Simple Summary
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) is a viable treatment for patients with peritoneal surface malignancies (PSM) who are not eligible for cytoreductive surgery (CRS). The literature suggests that a minimum of three PIPAC procedures (empirical standard treatment) should be administered to optimize oncological outcomes. However, many patients are not able to complete three treatments for various reasons. The aim of this retrospective study was to identify causes and predictive factors for incomplete PIPAC treatment and, hence, to improve patient selection.
Abstract
Background: Since 2011, Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) has emerged as a promising treatment option for patients with peritoneal surface malignancies (PSM) who are not eligible for cytoreductive surgery (CRS). Repeated minimal-invasive treatment is one of the key features and the current empirical standard treatment (ST) consists of at least three administrations over about three months. However, many patients are unable to complete the full course, limiting the potential benefits of PIPAC. Method: This retrospective, single-center study assessed the completion rate of ST and identified the main causes and predictive factors for discontinuation. This study also evaluated the feasibility, safety, and efficacy of PIPAC and investigated whether improved patient selection over the years has resulted in better oncological outcomes. Result: Data from 168 patients treated with PIPAC between January 2017 and March 2023 for a total of 336 procedures showed that only 29% completed ST. Multivariate analysis identified ascites >500 mL and a prior history of bowel obstruction as significant predictors of discontinuation. Conclusions: Patients with radiological or clinical signs of obstruction should not be considered for PIPAC treatment, and ascites increases the risk of incomplete treatment. Larger studies are eagerly awaited to corroborate these findings and refine the selection criteria by disease entity.
1. Introduction
Peritoneal surface malignancies (PSMs) are a heterogeneous group of tumors that differ in incidence, sensitivity to systemic therapies, and prognosis. Compared to other metastatic sites, systemic chemotherapy is less effective for peritoneal metastases (PMs). This might have to do with different molecular subtypes and tumor biology. Furthermore, reduced blood supply to the peritoneum, high interstitial pressure, and the peritoneum–plasma barrier are important pharmacokinetic limitations preventing systemic treatments from reaching and targeting tumor cells. Therefore, in recent decades, new techniques for delivering chemotherapy have been developed to increase local control of peritoneal disease, with promising results [1,2].
Nowadays, cytoreductive surgery (CRS), mostly in combination with consecutive hyperthermic intraperitoneal chemotherapy (HIPEC) [3,4,5], appears to be the only therapeutic option with curative potential in selected patients with a low peritoneal disease burden [6,7].
Since 2011, Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) has been used as a treatment option for patients with PSM not eligible for CRS and HIPEC. This new technique increases drug concentrations in targeted peritoneal tissues while maintaining low plasma concentrations, thus limiting systemic toxicity [8,9,10]. Moreover, the concurrent performance of a diagnostic laparoscopy with peritoneal biopsies allows for close monitoring of the treatment response. The literature has demonstrated that encouraging oncological outcomes can be achieved with repeated PIPAC treatment [11,12,13]. However, many patients do not complete the standard treatment, thus probably limiting the benefits. The main aim of this study is to identify the rate of patients who received at least three PIPACs and to identify the most frequent causes and risk factors for PIPAC discontinuation. The secondary aim was to assess the feasibility, safety, and efficacy of PIPAC in terms of survival and regression of symptoms. Finally, we evaluated whether the progressive improvement in and increased attention to patient selection for PIPAC over the years has led to improved outcomes.
2. Materials and Methods
This is a retrospective single-center study based on a prospectively maintained database, which included patients undergoing PIPAC from January 2017 to March 2023 at the Department of Peritoneal and Retroperitoneal Surgery, Fondazione Policlinico Universitario Agostino Gemelli, IRCCS. Indication for PIPAC treatment was provided by a multidisciplinary team.
The inclusion criteria were age > 18 years, presence of primary peritoneal tumors (mesothelioma), or peritoneal carcinomatosis from a different primary tumor (colorectal, stomach, ovarian, pancreatic, hepatobiliary, appendix, breast, small intestine, and lung) not susceptible to CRS. The exclusion criteria were patients’ refusal, presence of retroperitoneal disease and/or other significant distant metastases at the time of the first PIPAC, presence of other concurrent cancer, a life expectancy of less than 3 months, inability to orally feed or the need for parenteral nutrition, and an Eastern Cooperative Oncology Group (ECOG) Performance Status > 2.
