SORL1-Mediated EGFR and FGFR4 Regulation Enhances Chemoresistance in Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Clinical Sample Collection and Human Transcriptomic Array (HTA)
2.3. Western Blot
2.4. RNA Extraction and Quantitative Real-Time PCR (QPCR)
2.5. Transient Transfection and Stable Knockdown of SORL1 Expression in Ovarian Cancer Cell Lines
2.6. Cell Viability, Apoptosis (Caspase-3/7 Activity), and Cell Cycle Analyses
2.7. Co-Immunoprecipitation (Co-IP)
2.8. Proximity Ligation Assay (PLA)
2.9. Animal Models
2.10. Statistical Analysis
3. Results
3.1. SORL1 Is Upregulated in Recurrent Tumors in Ovarian Cancer
3.2. SORL1 Protein Level Is Increased in Ovarian Cancer Cells That Survived Carboplatin Treatment
3.3. SORL1 Expression Is Upregulated in Ovarian Cancer in Comparison to the Normal Ovary
3.4. A Higher Level of SORL1 Expression Is Associated with Shorter Survival in Ovarian Cancer
3.5. Upregulated Expression of SORL1 Promotes Cell Proliferation and Resistance to Carboplatin Treatment in Ovarian Cancer Cell Lines
3.6. SORL1 Knockdown Inhibits Cell Proliferation and Improves Sensitivity to Carboplatin-Induced Apoptosis in Ovarian Cancer Cell Lines
3.7. SORL1 Regulates EGF and FGF Signaling Through Interactions with EGFR and FGFR4 in Ovarian Cancer Cell Lines
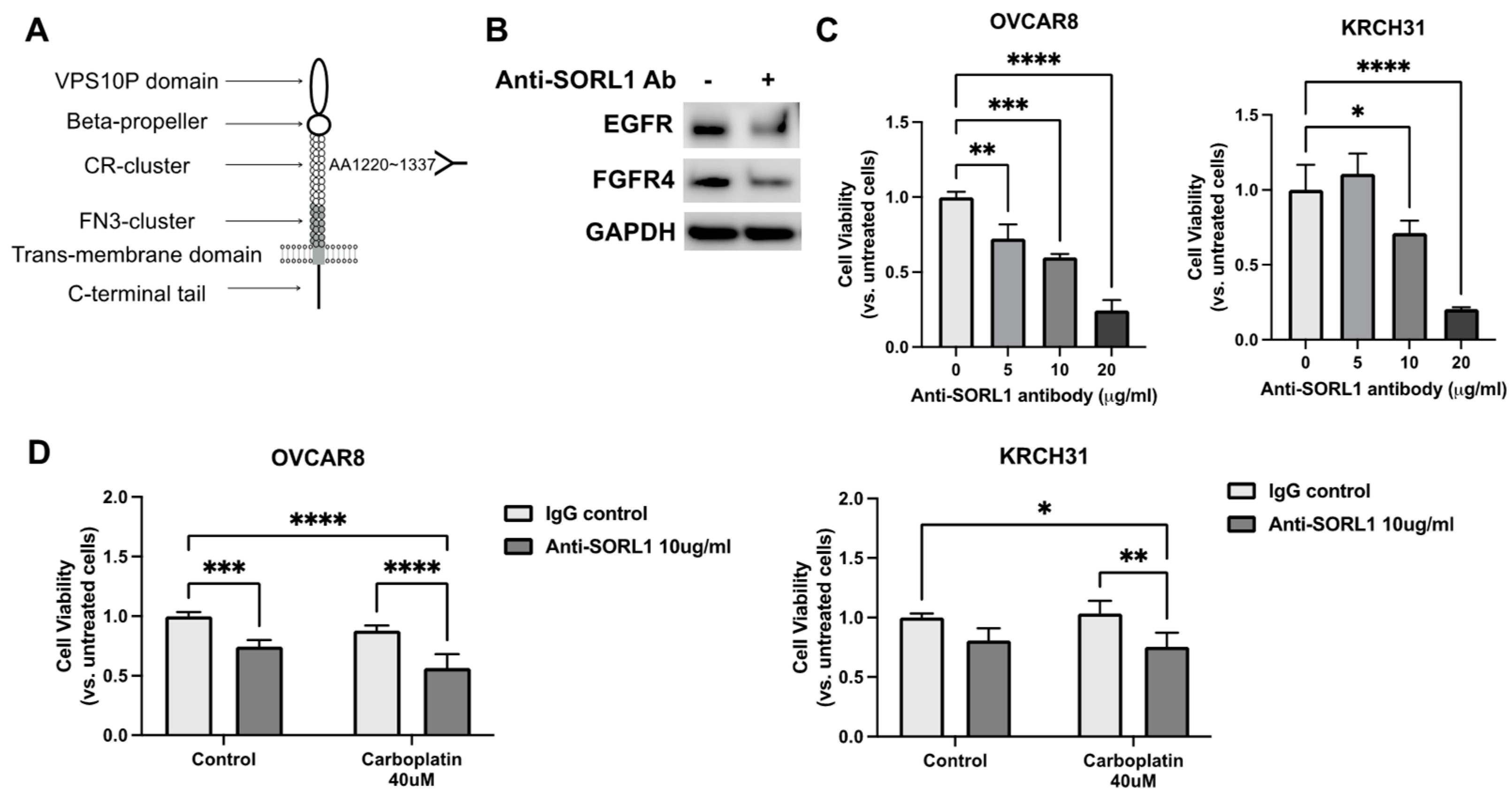
3.8. Anti-SORL1 Antibody Reduces Viability of Ovarian Cancer Cell Lines and Improves Chemosensitivity
3.9. SORL1 Knockdown Inhibits Tumor Growth in Xenograft Mouse Model of Ovarian Cancer and Downregulates EGFR and FGFR4
3.10. Treatment with FGF401, FGFR4-Specific Inhibitor, Enhances Sensitivity to Carboplatin of SORL1-Expressing Ovarian Cancer in Xenograft Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, O.M.; Reiche, J.; Schmidt, V.; Gotthardt, M.; Spoelgen, R.; Behlke, J.; von Arnim, C.A.; Breiderhoff, T.; Jansen, P.; Wu, X.; et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2005, 102, 13461–13466. [Google Scholar] [CrossRef] [PubMed]
- Mehmedbasic, A.; Christensen, S.K.; Nilsson, J.; Ruetschi, U.; Gustafsen, C.; Poulsen, A.S.; Rasmussen, R.W.; Fjorback, A.N.; Larson, G.; Andersen, O.M. SorLA complement-type repeat domains protect the amyloid precursor protein against processing. J. Biol. Chem. 2015, 290, 3359–3376. [Google Scholar] [CrossRef]
- Hung, C.; Tuck, E.; Stubbs, V.; van der Lee, S.J.; Aalfs, C.; van Spaendonk, R.; Scheltens, P.; Hardy, J.; Holstege, H.; Livesey, F.J. SORL1 deficiency in human excitatory neurons causes APP-dependent defects in the endolysosome-autophagy network. Cell Rep. 2021, 35, 109259. [Google Scholar] [CrossRef]
- Rogaeva, E.; Meng, Y.; Lee, J.H.; Gu, Y.; Kawarai, T.; Zou, F.; Katayama, T.; Baldwin, C.T.; Cheng, R.; Hasegawa, H.; et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007, 39, 168–177. [Google Scholar] [CrossRef]
