Cancer Therapy-Induced Cardiotoxicity: Results of the Analysis of the UK DEFINE Database
Simple Summary
Abstract
1. Introduction
2. Methods
- Chemotherapy: carboplatin, cisplatin, cyclophosphamide, docetaxel, doxorubicin, epirubicin, gemcitabine, methotrexate, paclitaxel, pemetrexed, vincristine, vinorelbine;
- Targeted therapy: afatinib, bevacizumab, erlotinib, gefitinib, nintedanib, osimertinib;
- Immunotherapy: atezolizumab, pembrolizumab.
| NSCLC Drugs | Number of Patients with C34 Diagnosis and Drug Use |
|---|---|
| Carboplatin | 20991 |
| Cisplatin | 8308 |
| Pemetrexed | 7839 |
| Etoposide | 7199 |
| Gemcitabine | 7015 |
| Vinorelbine | 6336 |
| Pembrolizumab | 3332 |
| Docetaxel | 2403 |
| Erlotinib | 2271 |
| Cyclophosphamide | 1657 |
| Methotrexate | 1359 |
| Doxorubicin | 1212 |
| Vincristine | 1120 |
| Paclitaxel | 1110 |
| Atezolizumab | 995 |
| Gefitinib | 752 |
| Nintedanib | 574 |
| Afatinib | 570 |
| Celecoxib | 488 |
| Epirubicin | 401 |
| Topotecan | 367 |
| Osimertinib | 345 |
| Irinotecan | 329 |
| Bevacizumab | 310 |
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? npj Precis. Onc. 2023, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Kantarjian, H.M.; Kesselheim, A.S.; Sigal, E.V. New drug approvals in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Armando, R.G.; Gómez, D.L.M.; Gomez, D.E. New drugs are not enough-drug repositioning in oncology: An update. Int. J. Oncol. 2020, 56, 651–684. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Lombardi, P.; Filetti, M.; Duranti, S.; Pietragalla, A.; Fabi, A.; Lorusso, D.; Altamura, V.; Sterbini, F.P.; Scambia, G.; et al. Oncologic Drugs Approval in Europe for Solid Tumors: Overview of the Last 6 Years. Cancers 2022, 14, 889. [Google Scholar] [CrossRef]
- IQVIA. Global Oncology Trends 2023; IQVIA: Durham, NC, USA, 2023. [Google Scholar]
- Tan, C.; Tasaka, H.; Yu, K.-P.; Murphy, M.L.; Karnofsky, D.A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967, 20, 333–353. [Google Scholar] [CrossRef]
