Molecular Insights on Signaling Cascades in Breast Cancer: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Molecular Classification of Breast Cancer
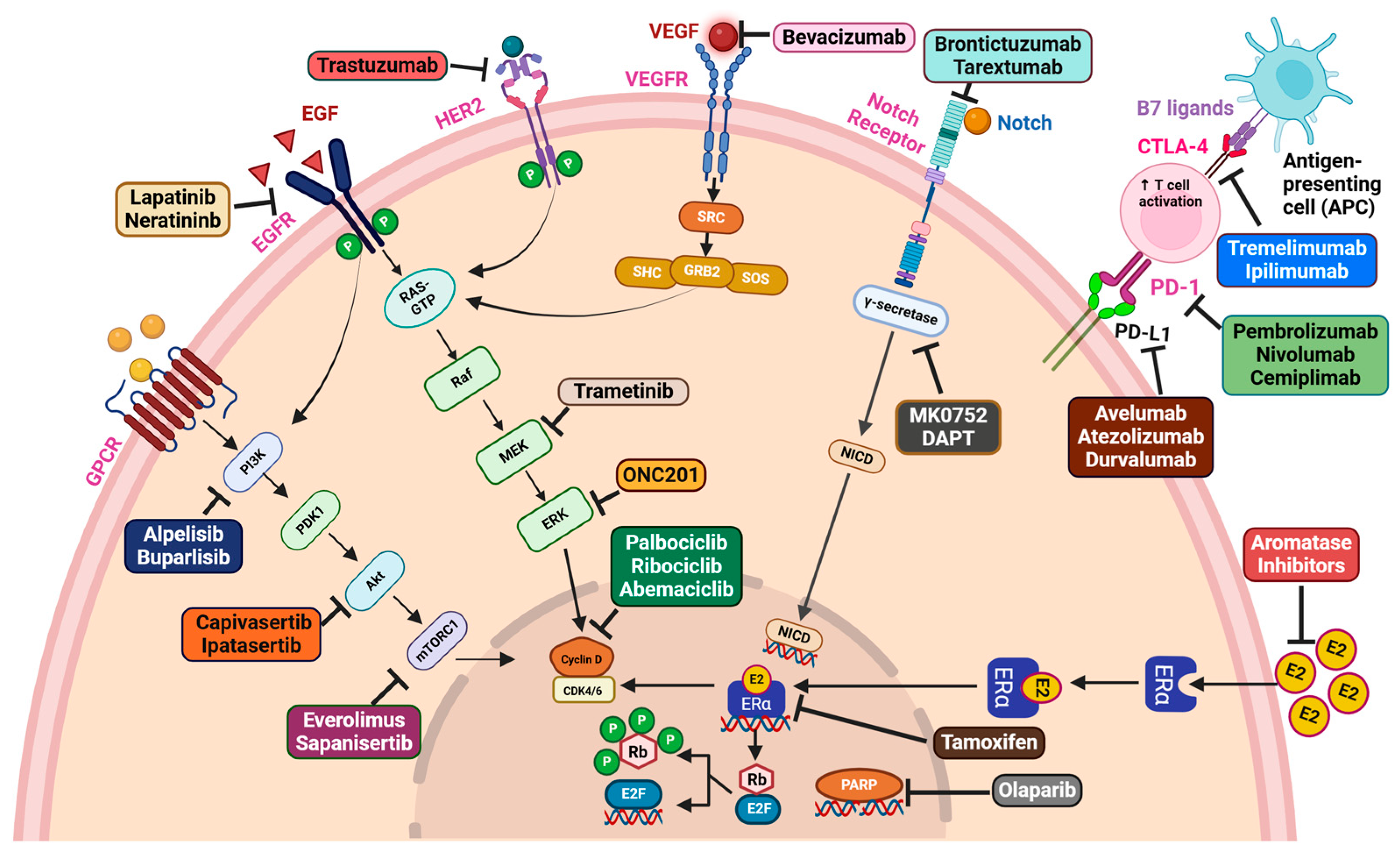
3. Novel Treatment Regimens in Breast Cancer
3.1. Chemotherapy
3.2. Immunotherapy
3.3. Targeted Therapy
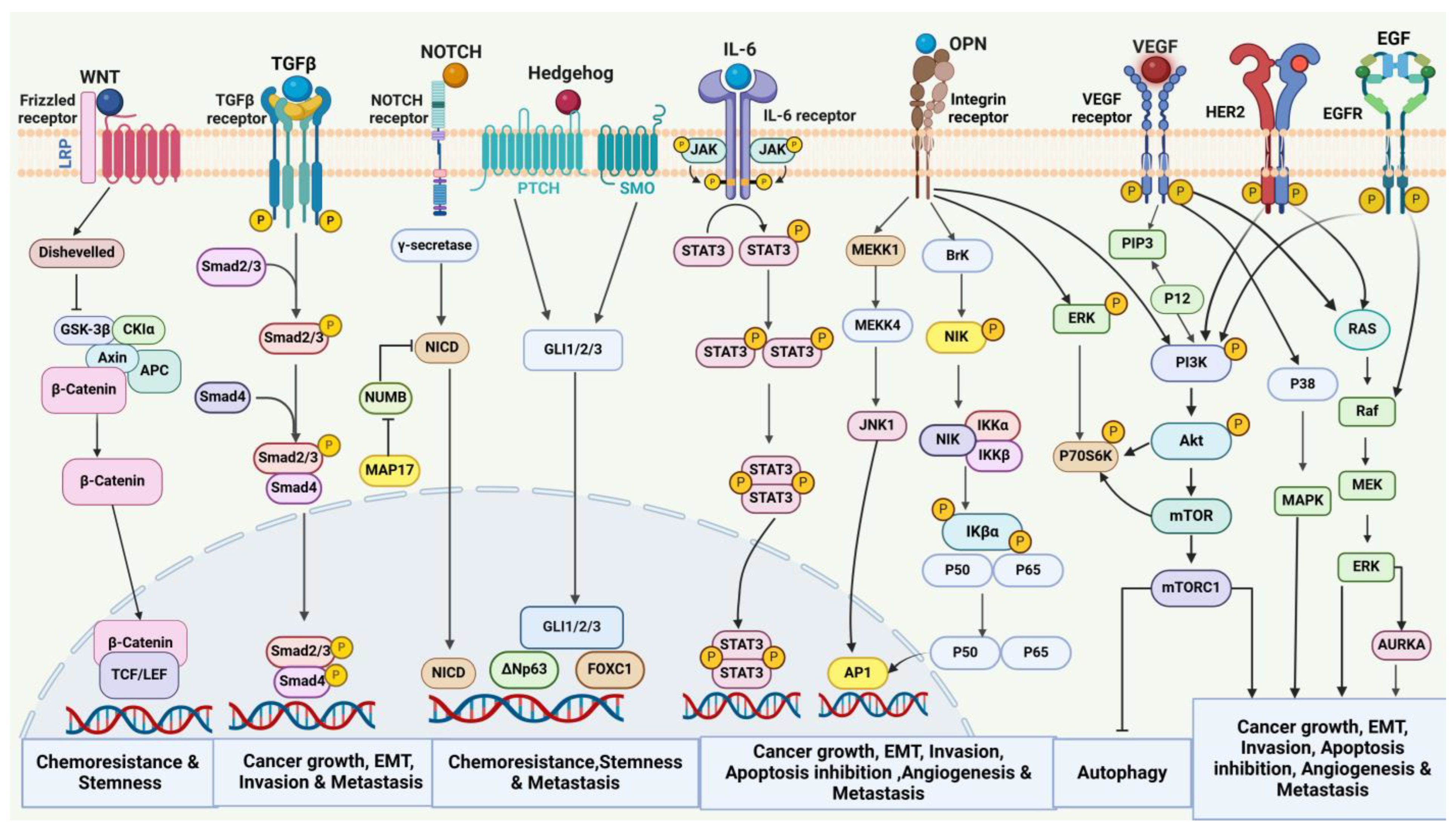
4. Oncogenic Signaling in Breast Cancer
4.1. Involvement of EMT in Breast Cancer Invasion and Metastasis
4.2. Angiogenesis
4.2.1. VEGF-Dependent Angiogenesis
4.2.2. VEGF-Independent Angiogenesis
4.3. Cancer Stem Cells
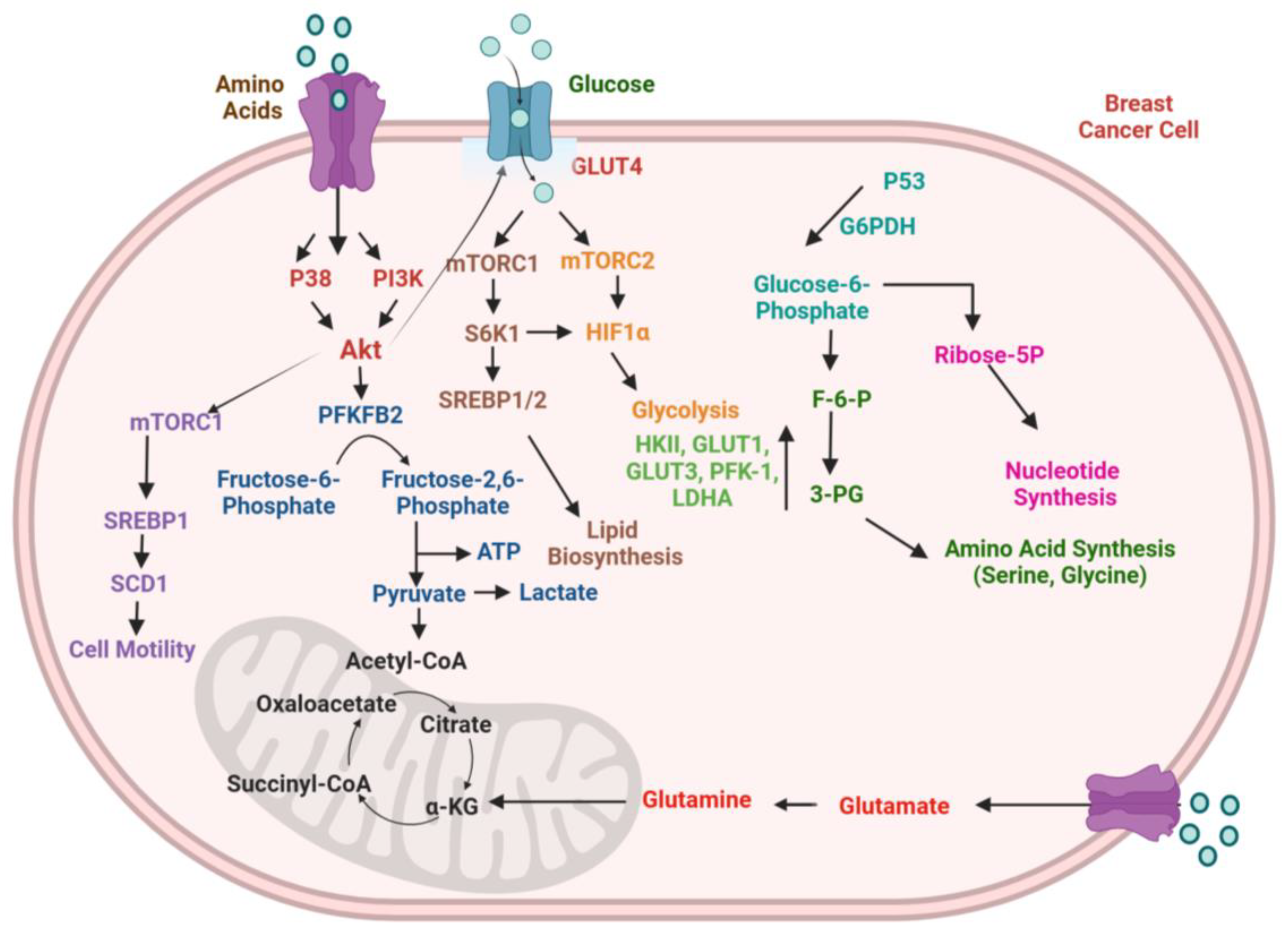
4.4. Tumor Metabolism
4.4.1. Glucose Metabolism
4.4.2. Lipid Metabolism
4.4.3. Immunometabolism
4.5. Autophagy
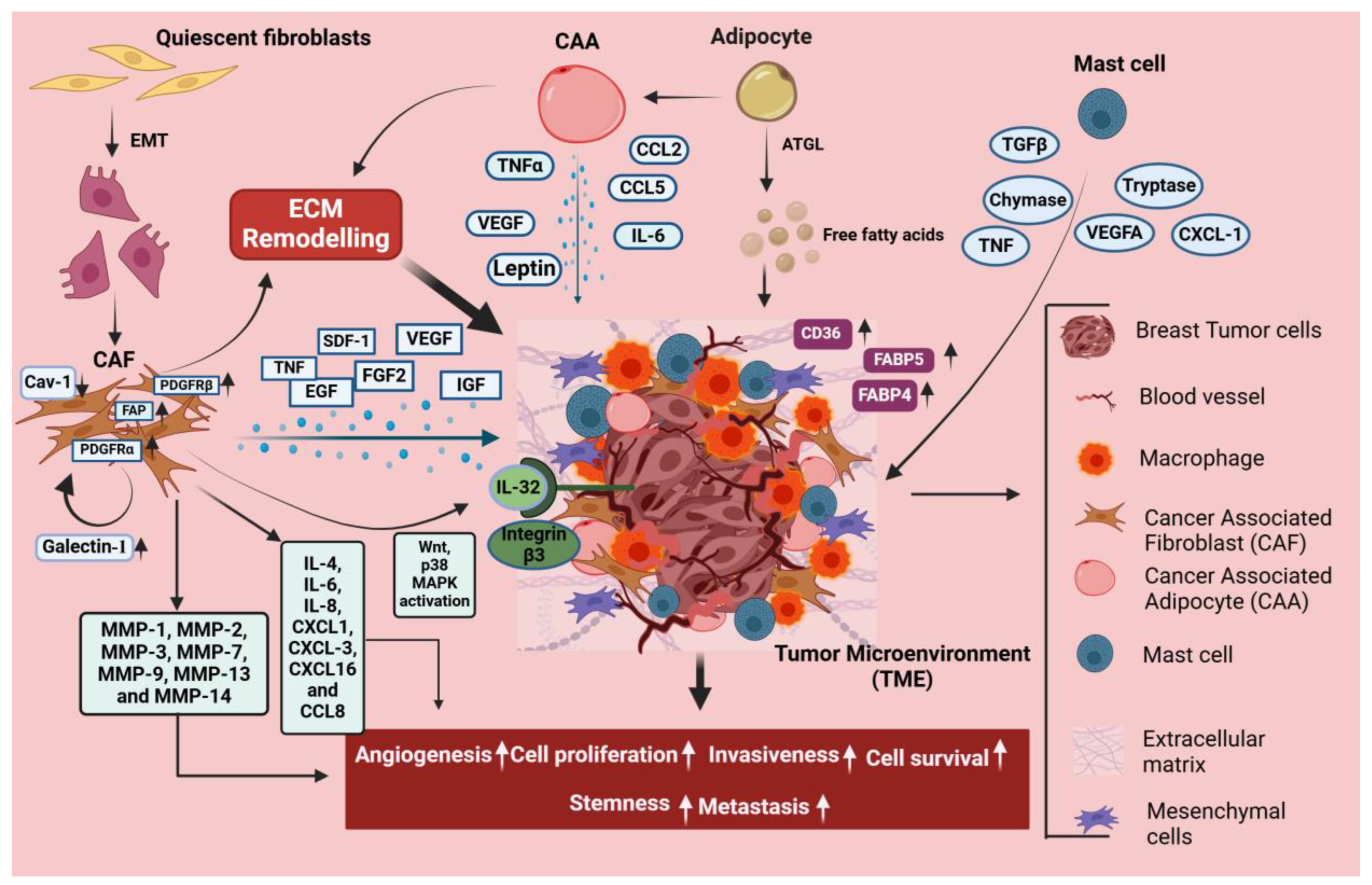
5. Stromal Cell-Mediated Signaling in Breast Cancer
5.1. CAFs
5.2. Adipocytes
5.3. Mast Cells
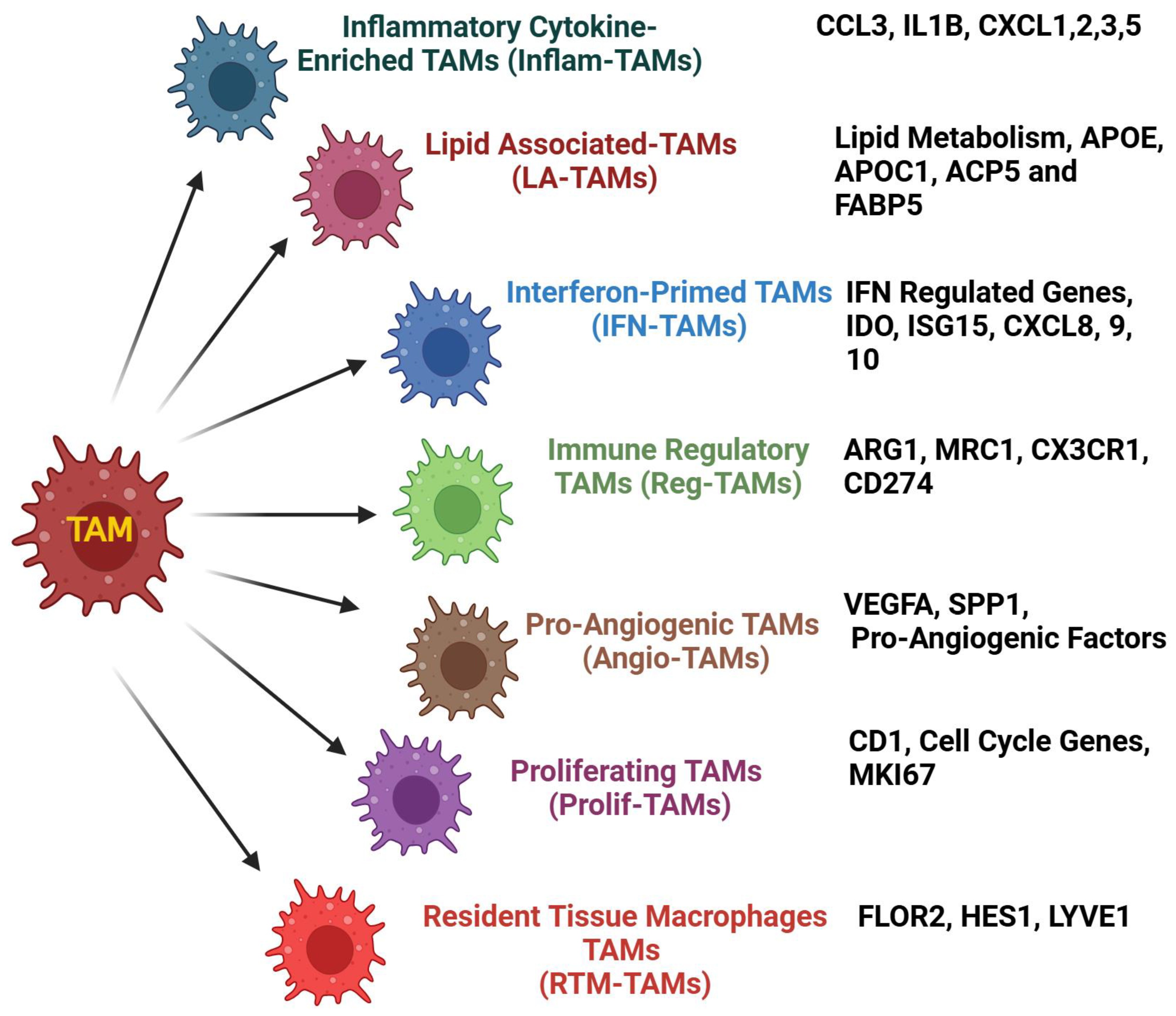
5.4. TAMs
6. Therapy Failure and Resistance
7. Epigenetic-Mediated Signaling
8. Exosomal CircRNA-, miRNA-, and lncRNA-Mediated Signaling in Breast Cancer
8.1. Exosomal Circular RNAs in Breast Cancer Pathogenesis
8.2. Oncogenic and Tumor Suppressor miRNAs in Breast Cancer Progression
8.3. Role of lncRNA-Mediated Signaling Pathways in Breast Cancer Development
9. Therapeutic Hotspots for Breast Cancer Treatment
10. Conclusions
11. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The Global Decrease in Cancer Mortality: Trends and Disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering Breast Cancer: From Biology to the Clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Karagiannakos, A.; Adamaki, M.; Tsintarakis, A.; Vojtesek, B.; Fåhraeus, R.; Zoumpourlis, V.; Karakostis, K. Targeting Oncogenic Pathways in the Era of Personalized Oncology: A Systemic Analysis Reveals Highly Mutated Signaling Pathways in Cancer Patients and Potential Therapeutic Targets. Cancers 2022, 14, 664. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.; Mietzsch, F.; Seibold, P.; Schneeweiss, A.; Schirmacher, P.; Chang-Claude, J.; Peter Sinn, H.; Aulmann, S. Intrinsic Breast Cancer Subtypes Defined by Estrogen Receptor Signalling-Prognostic Relevance of Progesterone Receptor Loss. Mod. Pathol. 2013, 26, 1161–1171. [Google Scholar] [CrossRef]
- Won, K.-A.; Spruck, C. Triple-negative Breast Cancer Therapy: Current and Future Perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A Double-Edged Sword in Cancer Treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic Agents for the Treatment of Metastatic Breast Cancer: An Update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Kim, S.-J.; Shao, F.; Ho, J.W.K.; Wong, K.U.; Miao, Z.; Hao, D.; Zhao, M.; Xu, J.; et al. Cisplatin Prevents Breast Cancer Metastasis through Blocking Early EMT and Retards Cancer Growth Together with Paclitaxel. Theranostics 2021, 11, 2442–2459. [Google Scholar] [CrossRef]
- Zheng, M.; Mei, Z.; Junaid, M.; Tania, M.; Fu, J.; Chen, H.-C.; Khan, M.A. Synergistic Role of Thymoquinone on Anticancer Activity of 5-Fluorouracil in Triple Negative Breast Cancer Cells. Anticancer. Agents Med. Chem. 2022, 22, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Iwata, H.; Taira, N.; Masuda, N.; Takahashi, M.; Yoshinami, T.; Ueno, T.; Toyama, T.; Yamanaka, T.; Takano, T.; et al. Pertuzumab Retreatment for HER2-Positive Advanced Breast Cancer: A Randomized, Open-Label Phase III Study (PRECIOUS). Cancer Sci. 2022, 113, 3169–3179. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in Breast Cancer: An Overview of Current Strategies and Perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-Line Atezolizumab plus Nab-Paclitaxel for Unresectable, Locally Advanced, or Metastatic Triple-Negative Breast Cancer: IMpassion130 Final Overall Survival Analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary Results from IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel with or without Atezolizumab for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Rugo, H.S.; Kabos, P.; Beck, J.T.; Jerusalem, G.; Wildiers, H.; Sevillano, E.; Paz-Ares, L.; Chisamore, M.J.; Chapman, S.C.; Hossain, A.M.; et al. Abemaciclib in Combination with Pembrolizumab for HR+ve, HER2- Metastatic Breast Cancer: Phase 1b Study. NPJ Breast Cancer 2022, 8, 118. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, J.S.; Yost, S.E.; Frankel, P.H.; Ruel, C.; Egelston, C.A.; Guo, W.; Padam, S.; Tang, A.; Martinez, N.; et al. Phase I/II Trial of Palbociclib, Pembrolizumab and Letrozole in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Eur. J. Cancer 2021, 154, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Nagini, S. Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, F.; Colantuoni, G.; Diana, A.; Mocerino, C.; Cartenì, G.; Lauria, R.; Febbraro, A.; Nuzzo, F.; Addeo, R.; Marano, O.; et al. Exemestane and Everolimus Combination Treatment of Hormone Receptor Positive, HER2 Negative Metastatic Breast Cancer: A Retrospective Study of 9 Cancer Centers in the Campania Region (Southern Italy) Focused on Activity, Efficacy and Safety. Mol. Clin. Oncol. 2018, 9, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized Phase II Trial of Everolimus in Combination with Tamoxifen in Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer with Prior Exposure to Aromatase Inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Schöffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A Phase Ib Study of Pictilisib (GDC-0941) in Combination with Paclitaxel, with and without Bevacizumab or Trastuzumab, and with Letrozole in Advanced Breast Cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.C.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Phase III Study of Veliparib with Carboplatin and Paclitaxel in HER2-Negative Advanced/Metastatic gBRCA-Associated Breast Cancer. Ann. Oncol. 2019, 30, v857–v858. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 1700. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Quader, S.; Cabral, H.; Ono, R. Interplay of EMT and CSC in Cancer and the Potential Therapeutic Strategies. Front. Pharmacol. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M. Transcriptional Regulation of EMT Transcription Factors in Cancer. Semin. Cancer Biol. 2023, 97, 21–29. [Google Scholar] [CrossRef]
- Mendonsa, A.M.; Na, T.-Y.; Gumbiner, B.M. E-Cadherin in Contact Inhibition and Cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Imani, S.; Hosseinifard, H.; Cheng, J.; Wei, C.; Fu, J. Prognostic Value of EMT-Inducing Transcription Factors (EMT-TFs) in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 28587. [Google Scholar] [CrossRef]
- Wang, H.; Sang, M.; Geng, C.; Liu, F.; Gu, L.; Shan, B. MAGE-A Is Frequently Expressed in Triple Negative Breast Cancer and Associated with Epithelial-Mesenchymal Transition. Neoplasma 2016, 63, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, Y.; Wang, Y.; Wang, C.; Kang, T.; Rychahou, P.G.; Chi, Y.-I.; Evers, B.M.; Zhou, B.P. Interaction with Suv39H1 Is Critical for Snail-Mediated E-Cadherin Repression in Breast Cancer. Oncogene 2013, 32, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Feldker, N.; Ferrazzi, F.; Schuhwerk, H.; Widholz, S.A.; Guenther, K.; Frisch, I.; Jakob, K.; Kleemann, J.; Riegel, D.; Bönisch, U.; et al. Genome-Wide Cooperation of EMT Transcription Factor ZEB1 with YAP and AP-1 in Breast Cancer. EMBO J. 2020, 39, e103209. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling Pathways Governing Breast Cancer Stem Cells Behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, J.; Yarden, Y.; Fu, L. The Key Roles of Cancer Stem Cell-Derived Extracellular Vesicles. Signal Transduct. Target. Ther. 2021, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.; Nakshatri, H. Breast-Cancer Stem Cells-beyond Semantics. Lancet Oncol. 2012, 13, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Suzuki, C.; Shima, H.; Kida, K.; Adachi, S.; Yamamoto, S.; Narui, K.; Tanabe, M.; Shimizu, D.; Taniguchi, R.; et al. Aldehyde Dehydrogenase 1-Related Genes in Triple-Negative Breast Cancer Investigated Using Network Analysis. Anticancer Res. 2020, 40, 6733–6742. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, W.A.; Palma, P.V.B.; Sicchieri, R.D.; Villacis, R.a.R.; Mandarano, L.R.M.; Oliveira, T.M.G.; Antonio, H.M.R.; Andrade, J.M.; Muglia, V.F.; Rogatto, S.R.; et al. Transcription Factor Networks Derived from Breast Cancer Stem Cells Control the Immune Response in the Basal Subtype. Sci. Rep. 2017, 7, 2851. [Google Scholar] [CrossRef] [PubMed]
- Vesuna, F.; Lisok, A.; Kimble, B.; Raman, V. Twist Modulates Breast Cancer Stem Cells by Transcriptional Regulation of CD24 Expression. Neoplasia 2009, 11, 1318–1328. [Google Scholar] [CrossRef]