- Mishra, S.; Knupp, A.; Szabo, M.P.; Williams, C.A.; Kinoshita, C.; Hailey, D.W.; Wang, Y.; Andersen, O.M.; Young, J.E. The Alzheimer’s gene SORL1 is a regulator of endosomal traffic and recycling in human neurons. Cell Mol. Life Sci. 2022, 79, 162. [Google Scholar] [CrossRef] [PubMed]
- Knupp, A.; Mishra, S.; Martinez, R.; Braggin, J.E.; Szabo, M.; Kinoshita, C.; Hailey, D.W.; Small, S.A.; Jayadev, S.; Young, J.E. Depletion of the AD Risk Gene SORL1 Selectively Impairs Neuronal Endosomal Traffic Independent of Amyloidogenic APP Processing. Cell Rep. 2020, 31, 107719. [Google Scholar] [CrossRef]
- Pietilä, M.; Sahgal, P.; Peuhu, E.; Jäntti, N.Z.; Paatero, I.; Närvä, E.; Al-Akhrass, H.; Lilja, J.; Georgiadou, M.; Andersen, O.M.; et al. SORLA regulates endosomal trafficking and oncogenic fitness of HER2. Nat. Commun. 2019, 10, 2340. [Google Scholar] [CrossRef]
- Al-Akhrass, H.; Pietilä, M.; Lilja, J.; Vesilahti, E.; Anttila, J.M.; Haikala, H.M.; Munne, P.M.; Klefström, J.; Peuhu, E.; Ivaska, J. Sortilin-related receptor is a druggable therapeutic target in breast cancer. Mol. Oncol. 2022, 16, 116–129. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; Yang, B.; Xu, Q.; Zhu, X.; Hu, L.; Teng, Y. SORL1 stabilizes ABCB1 to promote cisplatin resistance in ovarian cancer. Funct. Integr. Genom. 2023, 23, 147. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Barnes, B.M.; Nelson, L.; Tighe, A.; Burghel, G.J.; Lin, I.-H.; Desai, S.; McGrail, J.C.; Morgan, R.D.; Taylor, S.S. Distinct transcriptional programs stratify ovarian cancer cell lines into the five major histological subtypes. Genome Med. 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Wilken, J.A.; Badri, T.; Cross, S.; Raji, R.; Santin, A.D.; Schwartz, P.; Branscum, A.J.; Baron, A.T.; I Sakhitab, A.; Maihle, N.J. EGFR/HER-targeted therapeutics in ovarian cancer. Future Med. Chem. 2012, 4, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, B.A.; Kirillova, I.; Richard, R.E.; Israeli, D.; Yablonka-Reuveni, Z. FGFR4 and its novel splice form in myogenic cells: Interplay of glycosylation and tyrosine phosphorylation. J. Cell Physiol. 2008, 215, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Mitsuhashi, N.; Shimizu, H.; Kimura, F.; Yoshidome, H.; Otsuka, M.; Kato, A.; Shida, T.; Okamura, D.; Miyazaki, M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 2012, 12, 56. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Futami, T.; Kawase, T.; Mori, K.; Asaumi, M.; Kihara, R.; Shindoh, N.; Kuromitsu, S. Identification of a novel oncogenic mutation of FGFR4 in gastric cancer. Sci. Rep. 2019, 9, 14627. [Google Scholar] [CrossRef]
- Zaid, T.M.; Yeung, T.-L.; Thompson, M.S.; Leung, C.S.; Harding, T.; Co, N.-N.; Schmandt, R.S.; Kwan, S.-Y.; Rodriguez-Aguay, C.; Lopez-Berestein, G.; et al. Identification of FGFR4 as a potential therapeutic target for advanced-stage, high-grade serous ovarian cancer. Clin. Cancer Res. 2013, 19, 809–820. [Google Scholar] [CrossRef]
