Advancing Cancer Drug Delivery with Nanoparticles: Challenges and Prospects in Mathematical Modeling for In Vivo and In Vitro Systems
Simple Summary
Abstract
1. Introduction
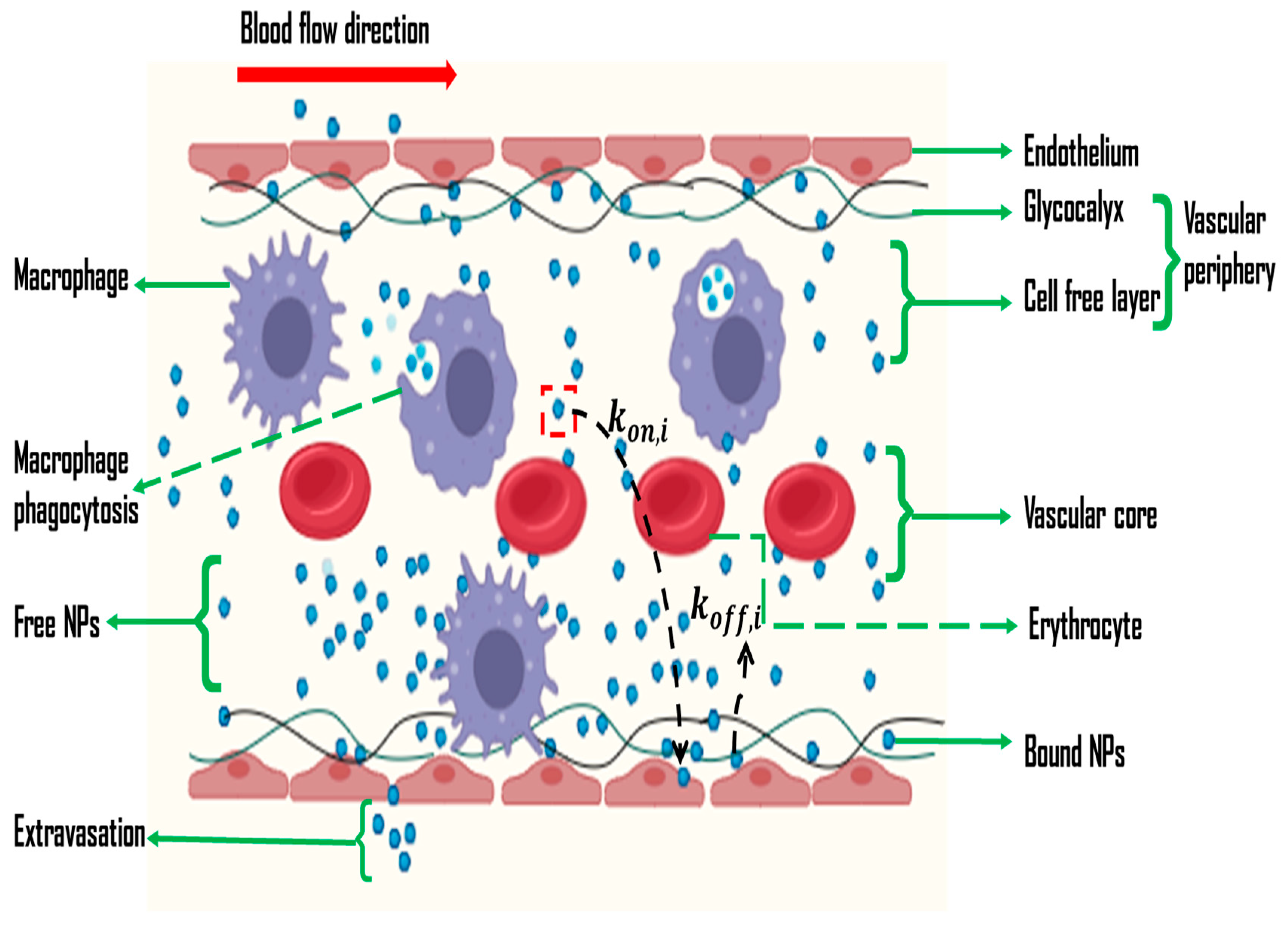
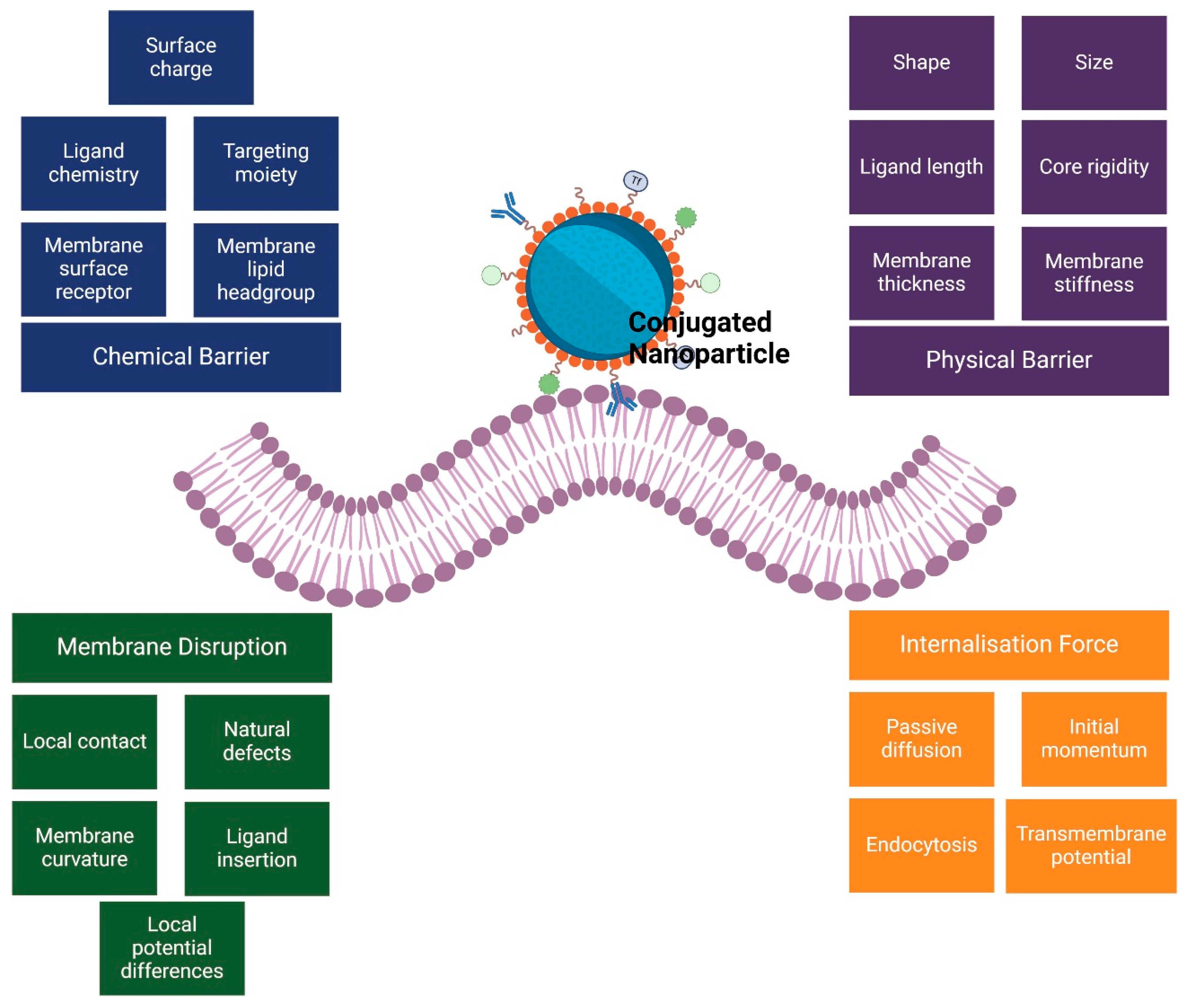
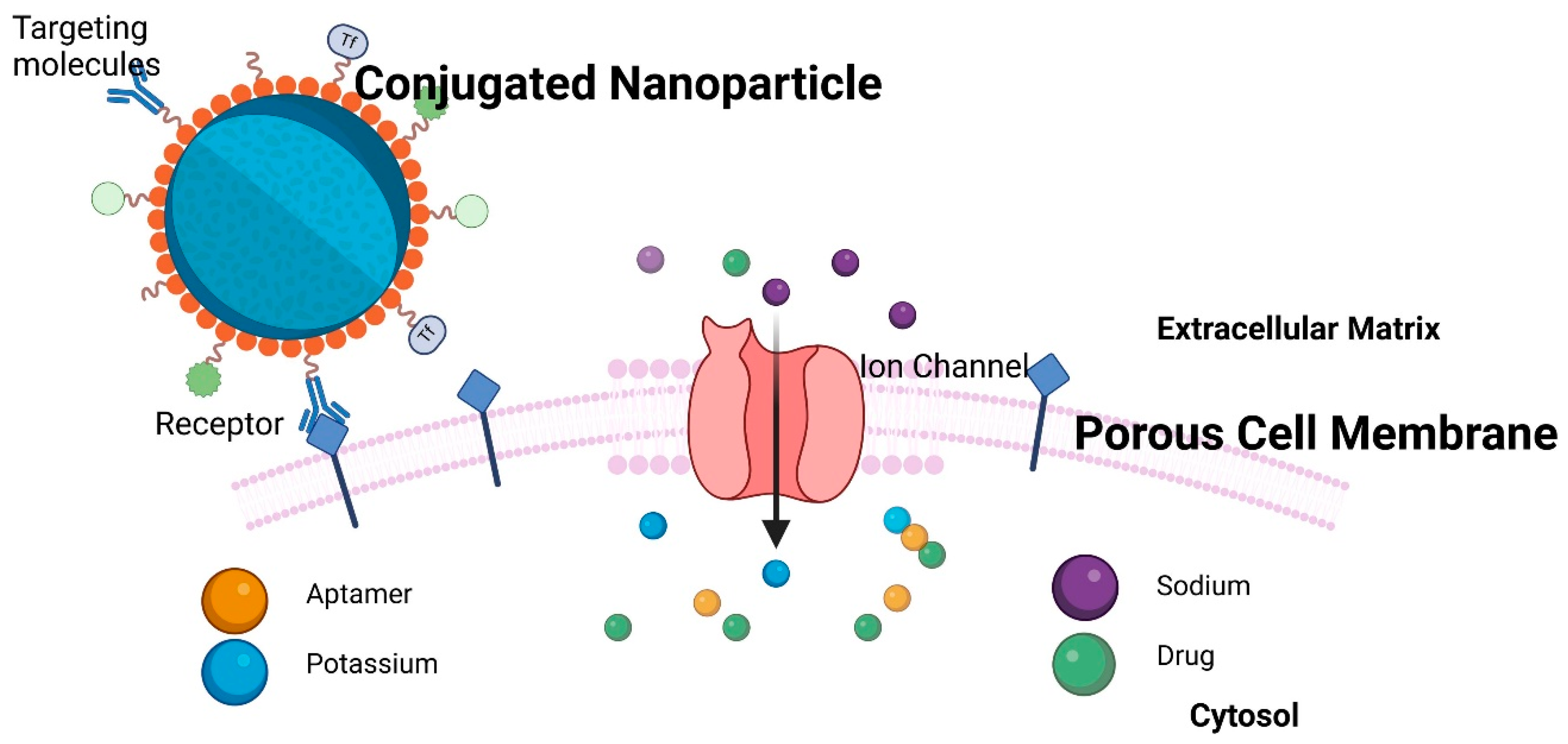
2. Challenges in In Vivo and In Vitro Nano-Based Cancer Drug Delivery
3. Nanoparticle-Based Cancer Drug Delivery Models
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.-L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Billaut, J.C.; André, V.; Kergosien, Y.; Tournamille, J.F. Optimization Issues in Chemotherapy Delivery. In Health Efficiency; Sarazin, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–118. [Google Scholar]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. [Google Scholar] [CrossRef]
- Bravo, M.; Fortuni, B.; Mulvaney, P.; Hofkens, J.; Uji-I, H.; Rocha, S.; Hutchison, J. Nanoparticle-mediated thermal Cancer therapies: Strategies to improve clinical translatability. J. Control. Release 2024, 372, 751–777. [Google Scholar] [CrossRef]
- Milligan, J.J.; Saha, S. A Nanoparticle’s Journey to the Tumor: Strategies to Overcome First-Pass Metabolism and Their Limitations. Cancers 2022, 14, 1741. [Google Scholar] [CrossRef] [PubMed]
- Horejs, C. It all comes down to the dose. Nat. Rev. Mater. 2020, 5, 728. [Google Scholar] [CrossRef]
- Tan, A.; Jeyaraj, R.; De Lacey, S.F. Chapter 7—Nanotechnology in neurosurgical oncology. In Nanotechnology in Cancer; Mathur, A.B., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 139–170. [Google Scholar]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Miao, L.; Zhong, G.; Lin, C.-H.; Dargazangy, R.; Alexander-Katz, A. Understanding the synergistic effect of physicochemical properties of nanoparticles and their cellular entry pathways. Commun. Biol. 2020, 3, 205. [Google Scholar] [CrossRef]
- Panigrahi, L.L.; Samal, P.; Sahoo, S.R.; Sahoo, B.; Pradhan, A.K.; Mahanta, S.; Rath, S.K.; Arakha, M. Nanoparticle-mediated diagnosis, treatment, and prevention of breast cancer. Nanoscale Adv. 2024, 6, 3699–3713. [Google Scholar] [CrossRef]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef]
