Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer: Oncological Potential and Limitations as the Primary Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Technique
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Perioperative Outcomes
3.3. Pathological Outcomes
3.4. Oncological Outcomes
3.5. Prognostic Factors
3.6. Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen Deprivation Therapy |
| BCR | Biochemical Recurrence |
| bGS | Biopsy Gleason Score |
| BRFS | Biochemical Recurrence-Free Survival |
| CA | California |
| CSS | Cancer-Specific Survival |
| EBRT | External Beam Radiotherapy |
| ePLND | Extended Pelvic Lymph Node Dissection |
| EZR | Easy R (Statistical Software) |
| GS | Gleason Score |
| IQR | Interquartile Range |
| LVI | Lymphovascular Invasion |
| MFS | Metastasis-Free Survival |
| NCCN | National Comprehensive Cancer Network |
| OS | Overall Survival |
| PCa | Prostate Cancer |
| pGS | Pathological Gleason Score |
| pN1 | Pathological Node-Positive |
| pT | Pathological T Stage |
| PSA | Prostate-Specific Antigen |
| PSMs | Positive Surgical Margins |
| RARP | Robot-Assisted Radical Prostatectomy |
| RP | Radical Prostatectomy |
| RT | Radiotherapy |
| SPCG-15 | Scandinavian Prostate Cancer Group Trial Number 15 |
| UC | Urinary Continence |
| USA | United States of America |
| VHR | Very High-Risk |
References
- Lowrance, W.T.; Elkin, E.B.; Yee, D.S.; Feifer, A.; Ehdaie, B.; Jacks, L.M.; Atoria, C.L.; Zelefsky, M.J.; Scher, H.I.; Scardino, P.T.; et al. Locally advanced prostate cancer: A population-based study of treatment patterns. BJU Int. 2012, 109, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology: Prostate Cancer; Version 2; National Comprehensive Cancer Network (NCCN): Plymouth Meeting, PA, USA, 2026. [Google Scholar]
- European Association of Urology (EAU) Guidelines, 2025th ed.; European Association of Urology (EAU): London, UK, 2025.
