Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Definition of Resectable PDAC
2.3. Treatment Strategy
2.4. Clinical Data
2.5. Statistical Analysis
3. Results
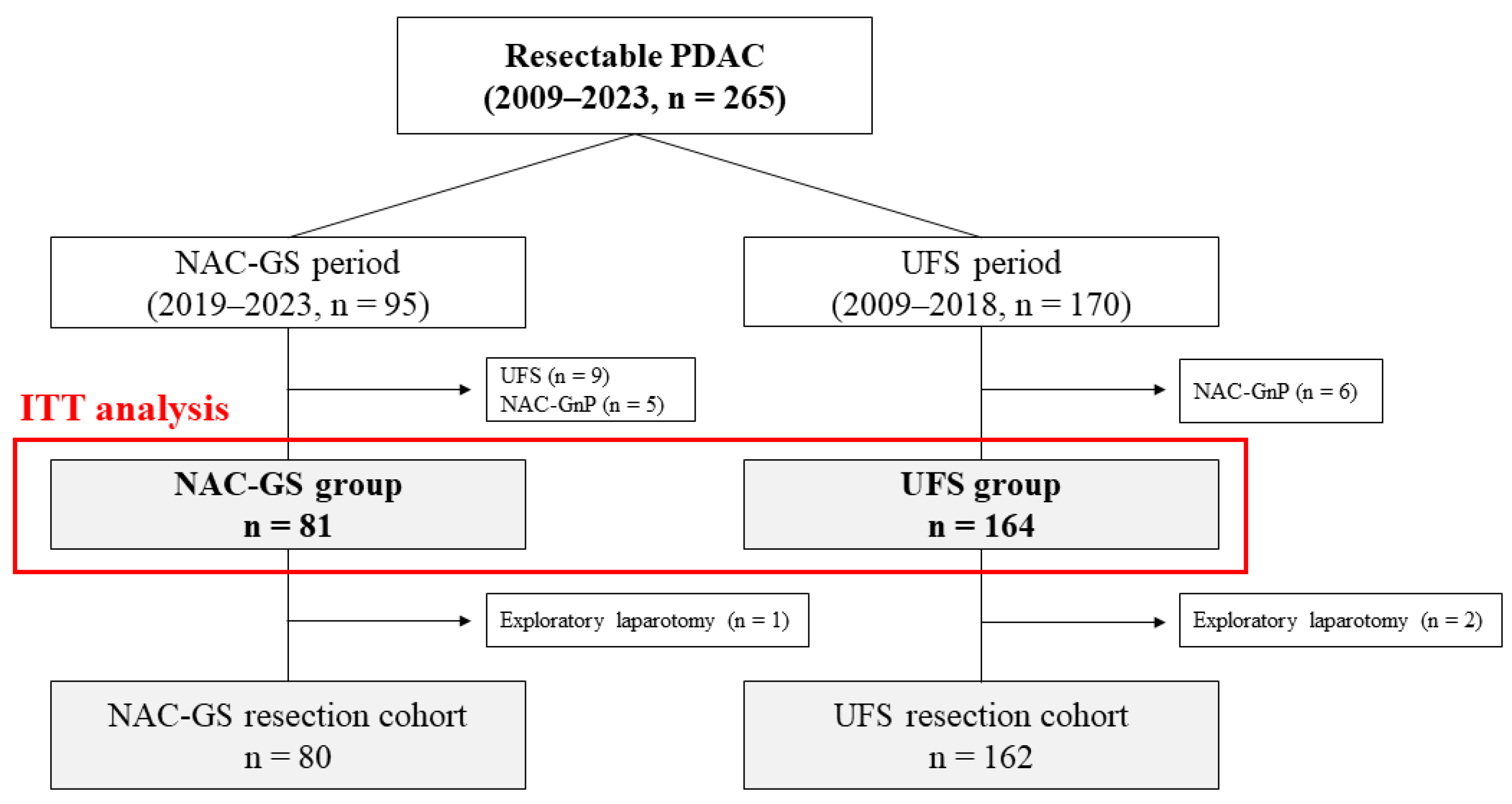
3.1. Study Cohort
3.2. Efficacy of NAC-GS
3.3. Patient Characteristics and Short-Term Outcomes
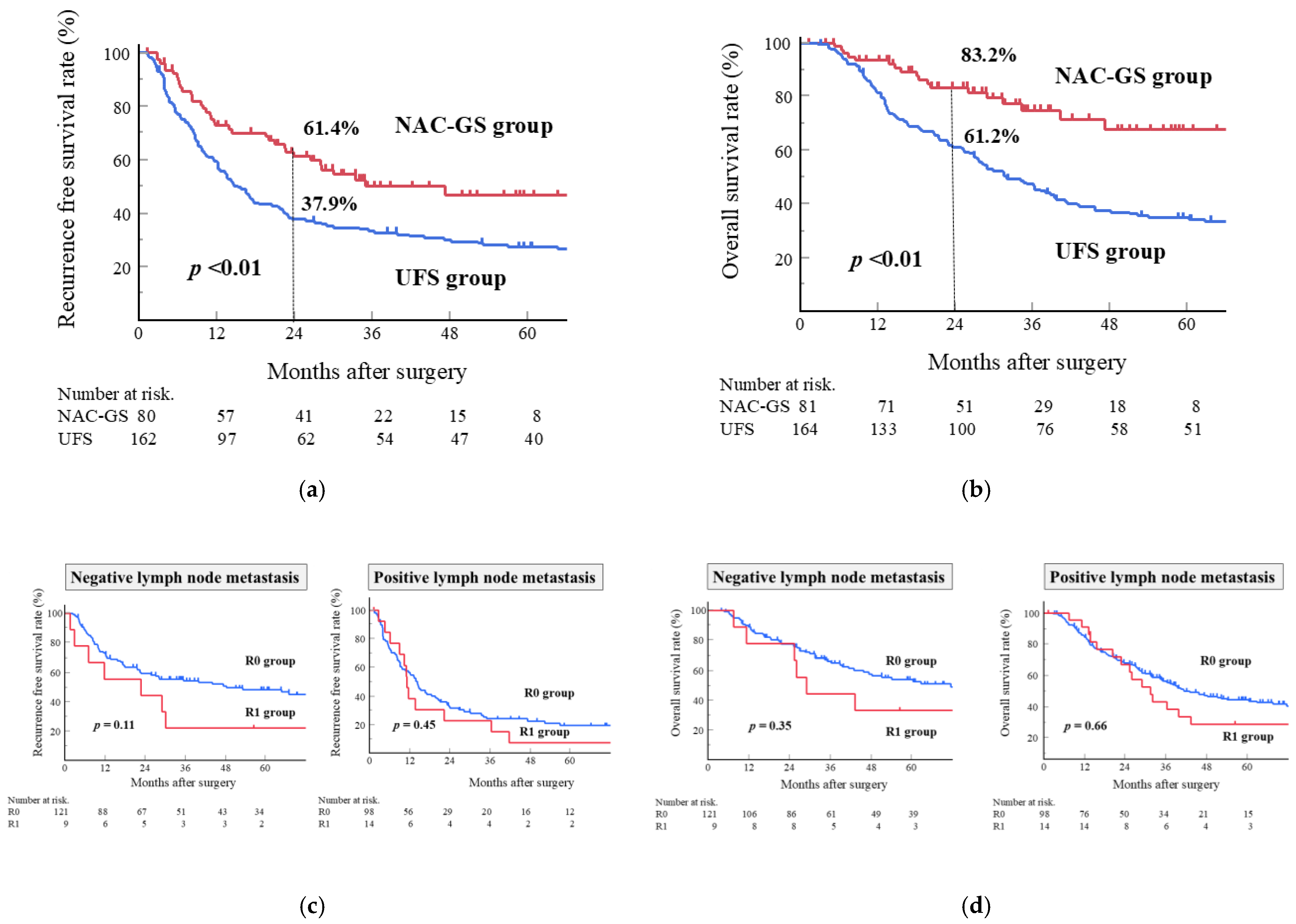
3.4. Long-Term Outcomes
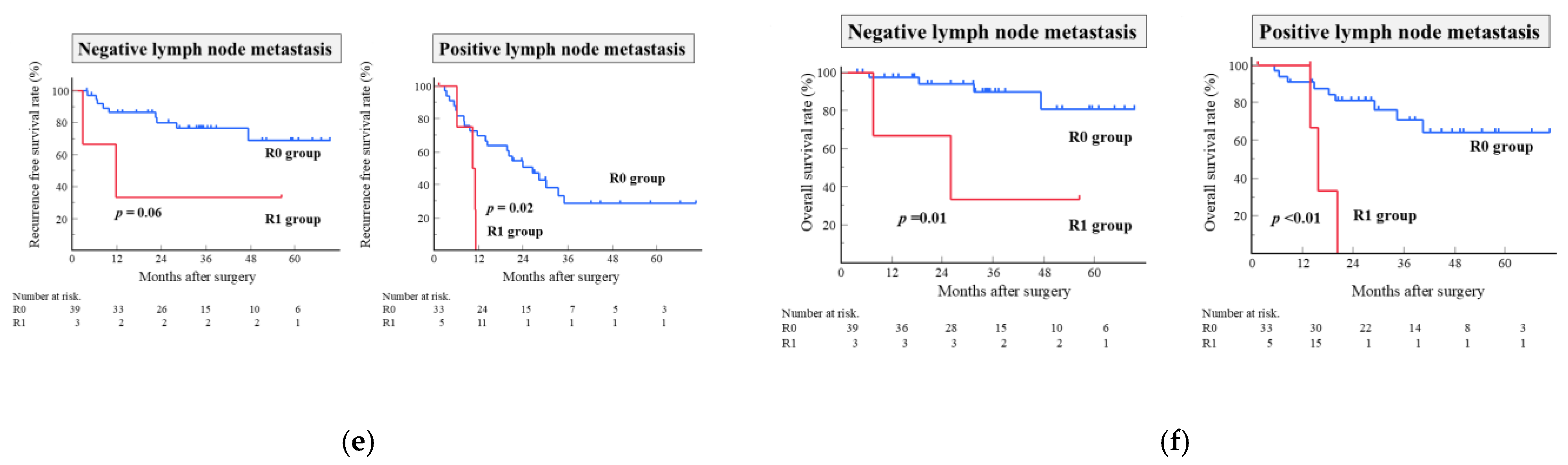
3.5. Risk Factors Associated with Survival
3.6. Risk Factors Associated with Early Recurrence Within Six Months
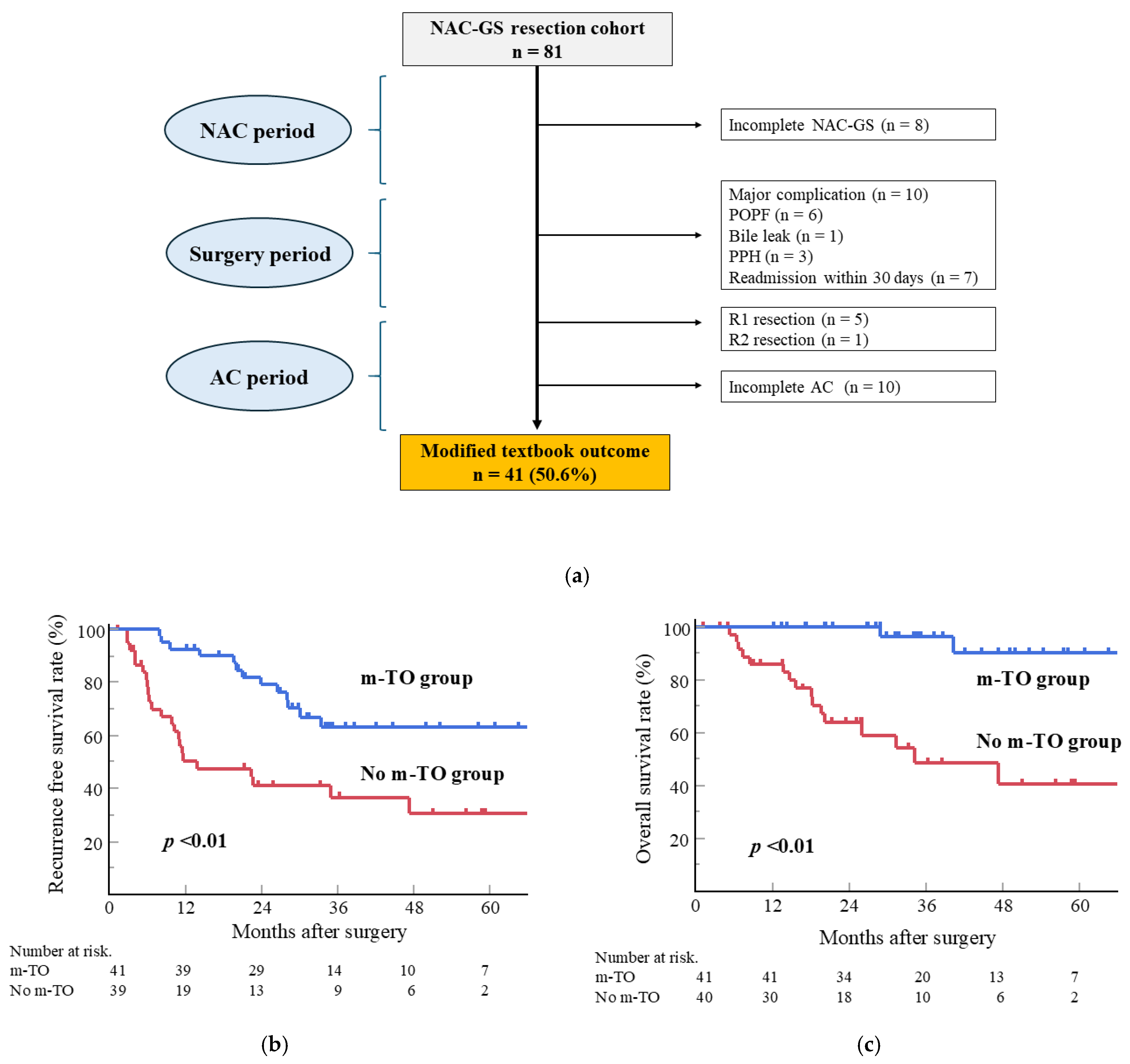
3.7. Impact of Modified TO in Patients with NAC-GS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, T.; Nara, S.; Ban, D.; Mizui, T.; Miyata, A.; Esaki, M. Neoadjuvant gemcitabine and S-1 in pancreatic ductal adenocarcinoma: Effects on nutritional status and pancreaticoduodenectomy outcomes. Surgery 2025, 180, 109026. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.H.; Muhammad, N.; Tarique, M.; Usmani, D.; Naz, H.; Sarode, A. The Role of Cancer-Specific Target Antigens in CAR T Cell Therapy in Hematological Malignancies. Curr. Tissue Microenviron. Rep. 2024, 5, 61–67. [Google Scholar] [CrossRef]
- Hirashita, T.; Tada, K.; Nagasawa, Y.; Orimoto, H.; Kawamura, M.; Fujinaga, A.; Takayama, H.; Kawano, Y.; Masuda, T.; Endo, Y.; et al. Benefits of neoadjuvant chemotherapy with gemcitabine plus S-1 for resectable pancreatic ductal adenocarcinoma. Mol. Clin. Oncol. 2025, 22, 18. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Pancreatic adenocarcinoma; Version 2.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Motoi, F.; Unno, M. Neoadjuvant treatment for resectable pancreatic adenocarcinoma: What is the best protocol? Ann. Gastroenterol. Surg. 2020, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Toyama, H.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; et al. Neoadjuvant Chemotherapy with Gemcitabine and S-1 versus Upfront Surgery for Resectable Pancreatic Cancer: Results of the Randomized Phase II/III Prep-02/JSAP05 Trial. Ann. Surg. 2025. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- van Roessel, S.; Mackay, T.M.; van Dieren, S.; van der Schelling, G.P.; Nieuwenhuijs, V.B.; Bosscha, K.; van der Harst, E.; van Dam, R.M.; Liem, M.S.L.; Festen, S.; et al. Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Ann. Surg. 2020, 271, 155–162. [Google Scholar] [CrossRef]
- Sweigert, P.J.; Ramia, J.M.; Villodre, C.; Carbonell-Morote, S.; De-la-Plaza, R.; Serradilla, M.; Pawlik, T.M. Textbook Outcomes in Liver Surgery: A Systematic Review. J. Gastrointest. Surg. 2023, 27, 1277–1289. [Google Scholar] [CrossRef]
- Dawood, Z.S.; Khalil, M.; Waqar, U.; Banani, I.; Alidina, Z.; Pawlik, T.M. Use of textbook outcome as a quality metric in hepatopancreaticobiliary surgery: A systematic review and meta-analysis. J. Gastrointest. Surg. 2025, 29, 102005. [Google Scholar] [CrossRef]
- Society, J.P. General Rules for the Study of Pancreatic Cancer, 7th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2016. [Google Scholar]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-Del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Duffey, P.L.; DeVita, V.T., Jr.; Wesley, M.N.; Hubbard, S.M.; Young, R.C. The calculation of actual or received dose intensity: A comparison of published methods. J. Clin. Oncol. 1991, 9, 2042–2051. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliográficas (Engl. Ed.) 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Evans, D.B.; Rich, T.A.; Byrd, D.R.; Cleary, K.R.; Connelly, J.H.; Levin, B.; Charnsangavej, C.; Fenoglio, C.J.; Ames, F.C. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. 1992, 127, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Umeda, Y.; Fuji, T.; Yasui, K.; Yamada, M.; Kimura, J.; Fujiwara, T. Role of robotic surgery as an element of Enhanced Recovery After Surgery protocol in patients undergoing pancreatoduodenectomy. J. Gastrointest. Surg. 2024, 28, 220–225. [Google Scholar] [CrossRef]