- Menna, P.; Salvatorelli, E. Primary Prevention Strategies for Anthracycline Cardiotoxicity: A Brief Overview. Chemotherapy 2017, 62, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; He, Y.; Hu, X. Cardio-Oncology: Mechanisms, Drug Combinations, and Reverse Cardio-Oncology. Int. J. Mol. Sci. 2022, 23, 10617. [Google Scholar] [CrossRef] [PubMed]
- Gollerkeri, A.; Harrold, L.; Rose, M.; Jain, D.; Burtness, B.A. Use of paclitaxel in patients with pre-existing cardiomyopathy: A review of our experience. Int. J. Cancer 2001, 93, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Steggink, L.C.; Van Veldhuisen, D.J.; Gietema, J.A.; van der Meer, P. Cardio-Oncology: Progress in Diagnosis and Treatment of Cardiac Dysfunction. Clin. Pharmacol. Ther. 2017, 101, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/39-all-cancers-fact-sheet.pdf (accessed on 30 March 2024).
- Mudd, T.W.; Khalid, M.; Guddati, A.K. Cardiotoxicity of chemotherapy and targeted agents. Am. J. Cancer Res. 2021, 11, 1132–1147. [Google Scholar]
- Economopoulou, P.; Kotsakis, A.; Kapiris, I.; Kentepozidis, N. Cancer therapy and cardiovascular risk: Focus on bevacizumab. Cancer Manag. Res. 2015, 7, 133–143. [Google Scholar] [CrossRef]
- Cheng, H.; Force, T. Why do Kinase Inhibitors Cause Cardiotoxicity and What can be Done About It? Progress. Cardiovasc. Dis. 2010, 53, 114–120. [Google Scholar] [CrossRef]
- Force, T.; Krause, D.S.; Van Etten, R.A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 2007, 7, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Russo, L.; Madsen, A.; Webster, J.; Becnel, L. Use of Real-World Evidence to Drive Drug Development Strategy and Inform Clinical Trial Design. Clin. Pharmacol. Ther. 2022, 111, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Iqbal, S.; Valdes, I.L.; Dresser, M.; Girish, S. Integrating real-world data to accelerate and guide drug development: A clinical pharmacology perspective. Clin. Transl. Sci. 2022, 15, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.; Le Roux, N.; Kalesnik-Orszulak, R.; Christian, J.; Hukkelhoven, M.; Rockhold, F.; O’Donnell, J. Real-World Evidence for Regulatory Decision-Making: Guidance from Around the World. Clin. Ther. 2022, 44, 420–437. [Google Scholar] [CrossRef]
