Imaging of Peripheral Intraneural Tumors: A Comprehensive Review for Radiologists
Simple Summary
Abstract
1. Introduction
2. Imaging Techniques and Their Appropriateness
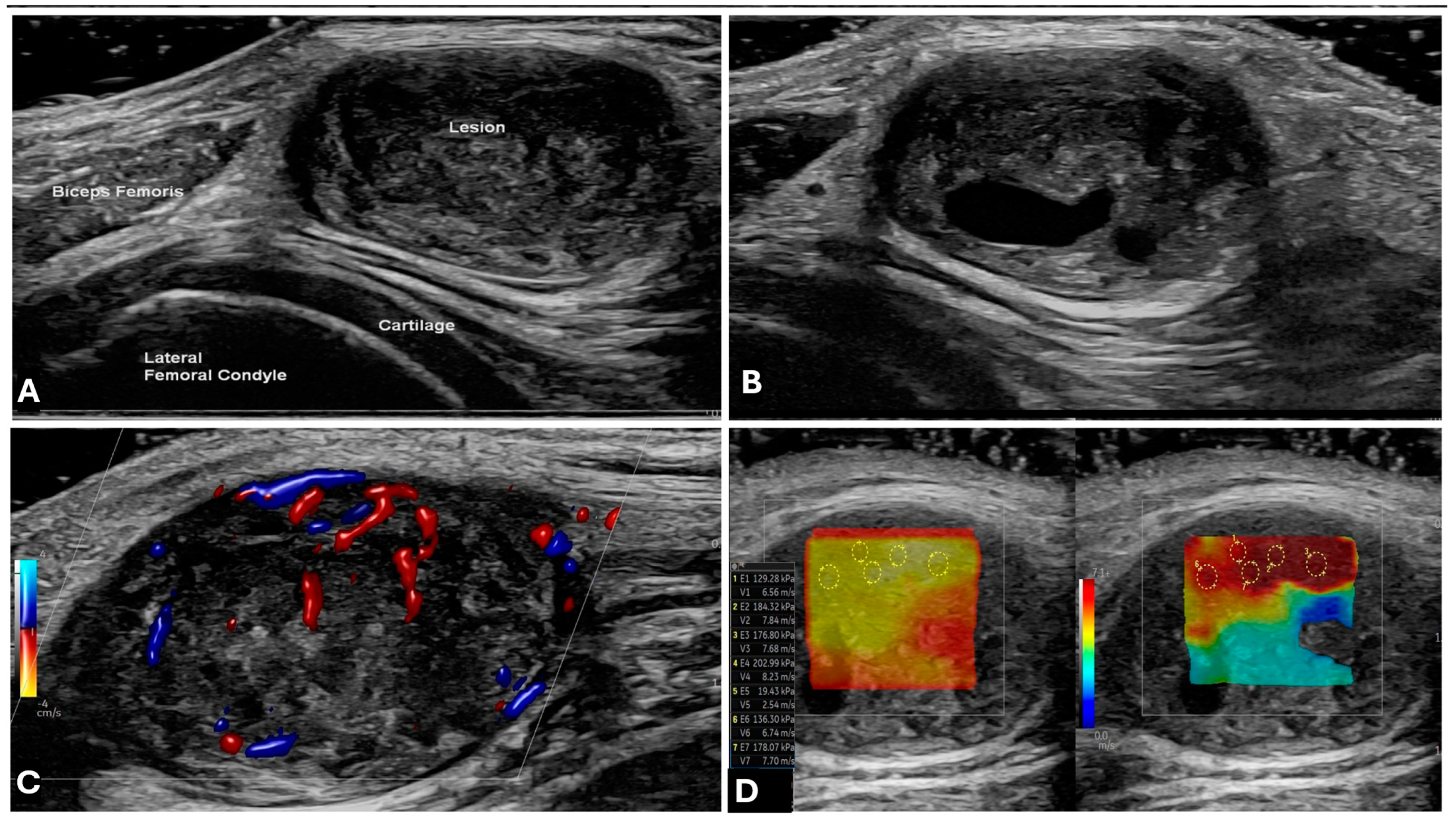
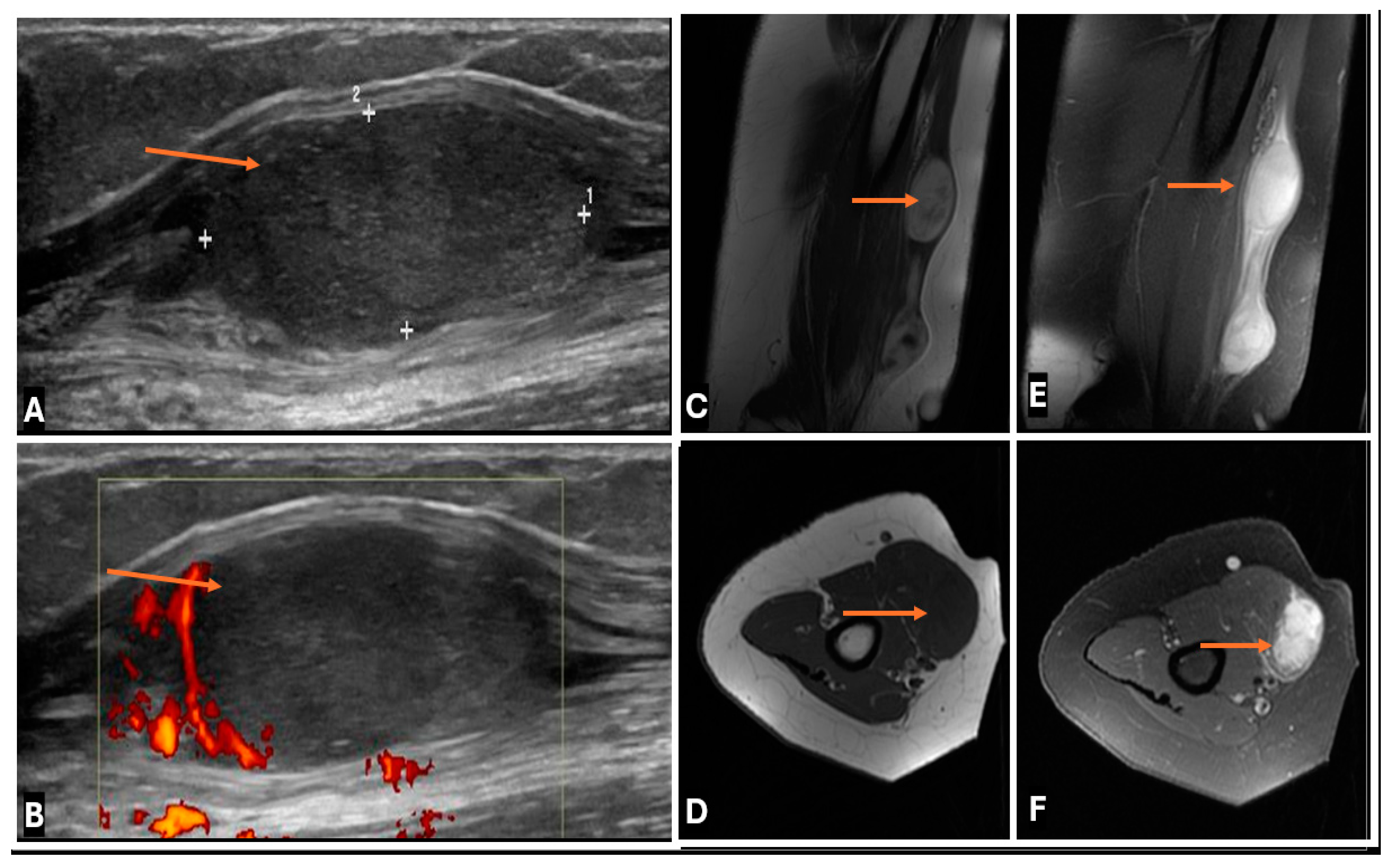
2.1. Ultrasound (US)
2.2. Computed Tomography (CT)
2.3. Magnetic Resonance Imaging (MRI)
2.4. PET-CT
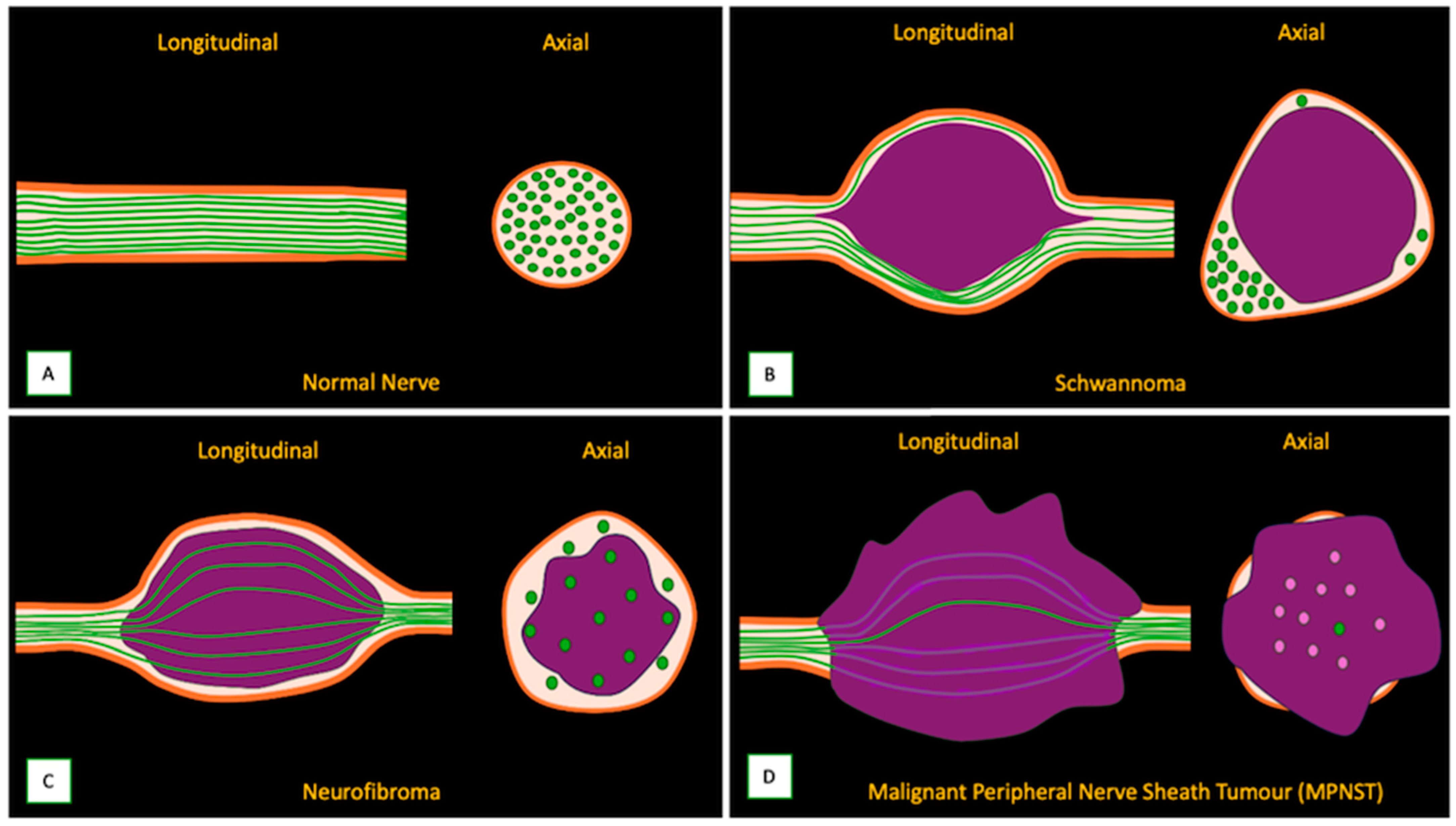
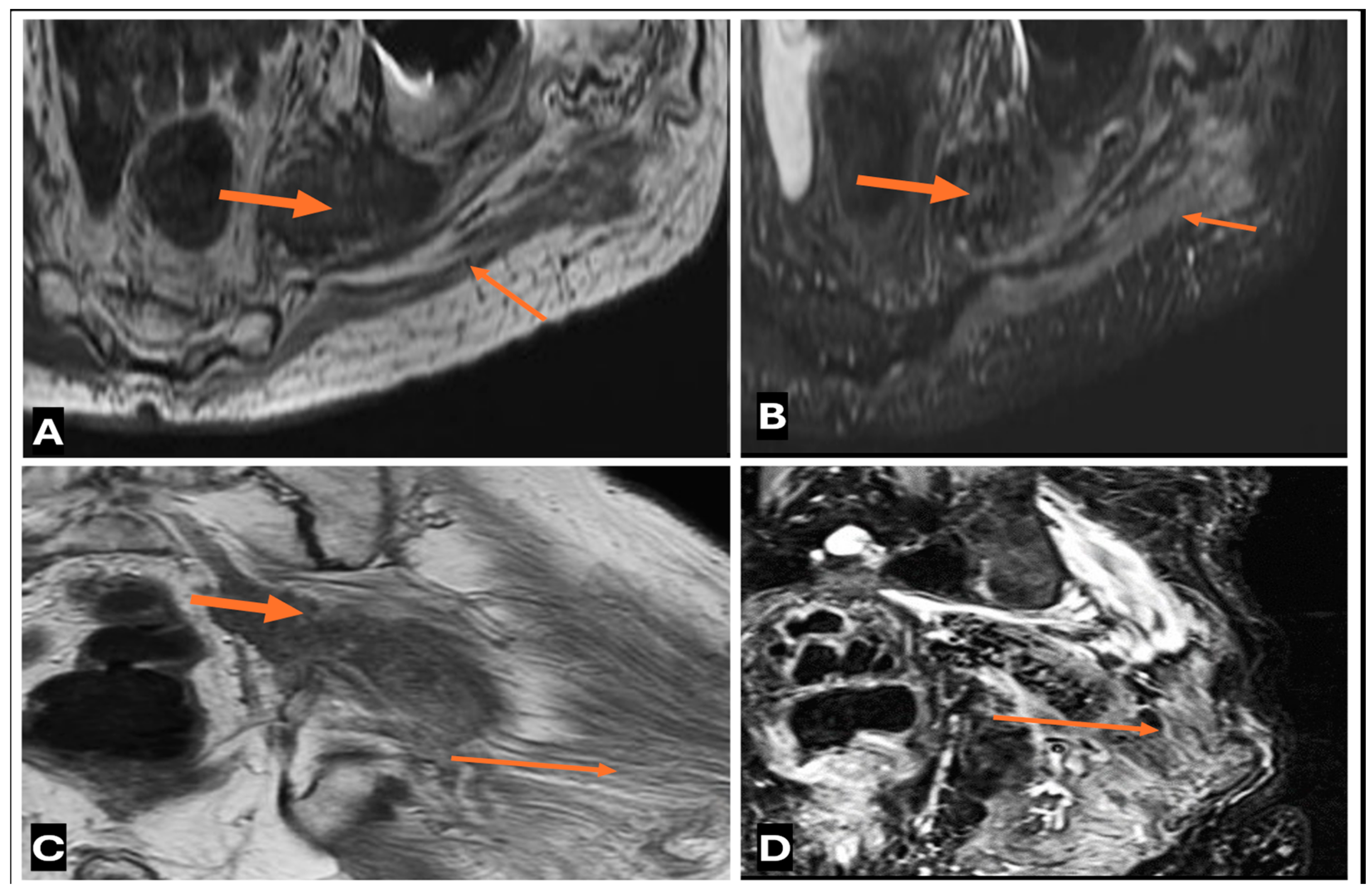