- Takagi, K.; Umeda, Y.; Yoshida, R.; Yagi, T.; Fujiwara, T. Robotic Radical Antegrade Modular Pancreatosplenectomy Using the Supracolic Anterior Superior Mesenteric Artery Approach. J. Gastrointest. Surg. 2021, 25, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Castillo, C.F.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic ductal adenocarcinoma: Is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef]
- Smaglo, B.G. Role for Neoadjuvant Systemic Therapy for Potentially Resectable Pancreatic Cancer. Cancers 2023, 15, 2377. [Google Scholar] [CrossRef]
- Kitano, Y.; Inoue, Y.; Takeda, T.; Oba, A.; Ono, Y.; Sato, T.; Ito, H.; Ozaka, M.; Sasaki, T.; Sasahira, N.; et al. Clinical Efficacy of Neoadjuvant Chemotherapy with Gemcitabine plus S-1 for Resectable Pancreatic Ductal Adenocarcinoma Compared with Upfront Surgery. Ann. Surg. Oncol. 2023, 30, 5093–5102. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Mori, S.; Shimizu, T.; Tago, K.; Harada, N.; Park, K.H.; Sakuraoka, Y.; Shiraki, T.; Iso, Y.; Aoki, T.; et al. Clinical Significance of Neoadjuvant Chemotherapy With Gemcitabine Plus S-1 for Resectable Pancreatic Ductal Adenocarcinoma. In Vivo 2019, 33, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Tempero, M.A.; Pelzer, U.; O’Reilly, E.M.; Winter, J.; Oh, D.Y.; Li, C.P.; Tortora, G.; Chang, H.M.; Lopez, C.D.; Bekaii-Saab, T.; et al. Adjuvant nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Fard, A.H.; Sadeghi, R.; Saffari, S.E.; Hashemi Fard, S.M.; Aliakbarian, M. A meta-analysis of prognostic factors in patients with left-sided pancreatic cancer. Indian J. Cancer 2022, 59, 310–316. [Google Scholar] [CrossRef]
- Leonhardt, C.S.; Hank, T.; Pils, D.; Gustorff, C.; Sahora, K.; Schindl, M.; Verbeke, C.S.; Strobel, O.; Klaiber, U. Prognostic impact of resection margin status on survival after neoadjuvant treatment for pancreatic cancer: Systematic review and meta-analysis. Int. J. Surg. 2024, 110, 453–463. [Google Scholar] [CrossRef]