2.1. Outcomes Measures
Standard treatment (ST) was defined as patients receiving ≥ 3 PIPACs and was compared to patients who underwent only 1 or 2 PIPACs. The factors analyzed as predictors of PIPAC discontinuation included patient characteristics (age, gender, body mass index (BMI), ECOG [14], tumor characteristics (site of the primary tumor, synchronous/metachronous carcinomatosis, and Peritoneal Cancer Index (PCI) according to the Sugarbaker score [15]), symptoms before first PIPAC and after third PIPAC (ascites volume (ascites volume was measured at PIPAC1, based on the intraoperative aspirates), pain, nausea, dysphagia, and intestinal obstruction symptoms), and systemic chemotherapy treatment (number of chemotherapy lines, number of chemotherapy cycles, and bimodal treatment (bimodal treatment was defined as the patient being under systemic chemotherapy in the interval between PIPACs)). Reasons for PIPAC treatment discontinuation were also evaluated: disease progression (DP), patient’s wish, other medical reasons, conversion to curative CRS, impossible access to the abdominal cavity (non-access), death, negative histology, and PIPAC complications.
The secondary outcome was the evaluation of the efficacy of PIPAC treatment. Median overall survival (mOS) from PIPAC1 was compared between patients undergoing ST and those undergoing only 1 or 2 PIPAC procedures. Furthermore, the symptoms reported at PIPAC1 were compared with those after the completion of ST to assess the impact of PIPAC on symptoms.
To estimate the feasibility and safety of PIPAC, we evaluated the incidence of complications according to the CTCAEv5 classification [16] and 30-day and 90-day mortality rates among all PIPAC procedures.
Finally, we evaluated the evolution of oncological outcomes throughout our experience. To achieve this, we analyzed the annual differences in the percentage of patients who completed the standard treatment (ST) and compared the median overall survival of patients treated with PIPAC between January 2017 and December 2020 with those treated between January 2021 and March 2023.
2.2. Surgical Technique
The procedure was performed according to current recommendations and safety protocols [17,18,19].
Under general anesthesia, a 10–12 mm laparoscopic balloon trocar was placed in accordance with the open technique, and a capnoperitoneum of 12 mmHg at 37 °C was applied. Then, one or two 5 mm laparoscopic balloon trocars were placed. During the procedure, ascites was documented and eventually removed (with a sample sent for cytological examination), and a thorough laparoscopic exploration was performed to calculate the PCI and assess the extension of peritoneal disease. Finally, multiple biopsies were performed in different abdominal quadrants. The PIPAC treatment involved the delivery of a pressurized aerosol using a CAPNOPEN© nebulizer connected to a high-pressure injector, which was inserted into the 10–12 mm trocar with an inclination of 45° relative to the underlying peritoneum to optimize the diffusion of the chemotherapy within the abdominal cavity. The system was then kept in a steady state for 30 min (application time), and any remaining toxic aerosol was exhausted in a closed surgical smoke evacuation system to prevent its diffusion outside the abdominal cavity. Finally, the trocars were removed, and laparoscopy was completed without the need for abdominal drainage placement.
The combinations of chemotherapy used in our study were consistent with those reported in the literature: Oxaliplatin 92 mg/m2 was used for PM from colorectal and appendiceal carcinoma and for pseudomyxoma. The combination of Cisplatin 7.5 mg/m2 + Doxorubicin 1.5 mg/m2 was used for gastric, ovarian, pancreatic, and biliary tract peritoneal metastases, and mesothelioma. Since 2018, after the phase I study by Tempfer et al. [9], a higher dose of Cisplatin and Doxorubicin (10.5–2.1 mg/m2) has been adopted. In addition, in patients with peritoneal carcinomatosis from pancreatic carcinoma, the use of Nab-Paclitaxel 112.5 mg/m2 has been adopted since March 2022 within the controlled phase II Nab-PIPAC trial to evaluate the effectiveness of this drug [12,20].
Patients were scheduled for three PIPACs at 4–6-week intervals. In patients with bimodal treatment, the predefined washout period from systemic chemotherapy to PIPAC was 2 weeks, and 1 week from PIPAC to systemic chemotherapy.