3. MRI-Based Approach to an Intraneural Tumor
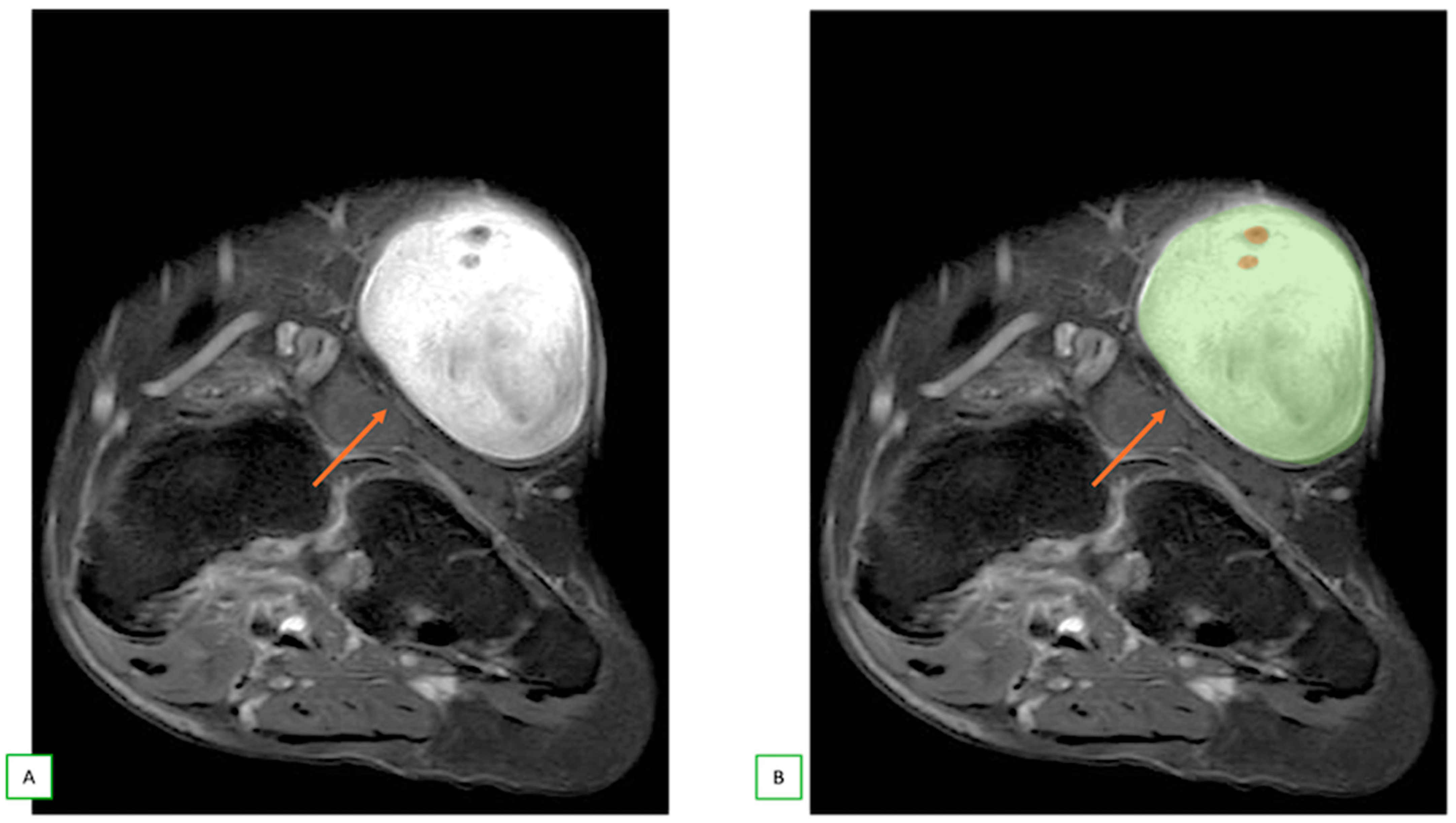
3.1. When to Suspect a Neurogenic Tumor on MRI
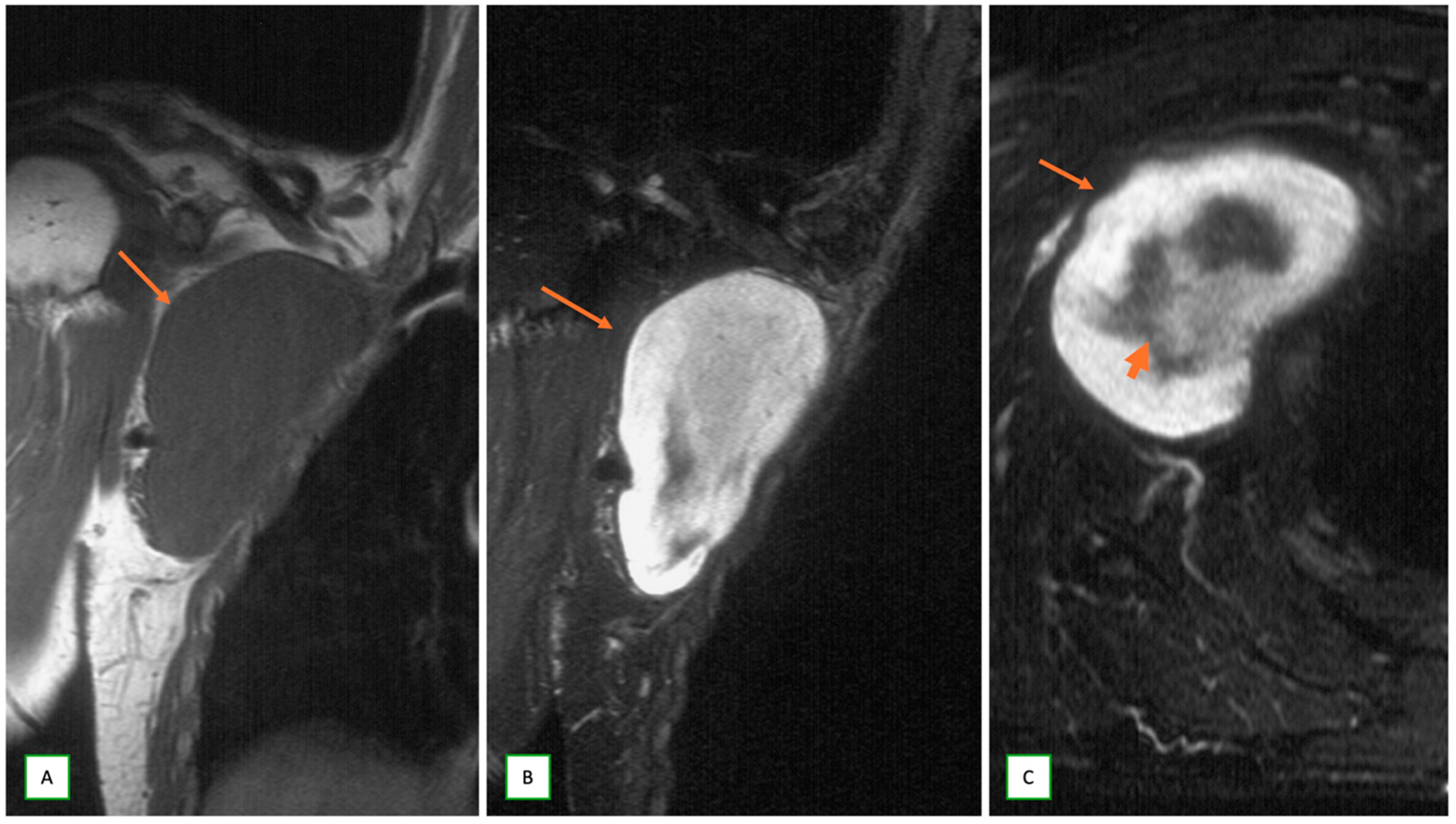
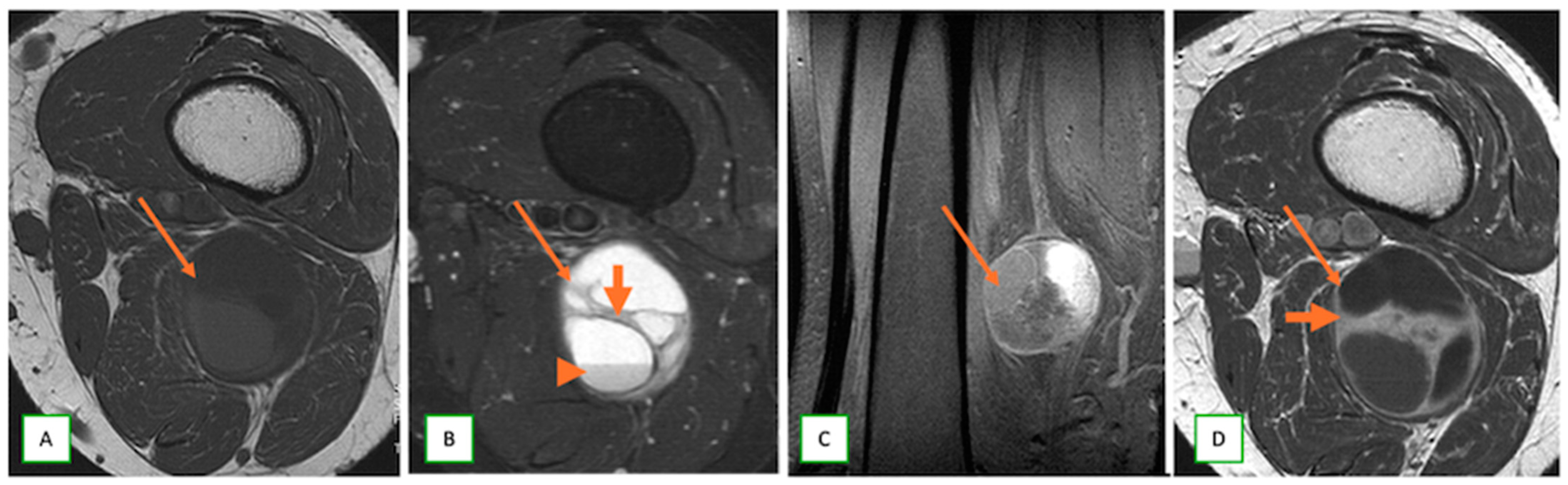
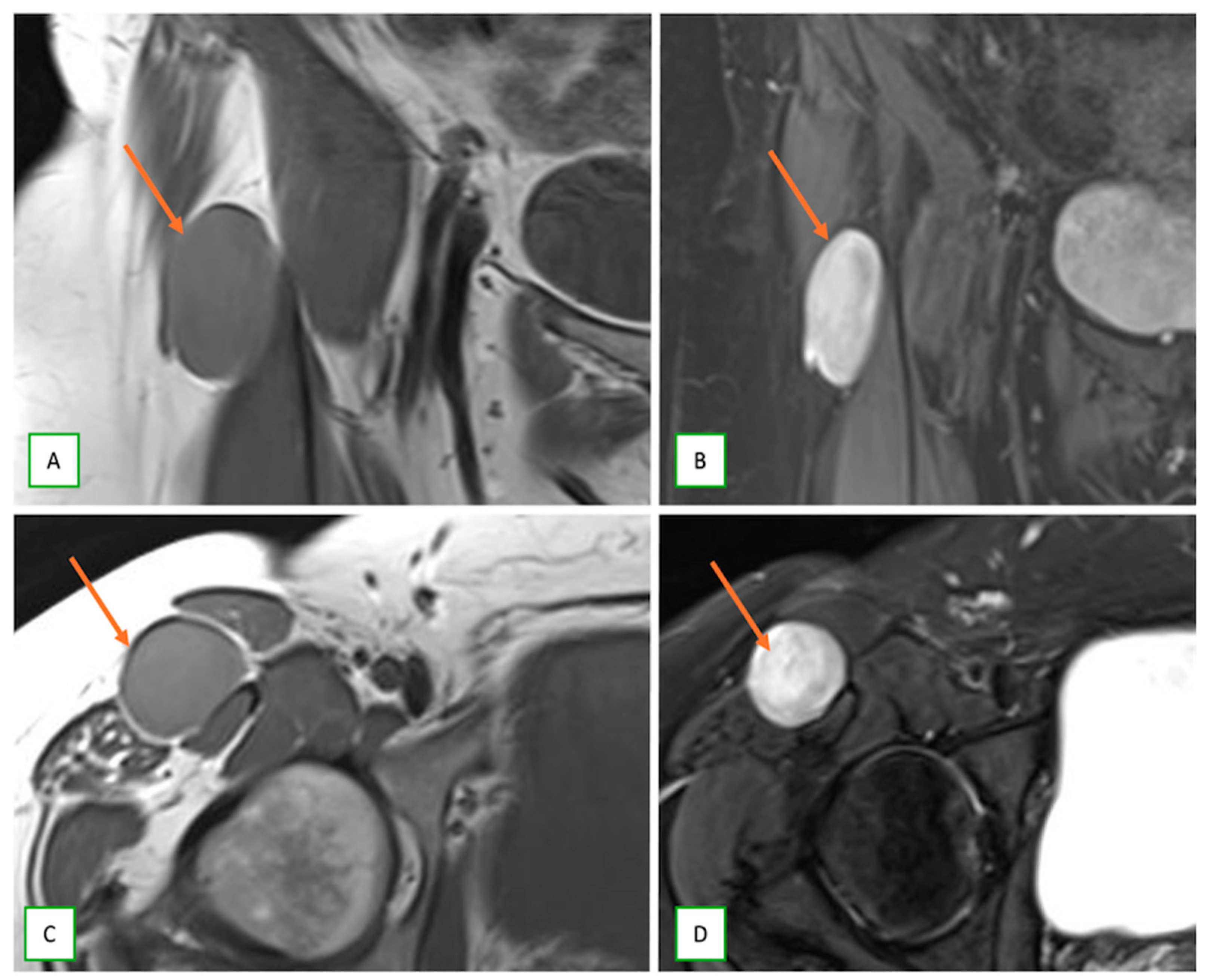
3.2. Differentiating Neurofibroma from Schwannoma on MRI
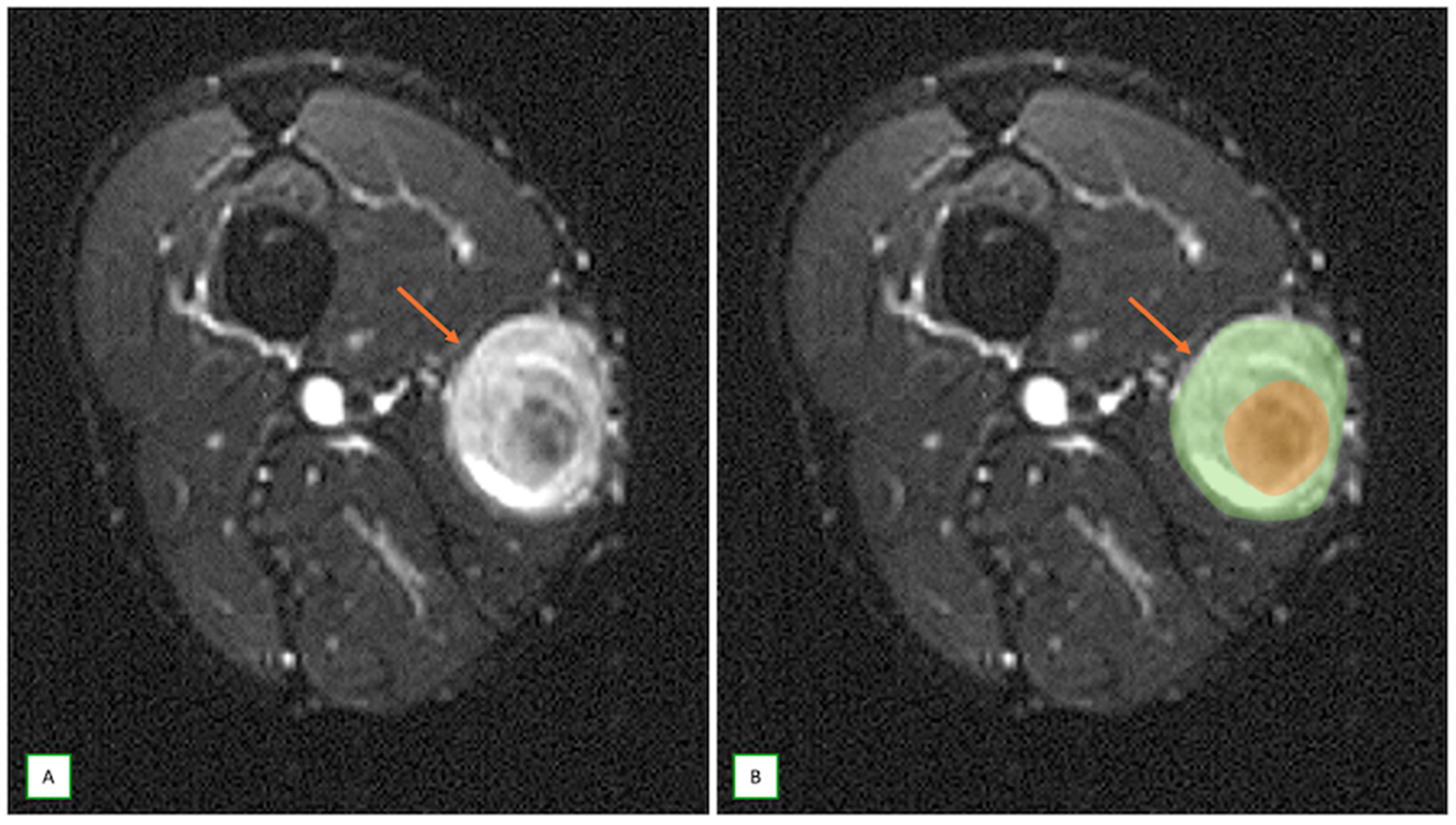
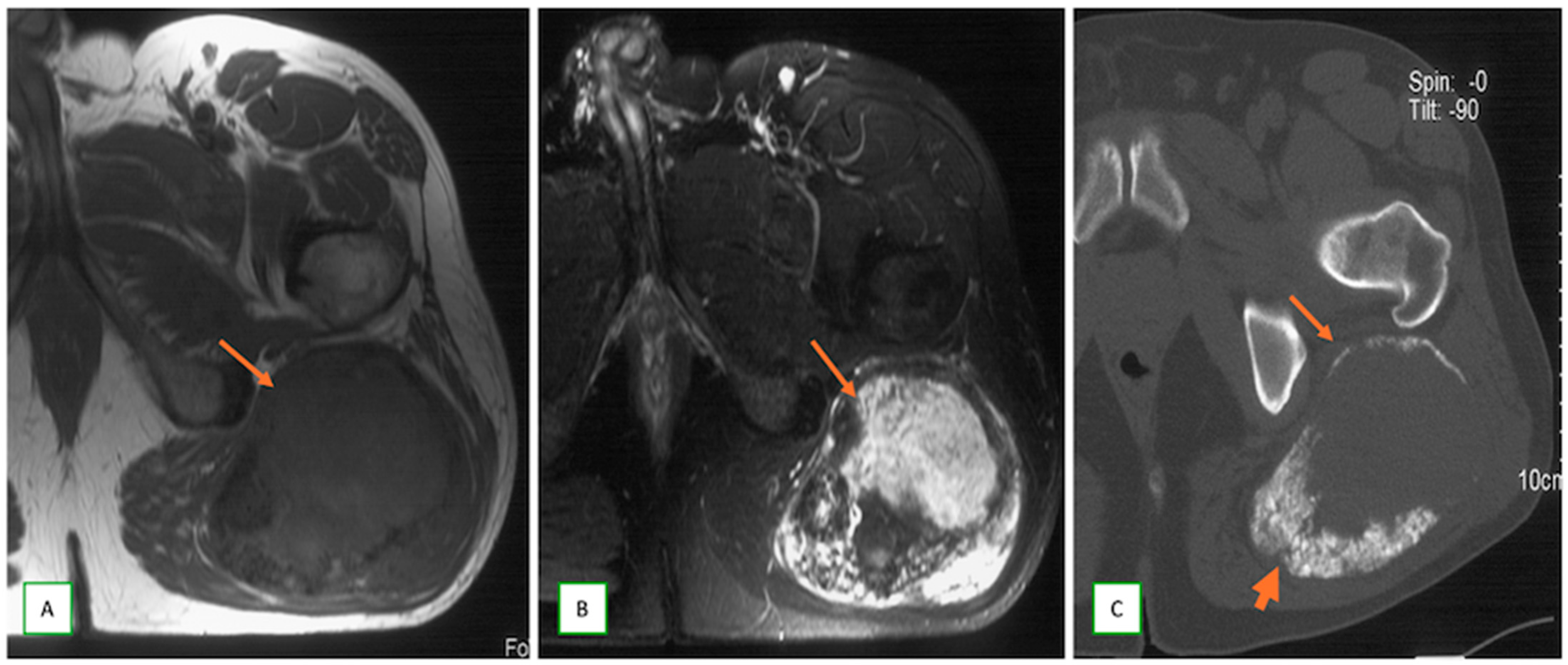