- Chan, S.H.Y.; Khatib, Y.; Webley, S.; Layton, D.; Salek, S. Identification of cardiotoxicity related to non-small cell lung cancer (NSCLC) treatments: A systematic review. Front. Pharmacol. 2023, 14, 1137983. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet Genom. 2012, 22, 310–318. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Qu, L.; Zhu, J. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Zhong, D. Comparing the adverse effects of platinum in combination with etoposide or irinotecan in previously untreated small-cell lung cancer patients with extensive disease: A network meta-analyses. Thorac. Cancer 2017, 8, 170–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nogami, N.; Hotta, K.; Segawa, Y.; Takigawa, N.; Hosokawa, S.; Oze, I.; Fujii, M.; Ichihara, E.; Shibayama, T.; Tada, A.; et al. Phase II study of irinotecan and amrubicin in patients with relapsed non-small cell lung cancer: Okayama Lung Cancer Study Group Trial 0402. Acta Oncol. 2012, 51, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A. Irinotecan in Non–Small-Cell Lung Cancer: Status of Ongoing Trials. Clin. Lung Cancer 2002, 4, S15–S22. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, Y.; Moriya, T.; Asaka-Amano, Y.; Kawashima, T.; Kurosu, K.; Tada, Y.; Nagao, K.; Kuriyama, T. Phase II study of weekly irinotecan and cisplatin for refractory or recurrent non-small cell lung cancer. Lung Cancer 2007, 58, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ren, X.; Chen, Y.; Li, Q.; Li, R.; Chen, Y.; Xia, S. Irinotecan-platinum combination therapy for previously untreated extensive-stage small cell lung cancer patients: A meta-analysis. BMC Cancer 2018, 18, 808. [Google Scholar] [CrossRef] [PubMed]
- BMA. COVID-19: Impact of the Pandemic on Healthcare Delivery; BMA: Edinburgh, UK, 2022. [Google Scholar]
- NHS England Digital. Cancer Registrations Statistics, England 2021-First Release, Counts Only. NHS England Digital. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-registration-statistics/england-2021---summary-counts-only (accessed on 20 April 2024).