- Storci, G.; Bertoni, S.; De Carolis, S.; Papi, A.; Nati, M.; Ceccarelli, C.; Pirazzini, C.; Garagnani, P.; Ferrarini, A.; Buson, G.; et al. Slug/β-Catenin-Dependent Proinflammatory Phenotype in Hypoxic Breast Cancer Stem Cells. Am. J. Pathol. 2013, 183, 1688–1697. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-High Breast Cancer Cells Promote Immune Suppression in the Lung Pre-Metastatic Niche via Exosomes and Support Cancer Progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villanueva, D.; Balamurugan, K.; Ali, H.R.; Kim, S.-R.; Sharan, S.; Johnson, R.C.; Merchant, A.S.; Caldas, C.; Landberg, G.; Sterneck, E. The C/EBPδ Protein Is Stabilized by Estrogen Receptor α Activity, Inhibits SNAI2 Expression and Associates with Good Prognosis in Breast Cancer. Oncogene 2016, 35, 6166–6176. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Mendoza-Villanueva, D.; Sharan, S.; Summers, G.H.; Dobrolecki, L.E.; Lewis, M.T.; Sterneck, E. C/EBPδ Links IL-6 and HIF-1 Signaling to Promote Breast Cancer Stem Cell-Associated Phenotypes. Oncogene 2019, 38, 3765–3780. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Senger, D.R.; Van de Water, L.; Brown, L.F.; Nagy, J.A.; Yeo, K.T.; Yeo, T.K.; Berse, B.; Jackman, R.W.; Dvorak, A.M.; Dvorak, H.F. Vascular Permeability Factor (VPF, VEGF) in Tumor Biology. Cancer Metastasis Rev. 1993, 12, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The Role of VEGF in Cancer-Induced Angiogenesis and Research Progress of Drugs Targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Qi, X.; Chen, Y.; Sun, B.; Dai, Y.; Gu, Y. PI3K/Akt and MAPK/ERK1/2 Signaling Pathways Are Involved in IGF-1-Induced VEGF-C Upregulation in Breast Cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh Behelgardi, M.; Zahri, S.; Gholami Shahvir, Z.; Mashayekhi, F.; Mirzanejad, L.; Asghari, S.M. Targeting Signaling Pathways of VEGFR1 and VEGFR2 as a Potential Target in the Treatment of Breast Cancer. Mol. Biol. Rep. 2020, 47, 2061–2071. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, Y.; Zheng, A.; Zhao, Y.; Wu, X.; Li, M.; Du, F.; Chen, Y.; Deng, S.; Chen, M.; et al. Targeting Hypoxia-Inducible Factors for Breast Cancer Therapy: A Narrative Review. Front. Pharmacol. 2022, 13, 1064661. [Google Scholar] [CrossRef]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-Driven Osteopontin Contributes to Breast Tumor Growth through Modulation of HIF1α-Mediated VEGF-Dependent Angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Thorat, D.; Soundararajan, G.; Pradhan, S.J.; Chakraborty, G.; Lohite, K.; Karnik, S.; Kundu, G.C. Semaphorin 3A Upregulates FOXO 3a-Dependent MelCAM Expression Leading to Attenuation of Breast Tumor Growth and Angiogenesis. Oncogene 2015, 34, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin Promotes Vascular Endothelial Growth Factor-Dependent Breast Tumor Growth and Angiogenesis via Autocrine and Paracrine Mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Qi, Y.; Gu, J.; Yu, D.; Lu, Y.; Liu, J.; Liang, X. Breast Cancer Cells-Derived Von Willebrand Factor Promotes VEGF-A-Related Angiogenesis through PI3K/Akt-miR-205-5p Signaling Pathway. Toxicol. Appl. Pharmacol. 2022, 440, 115927. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Chen, X.; Li, Y.; Bian, W.; Yao, C. San Huang Decoction Targets Aurora Kinase A to Inhibit Tumor Angiogenesis in Breast Cancer. Integr. Cancer Ther. 2020, 19, 1534735420983463. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Guan, S.; Hou, Y.; Qin, Y.; Zeng, H.; Yang, L.; Qiao, Y.; Liu, S.; Li, Q.; Jin, T.; et al. FOSL2 Promotes VEGF-Independent Angiogenesis by Transcriptionnally Activating Wnt5a in Breast Cancer-Associated Fibroblasts. Theranostics 2021, 11, 4975–4991. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hou, Y.; Zhou, X.; Lang, L.; Luo, H.; Sun, Y.; Wan, X.; Yuan, T.; Wang, R.; Liu, Y.; et al. Cancer-Associated Fibroblasts Facilitate Premetastatic Niche Formation through lncRNA SNHG5-Mediated Angiogenesis and Vascular Permeability in Breast Cancer. Theranostics 2022, 12, 7351–7370. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Wang, Z.; Kuang, X.; Shao, N.; Lin, Y. lncRNA NR2F1-AS1 Promotes Breast Cancer Angiogenesis through Activating IGF-1/IGF-1R/ERK Pathway. J. Cell. Mol. Med. 2020, 24, 8236. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, X.; Yang, Y.; Wei, B.; Li, Q.; Mao, G.; He, Y.; Li, Y.; Zheng, L.; Zhang, Q.; et al. Decylubiquinone Suppresses Breast Cancer Growth and Metastasis by Inhibiting Angiogenesis via the ROS/P53/ BAI1 Signaling Pathway. Angiogenesis 2020, 23, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Veine, D.M.; Livant, D.L. Therapeutic Inhibition of Breast Cancer Bone Metastasis Progression and Lung Colonization: Breaking the Vicious Cycle by Targeting A5β1 Integrin. Breast Cancer Res. Treat. 2016, 157, 489–501. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Deng, Q.; Yang, C.; Wang, D.; Jiang, G.; Yao, X.; He, X.; Ding, J.; Qiang, J.; et al. TEM8 Marks Neovasculogenic Tumor-Initiating Cells in Triple-Negative Breast Cancer. Nat. Commun. 2021, 12, 4413. [Google Scholar] [CrossRef]
- van Beijnum, J.R.; Huijbers, E.J.M.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular Vimentin Mimics VEGF and Is a Target for Anti-Angiogenic Immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Yan, L.; Wu, M.; Wang, T.; Yuan, H.; Zhang, X.; Zhang, H.; Li, T.; Pandey, V.; Han, X.; Lobie, P.E.; et al. Breast Cancer Stem Cells Secrete MIF to Mediate Tumor Metabolic Reprogramming That Drives Immune Evasion. Cancer Res. 2024, 84, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Jiang, C.; Dai, T.; Xu, R.; Zhou, X.; Su, X.; Zhao, X. Knockdown of XB130 Restrains Cancer Stem Cell-like Phenotype through Inhibition of Wnt/β-Catenin Signaling in Breast Cancer. Mol. Carcinog. 2019, 58, 1832–1845. [Google Scholar] [CrossRef]
- Ashad-Bishop, K.; Garikapati, K.; Lindley, L.E.; Jorda, M.; Briegel, K.J. Loss of Limb-Bud-and-Heart (LBH) Attenuates Mammary Hyperplasia and Tumor Development in MMTV-Wnt1 Transgenic Mice. Biochem. Biophys. Res. Commun. 2019, 508, 536–542. [Google Scholar] [CrossRef]
- Satriyo, P.B.; Bamodu, O.A.; Chen, J.-H.; Aryandono, T.; Haryana, S.M.; Yeh, C.-T.; Chao, T.-Y. Cadherin 11 Inhibition Downregulates β-Catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer. J. Clin. Med. 2019, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Li, C.; Wang, Y.; Matsukawa, A. The Chemokine Monocyte Chemoattractant Protein-1/CCL2 Is a Promoter of Breast Cancer Metastasis. Cell Mol. Immunol. 2023, 20, 714–738. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Zhang, Y.; Wu, S.; Li, H.; Sun, L.; Liu, Y.; Zhu, X.; Qiao, X.; Ma, Q.; Liu, C.; et al. KK-LC-1 as a Therapeutic Target to Eliminate ALDH+ve Stem Cells in Triple Negative Breast Cancer. Nat. Commun. 2023, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Guo, S.; Liu, C.; Zhao, Y.; Feng, C.; Liu, Y.; Wang, T.; Li, C. Overexpression of SDF-1 Activates the NF-κB Pathway to Induce Epithelial to Mesenchymal Transition and Cancer Stem Cell-like Phenotypes of Breast Cancer Cells. Int. J. Oncol. 2016, 48, 1085–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, S.; Lv, Q.; Zhao, Y.; Liu, C.; Sun, Y.; Xi, K.; Xiao, J.; Li, C. Wnt/β-Catenin Pathway Is Required for Epithelial to Mesenchymal Transition in CXCL12 over Expressed Breast Cancer Cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12357–12367. [Google Scholar] [PubMed]
- Zhang, R.; Dong, M.; Tu, J.; Li, F.; Deng, Q.; Xu, J.; He, X.; Ding, J.; Xia, J.; Sheng, D.; et al. PMN-MDSCs Modulated by CCL20 from Cancer Cells Promoted Breast Cancer Cell Stemness through CXCL2-CXCR2 Pathway. Signal Transduct. Target. Ther. 2023, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhang, M.; Sun, B.; Xu, C.; Yang, Y.; Zhang, Y.; Li, S.; Chen, G.; Chen, C.; Li, Y.; et al. LINC00115 Promotes Chemoresistant Breast Cancer Stem-like Cell Stemness and Metastasis through SETDB1/PLK3/HIF1α Signaling. Mol. Cancer 2024, 23, 60. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast Cancer Stem Cells Transition between Epithelial and Mesenchymal States Reflective of Their Normal Counterparts. Stem Cell Rep. 2013, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Pasani, S.; Sahoo, S.; Jolly, M.K. Hybrid E/M Phenotype(s) and Stemness: A Mechanistic Connection Embedded in Network Topology. J. Clin. Med. 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Abdollahi, B.; Wilkins, O.M.; Lu, H.; Chakraborty, P.; Ognjenovic, N.B.; Muller, K.E.; Jolly, M.K.; Christensen, B.C.; Hassanpour, S.; et al. Phenotypic Heterogeneity Driven by Plasticity of the Intermediate EMT State Governs Disease Progression and Metastasis in Breast Cancer. Sci. Adv. 2022, 8, eabj8002. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Bao, L.; Xue, Y.; Zhu, M.; Kumar, A.; Xing, C.; Wang, J.E.; Wang, Y.; Luo, W. ZMYND8 Protects Breast Cancer Stem Cells against Oxidative Stress and Ferroptosis through Activation of NRF2. J. Clin. Investig. 2024, 134, e171166. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, H.; Wang, J.; Huang, M.; Fu, D.; Qin, L.; Yin, Q. Energy Metabolism Pathways in Breast Cancer Progression: The Reprogramming, Crosstalk, and Potential Therapeutic Targets. Transl. Oncol. 2022, 26, 101534. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Pan, Y.; Chen, F. The Metabolic Landscape of Breast Cancer and Its Therapeutic Implications. Mol. Diagn. Ther. 2023, 27, 349–369. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Zhao, Q.; Fu, S.; Jin, J. Emerging Roles of Aerobic Glycolysis in Breast Cancer. Clin. Transl. Oncol. 2020, 22, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Liu, R.; Li, J.; Wang, Y.; Tan, L.; Li, X.-J.; Qian, X.; Zhang, C.; Xia, Y.; Xu, D.; et al. EGFR-Phosphorylated Platelet Isoform of Phosphofructokinase 1 Promotes PI3K Activation. Mol. Cell 2018, 70, 197–210.e7. [Google Scholar] [CrossRef]