- Hu, L.; Cong, L. Fibroblast growth factor 19 is correlated with an unfavorable prognosis and promotes progression by activating fibroblast growth factor receptor 4 in advanced-stage serous ovarian cancer. Oncol. Rep. 2015, 34, 2683–2691. [Google Scholar] [CrossRef]
- Mitra, A.K.; Davis, D.A.; Tomar, S.; Roy, L.; Gurler, H.; Xie, J.; Lantvit, D.D.; Cardenas, H.; Fang, F.; Liu, Y.; et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol. 2015, 138, 372–377. [Google Scholar] [CrossRef]
- Haley, J.; Tomar, S.; Pulliam, N.; Xiong, S.; Perkins, S.M.; Karpf, A.R.; Mitra, S.; Nephew, K.P.; Mitra, A.K. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget 2016, 7, 32810–32820. [Google Scholar] [CrossRef]
- Alvero, A.B.; O’Malley, D.; Brown, D.; Kelly, G.; Garg, M.; Chen, W.; Rutherford, T.; Mor, G. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer 2006, 106, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.; Lopez, S.; Zeybek, B.; Bellone, S.; Bonazzoli, E.; Pelligra, S.; Zammataro, L.; Manzano, A.; Manara, P.; Bianchi, A.; et al. Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer. Front. Oncol. 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kostylev, M.A.; Lee, S.; Strittmatter, S.M. Systematic and standardized comparison of reported amyloid-beta receptors for sufficiency, affinity, and Alzheimer’s disease relevance. J. Biol. Chem. 2019, 294, 6042–6053. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.N.; Finkler, N.; Edwards, R.P.; Garcia, A.A.; Crozier, M.; Irwin, D.H.; Barrett, E. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: Results from a phase II multicenter study. Int. J. Gynecol. Cancer 2005, 15, 785–792. [Google Scholar] [CrossRef]
- Schilder, R.J.; Sill, M.W.; Chen, X.; Darcy, K.M.; Decesare, S.L.; Lewandowski, G.; Lee, R.B.; Arciero, C.A.; Wu, H.; Godwin, A.K. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: A Gynecologic Oncology Group Study. Clin. Cancer Res. 2005, 11, 5539–5548. [Google Scholar] [CrossRef]
- Blank, S.V.; Christos, P.; Curtin, J.P.; Goldman, N.; Runowicz, C.D.; Sparano, J.A.; Liebes, L.; Chen, H.X.; Muggia, F.M. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: A phase II study based on surgical reassessment. Gynecol. Oncol. 2010, 119, 451–456. [Google Scholar] [CrossRef]
- Hirte, H.; Oza, A.; Swenerton, K.; Ellard, S.; Grimshaw, R.; Fisher, B.; Tsao, M.; Seymour, L. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149). Gynecol. Oncol. 2010, 118, 308–312. [Google Scholar] [CrossRef]