- Takagi, K.; Umeda, Y.; Yoshida, R.; Fuji, T.; Yasui, K.; Yagi, T.; Fujiwara, T. Role of Surgery for Pancreatic Ductal Adenocarcinoma in the Era of Multidisciplinary Treatment. J. Clin. Med. 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Mantzavinou, A.; Uppara, M.; Chan, J.; Patel, B. Robotic versus open pancreaticoduodenectomy, comparing therapeutic indexes; a systematic review. Int. J. Surg. 2022, 101, 106633. [Google Scholar] [CrossRef] [PubMed]
- Fuji, T.; Takagi, K.; Umeda, Y.; Yasui, K.; Yamada, M.; Nagai, Y.; Fujiwara, T. Impact of Robotic Surgery on Postoperative Pancreatic Fistula for High-Risk Pancreaticojejunostomy after Pancreatoduodenectomy. Dig. Surg. 2025, 42, 49–58. [Google Scholar] [CrossRef] [PubMed]
| Clinical Outcomes | n = 81 |
|---|---|
| Initial dose reduction | |
| GEM, yes | 13 (16.0) |
| S-1, yes | 25 (30.9) |
| GEM RDI, % | 77.3 (64.4–99.2) |
| S-1 RDI, % | 85.0 (66.7–100) |
| NAC completion | 73 (90.1) |
| Any adverse events | 76 (93.8) |
| ≥Grade 3 | 54 (66.7) |
| Neutropenia | 60 (74.1) |
| ≥Grade 3 | 48 (59.3) |
| Stomatitis | 15 (18.5) |
| ≥Grade 3 | 3 (3.7) |
| Constipation | 30 (37.0) |
| ≥Grade 3 | 0 (0) |
| Diarrhea | 6 (7.4) |
| ≥Grade 3 | 1 (1.2) |
| Skin rash | 28 (34.6) |
| ≥Grade 3 | 6 (7.4) |
| Interstitial pneumonia | 2 (2.5) |
| ≥Grade 3 | 1 (1.2) |
| RECIST | |
| CR/PR/SD/PD | 0 (0)/9 (11.1)/70 (86.4)/2 (2.5) |
| Evans grading system | |
| Grade I/IIa/IIb/III/IV | 8 (10.5)/54 (71.1)/12 (15.8)/0 (0)/2 (2.6) |
| Variables | NAC-GS (n = 81) | UFS (n = 164) | p-Value |
|---|---|---|---|
| Preoperative characteristics | |||
| Age, year | 73 (66–77) | 71 (65–76) | 0.35 |
| Sex (male/female) | 38 (46.9)/43 (53.1) | 99 (60.4)/65 (39.6) | 0.06 |
| BMI, kg/m2 | 23 (21–25) | 22 (20–24) | 0.05 |
| Tumor location (head/body & tail) | 44 (54.3)/37 (45.7) | 94 (57.3)/70 (42.7) | 0.68 |
| CEA at initial diagnosis, ng/mL | 2.1 (1.4–3.2) * WNL | 3.0 (1.9–4.1) * WNL | 0.001 |