- Moris, L.; Cumberbatch, M.G.; Van den Broeck, T.; Gandaglia, G.; Fossati, N.; Kelly, B.; Pal, R.; Briers, E.; Cornford, P.; De Santis, M.; et al. Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review. Eur. Urol. 2020, 77, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ciezki, J.P.; Weller, M.; Reddy, C.A.; Kittel, J.; Singh, H.; Tendulkar, R.; Stephans, K.L.; Ulchaker, J.; Angermeier, K.; Stephenson, A.; et al. A Comparison Between Low-Dose-Rate Brachytherapy With or Without Androgen Deprivation, External Beam Radiation Therapy With or Without Androgen Deprivation, and Radical Prostatectomy With or Without Adjuvant or Salvage Radiation Therapy for High-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; De Lorenzis, E.; Novara, G.; Fossati, N.; De Groote, R.; Dovey, Z.; Suardi, N.; Montorsi, F.; Briganti, A.; Rocco, B.; et al. Robot-assisted radical prostatectomy and extended pelvic lymph node dissection in patients with locally-advanced prostate cancer. Eur. Urol. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Dell’Oglio, P.; Rosiello, G.; Puliatti, S.; Brook, N.; Turri, F.; Larcher, A.; Beato, S.; Andras, I.; Wisz, P.; et al. Technical Refinements in Superextended Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer Patients at Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2021, 80, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Willemse, P.-P.M.; Van den Broeck, T.; Van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Boorjian, S.A.; Buyyounouski, M.K.; Chapin, B.F.; Chen, D.Y.T.; Cheng, H.H.; Chou, R.; Jacene, H.A.; Kamran, S.C.; Kim, S.K.; et al. Salvage Therapy for Prostate Cancer: AUA/ASTRO/SUO Guideline Part I: Introduction and Treatment Decision-Making at the Time of Suspected Biochemical Recurrence after Radical Prostatectomy. J. Urol. 2024, 211, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Fossati, N.; Wiegel, T.; D’Amico, A.; Hofman, M.S.; Gillessen, S.; Mottet, N.; Joniau, S.; Spratt, D.E. Management of Persistently Elevated Prostate-specific Antigen After Radical Prostatectomy: A Systematic Review of the Literature. Eur. Urol. Oncol. 