Additional procedures were performed based on the surgeon’s evaluation. Bowel resections were performed in the case of isolated sub-obstructive peritoneal lesions. Adnexectomy was performed in patients where ovarian lesions were the only site of progression or the lesions were symptomatic [21].
2.3. Postoperative Treatment and Follow-Up
Adverse events were recorded either during the hospitalization period or reported by patients during outpatient visits. Between each PIPAC administration, patients underwent routine follow-up assessments, including hematological, radiological, and oncological marker measurements.
These results were reviewed by a multidisciplinary team, and additional PIPAC treatments were administered only after confirming the absence of disease progression.
2.4. Statistical Analysis
Continuous variables are reported as mean (Standard Deviation—SD) or median (Minimum–Maximum). Categorical variables are reported as numbers and frequencies (%). Multivariable analyses were performed using multinomial logistic regression integrating with univariate p-values ≤ 0.1. The statistical power of the multivariate analysis was calculated by setting the effect size f2 to 0.15 and the alpha error probability (α) to 0.05. Kaplan–Meier curves were used for graphical representation of the results. Statistical significance was considered for p-values < 0.05, and confidence intervals (CIs) were established at 95%. Statistical analysis and graph processing were performed using GraphPad Prism 9, the IBM SPSS software v29.0.1.0 platform, Excel v16.54, and G*Power 3.1.
3. Results
A total of 336 PIPAC procedures were performed on 168 patients with PM. Disease entities were gastric cancer in 69 patients (41%), pancreatic cancer in 46 (27.5%), colorectal cancer in 30 (18%), hepatobiliary cancer in 12 (7%), and other primary sites (ovarian, breast, lung, small bowel, and mesothelioma) in 11 (6.5%). The reasons for PIPAC discontinuation are summarized in Table 1.

Table 1.
Reasons for PIPAC interruption before PIPAC3.
Presence of abdominal pain (OR = 0.469, 95% CI [0.230–0.956], p = 0.037) and bowel obstruction symptoms before PIPAC1 (OR = 0.249, 95% CI [0.072–0.862], p = 0.028) and ascites > 500 mL during the first treatment (OR = 0.366, 95% CI [0.157–0.853], p = 0.02) were demonstrated to be predictors of standard treatment discontinuation. No other significant predictors of treatment discontinuation were identified among the evaluated factors (Table 2).

Table 2.
Potential predictors for interruption of standard treatment.
Prior history of bowel obstruction symptoms (OR = 0.264, 95% CI [0.74–0.947], p = 0.041) and ascites > 500 mL (OR = 0.375, 95% CI [0.157–0.900], p = 0.028) were found to be independent predictive factors of discontinuation of PIPAC in a multivariable logistic regression (Table 3).

Table 3.
Multivariable logistic regression analysis of predictive factors.
The power of the multivariate analysis comparing the two groups was computed and determined to be 94%.
The median follow-up period was 9 months, with a median overall survival from PIPAC1 of 10 months in the entire study population. We observed a significant difference in median overall survival between patients who underwent ST (12 months) and those who received only 1–2 PIPAC procedures (7 months) (p: 0.0171) (Figure 1).
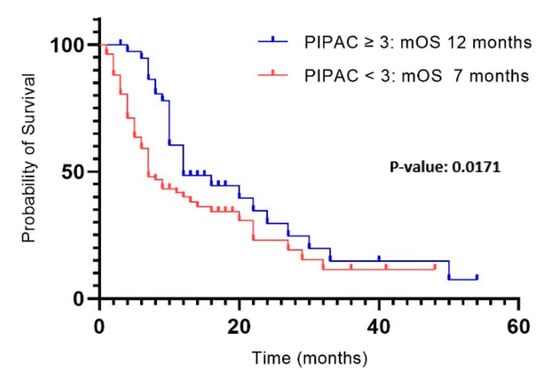
Figure 1.
Survival from PIPAC1 in patients undergoing ST and 1–2 PIPAC.
Interestingly, the ST group exhibited a significant decrease in ascites (p: 0.04), while no efficacy was observed in the regression of other symptoms (Table 4).

Table 4.
Symptoms before PIPAC1 and after PIPAC3.