| CEA at operation, ng/mL | 2.8 (2.0–4.0) * WNL | 3.0 (1.9–4.1) * WNL | 0.69 |
| CA19-9 at initial diagnosis, U/mL | 44 (18–139) | 65 (22–157) | 0.33 |
| CA19-9 at operation, U/mL | 23 (11–53) | 67 (22–156) | <0.001 |
| Tumor size at initial diagnosis, mm | 20 (17–27) | 22 (16–28) | 0.48 |
| Tumor size at operation, mm | 18 (15–23) | 22 (16–28) | <0.001 |
| Operative factors | |||
| Procedure (PD/DP/TP) | 41 (51.3)/34 (42.5)/5 (6.3) | 93 (57.4)/65 (40.1)/4 (2.5) | 0.29 |
| Portal vein resection | 15 (18.5) | 39 (23.8) | 0.41 |
| Operative time, min | 390 (297–457) | 368 (277–453) | 0.45 |
| Blood loss, mL | 210 (55–400) | 360 (150–610) | <0.001 |
| Minimally invasive surgery | 27 (33.3) | 2 (1.2) | <0.001 |
| Postoperative factors | |||
| Mortality | 0 (0) | 2 (1.2) | 1 |
| Major complication (CD ≥ IIIa) | 10 (12.4) | 46 (28.1) | 0.006 |
| POPF (≥grade B) | 6 (7.4) | 38 (23.2) | 0.002 |
| DGE (≥grade B) | 4 (5.0) | 10 (6.1) | 1 |
| Pathological factors * | |||
| Tumor size, mm | 19 (12–23) | 23 (18–29) | <0.001 |
| Lymph node metastasis | 38 (47.5) | 74 (45.7) | 0.89 |
| R0/R1 | 72 (90.0)/8 (10.0) | 147 (90.7)/15 (9.3) | 0.82 |
| DPM positive | 4 (5.0) | 11 (6.8) | 0.78 |
| PCM positive | 2 (2.5) | 6 (3.7) | 1 |
| Adjuvant Chemotherapy | |||
| Induction of AC | 72 (88.9) | 121 (73.8) | 0.008 |
| Induction of AC from surgery, days | 43 (31–54) | 56 (38–74) | <0.001 |
| AC completion | 56 (69.1) | 84 (51.2) | 0.009 |
| Oncological outcomes * | |||
| Recurrence | 33 (41.3) | 105 (64.8) | <0.001 |
| Recurrence within 6 months | 6 (7.5) | 36 (22.2) | 0.004 |
| Recurrence within 12 months | 19 (23.8) | 62 (38.3) | 0.03 |
| Local recurrence | 11 (13.8) | 24 (14.8) | 1 |
| Soft tissue | 10 (12.5) | 16 (9.9) | 0.52 |
| Remnant pancreas | 1 (1.3) | 9 (5.6) | 0.17 |
| Systemic recurrence | 21 (26.3) | 92 (56.8) | <0.001 |
| Liver | 8 (10.0) | 41 (25.3) | 0.006 |
| Lung | 6 (7.5) | 22 (13.6) | 0.20 |
| Bone | 0 (0) | 3 (1.9) | 0.55 |
| Peritoneal metastases | 5 (6.3) | 23 (14.2) | 0.09 |