- Deshmukh, R.; Sethi, P.; Singh, B.; Shiekmydeen, J.; Salave, S.; Patel, R.J.; Ali, N.; Rashid, S.; Elossaily, G.M.; Kumar, A. Recent Review on Biological Barriers and Host–Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies. Pharmaceutics 2024, 16, 1076. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Emerging Nanotherapeutic Approaches to Overcome Drug Resistance in Cancers with Update on Clinical Trials. Pharmaceutics 2022, 14, 866. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Lai, Y.; Xu, W.; Lei, S.; Chen, G.; Wang, Z. A computer-aided, heterodimer-based “triadic” carrier-free drug delivery platform to mitigate multidrug resistance in lung cancer and enhance efficiency. J. Colloid Interface Sci. 2025, 677 Pt B, 523–540. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Baracos, V.E. On the cusp of targeted therapy for cancer cachexia—What clinical benefits might we promise our patients? Nat. Rev. Clin. Oncol. 2024, 22, 8–9. [Google Scholar] [CrossRef]
- van Winkel, C.A.J.; Pierik, F.R.; Brouwers, A.H.; de Groot, D.J.A.; de Vries, E.G.; Lub-de Hooge, M.N. Molecular imaging supports the development of multispecific cancer antibodies. Nat. Rev. Clin. Oncol. 2024, 21, 852–866. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Miao, L.; Huang, L. Exploring the tumor microenvironment with nanoparticles. Cancer Treat. Res. 2015, 166, 193–226. [Google Scholar]
- Liu, Y.; Zhou, J.; Li, Q.; Li, L.; Jia, Y.; Geng, F.; Zhou, J.; Yin, T. Tumor microenvironment remodeling-based penetration strategies to amplify nanodrug accessibility to tumor parenchyma. Adv. Drug Deliv. Rev. 2021, 172, 80–103. [Google Scholar] [CrossRef]
- Joseph, J.F.; Gronbach, L.; García-Miller, J.; Cruz, L.M.; Wuest, B.; Keilholz, U.; Zoschke, C.; Parr, M.K. Automated Real-Time Tumor Pharmacokinetic Profiling in 3D Models: A Novel Approach for Personalized Medicine. Pharmaceutics 2020, 12, 413. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Boehm, J.S.; Karlsson, E.K.; Padron, E.; Seshadri, M.; Wallis, D.; Snyder, J.C. Precision preclinical modeling to advance cancer treatment. J. Natl. Cancer Inst. 2024, djae249. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Schwanke, R.C.; Siqueira, J.M.; Freitas, C.S.; Marcon, R.; Calixto, J.B. Non-clinical studies in the process of new drug development—Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz. J. Med. Biol. Res. 2016, 49, e5646. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Rubin, E.H.; Mehrotra, N.; Pinheiro, J.; Fernandes, L.L.; Roy, A.; Bailey, S.; de Alwis, D.P. Rendering the 3 + 3 Design to Rest: More Efficient Approaches to Oncology Dose-Finding Trials in the Era of Targeted Therapy. Clin. Cancer Res. 2016, 22, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Wang, M.; Webster, T.J. In vitro and ex vivo systems at the forefront of infection modeling and drug discovery. Biomaterials 2019, 198, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Humblet-Baron, S.; Duffy, D.; Goris, A. Human immune diversity: From evolution to modernity. Nat. Immunol. 2021, 22, 1479–1489. [Google Scholar] [CrossRef]
- Nguyen, L.N.M.; Ngo, W.; Lin, Z.P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S.M.; Chan, W.C.W. The mechanisms of nanoparticle delivery to solid tumours. Nat. Rev. Bioeng. 2024, 2, 201–213. [Google Scholar] [CrossRef]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.T.; Duong, V.-A. Recent advances in surface decoration of nanoparticles in drug delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Mujtaba, M.; Negi, A.; King, A.W.; Zare, M.; Kuncova-Kallio, J. Surface modifications of nanocellulose for drug delivery applications: A critical review. Curr. Opin. Biomed. Eng. 2023, 28, 100475. [Google Scholar] [CrossRef]
- Sun, X.; Hu, B. Mathematical modeling and computational prediction of cancer drug resistance. Brief. Bioinform. 2017, 19, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Saeed, S.M.; Deka, L.; Uddin, J.; Das, D.B. Applications of Machine Learning (ML) and Mathematical Modeling (MM) in Healthcare with Special Focus on Cancer Prognosis and Anticancer Therapy: Current Status and Challenges. Pharmaceutics 2024, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, L.; Agarwal, S.; Tanner, K. Preclinical models for drug discovery for metastatic disease. Cell 2023, 186, 1792–1813. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Geertsma, R.E.; Borchard, G. Regulatory safety evaluation of nanomedical products: Key issues to refine. Drug Deliv. Transl. Res. 2022, 12, 2042–2047. [Google Scholar] [CrossRef]
- Oualikene-Gonin, W.; Sautou, V.; Ezan, E.; Bastos, H.; Bellissant, E.; Belgodère, L.; Maison, P.; Ankri, J.; Scientific Advisory Board of the ANSM. Regulatory assessment of nano-enabled health products in public health interest. Position of the scientific advisory board of the French National Agency for the Safety of Medicines and Health Products. Front. Public Health 2023, 11, 1125577. [Google Scholar] [CrossRef]