Intraoperative complications occurred in two patients due to small bowel perforation during laparoscopic abdominal access. As for minor complications, CTCAE 1–2 adverse events were recorded in 47 procedures (13.98%). In detail, sixteen patients developed intense abdominal pain treated with analgesics. Twelve (3.57%) patients developed nausea or vomiting responsive to antiemetics. Ten (2.98%) patients developed a surgical site infection/dehiscence. Nine (2.68%) patients developed fever, and four of them were treated with antibiotics. Among the major complications, patients experienced CTCAE 3–4 adverse events in 13 procedures (3.86%). Four (1.19%) patients developed anemia and received a blood transfusion. Four (1.19%) patients had severe liver toxicity. Three (0.9%) patients developed neutropenia and two (0.6%) patients developed deep vein thrombosis. There were no severe complications needing reoperations or deaths during the in-hospital stay. The 30-day mortality rate was 1.78% (3 patients), while the 90-day mortality rate was 8.92% (15 patients).
Finally, a progressive and significant (p: 0.0404) increase in the number of patients receiving three PIPACs was documented between 2017 and 2023 (Table 5).

Table 5.
Patients who underwent ST between 2017 and 2020 and 2021 and 2023.
A significantly higher survival (p: 0.0001) was observed in patients treated up to 2020 compared to those treated in the following 3 years, with a mOS of 8 months (range 2–54) and 14 months (range 1–50), respectively (Figure 2).
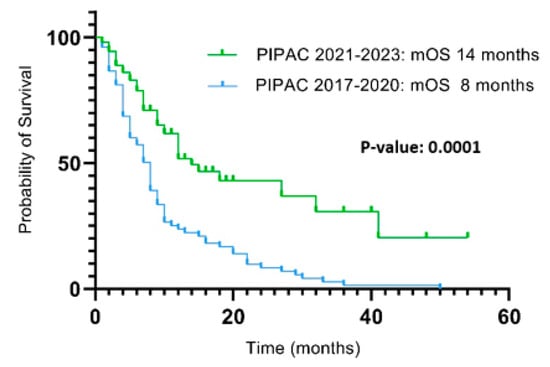
Figure 2.
Survival from PIPAC1 between 2017 and 2020 and 2021 and 2023.
4. Discussion
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC), combined with systemic chemotherapy, represents an intraperitoneal therapeutic option to increase chemotherapeutic agents’ penetration into peritoneal metastases (PMs) [12,22,23]. According to the literature [24,25,26] and our findings, repeated treatment (at least three PIPACs) resulted in improved survival. A better survival rate with an increasing number of PIPAC procedures is also documented [6,27,28]. This provides a rationale for considering PIPAC treatment earlier in the treatment course, either alone or in combination with first-line systemic chemotherapy when tumor cells are more chemo-sensitive. Indeed, in patients with PM from pancreatic cancer, we initiated treatment already in the first metastatic line, with 33% of patients completing the entire treatment schedule. This percentage was higher compared to patients with PM from gastric and colorectal cancer, in whom PIPAC was performed in more advanced stages of the disease (27% and 26%, respectively).
The key finding of our analysis was that a small percentage (29%) of patients were able to complete at least 3 PIPAC procedures, resulting in survival benefits. Our experience reported less favorable results compared to the literature. According to a recent meta-analysis, 39% of patients complete the standard treatment protocol, with higher percentages in colon cancer (47%) and ovarian cancer (42%), and lower percentages in gastric cancer (34%), as well as hepatobiliary and pancreatic cancers (34%) [12]. A plausible explanation for these results may be the inclusion of patients treated in the early stages of our experience, where we observed a higher incidence of failures in completing the standard treatment compared to more recent patients. This finding is correlated with a progressive improvement in the surgical team’s experience and the systematic discussion of all patients eligible for PIPAC in multidisciplinary meetings. This, combined with an increasing understanding of the biological behavior of peritoneal surface malignancies (PSMs) and the therapeutic potential of PIPAC, has led to a progressive improvement in patient selection and, consequently, to an increase in the number of patients completing three PIPAC procedures over the years (Table 5), resulting in a concomitant improvement in oncological outcomes in terms of survival (Figure 2).
