Association of Increased CT-Attenuation of Visceral Adipose Tissue After Surgery with Poor Survival Outcomes in Patients with Stage II–III Gastric Cancer: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurements of CT-Attenuation of Adipose Tissue
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlation Analysis
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Korean practice guidelines for gastric cancer 2022: An evidence-based, multidisciplinary approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, S.Y.; Lee, C.M.; Ahn, S.H.; Park, D.J.; Kim, H.H. Factors predicting peritoneal recurrence in advanced gastric cancer: Implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer 2014, 17, 529–536. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.; Sano, T. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Squires, M.H.; Poultsides, G.A.; Fields, R.C.; Schmidt, C.; Weber, S.M.; Votanopoulos, K.; Maithel, S.K.; et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J. Am. Coll. Surg. 2014, 219, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, Y.; Du, J.; Zou, Z.; Zhang, Y.; Wu, N.; Wang, Q.; Yuan, L.; Liu, B. Peritoneal metastasis in relation to outcome and therapeutic strategy in gastric cancer. Transl. Cancer Res. 2017, 6, 149–156. [Google Scholar] [CrossRef]
- Szor, D.J.; Pereira, M.A.; Ramos, M.; Nigro, B.C.; Dias, A.R.; Ribeiro, U., Jr. Peritoneal recurrence in gastric cancer after curative gastrectomy: Risk factors and predictive score model. J. Gastrointest. Surg. 2024, 29, 101850. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Ohara, M.; Domen, H.; Shichinohe, T.; Hirano, S.; Ishizaka, M. Differences in risk factors between patterns of recurrence in patients after curative resection for advanced gastric carcinoma. World J. Surg. Oncol. 2013, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Son, M.W.; Chung, I.K.; Cho, Y.S.; Lee, M.S.; Lee, S.M. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer 2020, 23, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, H.; Zhou, H.; Cheng, Z.; Liu, G.; Huang, C.; Dou, R.; Liu, F.; You, X. Galectin-1 promotes gastric cancer peritoneal metastasis through peritoneal fibrosis. BMC Cancer 2023, 23, 559. [Google Scholar] [CrossRef]
- Hamabe-Horiike, T.; Harada, S.I.; Yoshida, K.; Kinoshita, J.; Yamaguchi, T.; Fushida, S. Adipocytes contribute to tumor progression and invasion of peritoneal metastasis by interacting with gastric cancer cells as cancer associated fibroblasts. Cancer Rep. 2023, 6, e1647. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, P.; Lv, J.; Ge, H.; Yan, Z.; Huang, S.; Li, B.; Xu, H.; Yang, L.; Xu, Z.; et al. Peritoneal high-fat environment promotes peritoneal metastasis of gastric cancer cells through activation of NSUN2-mediated ORAI2 m5C modification. Oncogene 2023, 42, 1980–1993. [Google Scholar] [CrossRef]