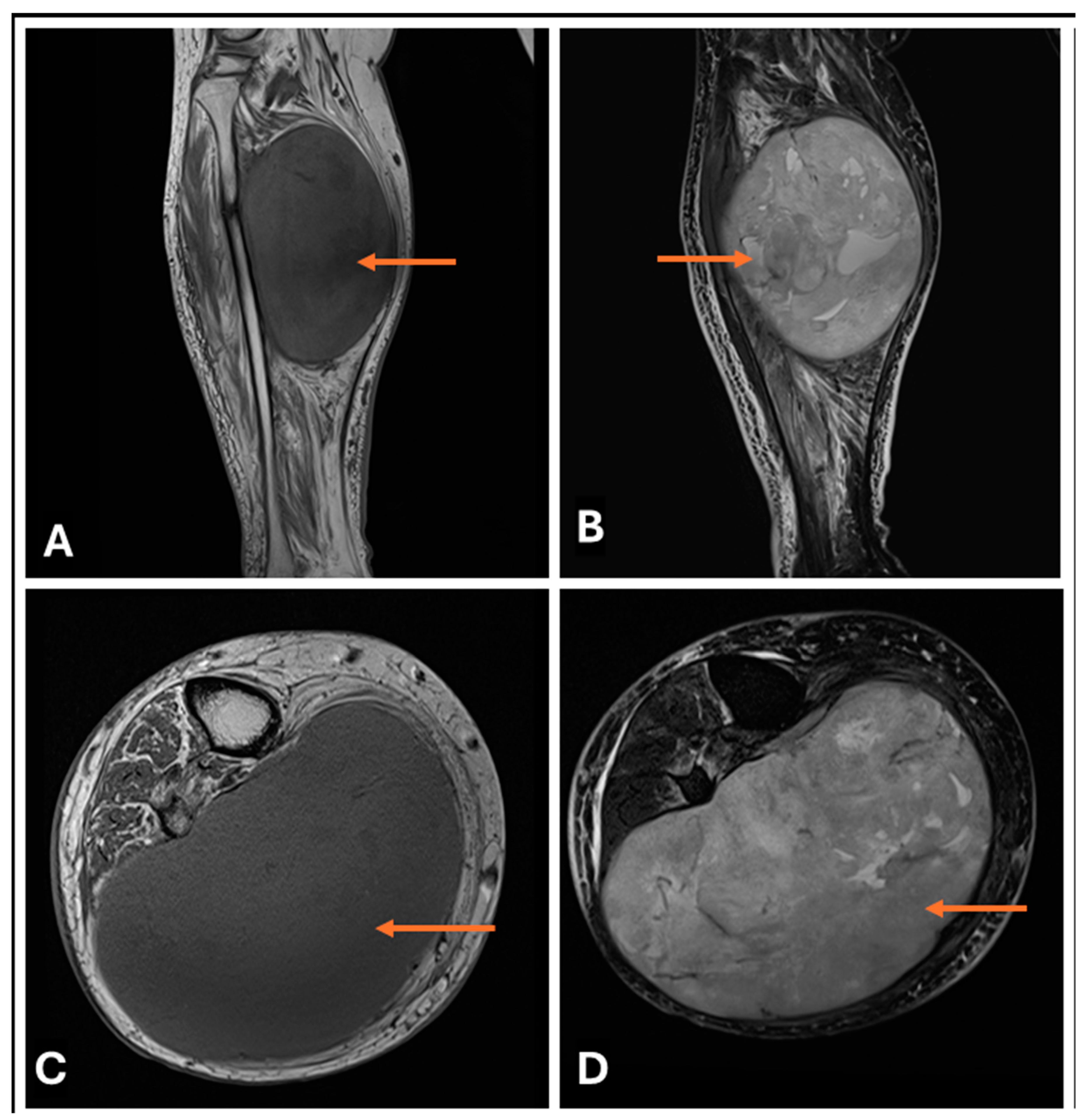
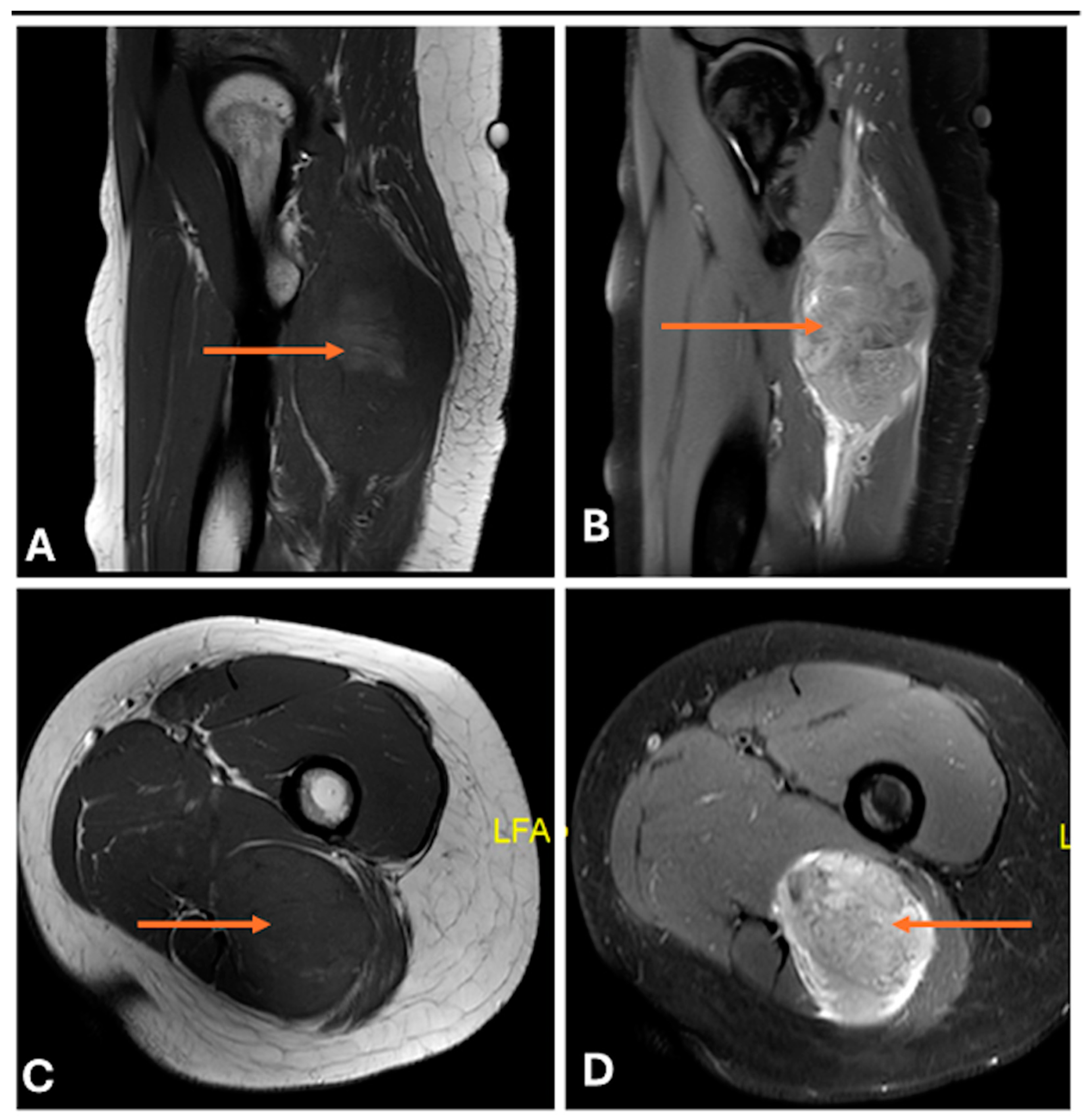
3.3. Differentiating Between Benign and Malignant PNSTs
3.4. When to Suspect Malignant Transformation in a Benign PNST
4. Imaging Differential Diagnosis
- Dermal nerve sheath myxomas or neurothekeomas occur as small, painless nodules in young adults; they appear hypoechoic on USG and markedly hyperintense on T2-w images (Figure 14).