- Novellasdemunt, L.; Tato, I.; Navarro-Sabate, A.; Ruiz-Meana, M.; Méndez-Lucas, A.; Perales, J.C.; Garcia-Dorado, D.; Ventura, F.; Bartrons, R.; Rosa, J.L. Akt-Dependent Activation of the Heart 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase (PFKFB2) Isoenzyme by Amino Acids. J. Biol. Chem. 2013, 288, 10640–10651. [Google Scholar] [CrossRef] [PubMed]
- Samih, N.; Hovsepian, S.; Aouani, A.; Lombardo, D.; Fayet, G. Glut-1 Translocation in FRTL-5 Thyroid Cells: Role of Phosphatidylinositol 3-Kinase and N-Glycosylation. Endocrinology 2000, 141, 4146–4155. [Google Scholar] [CrossRef]
- Garrido, P.; Morán, J.; Alonso, A.; González, S.; González, C. 17β-Estradiol Activates Glucose Uptake via GLUT4 Translocation and PI3K/Akt Signaling Pathway in MCF-7 Cells. Endocrinology 2013, 154, 1979–1989. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Cortes-Funes, H.; Gomez, H.L.; Ciruelos, E.M. The Phosphatidyl Inositol 3-Kinase/AKT Signaling Pathway in Breast Cancer. Cancer Metastasis Rev. 2010, 29, 751–759. [Google Scholar] [CrossRef]
- Elwy, F.; Helwa, R.; El Leithy, A.A.; Shehab El din, Z.; Assem, M.M.; Hassan, N.H.A. PIK3CA Mutations in HER2-Positive Breast Cancer Patients; Frequency and Clinicopathological Perspective in Egyptian Patients. Asian Pac. J. Cancer Prev. 2017, 18, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mayer, J.A.; Mazumdar, A.; Fertuck, K.; Kim, H.; Brown, M.; Brown, P.H. Estrogen Induces C-Myc Gene Expression via an Upstream Enhancer Activated by the Estrogen Receptor and the AP-1 Transcription Factor. Mol. Endocrinol. 2011, 25, 1527–1538. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current Medical Treatment of Estrogen Receptor-Positive Breast Cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of mTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Abdullah, N.; Thowfeik, F.S.; Altorki, N.K.; McGraw, T.E. Distinct Akt Phosphorylation States Are Required for Insulin Regulated Glut4 and Glut1-Mediated Glucose Uptake. eLife 2017, 6, e26896. [Google Scholar] [CrossRef]
- Albert, V.; Svensson, K.; Shimobayashi, M.; Colombi, M.; Muñoz, S.; Jimenez, V.; Handschin, C.; Bosch, F.; Hall, M.N. mTORC2 Sustains Thermogenesis via Akt-Induced Glucose Uptake and Glycolysis in Brown Adipose Tissue. EMBO Mol. Med. 2016, 8, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Ghanavat, M.; Shahrouzian, M.; Deris Zayeri, Z.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging Deeper through Glucose Metabolism and Its Regulators in Cancer and Metastasis. Life Sci. 2021, 264, 118603. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant P53 in Cancer: From Molecular Mechanism to Therapeutic Modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting Glucose Metabolism to Suppress Cancer Progression: Prospective of Anti-Glycolytic Cancer Therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Yang, H.; Liang, T.; Bai, X. Hurdle or Thruster: Glucose Metabolism of T Cells in Anti-Tumour Immunity. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189022. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Gatenbee, C.; Robertson-Tessi, M.; Bravo, R.; Dhillon, J.; Balagurunathan, Y.; Berglund, A.; Vishvakarma, N.; Ibrahim-Hashim, A.; Choi, J.; et al. Acidity Promotes Tumour Progression by Altering Macrophage Phenotype in Prostate Cancer. Br. J. Cancer 2019, 121, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic Acid and an Acidic Tumor Microenvironment Suppress Anticancer Immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.-L. Lipid Metabolic Reprogramming in Tumor Microenvironment: From Mechanisms to Therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, K.; Liu, Z.; Zhao, R.; Li, F. Fatty Acid Metabolism of Immune Cells: A New Target of Tumour Immunotherapy. Cell Death Discov. 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in Cell Death, Autophagy, and Disease. Autophagy 2023, 19, 2621. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.W.; Amante, J.J.; Chhoy, P.; Elaimy, A.L.; Liu, H.; Zhu, L.J.; Baer, C.E.; Dixon, S.J.; Mercurio, A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell 2019, 51, 575–586.e4. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Xu, Y.; Yuan, C.; Sheng, X.; Wu, Z.; Peng, J.; Wang, Y.; Lin, Y.; Zhou, L.; Xu, S.; et al. Predictive and Prognostic Impact of Ferroptosis-Related Genes ACSL4 and GPX4 on Breast Cancer Treated with Neoadjuvant Chemotherapy. EBioMedicine 2021, 71, 103560. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Liu, C.; Wang, L.; Wu, J.; Sun, C.; Wu, Q. Reprogramming of Lipid Metabolism Mediates Crosstalk, Remodeling, and Intervention of Microenvironment Components in Breast Cancer. Int. J. Biol. Sci. 2024, 20, 1884–1904. [Google Scholar] [CrossRef]
- Ge, D.; Gao, J.; Han, L.; Li, Y.; Liu, H.-H.; Yang, W.-C.; Chang, F.; Liu, J.; Yu, M.; Zhao, J. Novel Effects of Sphingosylphosphorylcholine on the Apoptosis of Breast Cancer via Autophagy/AKT/P38 and JNK Signaling. J. Cell Physiol. 2019, 234, 11451–11462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Liu, Y.; Su, P.; Zhang, J.; Wang, X.; Sun, M.; Chen, B.; Zhao, W.; Wang, L.; et al. SREBP1, Targeted by miR-18a-5p, Modulates Epithelial-Mesenchymal Transition in Breast Cancer via Forming a Co-Repressor Complex with Snail and HDAC1/2. Cell Death Differ. 2019, 26, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Chen, Z.; Feng, H.; Chen, Y.; Zhang, C.; Yu, J.; Luo, Y.; Zhao, L.; Jiang, X.; Shi, F. Sphingomyelin Synthase 2 Promotes an Aggressive Breast Cancer Phenotype by Disrupting the Homoeostasis of Ceramide and Sphingomyelin. Cell Death Dis. 2019, 10, 157. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Ye, Q. Metabolism and Immunity in Breast Cancer. Front. Med. 2021, 15, 178–207. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, K.E.; Hongo, R.; Ye, X.; Young, K.; Carbonell, K.; Healey, D.C.C.; Siska, P.J.; Barone, S.; Roe, C.E.; Smith, C.C.; et al. CD28 Costimulation Drives Tumor-Infiltrating T Cell Glycolysis to Promote Inflammation. JCI Insight 2020, 5, e138729. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 Alters T-Cell Metabolic Reprogramming by Inhibiting Glycolysis and Promoting Lipolysis and Fatty Acid Oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Taha, R.Z.; Sasidharan Nair, V.; Alajez, N.M.; Elkord, E. PD-L1 Blockade by Atezolizumab Downregulates Signaling Pathways Associated with Tumor Growth, Metastasis, and Hypoxia in Human Triple Negative Breast Cancer. Cancers 2019, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Koo, J.S. The Role of Autophagy in Breast Cancer Metastasis. Biomedicines 2023, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Missiroli, S.; Morciano, G.; Perrone, M.; Mantovani, C.M.; Anania, G.; Fiorica, F.; Pinton, P.; Giorgi, C. Understanding the Role of Autophagy in Cancer Formation and Progression Is a Real Opportunity to Treat and Cure Human Cancers. Cancers 2021, 13, 5622. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in Inflammation, Infection, and Immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Signaling Pathways of Autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Wu, Y.; Jin, F.; Xia, Y.; Liu, X. Genetic and Epigenetic Silencing of the Beclin 1 Gene in Sporadic Breast Tumors. BMC Cancer 2010, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of Autophagy and Inhibition of Tumorigenesis by Beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Kreuzer, J.; Kumsta, C.; Wu, L.; Kamer, K.J.; Cedillo, L.; Zhang, Y.; Li, S.; Kacergis, M.C.; Webster, C.M.; et al. Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell 2019, 177, 299–314.e16. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, M.; Chen, J.-H.; Wang, Z.; Du, X.-F.; Liu, P.-X.; Li, W.-H. Osteopontin Knockdown Inhibits Av,Β3 Integrin-Induced Cell Migration and Invasion and Promotes Apoptosis of Breast Cancer Cells by Inducing Autophagy and Inactivating the PI3K/Akt/mTOR Pathway. Cell Physiol. Biochem. 2014, 33, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-G.; Kwon, Y.-S.; Nam, K.-S.; Kim, S.Y.; Hwang, I.H.; Kim, S.; Jang, H. Chaga Mushroom Extract Induces Autophagy via the AMPK-mTOR Signaling Pathway in Breast Cancer Cells. J. Ethnopharmacol. 2021, 274, 114081. [Google Scholar] [CrossRef]