2021, 4, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow. Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sharma, R.; Moschovas, M.C.; Saikali, S.; Gamal, A.; Sandri, M.; Rogers, T.; Patel, V. Prognostic significance of perineural invasion and lymphovascular invasion following robot-assisted radical prostatectomy with negative surgical margins: A retrospective study from a high-volume center. World J. Urol. 2025, 43, 536. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or Early Salvage Radiotherapy for the Treatment of Localised and Locally Advanced Prostate Cancer: A Prospectively Planned Systematic Review and Meta-Analysis of Aggregate Data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Chierigo, F.; Wenzel, M.; Würnschimmel, C.; Flammia, R.S.; Horlemann, B.; Tian, Z.; Saad, F.; Chun, F.K.H.; Graefen, M.; Gallucci, M.; et al. Survival after radical Prostatectomy versus radiation therapy in High Risk and Very High-Risk Prostate Cancer. J. Urol. 2022, 207, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.L.; Patel, N.; Faiena, I.; Radadia, K.D.; Moore, D.F.; Elsamra, S.E.; Singer, E.A.; Stein, M.N.; Eastham, J.A.; Scardino, P.T.; et al. Comparative Effectiveness of Radical Prostatectomy with Adjuvant Radiotherapy versus Radiotherapy plus Androgen Deprivation Therapy for Men with Advanced Prostate Cancer. Cancer 2018, 124, 4010–4022. [Google Scholar] [CrossRef] [PubMed]
- SPCG. 15 Trial. Surgery Versus Radiotherapy for Locally Advanced Prostate Cancer. Identifier: NCT02102477. Available online: https://clinicaltrials.gov (accessed on 10 August 2025).
- Greenberger, B.A.; Zaorsky, N.G.; Den, R.B. Comparison of Radical Prostatectomy versus Radiation and Androgen Deprivation Therapy Strategies as Primary Treatment for High-Risk Localized Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2020, 6, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, H.P.; Arnow, K.D.; Trickey, A.W.; Leppert, J.T.; Wren, S.M.; Morris, A.M. Assessment of second primary cancer risk among men receiving primary radiotherapy vs surgery for the treatment of prostate cancer. JAMA Netw. Open 2022, 5, e2223025. [Google Scholar] [CrossRef] [PubMed]