- Murphy, R.A.; Register, T.C.; Shively, C.A.; Carr, J.J.; Ge, Y.; Heilbrun, M.E.; Cummings, S.R.; Koster, A.; Nevitt, M.C.; Satterfield, S.; et al. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 109–117. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, J.W.; Jang, S.H.; Lee, H.J.; Lee, J.H.; Oh, M.H.; Lee, S.M. Prognostic significance of imaging features of peritumoral adipose tissue in FDG PET/CT of patients with colorectal cancer. Eur. J. Radiol. 2021, 145, 110047. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, S.M.; Chung, Y.A. Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pancreatic adenocarcinoma. Clin. Radiol. 2018, 73, 1056.e1–1056.e10. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Moctezuma-Velazquez, C.; Meza-Junco, J.; Baracos, V.E.; DunichandHoedl, A.R.; Ghosh, S.; Sarlieve, P.; Owen, R.J.; Kneteman, N.; Montano-Loza, A.J. Visceral adipose tissue radiodensity is linked to prognosis in hepatocellular carcinoma patients treated with selective internal radiation therapy. Cancers 2020, 12, 356. [Google Scholar] [CrossRef]
- Song, G.J.; Ahn, H.; Son, M.W.; Yun, J.H.; Lee, M.S.; Lee, S.M. Adipose tissue quantification improves the prognostic value of GLIM criteria in advanced gastric cancer patients. Nutrients 2024, 16, 728. [Google Scholar] [CrossRef]
- Park, K.B.; Kwon, O.K.; Yu, W. Midterm body composition changes after open distal gastrectomy for early gastric cancer. Ann. Surg. Treat. Res. 2018, 95, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.A.; Heo, S.J.; et al. Marked loss of muscle, visceral fat, or subcutaneous fat after gastrectomy predicts poor survival in advanced gastric cancer: Single-center study from the CLASSIC trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Lee, J.W.; Yoo, I.D.; Hong, S.P.; Kang, B.; Kim, J.S.; Kim, Y.K.; Bae, S.H.; Jang, S.J.; Lee, S.M. Prognostic impact of visceral adipose tissue imaging parameters in patients with cholangiocarcinoma after surgical resection. Int. J. Mol. Sci. 2024, 25, 3939. [Google Scholar] [CrossRef]
- Sugawara, K.; Taguchi, S.; Gonoi, W.; Hanaoka, S.; Shiomi, S.; Kishitani, K.; Uemura, Y.; Akamatsu, N.; Inui, S.; Tanaka, K.; et al. Integrated impact of multiple body composition parameters on overall survival in gastrointestinal or genitourinary cancers: A descriptive cohort study. JPEN J. Parenter. Enteral. Nutr. 2024, 48, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, G.; Yeh, T.S. Visceral-to-subcutaneous fat ratio independently predicts the prognosis of locally advanced gastric cancer—Highlighting the role of adiponectin receptors and PPARα, β/δ, γ. Eur. J. Surg. Oncol. 2021, 47, 3064–3073. [Google Scholar] [CrossRef]