- Wang, B.; Mao, J.-H.; Wang, B.-Y.; Wang, L.-X.; Wen, H.-Y.; Xu, L.-J.; Fu, J.-X.; Yang, H. Exosomal miR-1910-3p Promotes Proliferation, Metastasis, and Autophagy of Breast Cancer Cells by Targeting MTMR3 and Activating the NF-κB Signaling Pathway. Cancer Lett. 2020, 489, 87–99. [Google Scholar] [CrossRef]
- Deng, G.; Zeng, S.; Qu, Y.; Luo, Q.; Guo, C.; Yin, L.; Han, Y.; Li, Y.; Cai, C.; Fu, Y.; et al. BMP4 Promotes Hepatocellular Carcinoma Proliferation by Autophagy Activation through JNK1-Mediated Bcl-2 Phosphorylation. J. Exp. Clin. Cancer Res. 2018, 37, 156. [Google Scholar] [CrossRef]
- Li, Q.; Zan, L. Knockdown of ATG4A Inhibits Breast Cancer Progression and Promotes Tamoxifen Chemosensitivity by Suppressing Autophagy. Mol. Med. Rep. 2022, 25, 101. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal Cells in Tumor Microenvironment and Breast Cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, X.; Wang, L.; Hong, X.; Yang, J. Metabolic Reprogramming and Crosstalk of Cancer-Related Fibroblasts and Immune Cells in the Tumor Microenvironment. Front. Endocrinol. 2022, 13, 988295. [Google Scholar] [CrossRef]
- Jezierska-Drutel, A.; Rosenzweig, S.A.; Neumann, C.A. Role of Oxidative Stress and the Microenvironment in Breast Cancer Development and Progression. Adv. Cancer Res. 2013, 119, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nogueira, P.; Fuster, G.; Gutierrez-Uzquiza, Á.; Gascón, P.; Carbó, N.; Bragado, P. Cancer-Associated Fibroblasts in Breast Cancer Treatment Response and Metastasis. Cancers 2021, 13, 3146. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, C.; Qin, Z. Metabolic Reprogramming of Cancer-Associated Fibroblasts and Its Effect on Cancer Cell Reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef] [PubMed]
- Luga, V.; Wrana, J.L. Tumor-Stroma Interaction: Revealing Fibroblast-Secreted Exosomes as Potent Regulators of Wnt-Planar Cell Polarity Signaling in Cancer Metastasis. Cancer Res. 2013, 73, 6843–6847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Xu, F.; Yin, Y.; Zhu, L.; Zhou, F. Galectin-1 Knockdown in Carcinoma-Associated Fibroblasts Inhibits Migration and Invasion of Human MDA-MB-231 Breast Cancer Cells by Modulating MMP-9 Expression. Acta Biochim. Biophys. Sin. 2016, 48, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Dasgupta, A.; Nguyen, K.H.; Liu, C.; Kovatich, A.J.; Schwartz, G.F.; Pestell, R.G.; Sotgia, F.; Rui, H.; Lisanti, M.P. Stromal Caveolin-1 Levels Predict Early DCIS Progression to Invasive Breast Cancer. Cancer Biol. Ther. 2009, 8, 1071–1079. [Google Scholar] [CrossRef]
- Sloan, E.K.; Ciocca, D.R.; Pouliot, N.; Natoli, A.; Restall, C.; Henderson, M.A.; Fanelli, M.A.; Cuello-Carrión, F.D.; Gago, F.E.; Anderson, R.L. Stromal Cell Expression of Caveolin-1 Predicts Outcome in Breast Cancer. Am. J. Pathol. 2009, 174, 2035–2043. [Google Scholar] [CrossRef]
- Wen, S.; Hou, Y.; Fu, L.; Xi, L.; Yang, D.; Zhao, M.; Qin, Y.; Sun, K.; Teng, Y.; Liu, M. Cancer-Associated Fibroblast (CAF)-Derived IL32 Promotes Breast Cancer Cell Invasion and Metastasis via Integrin Β3-P38 MAPK Signalling. Cancer Lett. 2019, 442, 320–332. [Google Scholar] [CrossRef]
- Tchou, J.; Kossenkov, A.V.; Chang, L.; Satija, C.; Herlyn, M.; Showe, L.C.; Puré, E. Human Breast Cancer Associated Fibroblasts Exhibit Subtype Specific Gene Expression Profiles. BMC Med. Genom. 2012, 5, 39. [Google Scholar] [CrossRef]
- Choi, Y.P.; Lee, J.H.; Gao, M.-Q.; Kim, B.G.; Kang, S.; Kim, S.H.; Cho, N.H. Cancer-Associated Fibroblast Promote Transmigration through Endothelial Brain Cells in Three-Dimensional in Vitro Models. Int. J. Cancer 2014, 135, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.M.; Koo, J.S. Differential Expression of Cancer-Associated Fibroblast-Related Proteins According to Molecular Subtype and Stromal Histology in Breast Cancer. Breast Cancer Res. Treat. 2015, 149, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Östman, A.; Augsten, M. Cancer-Associated Fibroblasts and Tumor Growth—Bystanders Turning into Key Players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.-K.; Nguyen, T.-T.; Thanh, V.V.; Quang, T.L.; Minh, L.B.; Pham, V.H.; Ngoc, V.T.N.; et al. The Effects of Adipocytes on the Regulation of Breast Cancer in the Tumor Microenvironment: An Update. Cells 2019, 8, 857. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-Derived Fibroblasts Promote Tumor Progression and Contribute to the Desmoplastic Reaction in Breast Cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Attané, C.; Milhas, D.; Dirat, B.; Dauvillier, S.; Guerard, A.; Gilhodes, J.; Lazar, I.; Alet, N.; Laurent, V.; et al. Mammary Adipocytes Stimulate Breast Cancer Invasion through Metabolic Remodeling of Tumor Cells. JCI Insight 2017, 2, e87489. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.-F.; Brisson, L. Interaction between Adipose Tissue and Cancer Cells: Role for Cancer Progression. Cancer Metastasis Rev. 2021, 40, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, S. Multi-faceted Role of Cancer-associated Adipocytes in the Tumor Microenvironment (Review). Mol. Med. Rep. 2021, 24, 866. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, M.; Morel, M.; Ferrand, N.; Fellahi, S.; Bastard, J.-P.; Lamazière, A.; Larsen, A.K.; Béréziat, V.; Atlan, M.; Sabbah, M. Breast-Associated Adipocytes Secretome Induce Fatty Acid Uptake and Invasiveness in Breast Cancer Cells via CD36 Independently of Body Mass Index, Menopausal Status and Mammary Density. Cancers 2019, 11, 2012. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Yan, X.; Yan, F.; Sun, Y.; Zeng, J.; Waigel, S.; Yin, Y.; Fraig, M.M.; Egilmez, N.K.; et al. Circulating Adipose Fatty Acid Binding Protein Is a New Link Underlying Obesity-Associated Breast/Mammary Tumor Development. Cell Metab. 2018, 28, 689–705.e5. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Xing, L.; Tan, Y.; Sun, J.; Zeng, B.; Xiang, T.; Tan, J.; Ren, G.; Wang, Y. Utilization of Adipocyte-Derived Lipids and Enhanced Intracellular Trafficking of Fatty Acids Contribute to Breast Cancer Progression. Cell Commun. Signal 2018, 16, 32. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Guidolin, D.; Annese, T.; Tortorella, C.; Ruggieri, S.; Rega, S.; Zito, F.A.; Nico, B.; Ribatti, D. Spatial Distribution of Mast Cells and Macrophages around Tumor Glands in Human Breast Ductal Carcinoma. Exp. Cell Res. 2017, 359, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Majorini, M.T.; Colombo, M.P.; Lecis, D. Few, but Efficient: The Role of Mast Cells in Breast Cancer and Other Solid Tumors. Cancer Res. 2022, 82, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, C.; Kataoka, T.R.; Hirata, M.; Furuhata, A.; Suzuki, E.; Toi, M.; Tsuruyama, T.; Okayama, Y.; Haga, H. The Killer Cell Ig-like Receptor 2DL4 Expression in Human Mast Cells and Its Potential Role in Breast Cancer Invasion. Cancer Immunol. Res. 2015, 3, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhou, T.; Shi, H.; Yao, M.; Zhang, D.; Qian, H.; Zeng, Q.; Wang, Y.; Jin, F.; Chai, C.; et al. Progranulin Induces Immune Escape in Breast Cancer via Up-Regulating PD-L1 Expression on Tumor-Associated Macrophages (TAMs) and Promoting CD8+ve T Cell Exclusion. J. Exp. Clin. Cancer Res. 2021, 40, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, P.; Wang, J.; Zhang, J.; Ma, Q.; Jiang, Y.; Wu, Y.; Han, T.; Xiang, D. HLF Regulates Ferroptosis, Development and Chemoresistance of Triple-Negative Breast Cancer by Activating Tumor Cell-Macrophage Crosstalk. J. Hematol. Oncol. 2022, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Dai, W.; Guo, X.; Wang, K. LncRNA-SNHG1 Promotes Macrophage M2-like Polarization and Contributes to Breast Cancer Growth and Metastasis. Aging 2021, 13, 23169–23181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wei, H.; Wang, H.; Wang, Z.; Li, J.; Ou, Y.; Xiao, X.; Wang, W.; Chang, A.; Sun, W.; et al. Zeb1-Induced Metabolic Reprogramming of Glycolysis Is Essential for Macrophage Polarization in Breast Cancer. Cell Death Dis. 2022, 13, 206. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Yang, N.; Shi, Q.; Su, H.; Lin, T.; He, Z.; Wang, W.; Guo, H.; Shen, P. Crosstalk between IL-15Rα+ve Tumor-Associated Macrophages and Breast Cancer Cells Reduces CD8+ve T Cell Recruitment. Cancer Commun. 2022, 42, 536–557. [Google Scholar] [CrossRef]