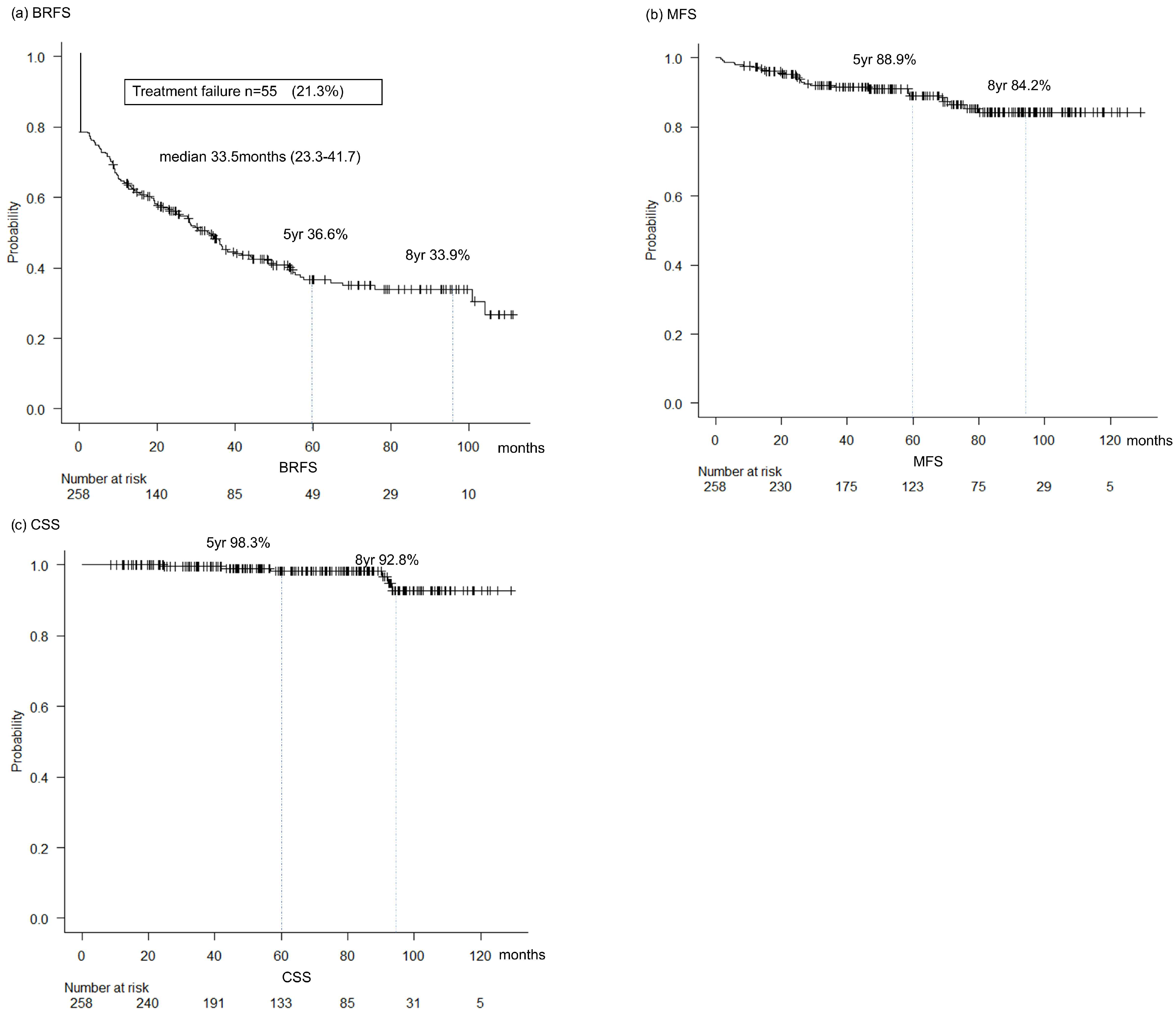
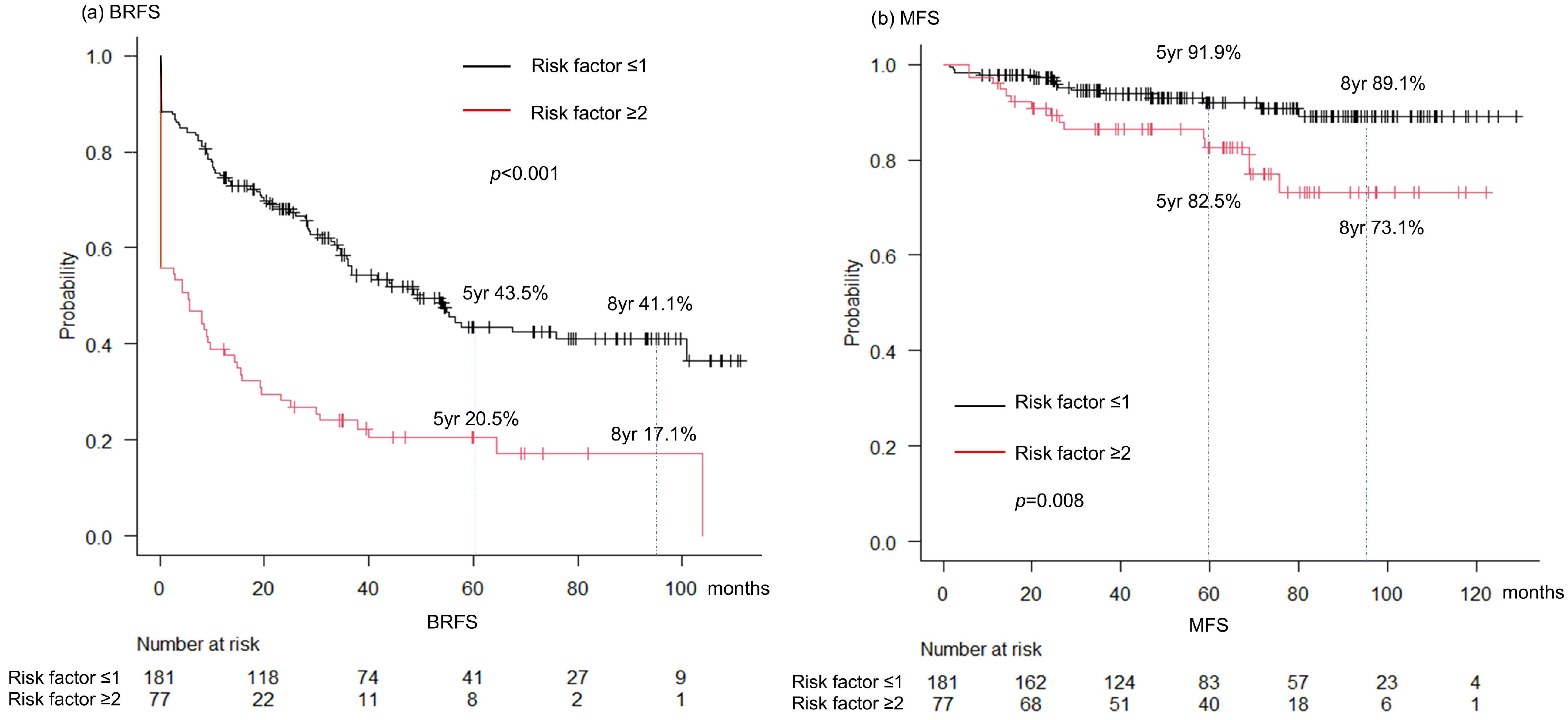
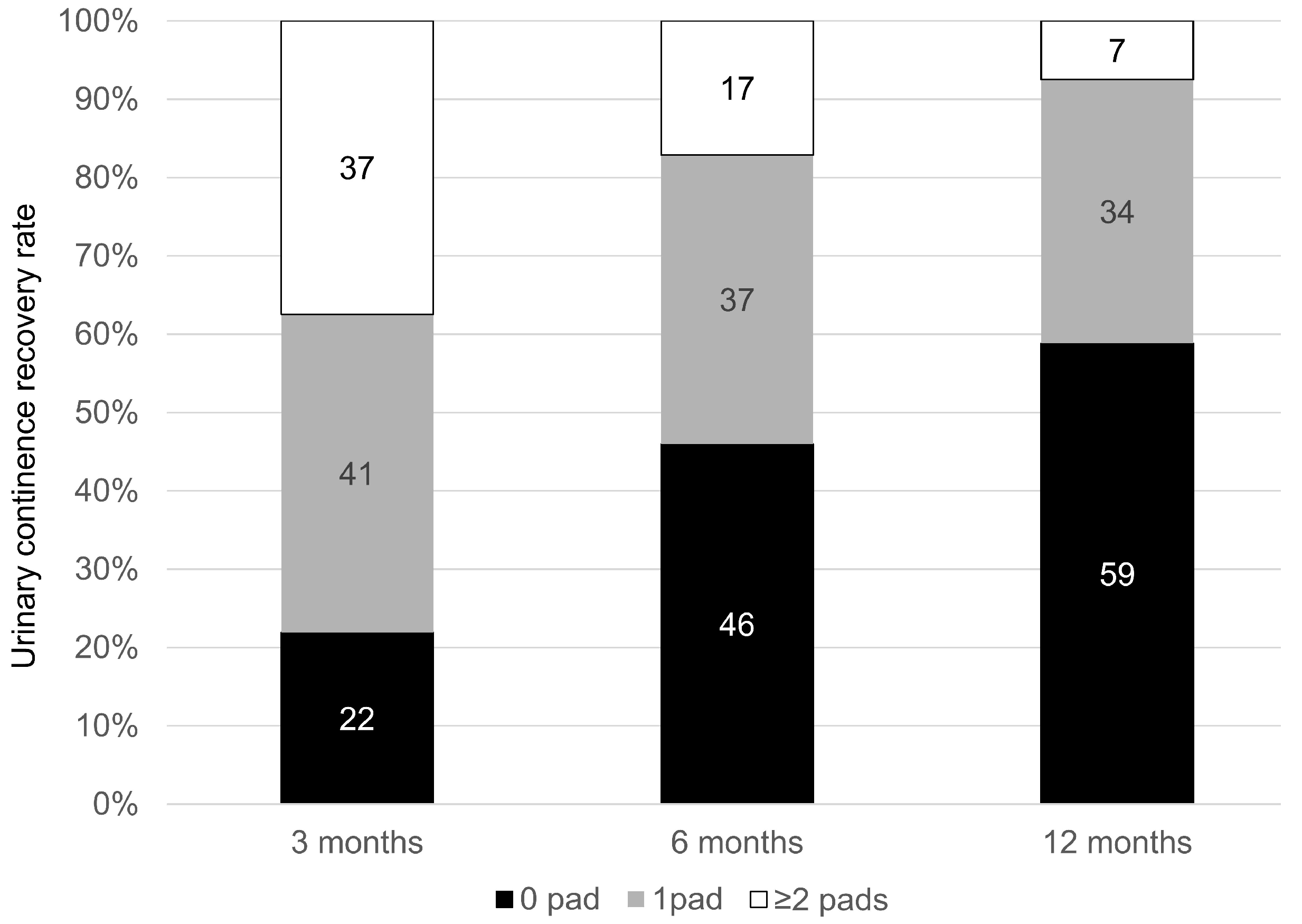
| Patients Characteristics | n = 258 |
|---|---|
| Age (yr)/median (IQR) | 69 (65–73) |
| PSA (ng/mL) median (IQR) | 14.6 (7.3–27.4) |
| Biopsy ISUP Gleason Grading (%) | |
| 1 | 2 (0.8) |
| 2 | 9 (3.5) |
| 3 | 7 (2.7) |
| 4 | 77 (29.8) |
| 5 | 163 (63.2) |
| Primary Gleason grade 5 (%) | 81 (31.4) |
| >4 biopsy cores with GS 8–10 (%) | 113 (46.1) |
| Clinical stage (%) | |
| ≤cT2 | 109 (42.1) |
| cT3a | 113 (43.6) |
| cT3b | 37 (14.3) |
| cT4 | 0 (0) |
| Follow up period (months)/median (IQR) | 60.6 (38.8–87.3) |
| Perioperative Outcomes | n = 258 |
|---|---|
| Operative time (min) median (IQR) | 288 (239–338) |
| Console time (min) median (IQR) | 237 (189–280) |
| Blood loss (mL) median (IQR) | 100 (10–200) |
| Postoperative complications (%) | 91 (35.1%) |
| Clavien-Dindo classification (%) | |
| I | 25 (9.7%) |
| II | 39 (15.1%) |
| IIIa | 9 (3.5%) |
| IIIb | 18 (6.9%) |
| IV | 0 (0%) |
| V | 0 (0%) |
| Postoperative complications | |
| IIIa Urethral stenosis | 1 |
| Ileus | 1 |
| Wound hernia | 4 |
| Infection | 2 |
| Vesicorectal fistula | 1 |
| IIIb Inguinal hernia | 13 |
| Urethral stenosis | 2 |
| Infection of lymphocele | 2 |
| Wound hernia | 1 |
| Pathological Outcomes | n = 258 |
|---|---|
| Pathological stage (%) | |
| ≤pT2 | 94 (36.4) |
| pT3a | 87 (33.7) |
| pT3b | 76 (29.5) |
| pT4 | 1 (0.4) |
| Pathological Gleason score (%) | |
| 1 | 0 (0) |
| 2 | 17 (6.6) |
| 3 | 29 (11.2) |
| 4 | 58 (22.5) |
| 5 | 154 (59.7) |
| Lymphovascular invasion (%) | 154 (65.5) |
| Positive surgical margins (%) | 97 (37.6) |
| pN1 | 70 (27.1) |
| Number of nodes removed /median (IQR) | 19 (13–26) |
| Number of positive nodes /median (IQR) | 1 (1–3) |
| Specimen confined disease (%) | 129 (50.0) |