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Weinstock, C.; Blumenthal, G.M.; Cheng, J.; He, K.; Zhuang, L.; Zhao, H.; Charlab, R.; Fan, I.; Keegan, P.; et al. Osimertinib for the Treatment of Metastatic EGFR T790M Mutation-Positive Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 2131–2135. [Google Scholar] [CrossRef]
- Fu, C.-M.; Li, L.-C.; Lee, Y.-T.; Wang, S.-W.; Hsu, C.-N. Apixaban vs. Warfarin in Atrial Fibrillation Patients with Chronic Kidney Disease. Front. Cardiovasc. Med. 2021, 8, 752468. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Keeling, D.; Baglin, T.; Tait, C.; Watson, H.; Perry, D.; Baglin, C.; Kitchen, S.; Makris, M. Guidelines on oral anticoagulation with warfarin–fourth edition. Br. J. Haematol. 2011, 154, 311–324. [Google Scholar] [CrossRef]
- SIGN. Antithrombotics: Indications and Management. A National Clinical Guideline. Scottish Intercollegiate Guidelines Network (SIGN). 2013. Available online: https://www.sign.ac.uk/media/1067/sign129.pdf (accessed on 8 March 2024).
- Prystowsky, E.N.; Padanilam, B.J.; Fogel, R.I. Treatment of Atrial Fibrillation. JAMA 2015, 314, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Blanchard, D.G. Diagnosis and Treatment of Atrial Fibrillation. Am. Fam. Physician 2016, 94, 442–452. [Google Scholar]
- Rehman, B.; Sanchez, D.P.; Shah, S. Atenolol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539844/ (accessed on 18 July 2023).
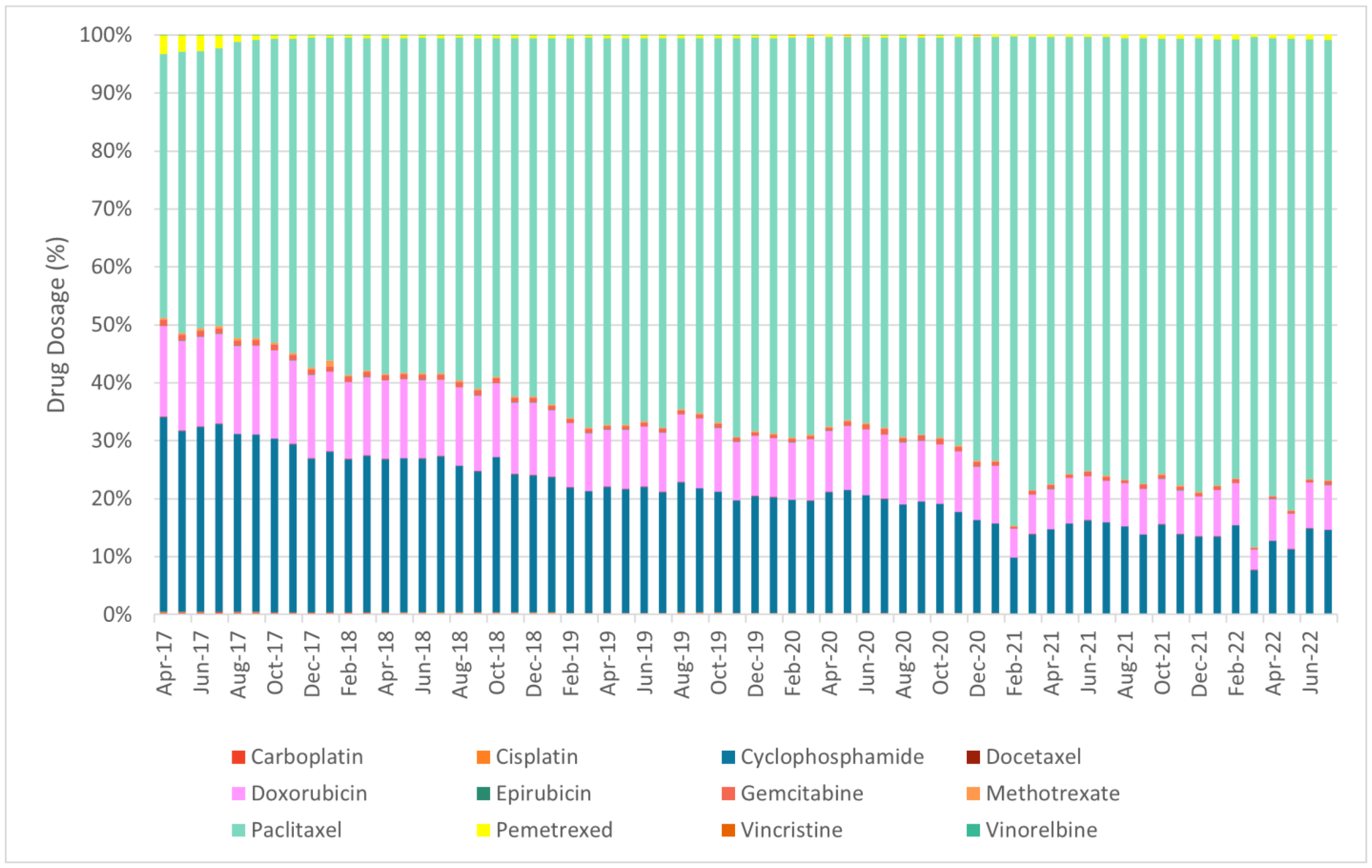
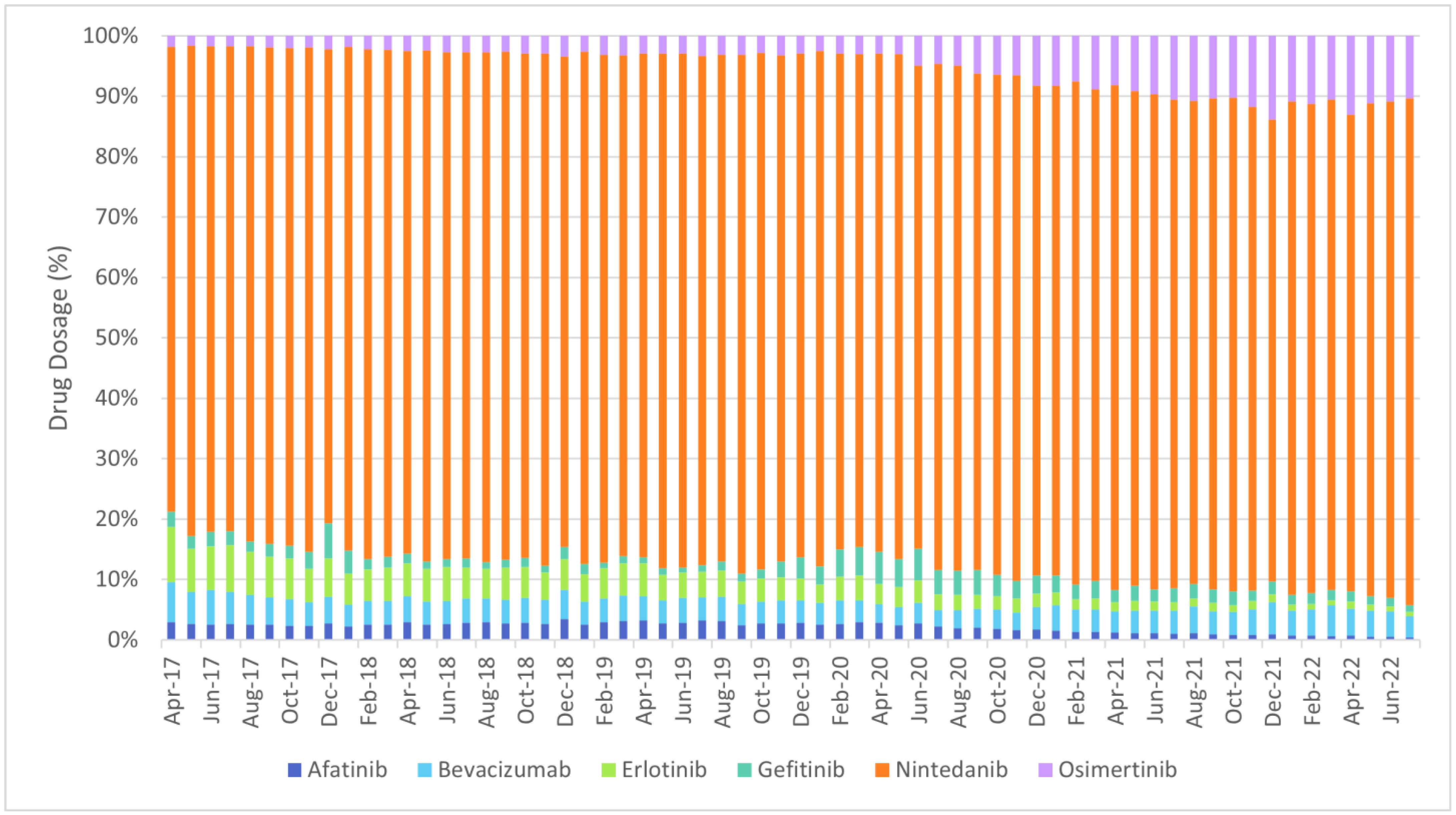
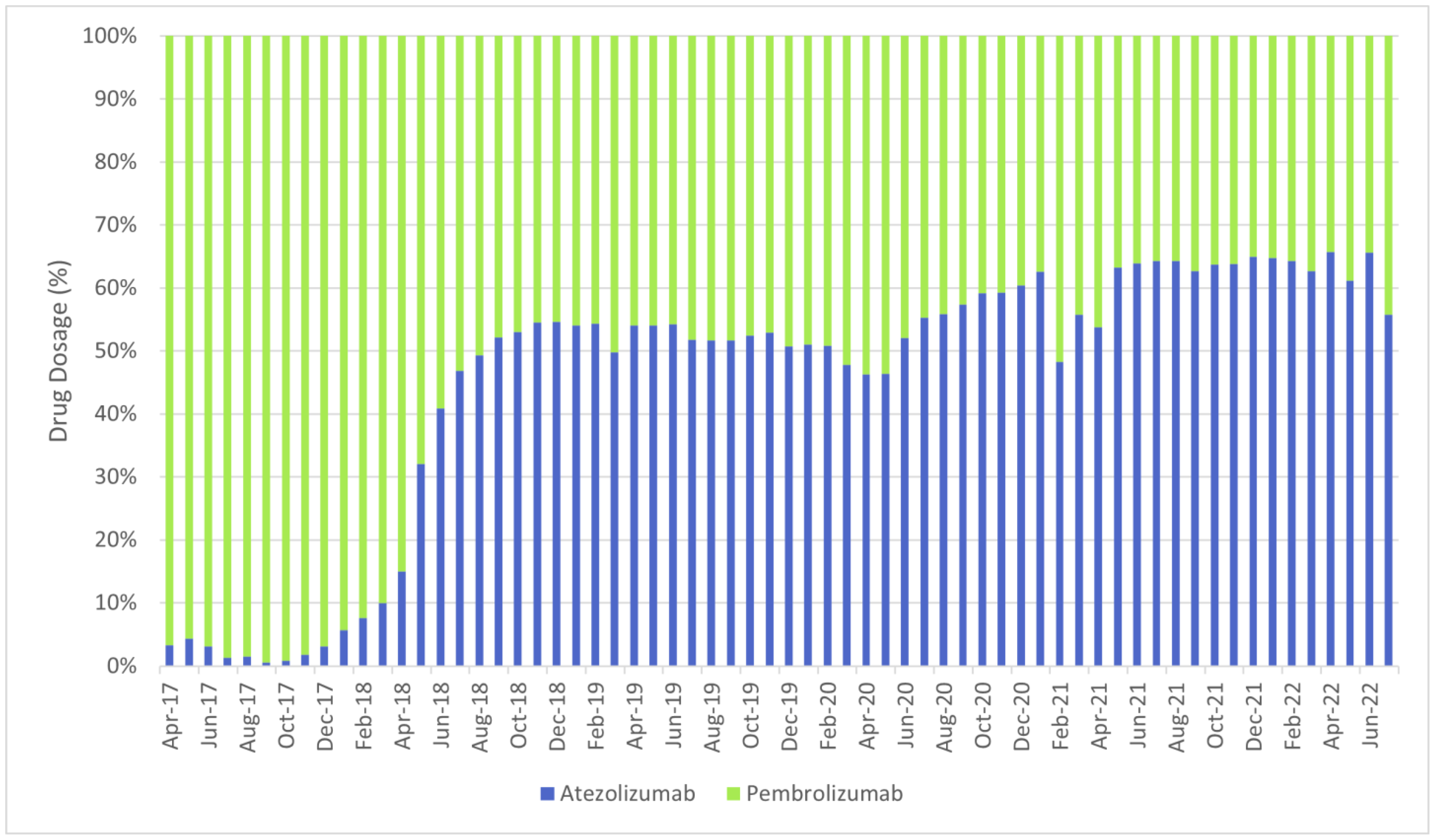
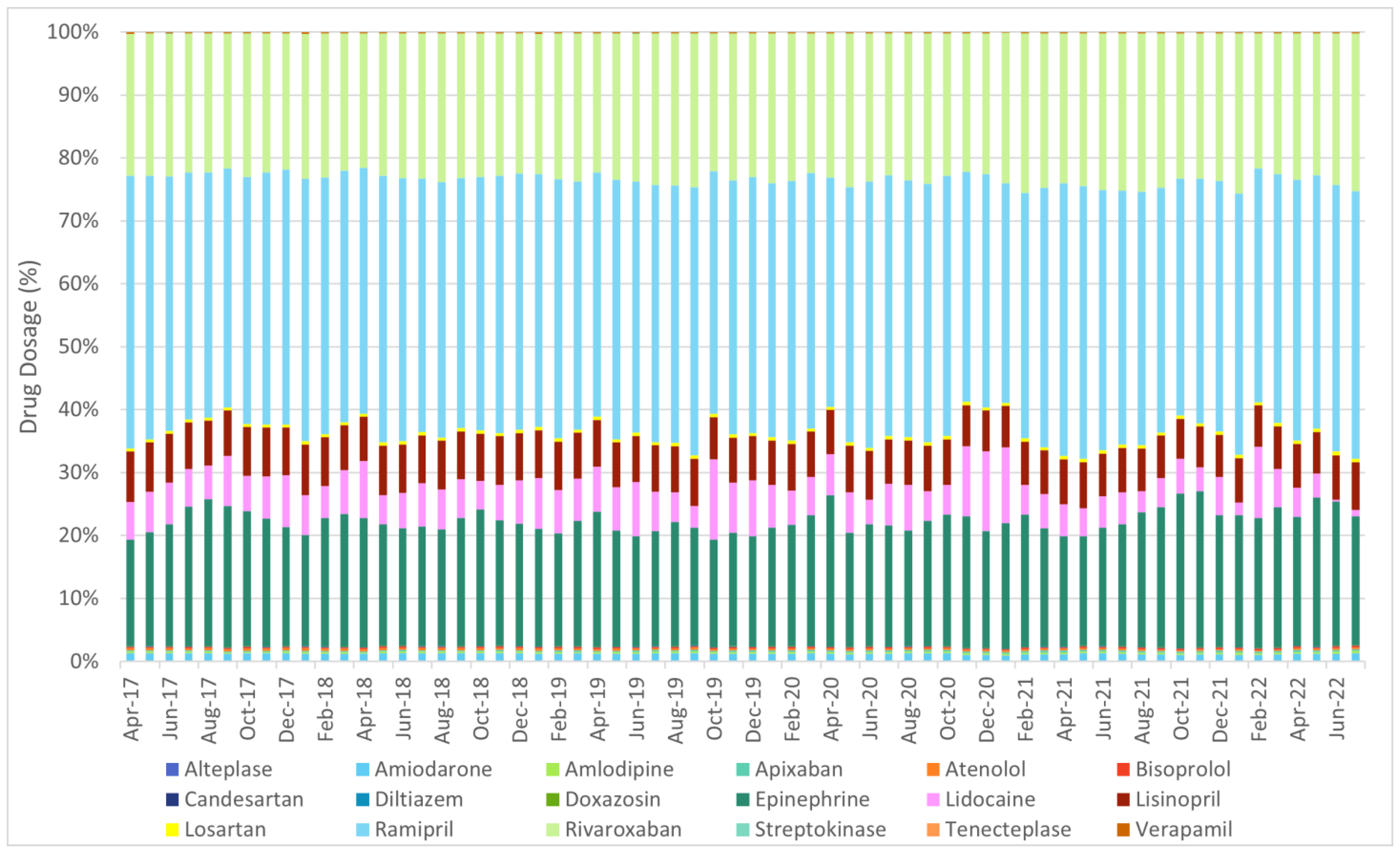
 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alteplase | Amiodarone | Amlodipine | Apixaban | Atenolol | Bisoprolol | Candesartan | Diltiazem | Doxazosin | Epinephrine | Lidocaine | Lisinopril | Losartan | Ramipril | Rivaroxaban | Streptokinase | Tenecteplase | Verapamil | |
| Afatinib | −0.061 | 0.206 | −0.372 ** | −0.565 *** | 0.354 ** | −0.195 | −0.357 ** | 0.279 * | −0.331 ** | −0.407 *** | 0.271 * | 0.147 | −0.322 ** | −0.146 | −0.341 ** | 0.233 | 0.308 * | 0.345 ** |