- 2.
- 3.
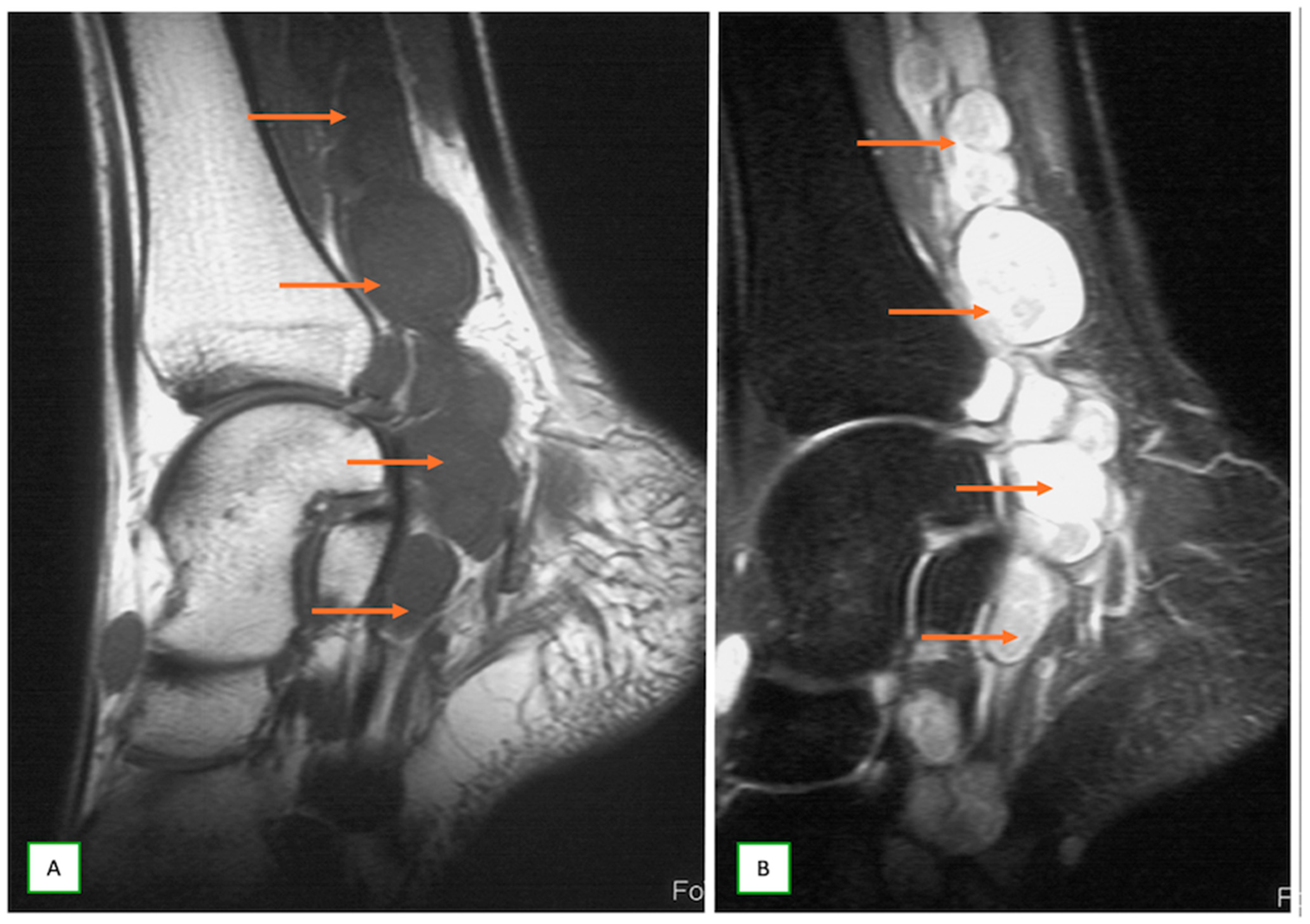
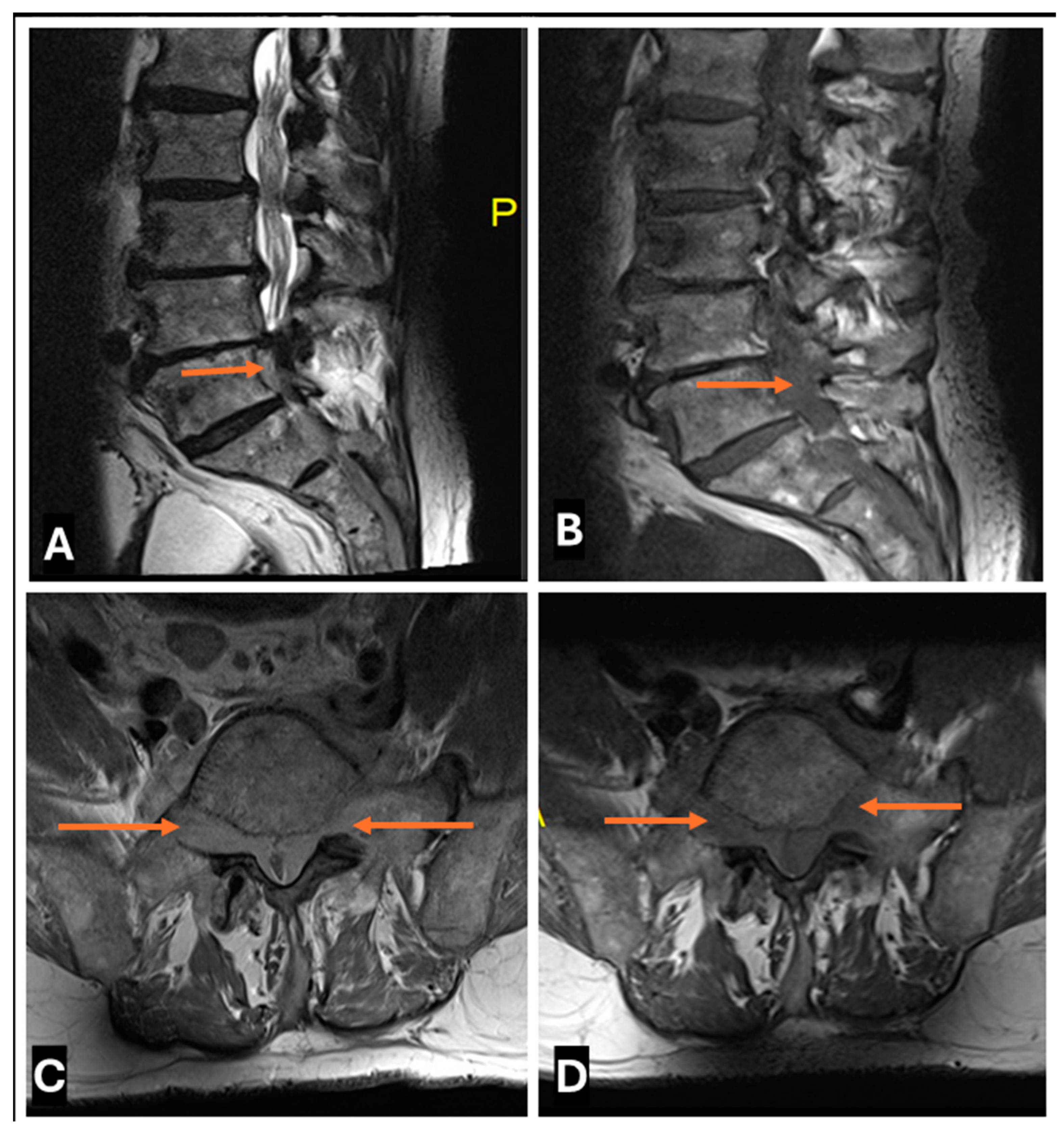
- Intraneural ganglion cysts usually occur near joints; are particularly common in the common peroneal nerve at the knee, owing to the intraarticular branch of the proximal tibiofibular joint; and appear as tubular, multiloculated cystic lesions following the course of the nerve on MRI [60,61] (Figure 16).
- 4.
- 5.
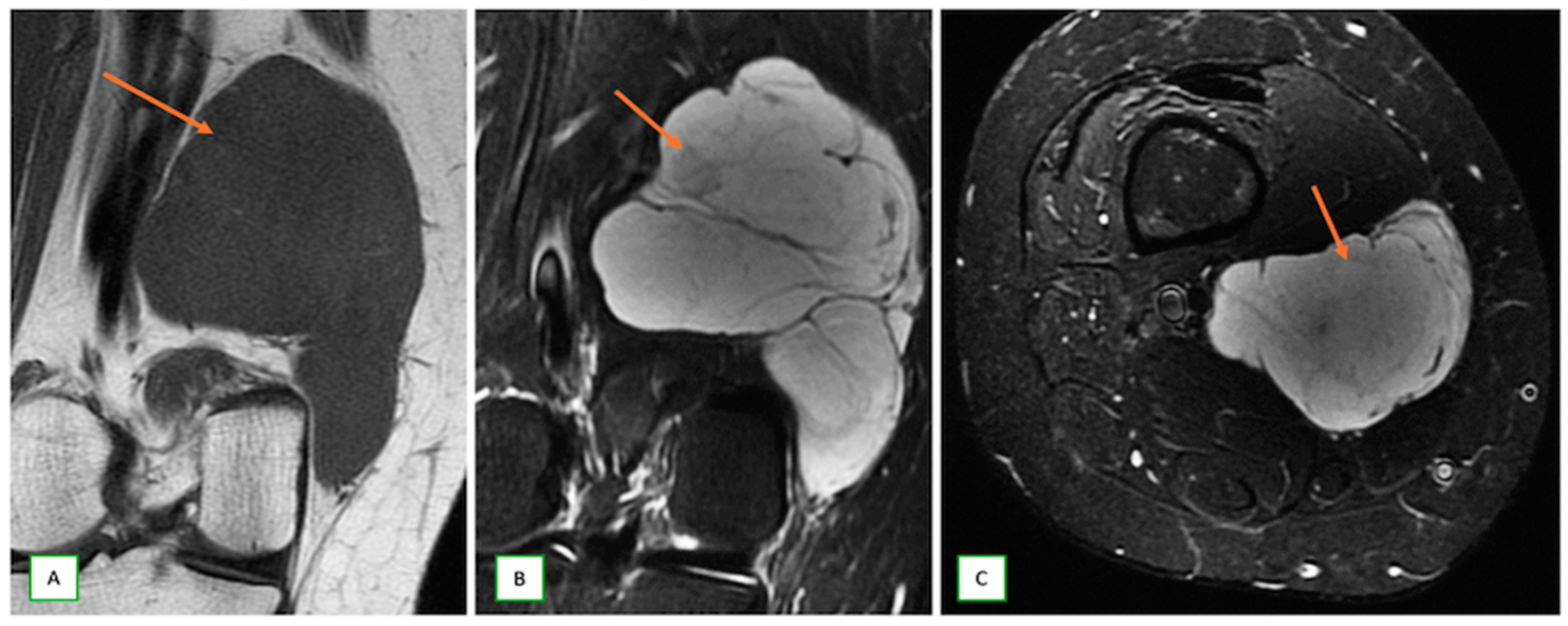
- Benign triton tumors, or neuromuscular choristomas, are rare congenital lesions with a mix of neural and striated muscle elements, usually appearing as fusiform nerve enlargements with heterogeneous signals on MRI and often mimicking an aggressive lesion. They typically present in the first two decades (Figure 18).