- Shang, J.R.; Zhu, J.; Bai, L.; Kulabiek, D.; Zhai, X.X.; Zheng, X.; Qian, J. Adipocytes impact on gastric cancer progression: Prognostic insights and molecular features. World J. Gastrointest. Oncol. 2024, 16, 3011–3031. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lin, K.; Zhao, Y.; Wu, Q.; Chen, D.; Wang, J.; Liang, Y.; Li, J.; Hu, J.; Wang, H.; et al. Adipocytes fuel gastric cancer omental metastasis via PITPNC1-mediated fatty acid metabolic reprogramming. Theranostics 2018, 8, 5452–5468. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, D.; Kinoshita, J.; Yamaguchi, T.; Nakamura, Y.; Gunjigake, K.; Ohama, T.; Sato, K.; Yamamoto, M.; Tsukamoto, T.; Nomura, S.; et al. Established fibrous peritoneal metastasis in an immunocompetent mouse model similar to clinical immune microenvironment of gastric cancer. BMC Cancer 2020, 20, 1014. [Google Scholar] [CrossRef]
- Bian, L.; Wu, D.; Chen, Y.; Ni, J.; Qu, H.; Li, Z.; Chen, X. Associations of radiological features of adipose tissues with postoperative complications and overall survival of gastric cancer patients. Eur. Radiol. 2022, 32, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.H.; Juliani, F.L.; Carrilho, L.A.O.; Pozzuto, L.; Padilha, D.M.H.; Silveira, M.N.; Costa, F.O.; Macedo, L.T.; da Cunha Júnior, A.D.; Mendes, M.C.S.; et al. Abdominal adiposity as a prognosis biomarker of clinical outcome in metastatic colorectal cancer. Nutrition 2023, 107, 111913. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.T.; Olsson, L.T.; Sanchez, A.; Knezevic, A.; Akin, O.; Scott, J.M.; Hakimi, A.A.; Russo, P.; Caan, B.J.; Mourtzakis, M.; et al. Radiodensities of Skeletal Muscle and Visceral Adipose Tissues Are Prognostic Factors in Clear-Cell Renal Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2024, 33, 1375–1382. [Google Scholar] [CrossRef]
- Li, L.M.; Feng, L.Y.; Liu, C.C.; Huang, W.P.; Yu, Y.; Cheng, P.Y.; Gao, J.B. Can visceral fat parameters based on computed tomography be used to predict occult peritoneal metastasis in gastric cancer? World J. Gastroenterol. 2023, 29, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Matsumoto, Y.; Toyozumi, T.; Otsuka, R.; Shiraishi, T.; Morishita, H.; Makiyama, T.; Nishioka, Y.; Yamada, M.; Hirata, A.; et al. High Subcutaneous Adipose Tissue Radiodensity Predicts Poor Prognosis in Patients With Gastric Cancer. Cancer Diagn. Progn. 