| Baseline Characteristics | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| PSA at diagnosis | ||||||
| <10 | 1.00 | 1.00 | ||||
| 10–20 | 1.46 | 0.62–3.42 | 0.384 | 1.50 | 0.57–3.96 | 0.413 |
| 20–40 | 1.53 | 0.69–3.42 | 0.300 | 1.88 | 0.74–4.74 | 0.181 |
| 40< | 4.00 | 1.65–9.72 | 0.002 | 4.91 | 1.69–14.2 | 0.0034 |
| cT stage ≤ T2a | 1.00 | 1.00 | ||||
| T2b-c | 0.96 | 0.28–3.37 | 0.953 | 0.77 | 0.21–2.82 | 0.690 |
| T3a | 2.85 | 1.11–7.36 | 0.030 | 2.80 | 1.00–7.81 | 0.049 |
| T3b | 6.60 | 2.27–19.2 | <0.001 | 8.61 | 2.61–28.4 | <0.001 |
| GG ≥ 4 | 4.94 | 0.64–37.9 | 0.125 | 9.91 | 1.12–87.6 | 0.039 |
| >4 cores of GG 4 | 2.91 | 1.52–5.57 | <0.001 | 2.88 | 1.38–6.04 | 0.005 |
| Primary Gleason grade 5 | 0.69 | 0.35–1.36 | 0.29 | 0.99 | 0.45–2.21 | 0.987 |
| Baseline Characteristics | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| PSA at diagnosis | ||||||
| <10 | 1.00 | 1.00 | ||||
| 10–20 | 1.36 | 0.90–2.06 | 0.15 | 1.03 | 0.66–1.62 | 0.889 |
| 20–40 | 0.96 | 0.63–1.45 | 0.84 | 0.85 | 0.55–1.32 | 0.468 |
| 40< | 1.66 | 1.02–2.72 | 0.043 | 1.19 | 0.69–2.05 | 0.530 |
| pT stage T2 | 1.00 | 1.00 | ||||
| T3a | 2.03 | 1.34–3.08 | <0.001 | 1.64 | 1.04–2.61 | 0.035 |
| T3b | 3.29 | 2.18–4.99 | <0.001 | 2.12 | 1.29–3.46 | 0.003 |
| T4 | 9.65 | 1.30–71.4 | 0.026 | 4.94 | 0.63–39.0 | 0.130 |
| Prostatectomy GG ≥ 4 | 1.50 | 0.95–2.38 | 0.085 | 1.05 | 0.64–1.70 | 0.856 |
| LVI positive | 2.68 | 1.80–3.98 | <0.001 | 1.72 | 1.10–2.69 | 0.017 |
| Resection margin positive | 1.41 | 1.02–1.95 | 0.038 | 1.02 | 0.71–1.48 | 0.900 |
| pN1 (vs. pN0) | 2.92 | 2.09–4.08 | <0.001 | 1.79 | 1.20–2.67 | 0.005 |
| Baseline Characteristics | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| PSA at diagnosis | ||||||
| <10 | 1.00 | 1.00 | ||||
| 10–20 | 0.80 | 0.30–2.14 | 0.657 | 0.46 | 0.15–1.38 | 0.165 |
| 20–40 | 0.53 | 0.19–1.51 | 0.233 | 0.34 | 0.10–1.13 | 0.078 |
| 40< | 1.40 | 0.52–3.73 | 0.503 | 0.47 | 0.15–1.48 | 0.197 |
| pT stage T2 | 1.00 | 1.00 | ||||
| T3a | 3.13 | 1.00–9.83 | 0.051 | 2.35 | 0.61–8.97 | 0.212 |