- 6.
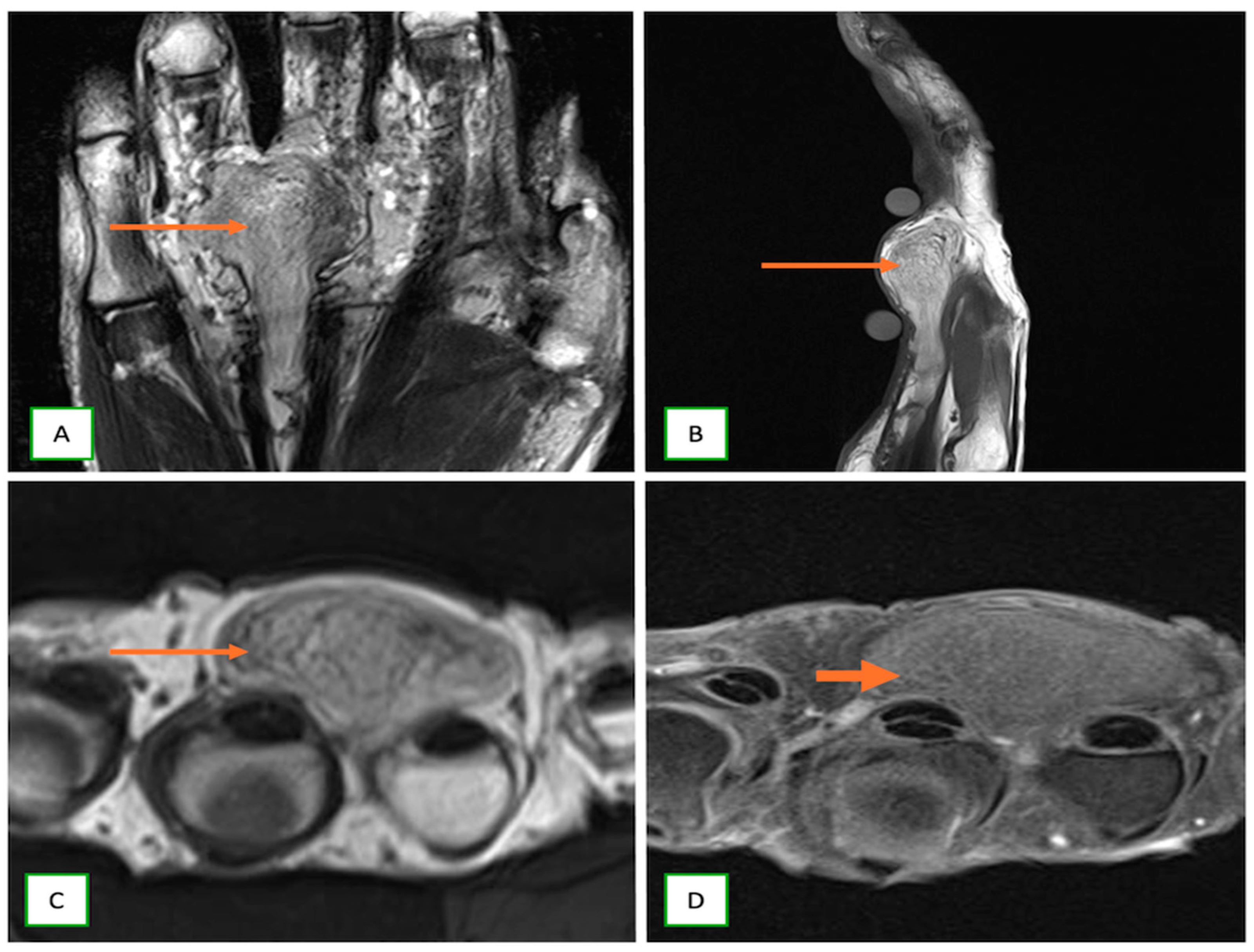
- Ganglioneuromas are rare, benign tumors of the sympathetic nervous system that are commonly identified incidentally and appear as well-defined, homogeneous masses, containing calcifications and showing mild to moderate enhancement on CT/MR in the posterior mediastinum, retroperitoneum, or adrenal gland (Figure 19).
- 7.
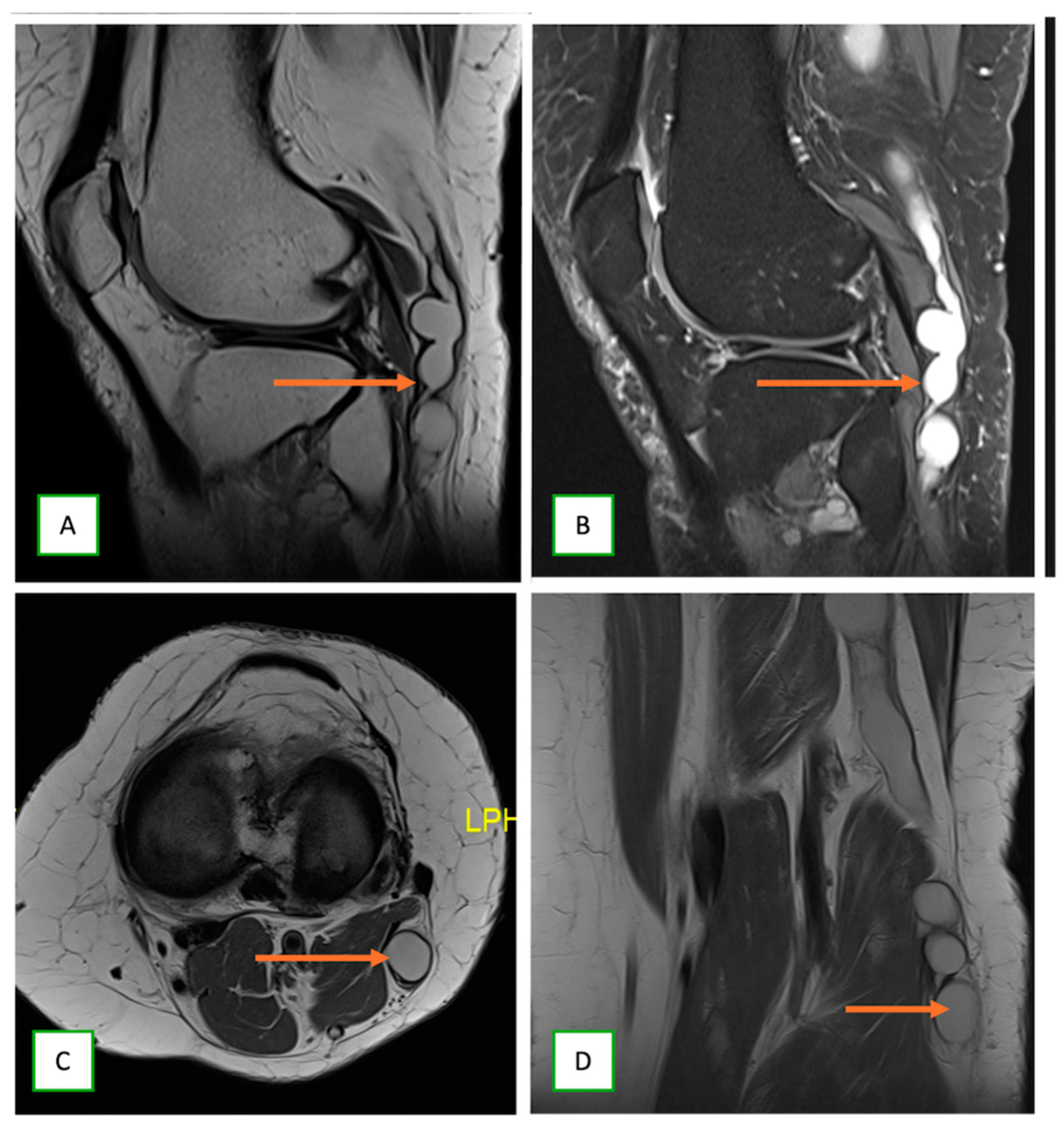
- Neurolymphomatosis describes intraneural spread of lymphoma and appears as diffuse enlargement of nerves, plexuses, spinal cord, and cauda equina. It shows more homogeneous enhancement than PNSTs and may be associated with lymphadenopathy or a known history of lymphoma [63] (Figure 20 and Figure 21).
- 8.
- 9.
- Traumatic neuromas: These neuromas occur at the site of a nerve injury or amputation and appear as a focal mass in continuity with the injured nerve, often with surrounding scarring [51].
- 10.
- Amyloidoma are rare and feature tumor-like amyloid deposition in peripheral nerves. They can mimic PNST but often show more diffuse nerve involvement [65].
- 11.
- Hybrid nerve sheath tumors, such as schwannoma–neurofibroma or schwannoma–perineurioma combinations, present with complex imaging features that can mimic more aggressive tumors; a definitive diagnosis necessitates immunohistochemistry and histopathological analysis (Figure 23).
- 12.
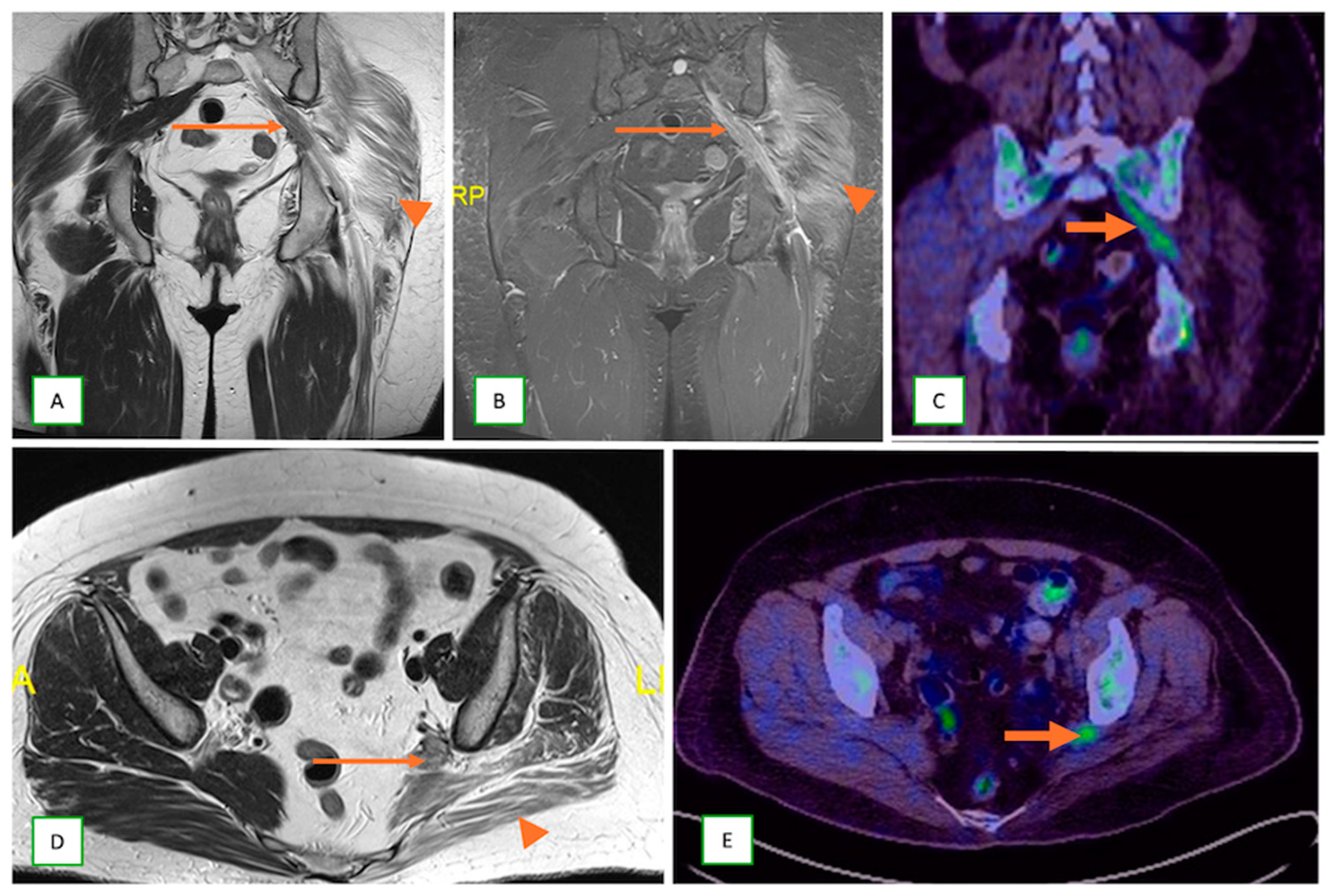
- Metastatic lesions: These include perineural spread of the tumor or metastasis to peripheral nerves by an adjacent or distant primary tumor. They are commonly observed with head and neck cancers, such as squamous cell carcinoma. They typically appear as linear thickening and enhancement extending along the course of the affected nerve on MRI with end muscle edema and appear FDG-avid on PET-CT [66] (Figure 24 and Figure 25).