2024, 4, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.A.D.; Moraes, T.F.; Anjos, B.H.L.; Alencar, N.R.G.; Chang, T.C.; Santana, B.; Menezes, V.O.; Vieira, L.O.; Brandão, S.C.S.; Salvino, M.A.; et al. Association between increased Subcutaneous Adipose Tissue Radiodensity and cancer mortality: Automated computation, comparison of cancer types, gender, and scanner bias. Appl. Radiat. Isot. 2024, 205, 111181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Caan, B.J.; Chen, W.Y.; Irwin, M.L.; Prado, C.M.; Cespedes Feliciano, E.M. Adipose tissue radiodensity and mortality among patients with nonmetastatic breast cancer. Clin. Nutr. 2022, 41, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, M.; Gevaert, O.; Aberle, M.; Olde Damink, S.W.; van Dijk, D.; Rensen, S.S. Tumor metabolic activity is associated with subcutaneous adipose tissue radiodensity and survival in non-small cell lung cancer. Clin. Nutr. 2024, 43, 1809–1815. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, E.J.; Yang, J.Y.; Park, K.B.; Kwon, O.K. Comparison of the prognosis of upper-third gastric cancer with that of middle and lower-third gastric cancer. J. Gastric Cancer 2024, 24, 159–171. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyashiro, I.; Yano, M.; Kishi, K.; Motoori, M.; Shingai, T.; Noura, S.; Ohue, M.; Ohigashi, H.; Ishikawa, O. Visceral fat changes after distal gastrectomy according to type of reconstruction procedure for gastric cancer. World J. Surg. Oncol. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Rivas, L.; Kurland, K.; Chen, S.; Sparks, A.; Lin, P.P.; Vaziri, K. National trends in total vs subtotal gastrectomy for middle and distal third gastric cancer. Am. J. Surg. 2020, 219, 691–695. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hsu, C.H.; Fan, H.L.; Liao, G.S.; Chen, T.W.; Chan, D.C. Prophylactic hyperthermic intraperitoneal chemotherapy for patients with clinical T4 gastric cancer. Eur. J. Surg. Oncol. 2022, 48, 1972–1979. [Google Scholar] [CrossRef]
- Jin, Z.J.; Lu, S.; Shi, M.; Yuan, H.; Yang, Z.Y.; Liu, W.T.; Ni, Z.T.; Yao, X.X.; Hua, Z.C.; Feng, R.H.; et al. Perioperative systemic and prophylactic intraperitoneal chemotherapy for type 4/large type 3 gastric cancer: DRAGON-10. Future Oncol. 2024, 20, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Giacopuzzi, S.; Vittimberga, G.; De Pascale, S.; Pastorelli, E.; Gelmini, R.; Viganò, J.; Graziosi, L.; Vagliasindi, A.; Rosa, F.; et al. Clinical outcomes of patients with complicated post-operative course after gastrectomy for cancer: A GIRCG study using the GASTRODATA registry. Updates Surg. 2023, 75, 419–427. [Google Scholar] [CrossRef]
- D’Souza, J.; McCombie, A.; Roberts, R. The influence of short-term postoperative outcomes on overall survival after gastric cancer surgery. ANZ J. Surg. 2023, 93, 2875–2884. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Number of Patients (%) | Median (Range) | |
|---|---|---|---|
| Age (years) | 60 (33–88) | ||
| Sex | Men | 174 (71.6%) | |
| Women | 69 (28.4%) | ||
| Histopathological classification | Papillary/tubular type | 75 (30.9%) | |
| Poorly cohesive type | 148 (60.9%) | ||
| Mucinous | 12 (4.9%) | ||