- Kundu, M.; Butti, R.; Panda, V.K.; Malhotra, D.; Das, S.; Mitra, T.; Kapse, P.; Gosavi, S.W.; Kundu, G.C. Modulation of the Tumor Microenvironment and Mechanism of Immunotherapy-Based Drug Resistance in Breast Cancer. Mol. Cancer 2024, 23, 92. [Google Scholar] [CrossRef]
- Chen, X.; Yang, M.; Yin, J.; Li, P.; Zeng, S.; Zheng, G.; He, Z.; Liu, H.; Wang, Q.; Zhang, F.; et al. Tumor-Associated Macrophages Promote Epithelial-Mesenchymal Transition and the Cancer Stem Cell Properties in Triple-Negative Breast Cancer through CCL2/AKT/β-Catenin Signaling. Cell Commun. Signal 2022, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-S.; Tseng, H.-Y.; Chen, Y.-A.; Shen, P.-C.; Al Haq, A.T.; Chen, L.-M.; Tung, Y.-C.; Hsu, H.-L. MCT-1/miR-34a/IL-6/IL-6R Signaling Axis Promotes EMT Progression, Cancer Stemness and M2 Macrophage Polarization in Triple-Negative Breast Cancer. Mol. Cancer 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Bill, R.; Wirapati, P.; Messemaker, M.; Roh, W.; Zitti, B.; Duval, F.; Kiss, M.; Park, J.C.; Saal, T.M.; Hoelzl, J.; et al. CXCL9:SPP1 Macrophage Polarity Identifies a Network of Cellular Programs That Control Human Cancers. Science 2023, 381, 515–524. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Guo, Z.; Yu, J.; Tan, J.; Simons, D.L.; Hu, K.; Liu, X.; Zhou, Q.; Zheng, Y.; et al. PD-L1-Expressing Tumor-Associated Macrophages Are Immunostimulatory and Associate with Good Clinical Outcome in Human Breast Cancer. Cell Rep. Med. 2024, 5, 101420. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 Derived from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via Activating NF-κB/SOX4 Signaling. Cell Death Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, N.; Li, J.; Jin, Q.; Jin, J.; Guo, J.; Wei, X.; Wang, X.; Yao, L.; Meng, D.; et al. Tumor Cell SPTBN1 Inhibits M2 Polarization of Macrophages by Suppressing CXCL1 Expression. J. Cell Physiol. 2024, 239, 97–111. [Google Scholar] [CrossRef]
- Anstee, J.E.; Feehan, K.T.; Opzoomer, J.W.; Dean, I.; Muller, H.P.; Bahri, M.; Cheung, T.S.; Liakath-Ali, K.; Liu, Z.; Choy, D.; et al. LYVE-1+ve Macrophages Form a Collaborative CCR5-Dependent Perivascular Niche That Influences Chemotherapy Responses in Murine Breast Cancer. Dev. Cell 2023, 58, 1548–1561.e10. [Google Scholar] [CrossRef]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-Derived Lactate Induces M2 Macrophage Polarization via the Activation of the ERK/STAT3 Signaling Pathway in Breast Cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gan, M.; Jin, X.; Dai, M.; Wang, Y.; Lei, Y.; Lin, Z.; Ming, J. miR-382 Inhibits Breast Cancer Progression and Metastasis by Affecting the M2 Polarization of Tumor-associated Macrophages by Targeting PGC-1α. Int. J. Oncol. 2022, 61, 126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Yu, L.; Hu, Z.-L.; Fang, Y.-F.; Shen, Y.-Y.; Song, M.-F.; Chen, Y. Regulation of CCL2 by EZH2 Affects Tumor-Associated Macrophages Polarization and Infiltration in Breast Cancer. Cell Death Dis. 2022, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Pe, K.C.S.; Saetung, R.; Yodsurang, V.; Chaotham, C.; Suppipat, K.; Chanvorachote, P.; Tawinwung, S. Triple-Negative Breast Cancer Influences a Mixed M1/M2 Macrophage Phenotype Associated with Tumor Aggressiveness. PLoS ONE 2022, 17, e0273044. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Zhao, L.; Shao, S.; Ning, Q.; Jing, X.; Zhang, Y.; Zhao, F.; Liu, X.; Gu, S.; et al. VEGFA/NRP-1/GAPVD1 Axis Promotes Progression and Cancer Stemness of Triple-Negative Breast Cancer by Enhancing Tumor Cell-Macrophage Crosstalk. Int. J. Biol. Sci. 2024, 20, 446–463. [Google Scholar] [CrossRef]
- Chu, J.; Hu, X.-C.; Li, C.-C.; Li, T.-Y.; Fan, H.-W.; Jiang, G.-Q. KLF14 Alleviated Breast Cancer Invasion and M2 Macrophages Polarization through Modulating SOCS3/RhoA/Rock/STAT3 Signaling. Cell Signal 2022, 92, 110242. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, L.; Peng, X.; Tu, J.; Li, S.; He, X.; Li, F.; Qiang, J.; Dong, H.; Deng, Q.; et al. IL1R2 Blockade Alleviates Immunosuppression and Potentiates Anti-PD-1 Efficacy in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 2282–2296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, Z.; Li, B.; Li, J.; Ou, Y.; Yu, X.; Zhang, Z.; Liu, S.; Fu, X.; Jin, H.; et al. Lipid-Associated Macrophages in the Tumor-Adipose Microenvironment Facilitate Breast Cancer Progression. Oncoimmunology 2022, 11, 2085432. [Google Scholar] [CrossRef]
- Ziauddin, M.F.; Hua, D.; Tang, S.-C. Emerging Strategies to Overcome Resistance to Endocrine Therapy for Breast Cancer. Cancer Metastasis Rev. 2014, 33, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qin, T.; Bi, Z.; Hong, H.; Ding, L.; Chen, J.; Wu, W.; Lin, X.; Fu, W.; Zheng, F.; et al. Rac1 Activates Non-Oxidative Pentose Phosphate Pathway to Induce Chemoresistance of Breast Cancer. Nat. Commun. 2020, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y.; Zhang, Y.; Ye, F.; Luo, D.; Li, Y.; Jin, Y.; Han, D.; Wang, Z.; Chen, B.; et al. HSPB1 Facilitates Chemoresistance through Inhibiting Ferroptotic Cancer Cell Death and Regulating NF-κB Signaling Pathway in Breast Cancer. Cell Death Dis. 2023, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yuan, X.; Jiang, Z.; Gan, D.; Ding, L.; Sun, Y.; Zhou, J.; Xu, L.; Liu, Y.; Wang, G. Regulation of AKT Phosphorylation by GSK3β and PTEN to Control Chemoresistance in Breast Cancer. Breast Cancer Res. Treat. 2019, 176, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, S.; Shi, Z.; Cao, L.; Liu, J.; Pan, T.; Zhou, D.; Zhang, J. Chemotherapy-Elicited Exosomal miR-378a-3p and miR-378d Promote Breast Cancer Stemness and Chemoresistance via the Activation of EZH2/STAT3 Signaling. J. Exp. Clin. Cancer Res. 2021, 40, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-M.; Wu, J.-F.; Luo, Q.-C.; Liu, Q.-F.; Wu, Q.-W.; Ye, G.-D.; She, H.-Q.; Li, B.-A. Pygo2 Activates MDR1 Expression and Mediates Chemoresistance in Breast Cancer via the Wnt/β-Catenin Pathway. Oncogene 2016, 35, 4787–4797. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Y.; Lou, C.; He, Y.; Zhang, Y.; Zhang, Q. FSTL1 Enhances Chemoresistance and Maintains Stemness in Breast Cancer Cells via Integrin Β3/Wnt Signaling under miR-137 Regulation. Cancer Biol. Ther. 2019, 20, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Jalalirad, M.; Haddad, T.C.; Salisbury, J.L.; Radisky, D.; Zhang, M.; Schroeder, M.; Tuma, A.; Leof, E.; Carter, J.M.; Degnim, A.C.; et al. Aurora-A Kinase Oncogenic Signaling Mediates TGF-β-Induced Triple-Negative Breast Cancer Plasticity and Chemoresistance. Oncogene 2021, 40, 2509–2523. [Google Scholar] [CrossRef] [PubMed]
- Chandra Jena, B.; Kanta Das, C.; Banerjee, I.; Das, S.; Bharadwaj, D.; Majumder, R.; Mandal, M. Paracrine TGF-Β1 from Breast Cancer Contributes to Chemoresistance in Cancer Associated Fibroblasts via Upregulation of the P44/42 MAPK Signaling Pathway. Biochem. Pharmacol. 2021, 186, 114474. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Al-Yahyaee, A.; Abdullah, N.; Sam, J.-E.; Al-Zeheimi, N.; Yaish, M.W.; Adham, S.A. Neuropilin-1 Promotes the Oncogenic Tenascin-C/Integrin Β3 Pathway and Modulates Chemoresistance in Breast Cancer Cells. BMC Cancer 2018, 18, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhang, C.; Chu, J.; Wu, Y.; Li, Y.; Liu, J.; Li, Q.; Li, S.; Shi, Q.; et al. Tamoxifen-Resistant Breast Cancer Cells Are Resistant to DNA-Damaging Chemotherapy Because of Upregulated BARD1 and BRCA1. Nat. Commun. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, N.; Yin, J.; Zhang, J.; Liu, H.; Guo, W.; Liu, M.; Liu, T.; Chen, D.; Luo, K.; et al. SRGN Crosstalks with YAP to Maintain Chemoresistance and Stemness in Breast Cancer Cells by Modulating HDAC2 Expression. Theranostics 2020, 10, 4290–4307. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Brady, A.F.; Frayling, I.M.; Hanson, H.; Tischkowitz, M.; Turnbull, C.; Side, L.; UK Cancer Genetics Group (UK-CGG). Consensus for Genes to Be Included on Cancer Panel Tests Offered by UK Genetics Services: Guidelines of the UK Cancer Genetics Group. J. Med. Genet. 2018, 55, 372–377. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Illert, A.-L.; Erbes, T.; Flotho, C.; Lübbert, M.; Duque-Afonso, J. The Epigenetics of Breast Cancer—Opportunities for Diagnostics, Risk Stratification and Therapy. Epigenetics 2022, 17, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lou, J. DNA Methylation Analysis. Methods Mol. Biol. 2019, 1894, 181–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-Based DNA Methylation as Biomarker for Breast Cancer: A Systematic Review. Clin. Epigenetics 2016, 8, 115. [Google Scholar] [CrossRef]