| T3b | 4.63 | 1.52–14.1 | 0.007 | 2.83 | 0.73–11.0 | 0.133 |
| T4 | NA | 0-NA | 0.998 | NA | 0.00-NA | 0.998 |
| Prostatectomy GG ≥ 4 | 5.33 | 0.73–39.2 | 0.100 | 2.37 | 0.31–17.9 | 0.403 |
| LVI positive | 7.79 | 1.83–33.2 | 0.006 | 5.00 | 1.12–22.3 | 0.035 |
| Resection margin positive | 1.43 | 0.68–2.97 | 0.343 | 0.84 | 0.34–2.06 | 0.697 |
| pN1 (vs pN0) | 1.43 | 0.66–3.08 | 0.361 | 0.50 | 0.20–1.23 | 0.130 |
| Persistent PSA | 5.97 | 2.85–12.5 | <0.001 | 5.66 | 2.29–14.0 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, N.; Shimbo, M.; Shishido, K.; Nobumori, S.; Sugihara, N.; Sawada, T.; Haga, S.; Arai, H.; Nishida, K.; Arai, O.; et al. Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer: Oncological Potential and Limitations as the Primary Treatment. Cancers 2025, 17, 3286. https://doi.org/10.3390/cancers17203286
Miura N, Shimbo M, Shishido K, Nobumori S, Sugihara N, Sawada T, Haga S, Arai H, Nishida K, Arai O, et al. Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer: Oncological Potential and Limitations as the Primary Treatment. Cancers. 2025; 17(20):3286. https://doi.org/10.3390/cancers17203286
Chicago/Turabian StyleMiura, Noriyoshi, Masaki Shimbo, Kensuke Shishido, Shota Nobumori, Naoya Sugihara, Takatora Sawada, Shunsuke Haga, Haruna Arai, Keigo Nishida, Osuke Arai, and et al. 2025. "Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer: Oncological Potential and Limitations as the Primary Treatment" Cancers 17, no. 20: 3286. https://doi.org/10.3390/cancers17203286
APA StyleMiura, N., Shimbo, M., Shishido, K., Nobumori, S., Sugihara, N., Sawada, T., Haga, S., Arai, H., Nishida, K., Arai, O., Onishi, T., Watanabe, R., Nishimura, K., Fukumoto, T., Miyauchi, Y., Kikugawa, T., Nishino, T., Endo, F., Hattori, K., & Saika, T. (2025). Robot-Assisted Radical Prostatectomy for Locally Advanced Prostate Cancer: Oncological Potential and Limitations as the Primary Treatment. Cancers, 17(20), 3286. https://doi.org/10.3390/cancers17203286