| Others | 8 (3.3%) | ||
| Lauren classification | Intestinal type | 72 (29.6%) | |
| Diffuse/indeterminate type | 171 (70.4%) | ||
| Tumor location | Upper | 59 (24.3%) | |
| Middle | 29 (11.9%) | ||
| Lower | 155 (63.8%) | ||
| Tumor size (cm) | 4.5 (0.7–18.0) | ||
| Surgery type | Subtotal gastrectomy | 171 (70.4%) | |
| Total gastrectomy | 69 (28.4%) | ||
| Proximal gastrectomy | 3 (1.2%) | ||
| Pathologic T stage | T2 | 36 (14.8%) | |
| T3 | 134 (55.1%) | ||
| T4 | 73 (30.0%) | ||
| Pathologic N stage | N0 | 68 (28.0%) | |
| N1 | 55 (22.6%) | ||
| N2 | 47 (19.3%) | ||
| N3 | 73 (30.0%) | ||
| TNM stage | Stage II | 126 (51.9%) | |
| Stage III | 117 (48.1%) | ||
| Adjuvant chemotherapy | Yes | 240 (98.8%) | |
| No | 3 (1.2%) | ||
| SAT HU on CT1 (HU) | −98.22 (−117.41–−58.90) | ||
| VAT HU on CT1 (HU) | −86.77 (−109.76–−53.86) | ||
| SAT HU on CT2 (HU) | −85.07 (−106.21–−41.55) | ||
| VAT HU on CT2 (HU) | −75.29 (−100.66–−54.68) | ||
| ΔSAT HU (%) | 12.4 (−22.8–65.0) | ||
| ΔVAT HU (%) | 14.7 (−34.3–48.9) | ||
| Clinicopathologial Factors | SAT HU on CT1 (HU) | VAT HU on CT1 (HU) | SAT HU on CT2 (HU) | VAT HU on CT2 (HU) | ΔSAT HU (%) | ΔVAT HU (%) | |
|---|---|---|---|---|---|---|---|
| Age | ≥60 years | −97.4 (−102.0–−90.1) | −86.9 (−93.2–−78.8) | −86.1 (−94.2–−74.6) | −72.3 (−78.2–−66.8) | 11.1 (4.1–22.1) | 15.0 (8.0–21.4) |
| <60 years | −98.8 (−103.8–−94.8) | −86.6 (−93.9–−79.7) | −71.4 (−78.7–−64.6) | −71.4 (−78.7–−64.6) | 12.6 (5.0–21.1) | 14.3 (8.2–22.4) | |
| p-value | 0.018 | 0.687 | <0.001 | 0.641 | 0.572 | 0.840 | |
| Sex | Men | −96.5 (−101.4–−90.2) | −86.4 (−93.2–−78.5) | −80.3 (−88.4–−70.1) | −74.2 (−78.4–−68.7) | 13.2 (4.8–22.6) | 14.6 (8.3–22.0) |
| Women | −103.1 (−105.7–−98.8) | −88.4 (−94.3–−80.7) | −91.8 (−98.0–−79.6) | −70.3 (−78.4–−65.2) | 11.6 (4.5–19.4) | 15.1 (8.0–22.8) | |
| p-value | <0.001 | 0.173 | 0.001 | 0.131 | 0.666 | 0.973 | |
| Histopathological classification | Papillary/tubular type | −97.6 (−101.8–−91.4) | −86.1 (−92.5–−78.8) | −84.3 (−94.2–−75.8) | −73.7 (−80.0–−66.3) | 9.5 (4.3–18.7) | 12.3 (6.6–21.4) |
| Poorly cohesive type | −101.8 (−107.3–−94.7) | −95.4 (−98.2–−85.1) | −95.3 (−98.8–−79.5) | −80.6 (−84.9–−67.7) | 13.7 (4.6–22.5) | 16.2 (8.9–22.6) | |
| Mucinous | −98.5 (−103.2–−92.5) | −86.7 (−93.2–−79.4) | −85.3 (−94.4–−72.8) | −71.4 (−77.7–−65.7) | 8.1 (3.6–10.6) | 12.1 (9.7–16.7) | |
| p-value | 0.114 | 0.099 | 0.353 | 0.231 | 0.209 | 0.276 | |
| Lauren classification | Intestinal type | −97.1 (−101.8–−90.0) | −85.9 (−93.0–−78.1) | −84.9 (−93.5–−76.2) | −71.6 (−77.9–−66.1) | 9.8 (5.0–20.8) | 14.1 (6.7–22.4) |
| Diffuse/indeterminate type | −98.7 (−103.3–−92.8) | −86.9 (−93.3–−79.5) | −80.1 (−88.5–−75.6) | −72.1 (−78.5–−65.6) | 13.2 (4.6–22.5) | 15.1 (9.2–22.0) | |