- Lu, A.; Wang, W.; Wang-Renault, S.-F.; Ring, B.Z.; Tanaka, Y.; Weng, J.; Su, L. 5-Aza-2′-Deoxycytidine Advances the Epithelial-Mesenchymal Transition of Breast Cancer Cells by Demethylating Sipa1 Promoter-Proximal Elements. J. Cell Sci. 2020, 133, jcs236125. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Fernandez, S.V.; Russo, P.A.; Fernbaugh, R.; Sheriff, F.S.; Lareef, H.M.; Garber, J.; Russo, I.H. 17-Beta-Estradiol Induces Transformation and Tumorigenesis in Human Breast Epithelial Cells. FASEB J. 2006, 20, 1622–1634. [Google Scholar] [CrossRef]
- Shi, B.; Liang, J.; Yang, X.; Wang, Y.; Zhao, Y.; Wu, H.; Sun, L.; Zhang, Y.; Chen, Y.; Li, R.; et al. Integration of Estrogen and Wnt Signaling Circuits by the Polycomb Group Protein EZH2 in Breast Cancer Cells. Mol. Cell Biol. 2007, 27, 5105–5119. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic Mechanisms in Breast Cancer Therapy and Resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Mo, K.; Kwon, H.; Choe, S.; Park, M.; Kwak, W.; Yoon, H. Epigenetic Regulation in Breast Cancer: Insights on Epidrugs. Epigenomes 2023, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- ENCODE Project Consortium. An Integrated Encyclopedia of DNA Elements in the Human Genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Gahan, P.B. Interplay between LncRNAs and microRNAs in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 8095. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Mao, A.; Liu, J.; Lu, J.; Ding, J.; Liu, W. Circular RNA Hsa_circ_0061825 (Circ-TFF1) Contributes to Breast Cancer Progression through Targeting miR-326/TFF1 Signalling. Cell Prolif. 2020, 53, e12720. [Google Scholar] [CrossRef] [PubMed]
- Adam-Artigues, A.; Garrido-Cano, I.; Carbonell-Asins, J.A.; Lameirinhas, A.; Simón, S.; Ortega-Morillo, B.; Martínez, M.T.; Hernando, C.; Constâncio, V.; Burgues, O.; et al. Identification of a Two-MicroRNA Signature in Plasma as a Novel Biomarker for Very Early Diagnosis of Breast Cancer. Cancers 2021, 13, 2848. [Google Scholar] [CrossRef] [PubMed]
- Giussani, M.; Ciniselli, C.M.; De Cecco, L.; Lecchi, M.; Dugo, M.; Gargiuli, C.; Mariancini, A.; Mancinelli, E.; Cosentino, G.; Veneroni, S.; et al. Circulating miRNAs as Novel Non-Invasive Biomarkers to Aid the Early Diagnosis of Suspicious Breast Lesions for Which Biopsy Is Recommended. Cancers 2021, 13, 4028. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-F.; Zhang, X.-Z.; Liu, B.-G.; Jia, G.-T.; Li, W.-L. Circular RNA Circ-ABCB10 Promotes Breast Cancer Proliferation and Progression through Sponging miR-1271. Am. J. Cancer Res. 2017, 7, 1566–1576. [Google Scholar] [PubMed]
- Zeng, K.; He, B.; Yang, B.B.; Xu, T.; Chen, X.; Xu, M.; Liu, X.; Sun, H.; Pan, Y.; Wang, S. The Pro-Metastasis Effect of circANKS1B in Breast Cancer. Mol. Cancer 2018, 17, 160. [Google Scholar] [CrossRef]
- Fu, B.; Liu, W.; Zhu, C.; Li, P.; Wang, L.; Pan, L.; Li, K.; Cai, P.; Meng, M.; Wang, Y.; et al. Circular RNA circBCBM1 Promotes Breast Cancer Brain Metastasis by Modulating miR-125a/BRD4 Axis. Int. J. Biol. Sci. 2021, 17, 3104–3117. [Google Scholar] [CrossRef]
- Xu, J.-H.; Wang, Y.; Xu, D. Hsa_circ_001569 Is an Unfavorable Prognostic Factor and Promotes Cell Proliferation and Metastasis by Modulating PI3K-AKT Pathway in Breast Cancer. Cancer Biomark. 2019, 25, 193–201. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Mao, L. Circular RNA Circ_0000442 Acts as a Sponge of MiR-148b-3p to Suppress Breast Cancer via PTEN/PI3K/Akt Signaling Pathway. Gene 2021, 766, 145113. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, Y.; Wu, L.; Ma, D. Comprehensive Circular RNA Profiling Reveals the Regulatory Role of the circRNA-000911/miR-449a Pathway in Breast Carcinogenesis. Int. J. Oncol. 2018, 52, 743–754. [Google Scholar] [CrossRef]
- Sereno, M.; Videira, M.; Wilhelm, I.; Krizbai, I.A.; Brito, M.A. miRNAs in Health and Disease: A Focus on the Breast Cancer Metastatic Cascade towards the Brain. Cells 2020, 9, 1790. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Ferns, G.A.; Talebian, S.; Nourbakhsh, M.; Avan, A.; Shahidsales, S. Role of Regulatory miRNAs of the PI3K/AKT Signaling Pathway in the Pathogenesis of Breast Cancer. Gene 2020, 737, 144459. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Miao, Y.; Shan, Y.; Liu, B.; Li, Y.; Zhao, L.; Jia, L. MiR-106b and miR-93 Regulate Cell Progression by Suppression of PTEN via PI3K/Akt Pathway in Breast Cancer. Cell Death Dis. 2017, 8, e2796. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Kazan, N.; Haciefendi, A.; Deveci Ozkan, A.; Ozdemir, K.; Ozen, M.; Kocer, H.B.; Yilmaz, F.; Kaleli, S.; Sahin, E.; et al. The Prognostic and Predictive Values of Differential Expression of Exosomal Receptor Tyrosine Kinases and Associated with the PI3K/AKT/mTOR Signaling in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Clin. Transl. Oncol. 2023, 25, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, N.; Hosseinpourfeizi, M.A.; Khojasteh, S.M.B.; Baradaran, B.; Safaralizadeh, R. Tumor Suppressive Activity of miR-424-5p in Breast Cancer Cells through Targeting PD-L1 and Modulating PTEN/PI3K/AKT/mTOR Signaling Pathway. Life Sci. 2020, 259, 118239. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Cheng, K.; Zhang, G.; Wang, X. miR-99a Directly Targets the mTOR Signalling Pathway in Breast Cancer Side Population Cells. Cell Prolif. 2014, 47, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Imani, S.; Wu, M.-Y.; Wu, R.-C. MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential. Cancers 2023, 15, 4723. [Google Scholar] [CrossRef]
- Lee, C.G.; McCarthy, S.; Gruidl, M.; Timme, C.; Yeatman, T.J. MicroRNA-147 Induces a Mesenchymal-to-Epithelial Transition (MET) and Reverses EGFR Inhibitor Resistance. PLoS ONE 2014, 9, e84597. [Google Scholar] [CrossRef]
- Song, C.; Liu, L.-Z.; Pei, X.-Q.; Liu, X.; Yang, L.; Ye, F.; Xie, X.; Chen, J.; Tang, H.; Xie, X. miR-200c Inhibits Breast Cancer Proliferation by Targeting KRAS. Oncotarget 2015, 6, 34968–34978. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Chen, L.; Xu, X.; Zhang, X.; Wang, B.; Min, L.; Liu, W. miRNA-200c Increases the Sensitivity of Breast Cancer Cells to Doxorubicin through the Suppression of E-Cadherin-Mediated PTEN/Akt Signaling. Mol. Med. Rep. 2013, 7, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.S.; Ryu, H.S.; Kim, N.; Kim, J.; Lee, E.; Moon, H.; Kim, K.H.; Jin, M.-S.; Kwon, N.H.; Kim, S.; et al. Tumor Suppressor miRNA-204-5p Regulates Growth, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Cancer Res. 2019, 79, 1520–1534. [Google Scholar] [CrossRef]
- An, X.; Liu, Y. HOTAIR in Solid Tumors: Emerging Mechanisms and Clinical Strategies. Biomed. Pharmacother. 2022, 154, 113594. [Google Scholar] [CrossRef]
- Born, L.J.; Chang, K.-H.; Shoureshi, P.; Lay, F.; Bengali, S.; Hsu, A.T.W.; Abadchi, S.N.; Harmon, J.W.; Jay, S.M. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv. Healthc. Mater. 2022, 11, e2002070. [Google Scholar] [CrossRef] [PubMed]
- Sadeghalvad, M.; Mansouri, K.; Mohammadi-Motlagh, H.-R.; Noorbakhsh, F.; Mostafaie, A.; Alipour, S.; Rezaei, N. Long Non-Coding RNA HOTAIR Induces the PI3K/AKT/mTOR Signaling Pathway in Breast Cancer Cells. Rev. Assoc. Med. Bras. (1992) 2022, 68, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Lu, J. The Roles of Long Noncoding RNAs in Breast Cancer Metastasis. Cell Death Dis. 2020, 11, 749. [Google Scholar] [CrossRef]
- Desouky, E.M.; Khaliefa, A.K.; Hozayen, W.G.; Shaaban, S.M.; Hasona, N.A. Signature of miR-21 and MEG-2 and Their Correlation with TGF-β Signaling in Breast Cancer. Hum. Exp. Toxicol. 2023, 42, 9603271231159799. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Olvera, S.I.; Aguilar-Arnal, L.; Cisneros-Villanueva, M.; Hidalgo-Miranda, A.; Marchat, L.A.; Salinas-Vera, Y.M.; Ramos-Payán, R.; Pérez-Plasencia, C.; Carlos-Reyes, Á.; Puente-Rivera, J.; et al. Breast Cancer Cells Reprogram the Oncogenic lncRNAs/mRNAs Coexpression Networks in Three-Dimensional Microenvironment. Cells 2022, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Maharati, A.; Moghbeli, M. Long Non-Coding RNAs as the Critical Regulators of PI3K/AKT, TGF-β, and MAPK Signaling Pathways during Breast Tumor Progression. J. Transl. Med. 2023, 21, 556. [Google Scholar] [CrossRef]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front. Endocrinol. 2019, 10, 245. [Google Scholar] [CrossRef]