| Atezolizumab | 0.495 *** | −0.380 ** | 0.545 *** | 0.955 *** | −0.781 *** | 0.312 * | 0.500 *** | −0.435 *** | 0.594 *** | 0.242 | −0.185 | −0.430 *** | 0.339 ** | 0.062 | 0.425 *** | −0.247 * | −0.222 | −0.596 *** |
| Bevacizumab | 0.329 ** | 0.039 | 0.692 *** | 0.773 *** | −0.362 ** | 0.561 *** | 0.611 *** | −0.082 | 0.674 *** | 0.524 *** | −0.192 | 0.027 | 0.572 *** | 0.423 *** | 0.588 *** | −0.076 | −0.253 * | −0.217 |
| Carboplatin | 0.275 * | 0.366 ** | 0.775 *** | 0.549 *** | 0.004 | 0.725 *** | 0.713 *** | 0.311 * | 0.753 *** | 0.530 *** | 0.196 | 0.400 ** | 0.721 *** | 0.645 *** | 0.820 *** | 0.056 | −0.136 | 0.200 |
| Cisplatin | −0.295 * | 0.648 *** | 0.175 | −0.394 ** | 0.741 *** | 0.326 ** | 0.156 | 0.713 *** | 0.129 | 0.256 * | 0.237 | 0.747 *** | 0.321 ** | 0.509 *** | 0.299 * | 0.248 * | 0.050 | 0.657 *** |
| Cyclophosphamide | −0.034 | 0.623 *** | 0.483 *** | 0.026 | 0.416 *** | 0.580 *** | 0.450 *** | 0.533 *** | 0.481 *** | 0.320 ** | 0.107 | 0.600 *** | 0.571 *** | 0.631 *** | 0.488 *** | 0.224 | 0.071 | 0.483 *** |
| Docetaxel | −0.393 ** | 0.783 *** | 0.172 | −0.425 *** | 0.786 *** | 0.450 *** | 0.226 | 0.876 *** | 0.158 | 0.159 | 0.196 | 0.806 *** | 0.351 ** | 0.569 *** | 0.282 * | 0.451 *** | 0.130 | 0.883 *** |
| Doxorubicin | 0.359 ** | 0.330 ** | 0.583 *** | 0.334 ** | 0.091 | 0.460 *** | 0.436 *** | 0.084 | 0.490 *** | 0.380 ** | 0.189 | 0.305 * | 0.541 *** | 0.466 *** | 0.517 *** | −0.133 | 0.023 | 0.093 |
| Epirubicin | −0.346 ** | 0.769 *** | 0.211 | −0.411 *** | 0.785 *** | 0.418 *** | 0.209 | 0.767 *** | 0.136 | 0.327 ** | 0.153 | 0.809 *** | 0.354 ** | 0.616 *** | 0.369 ** | 0.383 ** | 0.152 | 0.836 *** |
| Erlotinib | −0.449 *** | 0.497 *** | −0.425 *** | −0.896 *** | 0.808 *** | −0.179 | −0.394 ** | 0.537 *** | −0.466 *** | −0.195 | 0.209 | 0.521 *** | −0.228 | 0.050 | −0.310 * | 0.297 * | 0.273 * | 0.674 *** |
| Gefitinib | 0.551 *** | −0.423 *** | −0.091 | 0.193 | −0.409 *** | −0.257 * | −0.233 | −0.627 *** | −0.098 | −0.162 | 0.003 | −0.447 *** | −0.249 * | −0.329 ** | −0.240 | −0.377 ** | 0.179 | −0.491 *** |