| p-value | 0.111 | 0.672 | 0.070 | 0.935 | 0.273 | 0.384 | |
| Tumor location | Upper | −98.5 (−103.3–−93.3) | −89.0 (−94.0–−79.0) | −77.1 (−85.7–−63.6) | −67.0 (−74.0–−63.3) | 20.0 (13.4–32.2) | 22.4 (16.3–25.9) |
| Middle | −99.5 (−103.4–−95.2) | −86.2 (−93.2–−80.8) | −86.9 (−95.5–−73.5) | −71.5 (−77.1–−68.3) | 15.1 (6.4–21.3) | 16.1 (9.9–21.1) | |
| Lower | −97.6 (−102.4–−91.4) | −86.6 (−93.2–−78.7) | −87.9 (−96.2–−78.1) | −74.3 (−80.9–−67.0) | 8.1 (2.6–7.5) | 11.9 (6.3–18.4) | |
| p-value | 0.178 | 0.544 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Surgery type | Total gastrectomy | −98.0 (−103.1–−92.0) | −87.3 (−93.3–−80.7) | −76.8 (−86.0–−68.8) | −67.5 (−74.0–−61.4) | 20.0 (13.4–30.9) | 20.8 (14.4–25.6) |
| Subtotal gastrectomy | −98.2 (−102.9–−93.1) | −86.6 (−93.4–−79.2) | −88.3 (−96.3–−78.4) | −74.3 (−80.4–−67.1) | 8.3 (2.8–17.5) | 12.2 (6.3–18.7) | |
| Proximal gastrectomy | −103.3 (−104.1–−91.0) | −90.3 (−93.2–−74.0) | −87.2 (−89.5–−84.6) | −71.2 (−72.3–−68.5) | 13.5 (6.1–15.1) | 21.2 (6.4–22.4) | |
| p-value | 0.856 | 0.879 | <0.001 | <0.001 | <0.001 | <0.001 | |
| T stage | T2 | −98.9 (−103.8–−95.4) | −90.3 (−94.9–−83.4) | −93.7 (−96.0–−82.8) | −77.2 (−83.3–−71.2) | 7.5 (1.3–14.5) | 6.9 (1.1–9.3) |
| T3 | −97.5 (−102.8–−91.7) | −86.6 (−93.3–−80.1) | −84.4 (−93.9–−73.2) | −71.4 (−77.7–−65.2) | 12.0 (4.6–22.5) | 14.9 (82.2–22.1) | |
| T4 | −98.9 (−103.2–−91.9) | −84.9 (−92.5–−76.6) | −82.4 (−93.4–−68.1) | −70.0 (−77.8–−64.4) | 15.1 (5.6–24.8) | 14.4 (8.0–23.2) | |
| p-value | 0.549 | 0.047 | 0.002 | 0.003 | 0.008 | 0.007 | |
| N stage | N0 | −98.6 (−103.2–−92.9) | −86.1 (−93.7–−80.7) | −86.4 (−95.5–−78.9) | −72.7 (−79.0–−67.5) | 13.2 (4.7–18.1) | 12.8 (8.0–21.0) |
| N1 | −97.9 (−102.4–−91.8) | −87.4 (−94.2–−78.8) | −87.5 (−94.9–−75.6) | −74.4 (−80.9–−65.4) | 10.3 (2.9–21.5) | 13.2 (6.3–21.7) | |
| N2 | −99.7 (−102.9–−94.0) | −88.4 (−94.0–−79.9) | −88.1 (−95.7–−74.2) | −74.1 (−78.5–−67.4) | 9.5 (5.5–19.5) | 14.4 (8.6–19.8) | |
| N3 | −97.4 (−103.5–−89.0) | −86.6 (−92.5–−77.2) | −80.3 (−93.0–−70.3) | −70.3 (−77.8–−65.6) | 16.2 (4.9–25.2) | 16.9 (10.1–24.4) | |
| p-value | 0.716 | 0.744 | 0.072 | 0.065 | 0.136 | 0.292 | |
| TNM stage | Stage II | −99.0 (−103.1–−93.3) | −87.4 (−94.6–−80.1) | −88.4 (−95.3–−80.3) | −74.4 (−80.5–−68.8) | 10.0 (8.0–12.8) | 12.5 (7.0–20.8) |
| Stage III | −97.9 (−103.1–−89.9) | −84.8 (−91.5–−77.5) | −71.1 (−79.7–−66.6) | −70.1 (−76.7–−63.6) | 15.5 (5.7–25.2) | 16.4 (10.1–24.4) | |
| p-value | 0.421 | 0.063 | <0.001 | <0.001 | 0.001 | 0.014 | |
| Variables | RFS | Peritoneal RFS | OS | |||
|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | |
| SAT HU on CT1 (<−97.2 HU vs. ≥−97.2 HU) | 0.160 | 1.375 (0.881–2.144) | 0.305 | 1.368 (0.752–2.489) | 0.234 | 1.456 (0.787–2.695) |
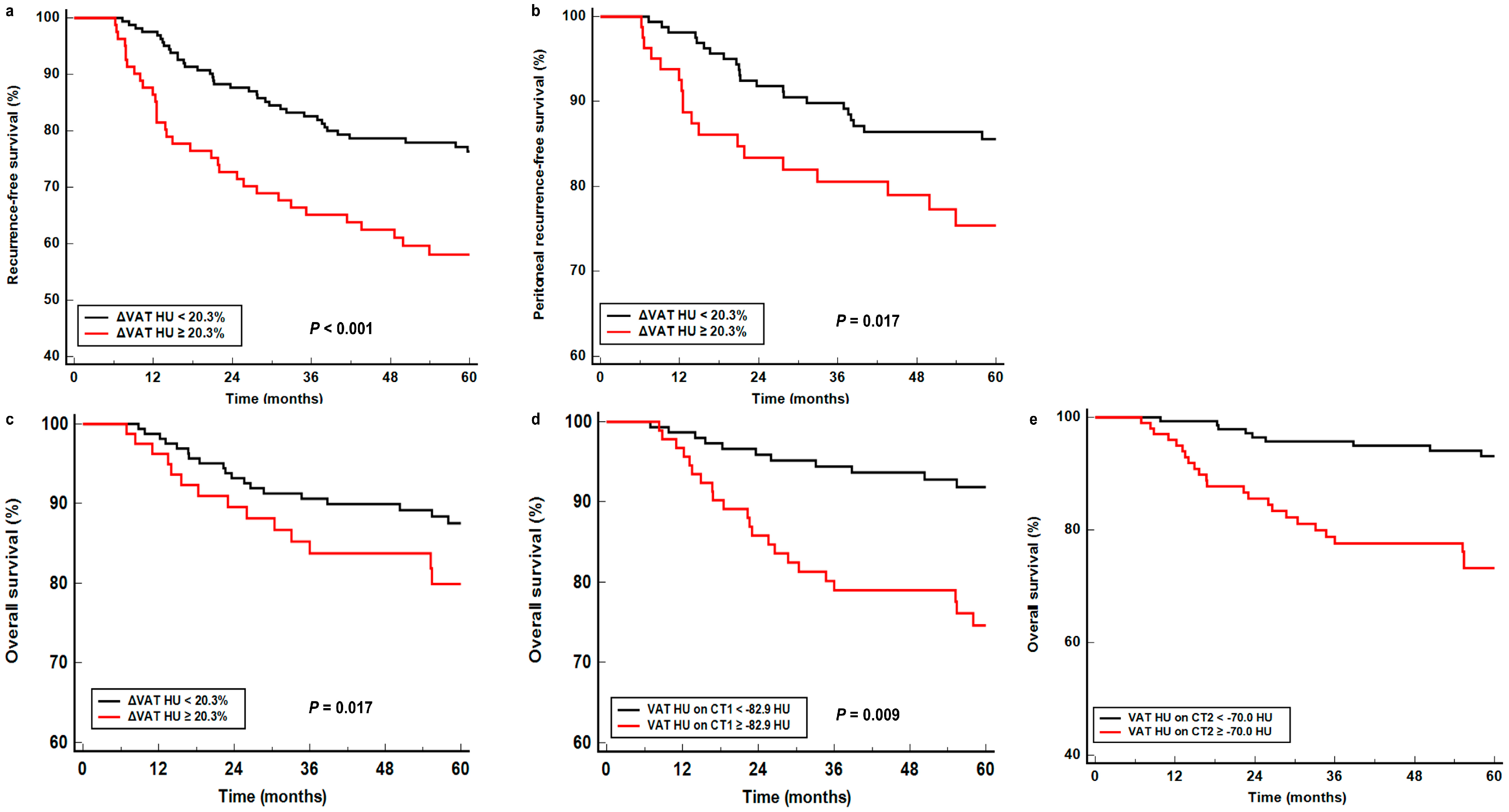