- Bertram-Ralph, E.; Amare, M. Factors affecting drug absorption and distribution. Anaesth. Intensive Care Med. 2023, 24, 221–227. [Google Scholar] [CrossRef]
- Abolhassani, H.; Eskandari, A.; Poor, A.S.; Zarrabi, A.; Khodadadi, B.; Karimifard, S.; Sahrayi, H.; Bourbour, M.; Yaraki, M.T. Nanobiotechnological approaches for breast cancer Management: Drug delivery systems and 3D In-Vitro models. Coord. Chem. Rev. 2024, 508, 215754. [Google Scholar] [CrossRef]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered tissues and strategies to overcome challenges in drug development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Butner, J.D.; Ramírez, J.R.; Chuang, Y.-L.; Noureddine, A.; Brinker, C.J.; Cristini, V.; Wang, Z. A mathematical model to predict nanomedicine pharmacokinetics and tumor delivery. Comput. Struct. Biotechnol. J. 2020, 18, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of Artificial Intelligence in Revolutionizing Drug Discovery. Fundam. Res. 2024. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. The significance of artificial intelligence in drug delivery system design. Adv. Drug Deliv. Rev. 2019, 151–152, 169–190. [Google Scholar] [CrossRef]
- Druedahl, L.C.; Price, W.N.; Minssen, T.; Sarpatwari, A. Use of Artificial Intelligence in Drug Development. JAMA Netw. Open 2024, 7, e2414139. [Google Scholar] [CrossRef]
- Dogra, P.; Butner, J.D.; Chuang, Y.-L.; Caserta, S.; Goel, S.; Brinker, C.J.; Cristini, V.; Wang, Z. Mathematical modeling in cancer nanomedicine: A review. Biomed. Microdevices 2019, 21, 40. [Google Scholar] [CrossRef]
- Salahshoori, I.; Golriz, M.; Nobre, M.A.; Mahdavi, S.; Malekshah, R.E.; Javdani-Mallak, A.; Jorabchi, M.N.; Khonakdar, H.A.; Wang, Q.; Mohammadi, A.H.; et al. Simulation-based approaches for drug delivery systems: Navigating advancements, opportunities, and challenges. J. Mol. Liq. 2024, 395, 123888. [Google Scholar] [CrossRef]
- Dai, X.; Chen, Y. Computational Biomaterials: Computational Simulations for Biomedicine. Adv. Mater. 2023, 35, e2204798. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Chitkara, D.; Mittal, A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm. Sin. B 2021, 11, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Clausen, J.R.; Rao, R.R.; Aidun, C.K. Nanoparticle diffusion in sheared cellular blood flow. J. Fluid Mech. 2019, 871, 636–667. [Google Scholar] [CrossRef]