| Gemcitabine | 0.288 * | −0.149 | 0.493 *** | 0.609 *** | −0.387 ** | 0.296 * | 0.410 *** | −0.273 * | 0.477 *** | 0.247 * | −0.011 | −0.111 | 0.371 ** | 0.219 | 0.424 *** | −0.246 | −0.189 | −0.366 ** |
| Methotrexate | 0.039 | 0.175 | 0.085 | −0.123 | 0.332 ** | 0.132 | 0.077 | 0.132 | 0.031 | 0.013 | 0.041 | 0.321 ** | 0.138 | 0.205 | 0.145 | 0.003 | 0.061 | 0.281 * |
| Nintedanib | 0.434 *** | −0.268 * | 0.512 *** | 0.818 *** | −0.641 *** | 0.330** | 0.451 *** | −0.327 ** | 0.548 *** | 0.198 | −0.210 | −0.332 ** | 0.355 ** | 0.094 | 0.338 ** | −0.245 | −0.191 | −0.478 *** |
| Osimertinib | 0.407 *** | −0.298 * | 0.572 *** | 0.906 *** | −0.666 *** | 0.340 ** | 0.510 *** | −0.408 *** | 0.575 *** | 0.386 ** | −0.256 * | −0.321 ** | 0.398 ** | 0.145 | 0.447 *** | −0.235 | −0.248 * | −0.536 |
| Paclitaxel | 0.466 *** | −0.242 | 0.584 *** | 0.843 *** | −0.570 *** | 0.397 ** | 0.514 *** | −0.270 * | 0.596 *** | 0.346 ** | −0.125 | −0.233 | 0.390 ** | 0.195 | 0.455 *** | −0.187 | −0.229 | −0.471 *** |
| Pembrolizumab | 0.500 *** | −0.433 *** | 0.454 *** | 0.879 *** | −0.759 *** | 0.224 | 0.380 ** | −0.471 *** | 0.494 *** | 0.123 | −0.206 | −0.485 *** | 0.217 | −0.026 | 0.320 * | −0.290 * | −0.194 | −0.635 *** |
| Pemetrexed | −0.141 | 0.181 | 0.128 | 0.011 | 0.082 | 0.133 | 0.091 | 0.061 | 0.069 | 0.224 | −0.153 | 0.163 | 0.127 | 0.233 | 0.131 | −0.007 | −0.129 | 0.106 |
| Vincristine | 0.499 *** | −0.468 *** | 0.332 ** | 0.744 *** | −0.708 *** | 0.106 | 0.193 | −0.541 *** | 0.342 ** | 0.151 | −0.159 | −0.488 *** | 0.090 | −0.091 | 0.205 | −0.372 ** | −0.195 | −0.607 *** |
| Vinorelbine | −0.452 *** | 0.760 *** | 0.189 | −0.409 *** | 0.779 *** | 0.401 ** | 0.241 | 0.825 *** | 0.156 | 0.177 | 0.175 | 0.780 *** | 0.383 ** | 0.545 *** | 0.310 * | 0.358 ** | 0.075 | 0.786 *** |
| Oncology Drug | Cardiology Drug That Was Most Associated with Each Oncology Drug | Cardiovascular Disease(s) That the Corresponding Cardiology Drug Was Assumed to Treat |
|---|---|---|
| Afatinib | Atenolol | Ischaemia/hypertension |