- Howell, A.; Howell, S.J. Tamoxifen Evolution. Br. J. Cancer 2023, 128, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 Study of Buparlisib (BKM120), a Pan-Class I PI3K Inhibitor, in Patients with Metastatic Triple-Negative Breast Cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Dent, R.; Im, S.-A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus Paclitaxel versus Placebo plus Paclitaxel as First-Line Therapy for Metastatic Triple-Negative Breast Cancer (LOTUS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulou, A.; Chatzinikolaou, S.; Panourgias, E.; Kaparelou, M.; Liontos, M.; Dimopoulos, M.-A.; Zagouri, F. The Emerging Role of Capivasertib in Breast Cancer. Breast 2022, 63, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Kline, C.L.B.; Prabhu, V.V.; Wagner, J.; Ishizawa, J.; Madhukar, N.; Lev, A.; Baumeister, M.; Zhou, L.; Lulla, A.; et al. Discovery and Clinical Introduction of First-in-Class Imipridone ONC201. Oncotarget 2016, 7, 74380–74392. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Patel, L.; Mayes, P.A.; Dicker, D.T.; Wu, G.S.; El-Deiry, W.S. Identification of TRAIL-Inducing Compounds Highlights Small Molecule ONC201/TIC10 as a Unique Anti-Cancer Agent That Activates the TRAIL Pathway. Mol. Cancer 2015, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual Inactivation of Akt and ERK by TIC10 Signals Foxo3a Nuclear Translocation, TRAIL Gene Induction, and Potent Antitumor Effects. Sci. Transl. Med. 2013, 5, 171ra17. [Google Scholar] [CrossRef] [PubMed]
- Ralff, M.D.; Kline, C.L.B.; Küçükkase, O.C.; Wagner, J.; Lim, B.; Dicker, D.T.; Prabhu, V.V.; Oster, W.; El-Deiry, W.S. ONC201 Demonstrates Antitumor Effects in Both Triple-Negative and Non-Triple-Negative Breast Cancers through TRAIL-Dependent and TRAIL-Independent Mechanisms. Mol. Cancer Ther. 2017, 16, 1290–1298. [Google Scholar] [CrossRef]
- Leung, E.Y.; Kim, J.E.; Askarian-Amiri, M.; Rewcastle, G.W.; Finlay, G.J.; Baguley, B.C. Relationships between Signaling Pathway Usage and Sensitivity to a Pathway Inhibitor: Examination of Trametinib Responses in Cultured Breast Cancer Lines. PLoS ONE 2014, 9, e105792. [Google Scholar] [CrossRef]
- Fedele, C.; Ran, H.; Diskin, B.; Wei, W.; Jen, J.; Geer, M.J.; Araki, K.; Ozerdem, U.; Simeone, D.M.; Miller, G.; et al. SHP2 Inhibition Prevents Adaptive Resistance to MEK Inhibitors in Multiple Cancer Models. Cancer Discov. 2018, 8, 1237–1249. [Google Scholar] [CrossRef]
- Royce, M.; Bachelot, T.; Villanueva, C.; Özgüroglu, M.; Azevedo, S.J.; Cruz, F.M.; Debled, M.; Hegg, R.; Toyama, T.; Falkson, C.; et al. Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: A Clinical Trial. JAMA Oncol. 2018, 4, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Petrossian, K.; Nguyen, D.; Lo, C.; Kanaya, N.; Somlo, G.; Cui, Y.X.; Huang, C.-S.; Chen, S. Use of Dual mTOR Inhibitor MLN0128 against Everolimus-Resistant Breast Cancer. Breast Cancer Res. Treat. 2018, 170, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Potter, D.A.; Salkeni, M.A.; Silverman, P.; Haddad, T.C.; Forget, F.; Awada, A.; Canon, J.-L.; Danso, M.; Lortholary, A.; et al. Sapanisertib Plus Exemestane or Fulvestrant in Women with Hormone Receptor-Positive/HER2-Negative Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Zubair, T.; Bandyopadhyay, D. Small Molecule EGFR Inhibitors as Anti-Cancer Agents: Discovery, Mechanisms of Action, and Opportunities. Int. J. Mol. Sci. 2023, 24, 2651. [Google Scholar] [CrossRef]
- Shah, M.; Nunes, M.R.; Stearns, V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor–Positive Advanced Breast Cancer? Oncology 2018, 32, 216–222. [Google Scholar] [PubMed]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical Landscape of LAG-3-Targeted Therapy. Immunooncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.-A.; Park, Y.H.; Delord, J.-P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and Durvalumab in Patients with Germline BRCA-Mutated Metastatic Breast Cancer (MEDIOLA): An Open-Label, Multicentre, Phase 1/2, Basket Study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Bernal, M.G.C.; Cervantes-Badillo, M.G.; Martínez-Herrera, J.F.; Lara-Torres, C.O.; Gerson-Cwilich, R.; Zentella-Dehesa, A.; Ibarra-Sánchez, M.d.J.; Esparza-López, J.; Montesinos, J.J.; Cortés-Morales, V.A.; et al. Biological Landscape of Triple Negative Breast Cancers Expressing CTLA-4. Front. Oncol. 2020, 10, 1206. [Google Scholar] [CrossRef]
- Loi, S.; Francis, P.A.; Zdenkowski, N.; Gebski, V.; Fox, S.B.; White, M.; Kiely, B.E.; Woodward, N.E.; Hui, R.; Redfern, A.D.; et al. Neoadjuvant Ipilimumab and Nivolumab in Combination with Paclitaxel Following Anthracycline-Based Chemotherapy in Patients with Treatment Resistant Early-Stage Triple-Negative Breast Cancer (TNBC): A Single-Arm Phase 2 Trial. J. Clin. Oncol. 2022, 40, 602. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Escuin-Ordinas, H.; Ibarrondo, F.J. Tremelimumab: Research and Clinical Development. Onco Targets Ther. 2016, 9, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, H.; Bulbule, A.; Kundu, G.C. Osteopontin: Role in Cell Signaling and Cancer Progression. Trends Cell Biol. 2006, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, Randomized, Single-Blinded, Multi-Center Phase II Trial of Two HER2 Peptide Vaccines, GP2 and AE37, in Breast Cancer Patients to Prevent Recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Heery, C.R.; Ibrahim, N.K.; Arlen, P.M.; Mohebtash, M.; Murray, J.L.; Koenig, K.; Madan, R.A.; McMahon, S.; Marté, J.L.; Steinberg, S.M.; et al. Docetaxel Alone or in Combination with a Therapeutic Cancer Vaccine (PANVAC) in Patients with Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Nimma, R.; Kundu, G.; Bulbule, A.; Kumar, T.V.S.; Gunasekaran, V.P.; Tomar, D.; Kumar, D.; Mane, A.; Gill, S.S.; et al. Tumor-Derived Osteopontin Drives the Resident Fibroblast to Myofibroblast Differentiation through Twist1 to Promote Breast Cancer Progression. Oncogene 2021, 40, 2002–2017. [Google Scholar] [CrossRef] [PubMed]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-Associated Macrophage Derived IL-6 Enriches Cancer Stem Cell Population and Promotes Breast Tumor Progression via Stat-3 Pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef]
- Panda, V.K.; Mishra, B.; Nath, A.N.; Butti, R.; Yadav, A.S.; Malhotra, D.; Khanra, S.; Mahapatra, S.; Mishra, P.; Swain, B.; et al. Osteopontin: A Key Multifaceted Regulator in Tumor Progression and Immunomodulation. Biomedicines 2024, 12, 1527. [Google Scholar] [CrossRef]
- Mishra, B.; Yadav, A.S.; Malhotra, D.; Mitra, T.; Sinsinwar, S.; Radharani, N.N.V.; Sahoo, S.R.; Patnaik, S.; Kundu, G.C. Chitosan Nanoparticle-Mediated Delivery of Curcumin Suppresses Tumor Growth in Breast Cancer. Nanomaterials 2024, 14, 1294. [Google Scholar] [CrossRef]

| Drug Name | Active Ingredient | Breast Cancer Type |
|---|---|---|
| KISQALI KISQALI FEMERA CO-PACK (CO-PACKAGE) | RIBOCICLIB SUCCINATE LETROZOLE; RIBOCICLIB SUCCINATE | HR+ve, HER2-ve breast cancer |
| ENHERTU | FAM TRASTUZUMAB DERUXTECAN-NXKI | HER2+ve breast cancer |
| TEPYLUTE | THIOTEPA | Breast adenocarcinoma |
| IBRANCE tablets | PALBOCICLIB | HR+ve, HER2-ve, advanced, or metastatic breast cancer |
| HALAVEN-injection | ERIBULIN MESYLATE | Metastatic breast cancer |
| TRUQAP | CAPIVASERTIB | HR+ve, HER2-ve, locally advanced metastatic breast cancer |
| ORSERDU | ELACESTRANT HYDROCHLORIDE | ER+ve, HER2-ve, ESR1-mutated, advanced, or metastatic breast cancer |
| VERZENIO with endocrine therapy (tamoxifen or an aromatase inhibitor or fulvestrant | ABEMACICLIB | HR+ve, HER2-ve, node-positive early and advanced, or metastatic breast cancer |
| LYNPARZA | OLAPARIB | Germline BRCA-mutated, HER2-ve breast cancer |
| KEYTRUDA | PEMBROLIZUMAB | Triple-negative breast cancer |
| TRODELVY | SACITUZUMAB GOVITECAN | Metastatic triple-negative breast cancer |
| S. No. | Breast Cancer Subtype | Therapy Details | Phase | Clinical Trial No. |
|---|---|---|---|---|
| 1 | TNBC | Pembrolizumab, axatilimab, radiation therapy | Phase 2 | NCT05491226 |
| 2 | TNBC | Utidelone (UTD1) plus capecitabine | Phase 2 | NCT06385990 |
| 3 | TNBC | Carboplatin, docetaxel, doxorubicin, cyclophosphamide, pembrolizumab | Phase 2 | NCT05645380 |
| 4 | TNBC | Fluzoparib + paclitaxel Epirubicin + cyclophosphamide | Phase 2 | NCT05834582 |
| 5 | TNBC | Sintilimab, anlotinib, nab-paclitaxel, carboplatin, epirubicin, cyclophosphamide | Phase 2 | NCT04877821 |
| 6 | TNBC | Capecitabine | Phase 2 | NCT04768426 |