- Lee, T.-R.; Choi, M.; Kopacz, A.M.; Yun, S.-H.; Liu, W.K.; Decuzzi, P. On the near-wall accumulation of injectable particles in the microcirculation: Smaller is not better. Sci. Rep. 2013, 3, 2079. [Google Scholar] [CrossRef]
- Peng, W.; Minli, B.; Jizu, L.; Chengzhi, H.; Yuyan, W. Numerical Investigation on the Turbulent Flow Characteristic of Nanofluids in a Horizontal Circular Tube. Numer. Heat Transf. Part A Appl. 2014, 66, 646–668. [Google Scholar] [CrossRef]
- Ahumada-Lazo, J.A.; Chen, R.H. Effects of nanoparticle concentration and peclet number on nanofluid droplet evaporation behavior. Int. J. Therm. Sci. 2022, 178, 107582. [Google Scholar] [CrossRef]
- Xu, Z.; Kleinstreuer, C. Direct nanodrug delivery for tumor targeting subject to shear-augmented diffusion in blood flow. Med. Biol. Eng. Comput. 2018, 56, 1949–1958. [Google Scholar] [CrossRef]
- Lin, C.; Kaper, H.J.; Li, W.; Splinter, R.; Sharma, P.K. Role of endothelial glycocalyx in sliding friction at the catheter-blood vessel interface. Sci. Rep. 2020, 10, 11855. [Google Scholar] [CrossRef]
- Kosmidis, K.; Dassios, G. Monte Carlo simulations in drug release. J. Pharmacokinet. Pharmacodyn. 2019, 46, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Zhang, X.P.; Zhao, Z.Q.; Guo, X.D. Dissipative Particle Dynamics Aided Design of Drug Delivery Systems: A Review. Mol. Pharm. 2020, 17, 1778–1799. [Google Scholar] [CrossRef]
- Galdi, I.; Lamberti, G. Drug release from matrix systems: Analysis by finite element methods. Heat Mass Transf. 2012, 48, 519–528. [Google Scholar] [CrossRef]
- Casalini, T.; Limongelli, V.; Schmutz, M.; Som, C.; Jordan, O.; Wick, P.; Borchard, G.; Perale, G. Molecular Modeling for Nanomaterial-Biology Interactions: Opportunities, Challenges, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 268. [Google Scholar] [CrossRef]
- Borówko, M.; Staszewski, T. Molecular Dynamics Simulations of Different Nanoparticles at Substrates. Int. J. Mol. Sci. 2024, 25, 4550. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, P.; Sun, H.; Dai, Y.; Liang, S.; Bai, X.; Feng, L. Optimization of Nanoparticles for Smart Drug Delivery: A Review. Nanomaterials 2021, 11, 2790. [Google Scholar] [CrossRef] [PubMed]