- 13.
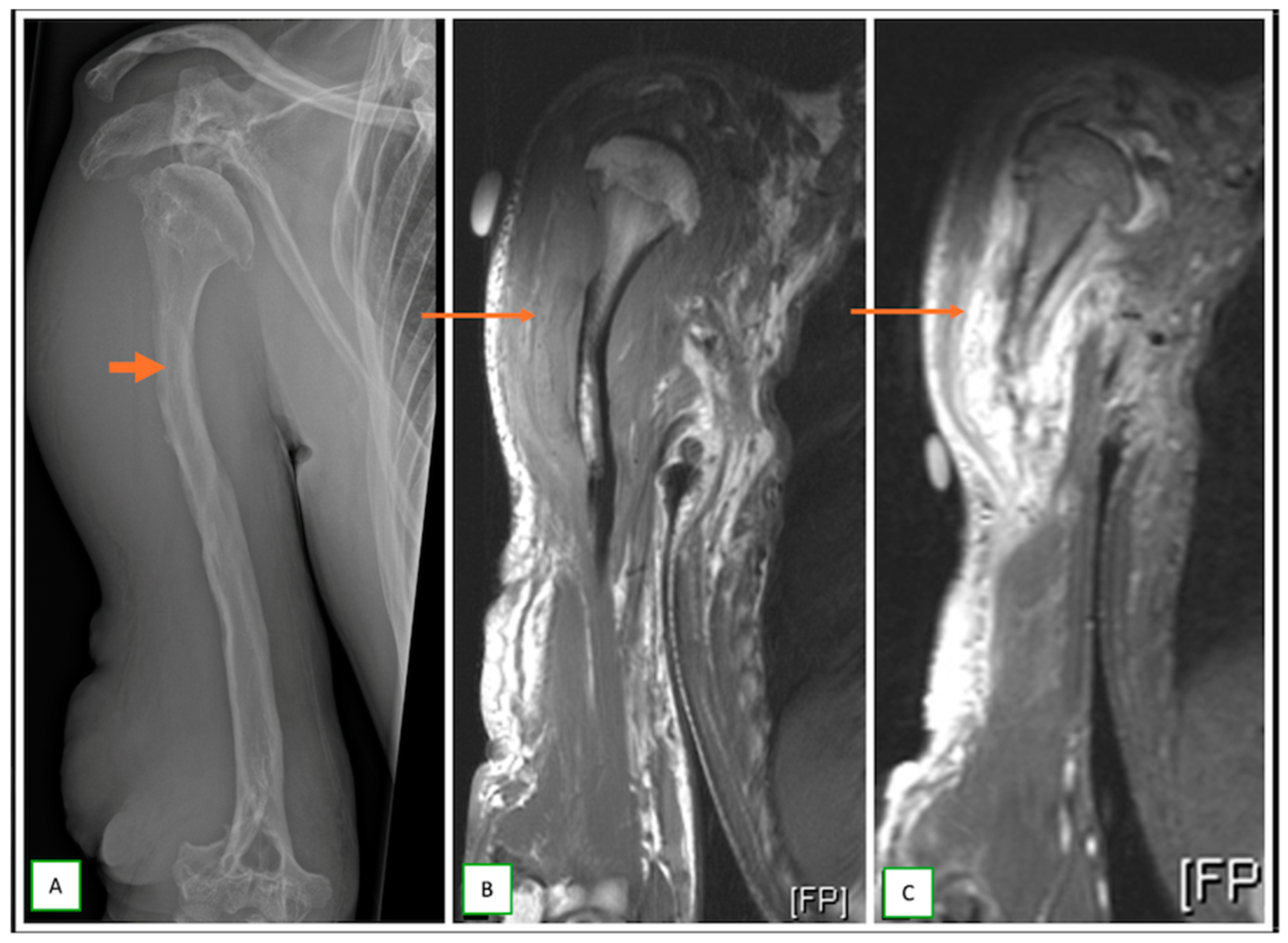
- Primary Sarcomas: Soft-tissue sarcomas are uncommon, accounting for just over 1% of adult malignancies. Synovial sarcoma, clear cell sarcoma, and epithelioid sarcomas are known to involve peripheral nerves. CT/MR reveals a large soft tissue mass with areas of necrosis or calcification and heterogeneous contrast enhancement. F-18 FDG uptake is useful for both tumor staging and treatment assessment (Figure 26, Figure 27 and Figure 28).
5. Multidisciplinary Team Approach in the Diagnosis and Treatment of Intraneural Tumors
5.1. Role of Interventional Musculoskeletal Radiologists
5.2. Role of Pathologist
5.3. Role of Musculoskeletal Oncologists
5.4. Role of Onco-Surgeon
5.5. Role of Radiation Oncologists
6. Biopsy and Treatment Considerations
7. Postoperative Imaging
8. Newer Imaging Advances
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferner, R.E.; Gutmann, D.H. International Consensus Statement on Malignant Peripheral Nerve Sheath Tumours in Neurofibromatosis 11. Cancer Res. 2002, 62, 1573–1577. [Google Scholar] [PubMed]
- Sunderland, S.S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13, 771–784. [Google Scholar] [CrossRef]
- Ziadi, A.; Saliba, I. Malignant peripheral nerve sheath tumour of intracranial nerve: A case series review. Auris Nasus Larynx 2010, 37, 539–545. [Google Scholar] [CrossRef]
- Kransdorf, M.J.; Murphey, M.D. Imaging of Soft Tissue Tumors, 3rd ed.; Wolters Kluwer Health Adis (ESP): Amsterdam, The Netherlands, 2013; 776p. [Google Scholar]
- Kransdorf, M.J. Malignant soft-tissue tumors in a large referral population: Distribution of diagnoses by age, sex, and location. Am. J. Roentgenol. 1995, 164, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Chee, D.W.Y.; Peh, W.C.G.; Shek, T.W.H. Pictorial Essay: Imaging of Peripheral Nerve Sheath Tumors. Can. Assoc. Radiol. J. 2011, 62, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Murphey, M.D.; Smith, W.S.; Smith, S.E.; Kransdorf, M.J.; Temple, H.T. From the Archives of the AFIP. RadioGraphics 1999, 19, 1253–1280. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.M.; Steffner, R.; Kwong, S.; Chin, A.; Boutin, R.D. MRI of Soft-Tissue Tumors: What to Include in the Report. RadioGraphics 2024, 44, e230086. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.; Isaac, A.; Bignotti, B.; Rossi, F.; Zaottini, F.; Martinoli, C. Nerve Tumors: What the MSK Radiologist Should Know. Semin. Musculoskelet. Radiol. 2019, 23, 76–84. [Google Scholar]
- Winter, N.; Dohrn, M.F.; Wittlinger, J.; Loizides, A.; Gruber, H.; Grimm, A. Role of high-resolution ultrasound in detection and monitoring of peripheral nerve tumor burden in neurofibromatosis in children. Childs Nerv. Syst. 2020, 36, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Telleman, J.A.; Stellingwerff, M.D.; Brekelmans, G.J.; Visser, L.H. Nerve ultrasound: A useful screening tool for peripheral nerve sheath tumors in NF1? Neurology 2017, 88, 1615–1622. [Google Scholar] [CrossRef]
- Lefebvre, G.; Le Corroller, T. Ultrasound and MR imaging of peripheral nerve tumors: The state of the art. Skelet. Radiol. 2023, 52, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Chiou, H.-J.; Chou, Y.-H.; Wang, H.-K.; Chiou, S.-Y.; Chang, C.-Y. Differentiation Between Schwannomas and Neurofibromas in the Extremities and Superficial Body. J. Ultrasound Med. 2008, 27, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.S.; Pisapia, J.M.; Ma, T.S.; Zager, E.L.; Heuer, G.G.; Khoury, V. Ultrasonographic Evaluation of Peripheral Nerves. World Neurosurg. 2016, 85, 333–339. [Google Scholar] [CrossRef]
- Pedro, M.T.; Antoniadis, G.; Scheuerle, A.; Pham, M.; Wirtz, C.R.; Koenig, R.W. Intraoperative high-resolution ultrasound and contrast-enhanced ultrasound of peripheral nerve tumors and tumorlike lesions. Neurosurg. Focus. 2015, 39, E5. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.G.; Cage, T.; Narvid, J.; Noss, R.; Chin, C.; Kliot, M. High-resolution ultrasonography and diffusion tensor tractography map normal nerve fascicles in relation to schwannoma tissue prior to resection. J. Neurosurg. 2014, 120, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, A.; Del Prever, E.B.; Cavallo, F.; Pozza, S.; Linari, A.; Lombardo, P.; Comandone, A.; Piana, R.; Faletti, C. Perfusion pattern and time of vascularisation with CEUS increase accuracy in differentiating between benign and malignant tumors in 216 musculoskeletal soft tissue masses. Eur. J. Radiol. 2015, 84, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, M.; Li, A.; Ye, X.; Li, C.; Xu, D. Diagnostic value of contrast-enhanced ultrasound for differential diagnosis of malignant and benign soft tissue masses: A meta-analysis. Ultrasound Med. Biol. 2020, 46, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Orsogna, N.; Porfiri, A.; Fioravanti, C.; D’Ambrosio, F. Elastographic and contrast-enhanced ultrasound features of a benign schwannoma of the common fibular nerve. J. Ultrasound 2013, 16, 135–138. [Google Scholar] [CrossRef]
- Gowda, K.H.; Mishra, G.V.; Phatak, S.V.; Pavanan, A.; Dhande, R.P. Role of ultrasonography and strain elastography findings in peripheral nerve sheath tumor: A narrative review. J. Datta Meghe Inst. Med. Sci. Univ. 2022, 17, 187–195. [Google Scholar] [CrossRef]
- Pilavaki, M.; Chourmouzi, D.; Kiziridou, A.; Skordalaki, A.; Zarampoukas, T.; Drevelengas, A. Imaging of peripheral nerve sheath tumors with pathologic correlation: Pictorial review. Eur. J. Radiol. 2004, 52, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Crist, J.; Hodge, J.R.; Frick, M.; Leung, F.P.; Hsu, E.; Gi, M.T.; Venkatesh, S.K. Magnetic Resonance Imaging Appearance of Schwannomas from Head to Toe: A Pictorial Review. J. Clin. Imaging Sci. 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, T.; Fisher, S.; Karri, S.; Ramzi, A.; Sharma, R.; Chhabra, A. Advanced MR Imaging of Peripheral Nerve Sheath Tumors Including Diffusion Imaging. Semin. Musculoskelet. Radiol. 2015, 19, 179–190. [Google Scholar]
- Chhabra, A.; Thakkar, R.S.; Andreisek, G.; Chalian, M.; Belzberg, A.J.; Blakeley, J.; Hoke, A.; Thawait, G.K.; Eng, J.; Carrino, J.A. Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. Am. J. Neuroradiol. 2013, 34, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Beaman, F.D.; Kransdorf, M.J.; Menke, D.M. Schwannoma: Radiologic-Pathologic Correlation. RadioGraphics 2004, 24, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.C.; Marais, M.D.; Flanigan, P.M.; Bydon, M.; Giannini, C.; Spinner, R.J.; Folpe, A.L. Malignant melanotic nerve sheath tumor. Am. J. Neuroradiol. 2022, 43, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Parham, D.M.; Lasater, O.E.; Chari, R.S.; Chen, G.; Fletcher, B.D. MR imaging differentiation of benign and malignant peripheral nerve sheath tumors: Use of the target sign. Pediatr. Radiol. 1997, 27, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Chhabra, A.; Blakely, J. Magnetic Resonance Neurography of Peripheral Nerve Tumors and Tumorlike Conditions. Neuroimaging Clin. North. Am. 2014, 24, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Wasa, J.; Nishida, Y.; Tsukushi, S.; Shido, Y.; Sugiura, H.; Nakashima, H.; Ishiguro, N. MRI Features in the Differentiation of Malignant Peripheral Nerve Sheath Tumors and Neurofibromas. Am. J. Roentgenol. 2010, 194, 1568–1574. [Google Scholar] [CrossRef]
- Derlin, T.; Tornquist, K.; Münster, S.; Apostolova, I.; Hagel, C.; Friedrich, R.E.; Wedegärtner, U.; Mautner, V.F. Comparative effectiveness of 18F-FDG PET/CT versus whole-body MRI for detection of malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Clin. Nucl. Med. 2013, 38, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, P.; Jonasson, L.; Maeder, P.; Thiran, J.-P.; Wedeen, V.J.; Meuli, R. Understanding Diffusion MR Imaging Techniques: From Scalar Diffusion-weighted Imaging to Diffusion Tensor Imaging and Beyond. RadioGraphics 2006, 26, S205–S223. [Google Scholar] [CrossRef]
- Demehri, S.; Belzberg, A.; Blakeley, J.; Fayad, L.M. Conventional and Functional MR Imaging of Peripheral Nerve Sheath Tumors: Initial Experience. Am. J. Neuroradiol. 2014, 35, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Blakeley, J.O.; Langmead, S.; Belzberg, A.J.; Fayad, L.M. Current status and recommendations for imaging in neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Skelet. Radiol. 2020, 49, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, B.; Descoteaux, M.; Mori, S.; Leemans, A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019, 32, e3785. [Google Scholar] [CrossRef]
- Gersing, A.S.; Cervantes, B.; Knebel, C.; Schwaiger, B.J.; Kirschke, J.S.; Weidlich, D.; Claudi, C.; Peeters, J.M.; Pfeiffer, D.; Rummeny, E.J. Diffusion tensor imaging and tractography for preoperative assessment of benign peripheral nerve sheath tumors. Eur. J. Radiol. 2020, 129, 109110. [Google Scholar] [CrossRef] [PubMed]
- Kasprian, G.; Amann, G.; Panotopoulos, J.; Schmidt, M.; Dominkus, M.; Trattnig, S.; Windhager, R.; Prayer, D.; Nöbauer-Huhmann, I. Peripheral nerve tractography in soft tissue tumors: A preliminary 3-tesla diffusion tensor magnetic resonance imaging study. Muscle Nerve 2015, 51, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Zhao, L.; Carrino, J.A.; Trueblood, E.; Koceski, S.; Shteriev, F.; Lenkinski, L.; Sinclair, C.D.J.; Andreisek, G. MR Neurography: Advances. Radiol. Res. Pract. 2013, 2013, 809568. [Google Scholar] [CrossRef] [PubMed]
- Yankeelov, T.E.; Gore, J.C. Dynamic contrast enhanced magnetic resonance imaging in oncology: Theory, data acquisition, analysis, and examples. Curr. Med. Imaging 2007, 3, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.L.; Lang, P. Bone and soft tissue tumors: The role of contrast agents for MR imaging. Eur. J. Radiol. 2000, 34, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.M.; Jacobs, M.A.; Wang, X.; Carrino, J.A.; Bluemke, D.A. Musculoskeletal Tumors: How to Use Anatomic, Functional, and Metabolic MR Techniques. Radiology 2012, 265, 340–356. [Google Scholar] [CrossRef]
- Fayad, L.M.; Wang, X.; Blakeley, J.O.; Durand, D.J.; Jacobs, M.A.; Demehri, S.; Subhawong, T.K.; Soldatos, T.; Barker, P.B. Characterization of peripheral nerve sheath tumors with 3T proton MR spectroscopy. Am. J. Neuroradiol. 2014, 35, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Golding, J.F.; Smith, M.; Calonje, E.; Jan, W.; Sanjayanathan, V.; O’Doherty, M. [18F] 2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumors (MPNSTs): A long-term clinical study. Ann. Oncol. 2008, 19, 390–394. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J. How We Read Oncologic FDG PET/CT. Cancer Imaging 2016, 16, 35. [Google Scholar] [CrossRef]
- Kim, J.W.; Roh, J.-L.; Kim, J.S.; Lee, J.H.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. 18F-FDG PET/CT surveillance at 3–6 and 12 months for detection of recurrence and second primary cancer in patients with head and neck squamous cell carcinoma. Br. J. Cancer 2013, 109, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Miller, E.; Lerman, H.; Even-Sapir, E. Benign Nonphysiologic Lesions with Increased 18F-FDG Uptake on PET/CT: Characterization and Incidence. Am. J. Roentgenol. 2007, 189, 1203–1210. [Google Scholar] [CrossRef]