Although PIPAC is not a technically demanding surgical procedure, it presents significant challenges related to the learning curve of patient selection and optimal indication. Therefore, a well-defined multidisciplinary team is crucial to accelerating the learning curve, reducing untoward outcomes in the early period and improving oncological results. We reported a disease progression rate of 55.8% during treatment, which was the primary reason for treatment discontinuation. This finding, consistent with that reported by Ezanno (PD rate of 49.1%) [26], underscores the palliative nature of PIPAC treatment and emphasizes the importance of careful patient selection for this approach.
The most noteworthy reason for the discontinuation of PIPAC in our series was conversion to CRS during treatment. In our cohort, 10% of patients achieved a sufficient reduction in peritoneal disease burden to undergo CRS, prompting the discontinuation of PIPAC. Among them, nine patients had gastric peritoneal metastases, while the remaining patients had peritoneal metastases from colorectal cancer. The literature reports varying conversion rates to CRS and HIPEC [29], ranging from 7.6%, as reported by the meta-analysis of Di Giorgio et al. [12], to 26%, as reported by Casella et al. [4]. Considering the results of our previously published series [30] and the results of this study, the primary field of application for PIPAC as a neoadjuvant procedure appears to be gastric cancer with peritoneal metastases (GCPM). These results may open new therapeutic horizons, suggesting that PIPAC, which was originally a palliative treatment, might represent a neoadjuvant treatment option before curative surgery in selected patients with GCPM. In this context, the ongoing prospective, two-arm, randomized multicenter phase III clinical study PIPAC_VEROne is assessing the potential benefits of a more “intensive” bidirectional chemotherapy plus PIPAC treatment approach for patients with peritoneal-only oligometastatic disease [31].
Our findings regarding the potential predictors of early PIPAC discontinuation were consistent with those reported by Balmer et al. [32], indicating that ascites > 500 mL or symptoms of bowel obstruction at the first PIPAC procedure were associated with a higher risk of treatment discontinuation. However, high-volume ascites should not be considered a contraindication for PIPAC. Indeed, our results also demonstrated a significant reduction in ascites among patients undergoing systemic therapy (ST), which significantly improves patients’ quality of life and aligns with one of the therapeutic goals of PIPAC [33,34].
Notably, factors such as age, gender, BMI, or ECOG status at first PIPAC did not influence PIPAC discontinuation. This suggests that PIPAC can be repeatedly applied even in patients with a certain degree of functional impairment. However, it should be noted that patients with an ECOG performance status > 2 are excluded a priori from treatment.
The main limitation of this study is the small number of patients who underwent at least three PIPAC procedures with consequent numerical heterogeneity within the two comparison groups. Additionally, the wide time window of patient’s treatment has led to temporal heterogeneity among the analyzed cases. On the other hand, this study represents the real-life experience of a high-volume center that initiated this treatment in the early stages of the technique’s development.
5. Conclusions
PIPAC can offer a survival advantage after completing at least three treatments, de-spite the high rate of disease progression that results in a low completion rate among patients. Therefore, the identification of predictive factors such as the presence of ascites greater than 500 mL and bowel obstructive symptoms is essential to improve patient selection and, consequently, enhance oncological outcomes. However, due to its low complication rate, treatment safety, and potential neoadjuvant role, it remains a viable option for a carefully selected group of patients. The ongoing PIPAC cohort studies, shall shortly deliver more information on optimized selection criteria for PIPAC treatment.