- Ekhator, C.; Qureshi, M.Q.; Zuberi, A.W.; Hussain, M.; Sangroula, N.; Yerra, S.; Devi, M.; Naseem, M.A.; Bellegarde, S.B.; Pendyala, P.R.; et al. Advances and Opportunities in Nanoparticle Drug Delivery for Central Nervous System Disorders: A Review of Current Advances. Cureus 2023, 15, e44302. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Sahebkar, A.; Shahlaei, M.; Moradi, S. Nano drug delivery systems: Molecular dynamic simulation. J. Mol. Liq. 2021, 332, 115823. [Google Scholar] [CrossRef]
- Akalin, A.A.; Dedekargınoğlu, B.; Choi, S.R.; Han, B.; Ozcelikkale, A. Predictive Design and Analysis of Drug Transport by Multiscale Computational Models Under Uncertainty. Pharm. Res. 2023, 40, 501–523. [Google Scholar] [CrossRef]
- Goldberg, E.; Scheringer, M.; Bucheli, T.D.; Hungerbühler, K. Critical Assessment of Models for Transport of Engineered Nanoparticles in Saturated Porous Media. Environ. Sci. Technol. 2014, 48, 12732–12741. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T. Experimental study and mathematical model of nanoparticle transport in porous media. Powder Technol. 2009, 192, 195–202. [Google Scholar] [CrossRef]
- Ju, B.; Dai, S.; Luan, Z.; Zhu, T.; Su, X.; Qiu, X. A study of wettability and permeability change caused by adsorption of nanometer structured polysilicon on the surface of porous media. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Melbourne, Australia, 8–10 October 2002. [Google Scholar] [CrossRef]
- Irfan, S.A.; Shafie, A.; Yahya, N.; Zainuddin, N. Mathematical Modeling and Simulation of Nanoparticle-Assisted Enhanced Oil Recovery—A Review. Energies 2019, 12, 1575. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Momeni, M.M.; Rahmim, A. Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework. Front. Oncol. 2021, 11, 655781. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Lux, J.; Anguy, Y. A Study of the Behavior of Implicit Pressure Explicit Saturation (IMPES) Schedules for Two-phase Flow in Dynamic Pore Network Models. Transp. Porous Media 2012, 93, 203–221. [Google Scholar] [CrossRef]
- Hofer, S.; Hofstätter, N.; Punz, B.; Hasenkopf, I.; Johnson, L.; Himly, M. Immunotoxicity of nanomaterials in health and disease: Current challenges and emerging approaches for identifying immune modifiers in susceptible populations. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1804. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Wang, Y.; Xia, T.; Zhao, Y.; Meng, H. Precision design of engineered nanomaterials to guide immune systems for disease treatment. Matter 2022, 5, 1162–1191. [Google Scholar] [CrossRef]