| Atezolizumab | Apixaban | Atrial fibrillation |
| Bevacizumab | Apixaban | Atrial fibrillation |
| Carboplatin | Rivaroxaban | Arterial/venous thromboembolic event |
| Cisplatin | Lisinopril | Hypertension/cardiac failure/arrest |
| Cyclophosphamide | Ramipril | Hypertension/cardiac failure/arrest |
| Docetaxel | Verapamil | Arrhythmia/hypertension |
| Doxorubicin | Amlodipine | Hypertension |
| Epirubicin | Verapamil | Arrhythmia/hypertension |
| Erlotinib | Atenolol | Ischaemia/hypertension |
| Gefitinib | Alteplase | Myocardial infarction |
| Gemcitabine | Apixaban | Atrial fibrillation |
| Methotrexate | Atenolol | Ischaemia/hypertension |
| Nintedanib | Apixaban | Atrial fibrillation |
| Osimertinib | Apixaban | Atrial fibrillation |
| Paclitaxel | Apixaban | Atrial fibrillation |
| Pembrolizumab | Apixaban | Atrial fibrillation |
| Pemetrexed | Ramipril | Hypertension/cardiac failure/arrest |
| Vincristine | Apixaban | Atrial fibrillation |
| Vinorelbine | Diltiazem | Hypertension |
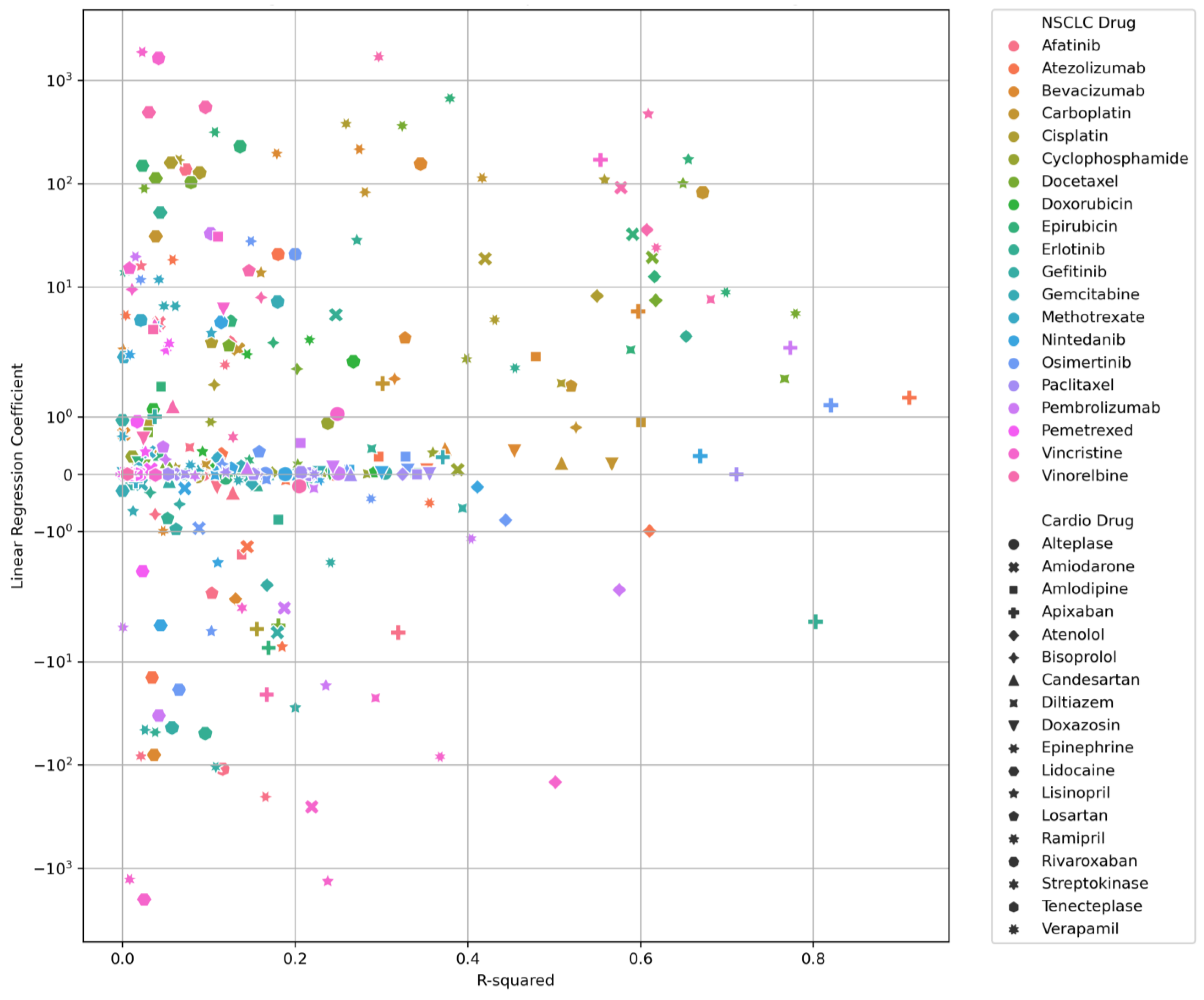
| Drug–Drug Pair | Linear Regression Analysis | |||
|---|---|---|---|---|
| NSCLC Drug | Cardiotoxicity Drug | Coefficient | Intercept | R2 Value |
| Atezolizumab | Apixaban | 1.337 | 4949.882 | 0.911 |
| Osimertinib | Apixaban | 1.204 | 5265.088 | 0.820 |
| Erlotinib | Apixaban | −4.095 | 9610.698 | 0.802 |
| Docetaxel | Verapamil | 5.540 | 1848.673 | 0.779 |
| Pembrolizumab | Apixaban | 2.595 | 3926.500 | 0.773 |
| Docetaxel | Diltiazem | 1.661 | −215.759 | 0.767 |
| Paclitaxel | Apixaban | 0.002 | 4595.091 | 0.711 |
| Epirubicin | Verapamil | 8.916 | 1077.783 | 0.699 |
| Vinorelbine | Diltiazem | 7.615 | −248.661 | 0.681 |
| Carboplatin | Rivaroxaban | 82.480 | 221,044.146 | 0.672 |
| Nintedanib | Apixaban | 0.316 | 1242.011 | 0.669 |
| Epirubicin | Lisinopril | 171.813 | 100,349.747 | 0.655 |
| Erlotinib | Atenolol | 3.340 | 5815.185 | 0.653 |
| Docetaxel | Lisinopril | 100.603 | 120,115.002 | 0.649 |
| Vinorelbine | Verapamil | 23.997 | 1988.937 | 0.618 |
| Docetaxel | Atenolol | 7.436 | 2241.347 | 0.618 |