- Debs, P.; Luna, R.; Fayad, L.M.; Ahlawat, S. MRI features of benign peripheral nerve sheath tumors: How do sporadic and syndromic tumors differ? Skelet. Radiol. 2024, 53, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Hochman, M.G. Soft-tissue tumors and tumorlike lesions: A systematic imaging approach. Radiology 2009, 253, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Kubiena, H.; Entner, T.; Schmidt, M.; Frey, M. Peripheral neural sheath tumors (PNST)—What a radiologist should know. Eur. J. Radiol. 2013, 82, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Caranci, F.; Briganti, F.; La Porta, M.; Antinolfi, G.; Cesarano, E.; Fonio, P.; Brunese, L.; Coppolino, F. Magnetic resonance imaging in brachial plexus injury. Musculoskelet Surg. 2013, 97 (Suppl. S2), S181–S190. [Google Scholar] [CrossRef]
- Abreu, E.; Aubert, S.; Wavreille, G.; Gheno, R.; Canella, C.; Cotten, A. Peripheral tumor and tumorlike neurogenic lesions. Eur. J. Radiol. 2013, 82, 38–50. [Google Scholar] [CrossRef]
- Farid, M.; Demicco, E.G.; Garcia, R.; Ahn, L.; Merola, P.R.; Cioffi, A.; Maki, R.G. Malignant peripheral nerve sheath tumors. Oncologist 2014, 19, 193–201. [Google Scholar] [CrossRef]
- Ahlawat, S.; Fayad, L.M. Imaging cellularity in benign and malignant peripheral nerve sheath tumors: Utility of the “target sign” by diffusion weighted imaging. Eur. J. Radiol. 2018, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Davidson, B.; Nadi, M.; Alotaibi, N.M.; Fehlings, M.G.; Gentili, F.; Valiante, T.A.; Tator, C.H.; Tymianski, M.; Guha, A.; et al. Management of peripheral nerve sheath tumors: 17 years of experience at Toronto Western Hospital. J. Neurosurg. 2018, 128, 1226–1234. [Google Scholar] [CrossRef]
- Zehou, O.; Fabre, E.; Zelek, L.; Sbidian, E.; Ortonne, N.; Banu, E.; Wolkenstein, P.; Valeyrie-Allanore, L. Chemotherapy for the treatment of malignant peripheral nerve sheath tumors in neurofibromatosis 1: A 10-year institutional review. Orphanet J. Rare Dis. 2013, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, M.; Høland, M.; Ågesen, T.H.; Brekke, H.R.; Liestøl, K.; Hall, K.S.; Mertens, F.; Picci, P.; Smeland, S.; Lothe, R.A. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro-Oncology 2013, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Smith, K.D.; Liu, J.; Lahat, G.; Myers, S.; Wang, W.L.; Zhang, W.; McCutcheon, I.E.; Slopis, J.M.; Lazar, A.J.; et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann. Surg. 2009, 249, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.R.; Baser, M.E.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef] [PubMed]
- LaFemina, J.; Qin, L.-X.; Moraco, N.H.; Antonescu, C.R.; Fields, R.C.; Crago, A.M.; Brennan, M.F.; Singer, S. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann. Surg. Oncol. 2013, 20, 66–72. [Google Scholar] [CrossRef]
- Spinner, R.J.; Hébert-Blouin, M.-N.; Rock, M.G.; Amrami, K.K. Extreme intraneural ganglion cysts. J. Neurosurg. 2011, 114, 217–224. [Google Scholar] [CrossRef]
- Lisovski, V.; Minderis, M. Intraneural ganglion cyst: A case report and a review of the literature. Acta Med. Litu. 2019, 26, 147–151. [Google Scholar] [CrossRef]
- Wilson, T.J.; Howe, B.M.; Stewart, S.A.; Spinner, R.J.; Amrami, K.K. Clinicoradiological features of intraneural perineuriomas obviate the need for tissue diagnosis. J. Neurosurg. 2018, 129, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Grisariu, S.; Avni, B.; Batchelor, T.T.; van den Bent, M.J.; Bokstein, F.; Schiff, D.; Kuittinen, O.; Chamberlain, M.C.; Roth, P.; Nemets, A.; et al. Neurolymphomatosis: An international primary CNS lymphoma collaborative group report. Blood J. Am. Soc. Hematol. 2010, 115, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Saida, T.; Sasaki, K.; Yoshida, M.; Kamimaki, T.; Nakajima, T. Fibrolipomatous hamartoma with macrodactyly and carpal tunnel syndrome. Radiol. Case Rep. 2022, 18, 335–338. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, G.A.; Broski, S.M.; Howe, B.M.; Spinner, R.J.; Amrami, K.K.; Dispenzieri, A.; Ringler, M.D. MRI of pathology-proven peripheral nerve amyloidosis. Skelet. Radiol. 2017, 46, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, K.G. Plexus and peripheral nerve metastasis. Handb. Clin. Neurol. 2018, 149, 257–279. [Google Scholar] [PubMed]
- Strakowski, J.A. Ultrasound-Guided Peripheral Nerve Procedures. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 687–715. [Google Scholar] [CrossRef]
- Pianta, M.; Chock, E.; Schlicht, S.; McCombe, D. Accuracy and complications of CT-guided core needle biopsy of peripheral nerve sheath tumors. Skelet. Radiol. 2015, 44, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.S.; Ehlers, M.E.; Lee, K.S. Preoperative Ultrasound-Guided Wire Localization of the Lateral Femoral Cutaneous Nerve. Oper Neurosurg 2017, 13, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Burks, S.S.; Jose, J.; Levi, A.D. Ultrasound-Guided Needle Localization Wires in Peripheral Nerve Injuries With Long Segmental Defects: Technical Case Report. Oper Neurosurg 2020, 20, E60–E65. [Google Scholar] [CrossRef] [PubMed]
- Bittman, R.W.; Peters, G.L.; Newsome, J.M.; Friedberg, E.B.; Mitchell, J.W.; Knight, J.M.; Prologo, J.D. Percutaneous Image-Guided Cryoneurolysis. Am. J. Roentgenol. 2018, 210, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Martell, B.; Jesse, M.K.; Lowry, P. CT-Guided Cryoablation of a Peripheral Nerve Sheath Tumor. J. Vasc. Interv. Radiol. 2016, 27, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Mrowczynski, O.; Mau, C.; Nguyen, D.T.; Sarwani, N.; Rizk, E.; Harbaugh, K. Percutaneous Radiofrequency Ablation for the Treatment of Peripheral Nerve Sheath Tumors: A Case Report and Review of the Literature. Cureus 2018, 10, e2534. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, M.; Yang, H.; Qian, Z.; Wang, G.; Wu, G.; Zhu, X.; Sun, Z. Preoperative embolization facilitating a posterior approach for the surgical resection of giant sacral neurogenic tumors. Oncol. Lett. 2013, 6, 251–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez, F.J.; Folpe, A.L.; Giannini, C.; Perry, A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012, 123, 295–319. [Google Scholar] [CrossRef]
- Martin, E.; Acem, I.; Grünhagen, D.J.; Bovée, J.V.M.G.; Verhoef, C. Prognostic Significance of Immunohistochemical Markers and Genetic Alterations in Malignant Peripheral Nerve Sheath Tumors: A Systematic Review. Front. Oncol. 2020, 10, 594069. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Villanueva-Meyer, J.E.; Goode, B.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Bastian, B.C.; Chamyan, G.; Maher, O.M.; Khatib, Z.; et al. The genetic landscape of ganglioglioma. Acta Neuropathol. Commun. 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, J.R.; Lamps, L.W.; McKenney, J.K. Rosai and Ackerman’s Surgical Pathology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumors: News and perspectives. Pathologica 2020, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Angelini, A. F|Musculoskeletal Oncology. In The EFORT White Book: “Orthopaedics and Traumatology in Europe”; Dennis Barber Ltd.: Lowestoft, UK, 2021. [Google Scholar] [PubMed]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.; Brodowicz, T.; Broto, J.M.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef]
- Chouliaras, K.; Patel, N.; Senehi, R.; Ethun, C.G.; Poultsides, G.; Grignol, V.; Gamblin, T.C.; Roggin, K.K.; Fields, R.C.; D’Agostino, R., Jr.; et al. Impact of resection margin on outcomes in high-grade soft tissue sarcomas of the extremity—A USSC analysis. J. Surg. Oncol. 2021, 123, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kroep, J.R.; Ouali, M.; Gelderblom, H.; Le Cesne, A.; Dekker, T.J.A.; Van Glabbeke, M.; Hogendoorn, P.C.W.; Hohenberger, P. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: An EORTC soft tissue and bone sarcoma group study. Ann. Oncol. 2011, 22, 207–214. [Google Scholar] [CrossRef]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Han, K.; Tiel, R.L.; Murovic, J.A.; Kline, D.G. Surgical outcomes of 654 ulnar nerve lesions. J. Neurosurg. 2003, 98, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Gundle, K.R.; Kafchinski, L.; Gupta, S.; Griffin, A.M.; Dickson, B.C.; Chung, P.W.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S.; Ferguson, P.C. Analysis of Margin Classification Systems for Assessing the Risk of Local Recurrence After Soft Tissue Sarcoma Resection. J. Clin. Oncol. 2018, 36, 704–709. [Google Scholar] [CrossRef]
- Ducic, I.; Fu, R.; Iorio, M.L. Innovative treatment of peripheral nerve injuries: Combined reconstructive concepts. Ann. Plast. Surg. 2012, 68, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Runu, R.; Ahmed, W.; Kumar, I.; Subash, A. A Retrospective Cohort Analysis of Limb Salvage Surgery Using Mega Prosthesis in Bone Tumors at a Tertiary Care Centre in Eastern India. Cureus 2022, 14, e28959. [Google Scholar] [PubMed]
- Hussein, M.; Gupta, H.; Ahuja, A.; Thaker, S. Imaging recommendations for soft tissue sarcomas: Model guidelines from diagnosis to post-treatment follow-up. Clin. Radiol. 2024, 79, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.L.M.; Delaney, T.F.; O’Sullivan, B.; Keus, R.B.; Le Pechoux, C.; Olmi, P.; Poulsen, J.-P.; Seddon, B.; Wang, D. Radiotherapy for management of extremity soft tissue sarcomas: Why, when, and where? Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 572–580. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Kaushal, A.; Citrin, D. The role of radiation therapy in the management of sarcomas. Surg. Clin. North. Am. 2008, 88, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Kepka, L.; DeLaney, T.F.; Suit, H.D.; Goldberg, S.I. Results of radiation therapy for unresected soft-tissue sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 852–859. [Google Scholar] [CrossRef]
- Schöffski, P.; Cornillie, J.; Wozniak, A.; Li, H.; Hompes, D. Soft tissue sarcoma: An update on systemic treatment options for patients with advanced disease. Oncol. Res. Treat. 2014, 37, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Okoye, C.C.; Patel, R.B.; Sahgal, A.; Foote, M.; Redmond, K.J.; Hofstetter, C.; Saigal, R.; Mossa-Basha, M.; Yuh, W.; et al. Stereotactic body radiotherapy for benign spinal tumors: Meningiomas, schwannomas, and neurofibromas. J. Radiosurgery SBRT 2019, 6, 167. [Google Scholar]
- Naghavi, A.O.; Fernandez, D.C.; Mesko, N.; Juloori, A.; Martinez, A.; Scott, J.G.; Shah, C.; Harrison, L.B. American Brachytherapy Society consensus statement for soft tissue sarcoma brachytherapy. Brachytherapy 2017, 16, 466–489. [Google Scholar] [CrossRef]
- Richards, P. Nerve injuries: Operative results for major nerve injuries, entrapments and tumors. D. G. Kline and A. R. Hudson. 265 × 187 mm. Pp. 611. Illustrated. 1995. Philadelphia, Pennsylvania: W. B. Saunders. £100. Br. J. Surg. 1996, 83, 716. [Google Scholar] [CrossRef]