Author Contributions
Conceptualization, M.A. and A.D.G.; methodology: A.D.G. and M.H.; software H.T.-F. and C.O.; validation: A.B.-Y., F.P. and A.D.G.; formal analysis, M.A. and F.S.; investigation: O.S., C.O., L.D., A.B. and A.B.-Y.; resources, A.D.G., F.P. and M.R.; data curation: C.O. and F.F.; writing—original draft preparation, M.A. and A.D.G.; writing—review and editing: O.S. and M.R.; supervision, A.D.G., M.H. and O.S.; project administration, M.A., A.D.G. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Fondazione Policlinico Gemelli, Rome (protocol code 4368, approved 16 December 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study at the moment of hospital admission.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors warmly thank Ministero della Salute, Ricerca Corrente 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Hida, K.; Ishibashi, H.; Sako, S.; Mizumoto, A.; Ichinose, M.; Padmanabhan, N.; Yoshida, S.; Yonemura, Y. Thir-ty-three long-term survivors after cytoreductive surgery in patients with peritoneal metastases from colorectal cancer: A ret-rospective descriptive study. World J. Surg. Oncol. 2021, 19, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macrì, A.; Gra-ziosi, L.; et al. Prognostic value of specific KRAS mutations in patients with colorectal peritoneal metastases. ESMO Open 2024, 9, 102976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casella, F.; Bencivenga, M.; Brancato, G.; Torroni, L.; Ridolfi, C.; Puccio, C.; Alloggio, M.; Meloni, F.; Fusario, D.; Marrelli, D.; et al. Bidirectional Approach with PIPAC and Systemic Chemotherapy for Patients with Synchronous Gastric Cancer Perito-neal Metastases (GCPM). Ann. Surg. Oncol. 2023, 30, 5733–5742. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemo-therapy with cytoreductive surgery: A systematic review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Santullo, F.; Abatini, C.; Attalla El Halabieh, M.; Ferracci, F.; Lodoli, C.; Barberis, L.; Giovinazzo, F.; Di Giorgio, A.; Pacelli, F. The Road to Technical Proficiency in Cytoreductive Surgery for Peritoneal Carcinomatosis: Risk-Adjusted Cumulative Summation Analysis. Front Surg. 2022, 18, 877970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gas-tric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal car-cinomatosis: A systematic review including evidence from Japan. Surg. Today 2021, 51, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.B.; Hilal, Z.; Dogan, A.; Petersen, M.; Rezniczek, G.A. Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peri-toneal metastasis. Eur. J. Surg. Oncol. 2018, 44, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Vizzielli, G.; Giudice, M.T.; Nardelli, F.; Costantini, B.; Salutari, V.; Inzani, F.S.; Zannoni, G.F.; Chiantera, V.; Di Giorgio, A.; Pacelli, F.; et al. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) Applied to Platinum-Resistant Recurrence of Ovarian Tumor: A Single-Institution Experience (ID: PARROT Trial). Ann. Surg. Oncol. 2024, 31, 1207–1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aulicino, M.; Orsini, C.; D’Annibale, G.; Barberis, L.; Catania, P.; Abatini, C.; Attalla El Halabieh, M.; Ferracci, F.; Lodoli, C.; Santullo, F.; et al. How to Implement Pressurized Intraperitoneal Aerosol Chemotherapy into a National Health System Sce-nario: A Single-Center Retrospective Analysis of Costs and Economic Sustainability at a High-Volume Italian Hospital. Cancers 2024, 16, 2637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Giorgio, A.; Macrì, A.; Ferracci, F.; Robella, M.; Visaloco, M.; De Manzoni, G.; Sammartino, P.; Sommariva, A.; Biacchi, D.; Roviello, F.; et al. 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Me-ta-Analysis. Cancers 2023, 15, 1125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugarbaker, P.H. Peritoneal carcinomatosis: Natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Perit. Carcinomatosis Drugs Dis. 1996, 81, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Perit. Carcinomatosis Drugs Dis. 1996, 82, 359–374. [Google Scholar]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017.