| VAT HU on CT1 (<−82.9 HU vs. ≥−82.9 HU) | 0.046 | 1.519 (1.007–2.372) | 0.048 | 1.826 (1.004–3.322) | 0.011 | 2.231 (1.203–4.136) |
| SAT HU on CT2 (<−82.3 HU vs. ≥−82.3 HU) | 0.020 | 2.405 (1.107–3.89) | 0.010 | 2.290 (1.222–4.290) | 0.010 | 2.438 (1.226–4.656) |
| VAT HU on CT2 (<−70.0 HU vs. ≥−70.0 HU) | <0.001 | 3.051 (1.924–4.838) | 0.001 | 2.892 (1.557–5.372) | <0.001 | 3.904 (2.017–7.557) |
| ΔSAT HU (<9.3% vs. ≥9.3%) | 0.002 | 2.438 (1.388–4.283) | 0.041 | 2.156 (1.034–4.495) | 0.028 | 2.380 (1.099–5.156) |
| ΔVAT HU (<20.3% vs. ≥20.3%) | <0.001 | 2.369 (1.516–3.702) | 0.020 | 2.044 (1.122–3.725) | 0.016 | 2.130 (1.149–3.947) |
| Variables | RFS | Peritoneal RFS | OS | |||
|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | |
| VAT HU on CT1 (<−82.9 HU vs. ≥−82.9 HU) | 0.190 | 1.403 (0.845–2.328) | 0.072 | 1.797 (0.939–3.632) | 0.049 | 2.016 (1.001–4.059) |
| ΔSAT HU (<9.3% vs. ≥9.3%) | 0.382 | 1.341 (0.695–2.589) | 0.753 | 1.146 (0.491–2.675) | 0.333 | 1.541 (0.612–3.701) |
| SAT HU on CT2 (<−82.3 HU vs. ≥−82.3 HU) | 0.101 | 1.555 (0.908–2.553) | 0.401 | 1.331 (0.682–2.600) | 0.235 | 1.518 (0.763–3.021) |
| VAT HU on CT2 (<−70.0 HU vs. ≥−70.0 HU) | 0.053 | 2.065 (0.996–3.387) | 0.066 | 1.992 (0.923–3.920) | 0.009 | 2.515 (1.267–4.991) |
| ΔVAT HU (<20.3% vs. ≥20.3%) | 0.002 | 2.437 (1.385–4.288) | 0.023 | 2.457 (1.135–5.318) | 0.043 | 2.204 (1.025–4.741) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Song, G.J.; Son, M.W.; Yun, J.H.; Lee, M.-S.; Lee, J.W. Association of Increased CT-Attenuation of Visceral Adipose Tissue After Surgery with Poor Survival Outcomes in Patients with Stage II–III Gastric Cancer: A Retrospective Cohort Study. Cancers 2025, 17, 235. https://doi.org/10.3390/cancers17020235
Lee SM, Song GJ, Son MW, Yun JH, Lee M-S, Lee JW. Association of Increased CT-Attenuation of Visceral Adipose Tissue After Surgery with Poor Survival Outcomes in Patients with Stage II–III Gastric Cancer: A Retrospective Cohort Study. Cancers. 2025; 17(2):235. https://doi.org/10.3390/cancers17020235
Chicago/Turabian StyleLee, Sang Mi, Geum Jong Song, Myoung Won Son, Jong Hyuk Yun, Moon-Soo Lee, and Jeong Won Lee. 2025. "Association of Increased CT-Attenuation of Visceral Adipose Tissue After Surgery with Poor Survival Outcomes in Patients with Stage II–III Gastric Cancer: A Retrospective Cohort Study" Cancers 17, no. 2: 235. https://doi.org/10.3390/cancers17020235
APA StyleLee, S. M., Song, G. J., Son, M. W., Yun, J. H., Lee, M.-S., & Lee, J. W. (2025). Association of Increased CT-Attenuation of Visceral Adipose Tissue After Surgery with Poor Survival Outcomes in Patients with Stage II–III Gastric Cancer: A Retrospective Cohort Study. Cancers, 17(2), 235. https://doi.org/10.3390/cancers17020235