| Epirubicin | Atenolol | 12.625 | 823.243 | 0.616 |
| Docetaxel | Amiodarone | 19.449 | 19,261.962 | 0.614 |
| Atezolizumab | Atenolol | −0.990 | 9483.021 | 0.610 |
| Vinorelbine | Lisinopril | 473.929 | 115,861.880 | 0.609 |
| Vinorelbine | Atenolol | 35.861 | 1778.631 | 0.607 |
| Carboplatin | Amlodipine | 0.908 | 2562.594 | 0.600 |
| Bevacizumab | Apixaban | 5.869 | 1829.886 | 0.597 |
| Epirubicin | Amiodarone | 32.446 | 15,888.632 | 0.591 |
| Epirubicin | Diltiazem | 2.474 | −330.944 | 0.589 |
| Vinorelbine | Amiodarone | 91.768 | 18,413.891 | 0.577 |
| Pembrolizumab | Atenolol | −2.024 | 10,352.082 | 0.575 |
| Carboplatin | Doxazosin | 0.168 | 483.829 | 0.567 |
| Cisplatin | Lisinopril | 109.250 | 140,300.077 | 0.558 |
| Vincristine | Apixaban | 170.599 | 4044.368 | 0.553 |
| Cisplatin | Atenolol | 8.214 | 3656.904 | 0.549 |
| Carboplatin | Bisoprolol | 0.814 | 2592.982 | 0.525 |
| Carboplatin | Losartan | 1.534 | 6331.187 | 0.519 |
| Carboplatin | Candesartan | 0.199 | 797.088 | 0.508 |
| Cisplatin | Diltiazem | 1.583 | 238.578 | 0.508 |
| Vincristine | Atenolol | −146.874 | 10,476.737 | 0.501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.H.Y.; Fitzpatrick, R.W.; Layton, D.; Webley, S.; Salek, S. Cancer Therapy-Induced Cardiotoxicity: Results of the Analysis of the UK DEFINE Database. Cancers 2025, 17, 311. https://doi.org/10.3390/cancers17020311
Chan SHY, Fitzpatrick RW, Layton D, Webley S, Salek S. Cancer Therapy-Induced Cardiotoxicity: Results of the Analysis of the UK DEFINE Database. Cancers. 2025; 17(2):311. https://doi.org/10.3390/cancers17020311
Chicago/Turabian StyleChan, Stefanie Ho Yi, Raymond W. Fitzpatrick, Deborah Layton, Sherael Webley, and Sam Salek. 2025. "Cancer Therapy-Induced Cardiotoxicity: Results of the Analysis of the UK DEFINE Database" Cancers 17, no. 2: 311. https://doi.org/10.3390/cancers17020311
APA StyleChan, S. H. Y., Fitzpatrick, R. W., Layton, D., Webley, S., & Salek, S. (2025). Cancer Therapy-Induced Cardiotoxicity: Results of the Analysis of the UK DEFINE Database. Cancers, 17(2), 311. https://doi.org/10.3390/cancers17020311