- Hubner, M.; Grass, F.; Teixeira-Farinha, H.; Pache, B.; Mathevet, P.; Demartines, N. Pressurized IntraPeritoneal Aerosol Chemotherapy—Practical aspects. Eur. J. Surg. Oncol. 2017, 43, 1102–1109. [Google Scholar] [CrossRef]
- Girardot-Miglierina, A.; Clerc, D.; Alyami, M.; Villeneuve, L.; Sgarbura, O.; Reymond, M.A.; Hubner, M.; the ISSPP PIPAC Study Group. Consensus statement on safety measures for pressurized intraperitoneal aerosol chemotherapy. Pleura Peritoneum 2021, 6, 139–149. [Google Scholar] [CrossRef]
- Hübner, M.; Alyami, M.; Villeneuve, L.; Cortés-Guiral, D.; Nowacki, M.; So, J.; Sgarbura, O. ISSPP PIPAC study group. Con-sensus guidelines for pressurized intraperitoneal aerosol chemotherapy: Technical aspects and treatment protocols. Eur. J. Surg. Oncol. 2022, 48, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Sgarbura, O.; Eveno, C.; Alyami, M.; Bakrin, N.; Guiral, D.C.; Ceelen, W.; Delgadillo, X.; Dellinger, T.; Di Giorgio, A.; Kefleye-sus, A.; et al. Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2022, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robella, M.; Hubner, M.; Sgarbura, O.; Reymond, M.; Khomiakov, V.; Di Giorgio, A.; Bhatt, A.; Bakrin, N.; Willaert, W.; Al-yami, M.; et al. Feasibility and safety of PIPAC combined with additional surgical procedures: PLUS study. Eur. J. Surg. Oncol. 2022, 48, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Abatini, C.; Attalla El Halabieh, M.; Vita, E.; Vizzielli, G.; Gallotta, V.; Pacelli, F.; Rotolo, S. From palliation to cure: PIPAC for peritoneal malignancies. Minerva Med. 2019, 110, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Sgarbura, O.; Villeneuve, L.; Alyami, M.; Bakrin, N.; Torrent, J.J.; Eveno, C.; Hübner, M.; ISSPP PIPAC study group. Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): Still standardized or on the verge of diversification? Eur. J. Surg. Oncol. 2021, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Farinha, H.; Hubner, M.; Abba, J.; Rao, R.G.; Willaert, W. Treatment response after pressurized intraperitoneal aerosol chemo-therapy (PIPAC) for peritoneal metastases of colorectal origin. Eur. J. Surg. Oncol. 2022, 48, E114. [Google Scholar] [CrossRef]
- Ezanno, A.C.; Malgras, B.; Pocard, M. Pressurized intraperitoneal aerosol chemotherapy, reasons for interrupting treatment: A systematic review of the literature. Pleura Peritoneum 2023, 8, 45–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kefleyesus, A.; Bhatt, A.; Escayola, C.; Khomyakov, V.; Hübner, M.; Reymond, M.A.; Thieme, R.; Sgarbura, O.; Willaert, W.; Ceelen, W.; et al. Descriptive review of current practices and prognostic factors in patients with ovarian cancer treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC): A multicentric, retrospective, cohort of 234 patients. Front Oncol. 2023, 13, 1204886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sindayigaya, R.; Dogan, C.; Demtröder, C.R.; Fischer, B.; Karam, E.; Buggisch, J.R.; Tempfer, C.B.; Lecomte, T.; Ouaissi, M.; Giger-Pabst, U. Clinical Outcome for Patients Managed with Low-Dose Cisplatin and Doxorubicin Delivered as Pressurized Intraperitoneal Aerosol Chemotherapy for Unresectable Peritoneal Metastases of Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytore-ductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Santullo, F.; Ferracci, F.; Abatini, C.; Attalla El Halabieh, M.; Lodoli, C.; D’Annibale, G.; Di Cesare, L.; D’Agostino, L.; Pecere, S.; Di Giorgio, A.; et al. Gastric cancer with peritoneal metastases: A single center outline and comparison of different surgical and intraperitoneal treatments. Langenbecks Arch. Surg. 2023, 408, 437. [Google Scholar] [CrossRef] [PubMed]
- Casella, F.; Bencivenga, M.; Rosati, R.; Fumagalli, U.R.; Marrelli, D.; Pacelli, F.; Macrì, A.; Donini, A.; Torroni, L.; Pavarana, M.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in multimodal therapy for patients with oligometastatic peritoneal gastric cancer: A randomized multicenter phase III trial PIPAC VEROne. Pleura Peritoneum 2022, 7, 135–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balmer, A.; Clerc, D.; Toussaint, L.; Sgarbura, O.; Taïbi, A.; Hübner, M.; Teixeira Farinha, H. Selection Criteria for Pressu-rized Intraperitoneal Aerosol Chemotherapy (PIPAC) Treatment in Patients with Peritoneal Metastases. Cancers 2022, 14, 2557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wong, L.C.K.; Sultana, R.; Lim, H.J.; Tan, J.W.; Tan, Q.X.; Wong, J.S.M.; Chia, C.S.; Ong, C.J. A systematic review on qua-lity of life (QoL) of patients with peritoneal metastasis (PM) who underwent pressurized intraperitoneal aerosol chemothe-rapy (PIPAC). Pleura Peritoneum 2022, 7, 39–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).