- Hasan, M.R.; Alsaiari, A.A.; Fakhurji, B.Z.; Molla, M.H.R.; Asseri, A.H.; Sumon, M.A.A.; Park, M.N.; Ahammad, F.; Kim, B. Application of Mathematical Modeling and Computational Tools in the Modern Drug Design and Development Process. Molecules 2022, 27, 4169. [Google Scholar] [CrossRef]
- Ashwini, T.; Narayan, R.; Shenoy, P.A.; Nayak, U.Y. Computational modeling for the design and development of nano based drug delivery systems. J. Mol. Liq. 2022, 368, 120596. [Google Scholar]
- Askin, S.; Burkhalter, D.; Calado, G.; El Dakrouni, S. Artificial Intelligence Applied to clinical trials: Opportunities and challenges. Heal. Technol. 2023, 13, 203–213. [Google Scholar] [CrossRef]
- Weissler, E.H.; Naumann, T.; Andersson, T.; Ranganath, R.; Elemento, O.; Luo, Y.; Freitag, D.F.; Benoit, J.; Hughes, M.C.; Khan, F.; et al. The role of machine learning in clinical research: Transforming the future of evidence generation. Trials 2021, 22, 537. [Google Scholar]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Afolaranmi, S.O.; Lastra, J.L.M.; Garcia, J.A.P. A comprehensive literature review of the applications of AI techniques through the lifecycle of industrial equipment. Discov. Artif. Intell. 2023, 3, 43. [Google Scholar] [CrossRef]
- De Spirito, M.; Palmieri, V.; Perini, G.; Papi, M. Bridging the Gap: Integrating 3D Bioprinting and Microfluidics for Advanced Multi-Organ Models in Biomedical Research. Bioengineering 2024, 11, 664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyayi, T.A.; Crous, A. Advancing Cancer Drug Delivery with Nanoparticles: Challenges and Prospects in Mathematical Modeling for In Vivo and In Vitro Systems. Cancers 2025, 17, 198. https://doi.org/10.3390/cancers17020198
Munyayi TA, Crous A. Advancing Cancer Drug Delivery with Nanoparticles: Challenges and Prospects in Mathematical Modeling for In Vivo and In Vitro Systems. Cancers. 2025; 17(2):198. https://doi.org/10.3390/cancers17020198
Chicago/Turabian StyleMunyayi, Tozivepi Aaron, and Anine Crous. 2025. "Advancing Cancer Drug Delivery with Nanoparticles: Challenges and Prospects in Mathematical Modeling for In Vivo and In Vitro Systems" Cancers 17, no. 2: 198. https://doi.org/10.3390/cancers17020198
APA StyleMunyayi, T. A., & Crous, A. (2025). Advancing Cancer Drug Delivery with Nanoparticles: Challenges and Prospects in Mathematical Modeling for In Vivo and In Vitro Systems. Cancers, 17(2), 198. https://doi.org/10.3390/cancers17020198