- Vergote, I.B.; Jimeno, A.; Joly, F.; Katsaros, D.; Coens, C.; Despierre, E.; Marth, C.; Hall, M.; Steer, C.B.; Colombo, N.; et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J. Clin. Oncol. 2014, 32, 320–326. [Google Scholar]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Schilder, R.J.; Hall, L.; Monks, A.; Handel, L.M.; Fornace, A.J., Jr.; Ozols, R.F.; Fojo, A.T.; Hamilton, T.C. Metallothionein gene expression and resistance to cisplatin in human ovarian cancer. Int. J. Cancer 1990, 45, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Neal, A.; Dibernardo, G.; Raheseparian, N.; Moatamed, N.A.; Memarzadeh, S. Efficacy of birinapant in combination with carboplatin in targeting platinum-resistant epithelial ovarian cancers. Int. J. Oncol. 2022, 60, 35. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Ryu, J.Y.; Cho, Y.J.; Choi, J.Y.; Choi, J.J.; Choi, C.H.; Sa, J.K.; Hwang, J.R.; Lee, J.W. The anti-tumor effects of AZD4547 on ovarian cancer cells: Differential responses based on c-Met and FGF19/FGFR4 expression. Cancer Cell Int. 2024, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Kim, J.Y.; Song, C.H.; Kim, M.; Do, Y.T.; Vo, T.T.L.; Choi, E.; Ha, E.; Seo, J.H.; Shin, S.-J. The FGFR Family Inhibitor AZD4547 Exerts an Antitumor Effect in Ovarian Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10817. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Adler, F.; Buhles, A.; Stamm, C.; Fairhurst, R.A.; Kiffe, M.; Sterker, D.; Centeleghe, M.; Wartmann, M.; Kinyamu-Akunda, J.; et al. FGF401, A First-In-Class Highly Selective and Potent FGFR4 Inhibitor for the Treatment of FGF19-Driven Hepatocellular Cancer. Mol. Cancer Ther. 2019, 18, 2194–2206. [Google Scholar] [CrossRef]
- Chan, S.L.; Schuler, M.; Kang, Y.K.; Yen, C.J.; Edeline, J.; Choo, S.P.; Lin, C.C.; Okusaka, T.; Weiss, K.H.; Macarulla, T.; et al. A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors. J. Exp. Clin. Cancer Res. 2022, 41, 189. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Bi, F.; Ge, Z.; Mansolf, M.; Hartwich, T.M.P.; Kolesnyk, V.; Yang, K.; Park, W.; Kim, D.; Grechukhina, O.; et al. SORL1-Mediated EGFR and FGFR4 Regulation Enhances Chemoresistance in Ovarian Cancer. Cancers 2025, 17, 244. https://doi.org/10.3390/cancers17020244
Jiang Z, Bi F, Ge Z, Mansolf M, Hartwich TMP, Kolesnyk V, Yang K, Park W, Kim D, Grechukhina O, et al. SORL1-Mediated EGFR and FGFR4 Regulation Enhances Chemoresistance in Ovarian Cancer. Cancers. 2025; 17(2):244. https://doi.org/10.3390/cancers17020244
Chicago/Turabian StyleJiang, Ziyan, Fangfang Bi, Zhiping Ge, Miranda Mansolf, Tobias M. P. Hartwich, Viktoriia Kolesnyk, Kevin Yang, Wonmin Park, Dongin Kim, Olga Grechukhina, and et al. 2025. "SORL1-Mediated EGFR and FGFR4 Regulation Enhances Chemoresistance in Ovarian Cancer" Cancers 17, no. 2: 244. https://doi.org/10.3390/cancers17020244
APA StyleJiang, Z., Bi, F., Ge, Z., Mansolf, M., Hartwich, T. M. P., Kolesnyk, V., Yang, K., Park, W., Kim, D., Grechukhina, O., Hui, P., Kim, S. W., & Yang-Hartwich, Y. (2025). SORL1-Mediated EGFR and FGFR4 Regulation Enhances Chemoresistance in Ovarian Cancer. Cancers, 17(2), 244. https://doi.org/10.3390/cancers17020244