| Lymph node | 3 (3.8) | 20 (12.4) | 0.04 |
| Others | 0 (0) | 3 (1.9) | 0.55 |
| RFS | OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | Univariate | Multivariate | |||||||||
| n | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age, year | |||||||||||||
| ≥75 | 84 | 1.31 | 0.95–1.81 | 0.10 | 1.31 | 0.92–1.86 | 0.13 | ||||||
| <75 | 158 | Ref | Ref | ||||||||||
| Sex | |||||||||||||
| Male | 134 | 1.52 | 1.10–2.09 | 0.01 | 1.63 | 1.15–2.32 | 0.006 | ||||||
| Female | 108 | Ref | Ref | ||||||||||
| BMI, kg/m2 | |||||||||||||
| ≥25 | 48 | 1.16 | 0.78–1.72 | 0.45 | 0.95 | 0.61–1.48 | 0.82 | ||||||
| <25 | 194 | Ref | Ref | ||||||||||
| Tumor location | |||||||||||||
| Head | 138 | 1.10 | 0.81–1.51 | 0.54 | 1.16 | 0.83–1.63 | 0.38 | ||||||
| Body & tail | 104 | Ref | Ref | ||||||||||
| CEA, ng/mL | |||||||||||||
| ≥5 | 36 | 0.80 | 0.50–1.28 | 0.35 | 0.87 | 0.53–1.44 | 0.60 | ||||||
| <5 | 203 | Ref | Ref | ||||||||||
| CA19-9, U/mL | |||||||||||||
| ≥40 | 123 | 1.88 | 1.36–2.58 | <0.001 | 1.66 | 1.18–2.35 | 0.004 | ||||||
| <40 | 117 | Ref | Ref | ||||||||||
| Operation time, h | |||||||||||||
| ≥7h | 89 | 1.03 | 0.75–1.42 | 0.86 | 1.18 | 0.83–1.67 | 0.35 | ||||||
| <7h | 153 | Ref | Ref | ||||||||||
| Blood loss, mL | |||||||||||||
| ≥500 | 70 | 1.74 | 1.26–2.41 | <0.001 | 2.12 | 1.50–2.99 | <0.001 | ||||||
| <500 | 172 | Ref | Ref | ||||||||||
| Surgical procedure | |||||||||||||
| PD or TP | 143 | 1.06 | 0.78–1.46 | 0.70 | 1.19 | 0.84–1.68 | 0.33 | ||||||
| DP | 99 | Ref | Ref | ||||||||||
| Minimally invasive surgery | |||||||||||||
| Yes | 29 | 0.28 | 0.13–0.60 | 0.001 | 0.18 | 0.06–0.56 | 0.003 | ||||||
| No | 213 | Ref | Ref | ||||||||||
| Portal vein resection | |||||||||||||
| Yes | 54 | 1.20 | 0.83–1.73 | 0.32 | 1.26 | 0.86–1.86 | 0.24 | ||||||
| No | 188 | Ref | Ref | ||||||||||
| Major complication (CD ≥ IIIa) | |||||||||||||
| Yes | 56 | 1.89 | 1.34–2.67 | <0.001 | 1.38 | 0.98–1.96 | 0.07 | 2.07 | 1.44–2.97 | <0.001 | 1.53 | 1.06–2.22 | 0.02 |