| 7 | TNBC | Anti-PD-1 monoclonal antibody, VEGFR2 tyrosine kinase inhibitor | Phase 2 | NCT05556200 |
| 8 | TNBC | Adebrelimab + stereotactic radiotherapy + nab-paclitaxel + carboplatin, adebrelimab + chemotherapy (nab-paclitaxel + carboplatin) | Phase 2 | NCT06165900 |
| 9 | TNBC | Epirubicin, cyclophosphamide, paclitaxel, carboplatin, | Phase 3 | NCT03876886 |
| 10 | TNBC | Deferoxamine plus chemotherapy | Phase 2 | NCT05300958 |
| 11 | TNBC | Tiragolumab, atezolizumab and chemotherapy | Phase 2 | NCT06175390 |
| 12 | TNBC | Epirubicin, CTX, paclitaxel, ddEpirubicin, ddCTX, paclitaxel (with carbo), carboplatin | Phase 3 | NCT04296175 |
| 13 | TNBC | BL-B01D1, eribulin, vinorelbine, gemcitabine, capecitabine | Phase 3 | NCT06382142 |
| 14 | TNBC | Olaparib, paclitaxel + carboplatin | Phase 2, 3 | NCT03150576 |
| 15 | TNBC | Sacituzumab govitecan-hziy (SG), Pembrolizumab, capecitabine | Phase 3 | NCT05633654 |
| 16 | TNBC | Albumin-bound paclitaxel + carboplatin, epirubicin + docetaxel | Phase 4 | NCT04136782 |
| 17 | TNBC | AZD6738, olaparib, durvalumab | Phase 2 | NCT03740893 |
| 18 | TNBC | Eribulin, LM-108, nab-paclitaxel, toripalimab | Phase 2 | NCT06387628 |
| 19 | TNBC | Tiragolumab, atezolizumab, ipilimumab | Phase 2 | NCT06342037 |
| 20 | TNBC | α-lactalbumin vaccine, zymosan | Phase 1 | NCT04674306 |
| 21 | TNBC | Ceralasertib, durvalumab, nab-paclitaxel | Phase 2 | NCT05582538 |
| 22 | TNBC | Capecitabine, talazoparib, pembrolizumab, inavolisib | Phase 2 | NCT04849364 |
| 23 | TNBC | Ribociclib, bicalutamide | Phase 1, 2 | NCT03090165 |
| 24 | TNBC | Camrelizumab plus famitinib with/without nab-palitaxel | Phase 2 | NCT05670925 |
| 25 | TNBC | Lenvatinib, pembrolizumab | Phase 1 | NCT04427293 |
| 26 | TNBC, stage IV breast cancer | Anti-HER2/HER3 dendritic cell vaccine, pembrolizumab | Phase 2 | NCT04348747 |
| 27 | TNBC (stage I, II, III breast cancer) | Carboplatin, cyclophosphamide, docetaxel, doxorubicin, paclitaxel, pembrolizumab | Phase 3 | NCT05929768 |
| 28 | Metastatic TNBC | Trilaciclib, pembrolizumab, gemcitabine, carboplatin | Phase 2 | NCT06027268 |
| 29 | Metastatic TNBC | Gemcitabine and carboplatin plus antibiotic (moxifloxacin); gemcitabine combined with carboplatin plus placebo | Phase 3 | NCT04722978 |
| 30 | TNBC, metastatic breast cancer | Sacituzumab govitecan | Phase 3 | NCT05552001 |
| 31 | TNBC, intermediate and high-risk luminal | Accelerated partial breast irradiation, chemotherapy | Phase 1, 2 | NCT02806258 |
| 32 | TNBC, ductal carcinoma in situ, lobular carcinoma in situ | Abemaciclib | Phase 2 | NCT03979508 |
| 33 | TNBC, HR+ve and HER2-ve breast cancer | Pembrolizumab, paclitaxel, carboplatin, cyclophosphamide, doxorubicin, capecitabine | Phase 2 | NCT04443348 |
| 34 | Invasive breast cancer (HR+ve, HER2-ve, or TNBC) | Cemiplimab, paclitaxel, carboplatin (not mandatory), doxorubicin, cyclophosphamide | Phase 2 | NCT04243616 |
| 35 | TNBC, stage III, IV, and Recurrent Breast Cancer | Avelumab, binimetinib, utomilumab, liposomal doxorubicin, sacituzumab govitecan | Phase 2 | NCT03971409 |
| 36 | Metastatic TNBC | Atezolizumab, bevacizumab, gemcitabine, carboplatin | Phase 2 | NCT04739670 |
| 37 | TNBC, metastatic breast cancer, HER2 -ve breast cancer | L-NMMA | Phase 2 | NCT05660083 |
| 38 | Metastatic TNBC, stage IV breast cancer | Ivermectin, balstilmab | Phase 1 & 2 | NCT05318469 |
| 39 | TNBC, metastatic breast cancer and ER-low breast cancer | Carboplatin, tocilizumab | Phase 2 | NCT05846789 |
| 40 | Breast cancers and metastatic AR+ve TNBC | Palbociclib, avelumab | Phase 1 | NCT04360941 |
| 41 | Unresectable or metastatic TNBC | Tobemstomig, pembrolizumab, nab-paclitaxel | Phase 2 | NCT05852691 |
| 42 | All types of breast cancer like HER2+ve and -ve and PR +ve, TNBC | ALX148, fam-trastuzumab deruxtecan-nxki, zanidatamab, tucatinib | Phase 1 | NCT05868226 |
| 43 | Breast cancer | Atezolizumab injection, bevacizumab, pertuzumab, trastuzumab | Phase 2 | NCT05180006 |
| 44 | Breast cancer | Nivolumab, ipilimumab | Phase 2 | NCT03815890 |
| 45 | HER2+ve, metastatic breast cancer | Inavolisib, Phesgo, taxane-based chemotherapy | Phase 3 | NCT05894239 |
| 46 | HER2+ve metastatic breast cancer | Atezolizumab + trastuzumab + vinorelbine | Phase 2 | NCT04759248 |
| 47 | Luminal breast cancer: HER2-ve HR+ve | Elacestrant, triptorelin | Phase 2 | NCT05982093 |
| 48 | Luminal A breast cancer | Breast irradiation (RT), endocrine therapy (ET): letrozole, anastrozole, exemestane, tamoxifen | Phase 3 | NCT04134598 |
| 49 | Luminal B/HER2-ve breast cancer | Dalpiciclib combined with aromatase inhibitors | Phase 2 | NCT05640778 |
| 50 | Ductal carcinoma in situ | Tamoxifen, exemestane, letrozole, anastrazole, testosterone + anastrazole, elacestrant, Z-endoxifen | Phase 2 | NCT06075953 |
| 51 | Ductal carcinoma in situ | Granulocyte–macrophage colony-stimulating factor, multi-epitope HER2 peptide vaccine H2NVAC | Phase 1 | NCT04144023 |
| 52 | Ductal carcinoma in situ, postmenopausal | Conjugated estrogens/bazedoxifene | Phase 2 | NCT02694809 |
| 53 | Invasive lobular carcinoma | Fulvestrant, repotrectinib | Phase 2 | NCT06408168 |
| 54 | Invasive breast lobular carcinoma | Neratinib | Phase 2 | NCT05919108 |
| 55 | HER2-ve breast cancer | Doxorubicin, cyclophosphamide Weekly paclitaxel, trastuzumab Pertuzumab | Phase 2 | NCT03412643 |
| 56 | ER+ve breast cancer, HER2-ve breast cancer, metastatic breast cancer | AI + CDK4/6i, SERD + CDK4/6i, mTOR inhibitor + AI, mTOR inhibitor + SERD, mTOR inhibitor + selective estrogen receptor modulator, PI3K inhibitor + SERD, PI3K inhibitor + AI, oral SERD | Phase 2 | NCT05826964 |
| 57 | HR+ve/HER2-ve metastatic breast cancer | Fulvestrant Capecitabine oral product | Phase 3 | NCT04263298 |
| 58 | HER2-low, HR+ve metastatic breast cancer | DB-1303/BNT323, capecitabine, paclitaxel, nab-paclitaxel | Phase 3 | NCT06018337 |
| 59 | ER+ve/HER2-ve metastatic breast cancer | Endocrine therapy combined with the local treatment of FES-negative lesions | Phase 3 | NCT06195709 |
| 60 | HER2-ve breast cancer | Cyclophosphamide, fludarabine, camrelizumab, chemotherapeutic drug, ADC, or PARP inhibitor | Phase 1 | NCT06121557 |
| 61 | HER2-ve breast cancer | Epirubicin, cyclophosphamide, docetaxel, paclitaxel | Phase 2 and 3 | NCT04576143 |
| 62 | HER2-ve breast cancer | Cyclophosphamide, Fludarabine, nab-paclitaxel, Gemcitabine, carboplatin | Phase 1 and 2 | NCT05981001 |
| 63 | HER2-ve breast cancer | Paclitaxel, carboplatin, Cyclophosphamide/doxorubicin | Phase 2 and 3 | NCT05889390 |
| 64 | HER2-ve breast cancer | Doxorubicin, epirubicin, cyclophosphamide, fludarabine Nab-paclitaxel | Phase 1 | NCT06121570 |
| 65 | ER+ve/HER2-ve breast cancer | Docetaxel, carboplatin, epirubicin, cyclophosphamide | Phase 3 | NCT05901428 |
| 66 | HR+ve/HER2-ve premenopausal breast cancer | Dalcelli, exemestane, gosserine Docetaxel, epirubicin hydrochloride, cyclophosphamide | Phase 2 and 3 | NCT06009627 |
| 67 | HER2-ve breast carcinoma, HR+ve | Cyclophosphamide, Doxorubicin, Durvalumab, paclitaxel | Phase 3 | NCT06058377 |
| 68 | HER2-ve early breast cancer | Liposomal doxorubicin, cyclophosphamide vs. docetaxel, cyclophosphamide | Phase 4 | NCT05302336 |
| 69 | HER2-ve breast cancer | Camrelizumab with vinorelbine and cisplatin | Phase 2 | NCT04848454 |
| 70 | HER2-ve breast carcinoma | Cyclophosphamide, Docetaxel | Phase 2 | NCT06042569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, V.K.; Mishra, B.; Mahapatra, S.; Swain, B.; Malhotra, D.; Saha, S.; Khanra, S.; Mishra, P.; Majhi, S.; Kumari, K.; et al. Molecular Insights on Signaling Cascades in Breast Cancer: A Comprehensive Review. Cancers 2025, 17, 234. https://doi.org/10.3390/cancers17020234
Panda VK, Mishra B, Mahapatra S, Swain B, Malhotra D, Saha S, Khanra S, Mishra P, Majhi S, Kumari K, et al. Molecular Insights on Signaling Cascades in Breast Cancer: A Comprehensive Review. Cancers. 2025; 17(2):234. https://doi.org/10.3390/cancers17020234
Chicago/Turabian StylePanda, Venketesh K., Barnalee Mishra, Samikshya Mahapatra, Biswajit Swain, Diksha Malhotra, Suryendu Saha, Sinjan Khanra, Priyanka Mishra, Sambhunath Majhi, Kavita Kumari, and et al. 2025. "Molecular Insights on Signaling Cascades in Breast Cancer: A Comprehensive Review" Cancers 17, no. 2: 234. https://doi.org/10.3390/cancers17020234
APA StylePanda, V. K., Mishra, B., Mahapatra, S., Swain, B., Malhotra, D., Saha, S., Khanra, S., Mishra, P., Majhi, S., Kumari, K., Nath, A. N., Saha, S., Jena, S., & Kundu, G. C. (2025). Molecular Insights on Signaling Cascades in Breast Cancer: A Comprehensive Review. Cancers, 17(2), 234. https://doi.org/10.3390/cancers17020234