- Date, R.; Muramatsu, K.; Ihara, K.; Taguchi, T. Advantages of intra-capsular micro-enucleation of schwannoma arising from extremities. Acta Neurochir. 2012, 154, 173–178, discussion 178. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Diagnosis and Treatment of Peripheral and Cranial Nerve Tumors with Expert Recommendations: An EUropean Network for RAre CANcers (EURACAN) Initiative. Cancers 2023, 15, 1930. [Google Scholar] [CrossRef]
- Levi, A.D.; Ross, A.L.; Cuartas, E.; Qadir, R.; Temple, H.T. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery 2010, 66, 833–840. [Google Scholar] [CrossRef]
- Kline, D.G.; Kim, D.; Midha, R.; Harsh, C.; Tiel, R. Management and results of sciatic nerve injuries: A 24-year experience. J. Neurosurg. 1998, 89, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.; Gillespie, A.; Tsokos, M.; Ondos, J.; Dombi, E.; Camphausen, K.; Widemann, B.C.; Kaushal, A. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front. Oncol. 2014, 4, 324. [Google Scholar] [CrossRef]
- Chang, J.H.; Shin, J.H.; Yamada, Y.J.; Mesfin, A.; Fehlings, M.G.; Rhines, L.D.; Sahgal, A. Stereotactic Body Radiotherapy for Spinal Metastases: What are the Risks and How Do We Minimize Them? Spine 2016, 41 (Suppl. S20), S238–S245. [Google Scholar] [CrossRef]
- Vaassen, P.; Dürr, N.R.; Rosenbaum, T. Treatment of Plexiform Neurofibromas with MEK Inhibitors: First Results with a New Therapeutic Option. Neuropediatrics 2022, 53, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.E.; Belzberg, A.J.; Crawford, J.R.; Hirbe, A.C.; Wang, Z.J. Treatment decisions and the use of MEK inhibitors for children with neurofibromatosis type 1-related plexiform neurofibromas. BMC Cancer 2023, 23, 553. [Google Scholar] [CrossRef]
- Maki, R.G.; D’Adamo, D.R.; Keohan, M.L.; Saulle, M.; Schuetze, S.M.; Undevia, S.D.; Livingston, M.B.; Cooney, M.M.; Hensley, M.L.; Mita, M.M.; et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J. Clin. Oncol. 2009, 27, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Catalanotti, F.; Solit, D.B.; Pulitzer, M.P.; Berger, M.F.; Scott, S.N.; Iyriboz, T.; Lacouture, M.E.; Panageas, K.S.; Wolchok, J.D.; Carvajal, R.D.; et al. Phase II trial of MEK inhibitor selumetinib (AZD6244, ARRY-142886) in patients with BRAFV600E/K-mutated melanoma. Clin. Cancer Res. 2013, 19, 2257–2264. [Google Scholar] [CrossRef]
- Vickers, A.J.; Thiruthaneeswaran, N.; Coyle, C.; Manoharan, P.; Wylie, J.; Kershaw, L.; Choudhury, A.; Mcwilliam, A. Does magnetic resonance imaging improve soft tissue sarcoma contouring for radiotherapy? BJR Open 2019, 1, 20180022. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Schmitz, F.; Grözinger, M.; Sedaghat, M. Malignant peripheral nerve sheath tumors in magnetic resonance imaging: Primary and recurrent tumor appearance, post-treatment changes, and metastases. Pol. J. Radiol. 2020, 85, e196–e201. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Van Goethem, J.W.M.; van den Hauwe, L.; Parizel, P.M. Imaging of the postoperative spine. Semin. Roentgenol. 2006, 41, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Masaryk, T.J.; Schrader, M.; Gentili, A.; Bohlman, H.; Modic, M.T. MR imaging of the postoperative lumbar spine: Assessment with gadopentetate dimeglumine. Am. J. Neuroradiol. 1990, 11, 771–776. [Google Scholar] [CrossRef]
- Brandão LAYoung Poussaint, T. Posterior Fossa Tumors. Neuroimaging Clin. N. Am. 2017, 27, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.M.Y.; Tyler, P.A.; Saifuddin, A.; Padhani, A.R. Diffusion-weighted imaging (DWI) in musculoskeletal MRI: A critical review. Skelet. Radiol. 2011, 40, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Belhawi, S.M.K.; Hoefnagels, F.W.A.; Baaijen, J.C.; Sanchez Aliaga, E.; Reijneveld, J.C.; Heimans, J.J.; Barkhof, F.; Vandertop, W.P.; De Witt Hamer, P.C. Early post-operative MRI overestimates residual tumor after resection of gliomas with no or minimal enhancement. Eur. Radiol. 2011, 21, 1526–1534. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 1996, 111, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Buchbender, C.; Heusner, T.A.; Lauenstein, T.C.; Bockisch, A.; Antoch, G. Oncologic PET/MRI, part 2: Bone tumors, soft-tissue tumors, melanoma, and lymphoma. J. Nucl. Med. 2012, 53, 1244–1252. [Google Scholar] [CrossRef]
- Stoll, G.; Wilder-Smith, E.; Bendszus, M. Imaging of the peripheral nervous system. Handb. Clin. Neurol. 2013, 115, 137–153. [Google Scholar]
- Peeken, J.C.; Spraker, M.B.; Knebel, C.; Dapper, H.; Pfeiffer, D.; Devecka, M.; Thamer, A.; Shouman, M.A.; Ott, A.; von Eisenhart-Rothe, R. Tumor grading of soft tissue sarcomas using MRI-based radiomics. EBioMedicine 2019, 48, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Jansma, C.Y.; Wan, X.; Acem, I.; Spaanderman, D.J.; Visser, J.J.; Hanff, D.; Taal, W.; Verhoef, C.; Klein, S.; Martin, E.; et al. Preoperative Classification of Peripheral Nerve Sheath Tumors on MRI Using Radiomics. Cancers 2024, 16, 2039. [Google Scholar] [CrossRef]
- Ristow, I.; Madesta, F.; Well, L.; Shenas, F.; Wright, F.; Molwitz, I.; Farschtschi, S.; Bannas, P.; Adam, G.; Mautner, V.F.; et al. Evaluation of magnetic resonance imaging-based radiomics characteristics for differentiation of benign and malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Neuro-Oncology 2022, 24, 1790–1798. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.-N.; Wang, M.-H.; Ni, Z.-Y.; Jiang, W.-H.; Chung, M.; Wei, C.-J.; Wang, Z.-C. Image-Based Differentiation of Benign and Malignant Peripheral Nerve Sheath Tumors in Neurofibromatosis Type 1. Front. Oncol. 2022, 12, 898971. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Velez, E.; Najafi, M.H.; Matcuk, G.; Gholamrezanezhad, A. PET imaging of peripheral nerve tumors. PET Clin. 2019, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rangger, C.; Haubner, R. Radiolabelled peptides for positron emission tomography and endoradiotherapy in oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C. Detection of Circulating Tumor DNA in Early-and Late-Stage Human. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging applications of bedside 3D printing in plastic surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef] [PubMed]









| MRI Sequence | Rationale |
|---|---|
| Axial T1-weighted 3.0-mm | Defines normal nerve anatomy; loss of signal from fat suggests nerve involvement. |
| Axial T2-weighted with fat saturation | Distinguishes tumors from fat and highlights intraneural cystic/oedematous components. |
| Axial T2-weighted STIR | Excellent for detecting nerve inflammation and cystic lesions. |
| Axial Diffusion-weighted imaging (DWI) | Detects highly cellular tumors; ADC values help distinguish benign vs. malignant tumors. |
| Coronal/Sagittal T2-weighted FS or STIR | Assesses nerve root and plexus involvement; highlights tumor–nerve relationship and extension along fascicles. |
| MR Neurography (MRN) | High-resolution nerve imaging (3D STIR/SPACE) to delineate fascicular architecture and perineural tissues. |
| Sagittal/Coronal Diffusion Tensor Imaging (DTI) | Quantifies nerve integrity; evaluates directionality of nerve fibers to identify disruption by the tumor. |
| Axial T1-weighted with Gadolinium FS | Enhances tumoral components and tumor delineation from surrounding tissues. |
| Sagittal/Coronal T1-weighted with Gadolinium FS | Assesses the extent of nerve involvement, especially post-treatment follow-up, to detect residual or recurrent tumor. |
| Dynamic Contrast-Enhanced (DCE) imaging | Evaluates perfusion characteristics of the tumor to differentiate benign from malignant based on enhancement kinetics. |
| Feature | Benign PNSTs (BPNSTs) | Malignant PNSTs (MPNSTs) |
|---|---|---|
| Size | Generally smaller (<5 cm). | Typically larger (>5 cm). |
| Margins | Well-defined, smooth margins. | Ill-defined, irregular margins. |
| Growth Rate | Slow-growing, stable over time. | Rapid growth or change in size over time. |
| T1-w Imaging | Isointense to slightly hypointense relative to muscle. | Isointense to hypointense relative to muscle, often heterogeneous. |
| T2-w Imaging | Hyperintense with possible “target sign” (central low, peripheral high signal). | Hyperintense, often heterogeneous without a clear “target sign”. |
| Enhancement Pattern | Homogeneous or mildly heterogeneous enhancement. | Markedly heterogeneous enhancement, often irregular or nodular. |
| Cystic Degeneration | Common, especially in schwannomas. | May be present but often associated with necrosis. |
| Peritumoral Oedema | Usually absent or minimal. | Frequently present with surrounding soft tissue oedema. |
| Invasion of Adjacent Structures | Absent; typically does not invade surrounding tissues. | Present; may invade adjacent muscles, bones, or neurovascular structures. |
| Bone Involvement | Absent; no bone destruction. | May cause bone erosion or destruction if adjacent to bony structures. |
| Nerve Enlargement | Fusiform enlargement, often uniform. | Irregular nerve enlargement, may show abrupt transitions. |
| Pain | Often less painful; pain is not a predominant feature. | Frequently associated with pain and neurological deficits. |
| Tinel’s Sign | May be positive, especially in schwannomas. | Can be positive, but often with more severe symptoms. |
| Clinical Context | Commonly associated with neurofibromatosis type 1 (NF1) or sporadic. | Often associated with neurofibromatosis type 1 (NF1) or history of prior radiation. |
| Specialty | Key Information Required |
|---|---|
| Interventional Radiologist (Pre-biopsy) |
|
| Surgeon (Surgical Planning) |
|
| Radiation Oncologist (Radiation Planning for MPNST) |
|
| Radiologist (Post-treatment Imaging) |
|
| Radiologist (Malignant Transformation of Benign Tumor) |
|
| Diagnosis: |
|
| Intervention: |
|
| Treatment Planning: |
|
| Management |
|
| Post-operative Care |
|
| General Recommendation |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirodkar, K.; Hussein, M.; Reddy, P.S.; Shah, A.B.; Raniga, S.; Pal, D.; Iyengar, K.P.; Botchu, R. Imaging of Peripheral Intraneural Tumors: A Comprehensive Review for Radiologists. Cancers 2025, 17, 246. https://doi.org/10.3390/cancers17020246
Shirodkar K, Hussein M, Reddy PS, Shah AB, Raniga S, Pal D, Iyengar KP, Botchu R. Imaging of Peripheral Intraneural Tumors: A Comprehensive Review for Radiologists. Cancers. 2025; 17(2):246. https://doi.org/10.3390/cancers17020246
Chicago/Turabian StyleShirodkar, Kapil, Mohsin Hussein, Pellakuru Saavi Reddy, Ankit B. Shah, Sameer Raniga, Devpriyo Pal, Karthikeyan P. Iyengar, and Rajesh Botchu. 2025. "Imaging of Peripheral Intraneural Tumors: A Comprehensive Review for Radiologists" Cancers 17, no. 2: 246. https://doi.org/10.3390/cancers17020246
APA StyleShirodkar, K., Hussein, M., Reddy, P. S., Shah, A. B., Raniga, S., Pal, D., Iyengar, K. P., & Botchu, R. (2025). Imaging of Peripheral Intraneural Tumors: A Comprehensive Review for Radiologists. Cancers, 17(2), 246. https://doi.org/10.3390/cancers17020246