| No | 186 | Ref | Ref | Ref | Ref | ||||||||
| Tumor size, mm | |||||||||||||
| >25 | 74 | 1.99 | 1.45–2.73 | <0.001 | 2.00 | 1.41–2.82 | <0.001 | ||||||
| ≤25 | 168 | Ref | Ref | ||||||||||
| Lymph node metastasis | |||||||||||||
| Yes | 112 | 2.03 | 1.48–2.77 | <0.001 | 1.94 | 1.42–2.66 | <0.001 | 1.79 | 1.28–2.52 | <0.001 | 1.69 | 1.20–2.38 | 0.003 |
| No | 130 | Ref | Ref | Ref | Ref | ||||||||
| R status | |||||||||||||
| R1 | 23 | 1.62 | 1.00–2.62 | 0.048 | 1.37 | 0.83–2.29 | 0.22 | ||||||
| R0 | 219 | Ref | Ref | ||||||||||
| Induction of NAC-GS | |||||||||||||
| Yes | 80 | 0.55 | 0.38–0.80 | 0.002 | 0.67 | 0.46–0.98 | 0.04 | 0.37 | 0.22–0.61 | <0.001 | 0.49 | 0.30–0.82 | 0.006 |
| No | 162 | Ref | Ref | Ref | Ref | ||||||||
| Induction of AC | |||||||||||||
| Yes | 193 | 0.42 | 0.30–0.61 | <0.001 | 0.38 | 0.24–0.63 | <0.001 | ||||||
| No | 49 | Ref | Ref | ||||||||||
| Completion of AC | |||||||||||||
| Yes | 140 | 0.28 | 0.21–0.39 | <0.001 | 0.32 | 0.23–0.44 | <0.001 | 0.22 | 0.16–0.32 | <0.001 | 0.27 | 0.19–0.38 | <0.001 |
| No | 102 | Ref | Ref | Ref | Ref | ||||||||
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| n | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Age, year | |||||||
| ≥75 | 84 | 1.05 | 0.53–2.11 | 0.88 | |||
| <75 | 158 | Ref | |||||
| Sex | |||||||
| Male | 134 | 1.77 | 0.88–3.57 | 0.11 | |||
| Female | 108 | Ref | |||||
| BMI, kg/m2 | |||||||
| ≥25 | 48 | 1.33 | 0.60–2.94 | 0.48 | |||
| <25 | 194 | Ref | |||||
| Tumor location | |||||||
| Head | 138 | 1.01 | 0.51–1.97 | 0.99 | |||
| Body & tail | 104 | Ref | |||||
| CEA, ng/ml | |||||||
| ≥5 | 36 | 1.47 | 0.62–3.51 | 0.38 | |||
| <5 | 203 | Ref | |||||
| CA19–9, U/mL | |||||||
| ≥40 | 123 | 3.11 | 1.48–6.55 | 0.003 | |||
| <40 | 117 | Ref | |||||
| Operation time, h | |||||||
| ≥7 h | 89 | 0.73 | 0.36–1.49 | 0.39 | |||
| <7 h | 153 | Ref | |||||
| Blood loss, mL | |||||||
| ≥500 | 70 | 1.89 | 0.94–3.77 | 0.07 | |||
| <500 | 172 | Ref | |||||
