.3390/cancers17203299