| Minimally invasive surgery | |||||||
| Yes | 29 | 0.32 | 0.07–1.40 | 0.13 | |||
| No | 213 | Ref | |||||
| Major complication (CD ≥ IIIa) | |||||||
| Yes | 56 | 2.46 | 1.21–5.02 | 0.01 | 1.75 | 0.82–3.72 | 0.14 |
| No | 186 | Ref | Ref | ||||
| Tumor size, mm | |||||||
| >25 | 74 | 3.13 | 1.58–6.20 | 0.001 | |||
| ≤25 | 168 | Ref | |||||
| Lymph node metastasis | |||||||
| Yes | 112 | 2.15 | 1.09–4.26 | 0.03 | 2.23 | 1.08–4.57 | 0.03 |
| No | 130 | Ref | Ref | ||||
| R status | |||||||
| R1 | 23 | 1.00 | 0.32–3.12 | 0.99 | |||
| R0 | 219 | Ref | |||||
| Induction of NAC-GS | |||||||
| Yes | 80 | 0.28 | 0.11–0.71 | 0.007 | 0.35 | 0.14–0.91 | 0.03 |
| No | 162 | Ref | Ref | ||||
| Induction of AC | |||||||
| Yes | 193 | 0.24 | 0.12–0.50 | <0.001 | 0.31 | 0.15–0.66 | 0.002 |
| No | 49 | Ref | Ref | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, K.; Takagi, K.; Fuji, T.; Nishiyama, T.; Nagai, Y.; Matsumoto, K.; Horiguchi, S.; Fujii, Y.; Otsuka, M.; Fujiwara, T. Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Cancers 2025, 17, 3287. https://doi.org/10.3390/cancers17203287
Yasui K, Takagi K, Fuji T, Nishiyama T, Nagai Y, Matsumoto K, Horiguchi S, Fujii Y, Otsuka M, Fujiwara T. Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Cancers. 2025; 17(20):3287. https://doi.org/10.3390/cancers17203287
Chicago/Turabian StyleYasui, Kazuya, Kosei Takagi, Tomokazu Fuji, Takeyoshi Nishiyama, Yasuo Nagai, Kazuyuki Matsumoto, Shigeru Horiguchi, Yuki Fujii, Motoyuki Otsuka, and Toshiyoshi Fujiwara. 2025. "Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma" Cancers 17, no. 20: 3287. https://doi.org/10.3390/cancers17203287
APA StyleYasui, K., Takagi, K., Fuji, T., Nishiyama, T., Nagai, Y., Matsumoto, K., Horiguchi, S., Fujii, Y., Otsuka, M., & Fujiwara, T. (2025). Impact of Neoadjuvant Chemotherapy with Gemcitabine Plus S-1 in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Cancers, 17(20), 3287. https://doi.org/10.3390/cancers17203287





