Ongoing Failure to Deliver Guideline-Concordant Care for Patients with Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. GCC Definition
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
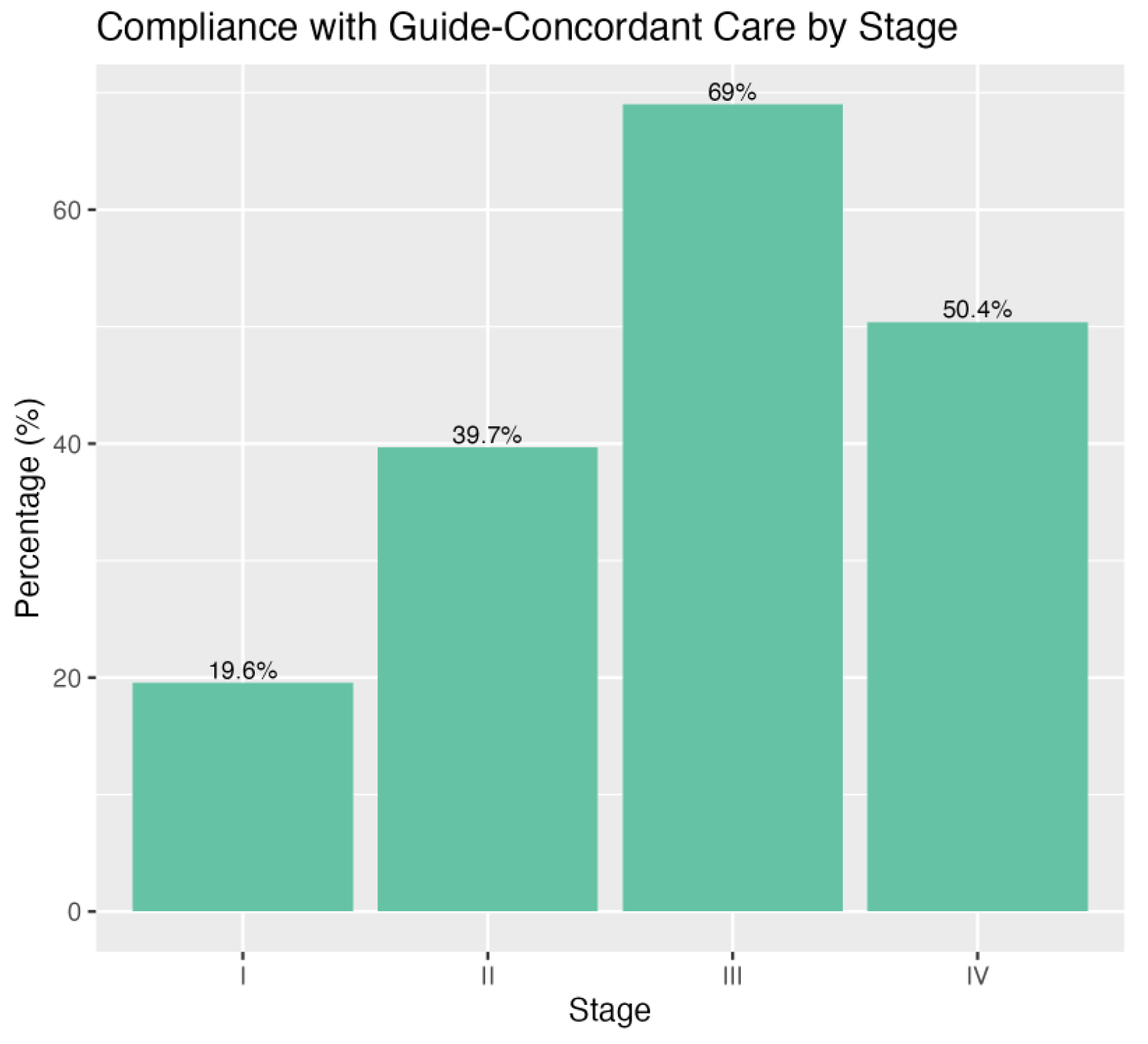
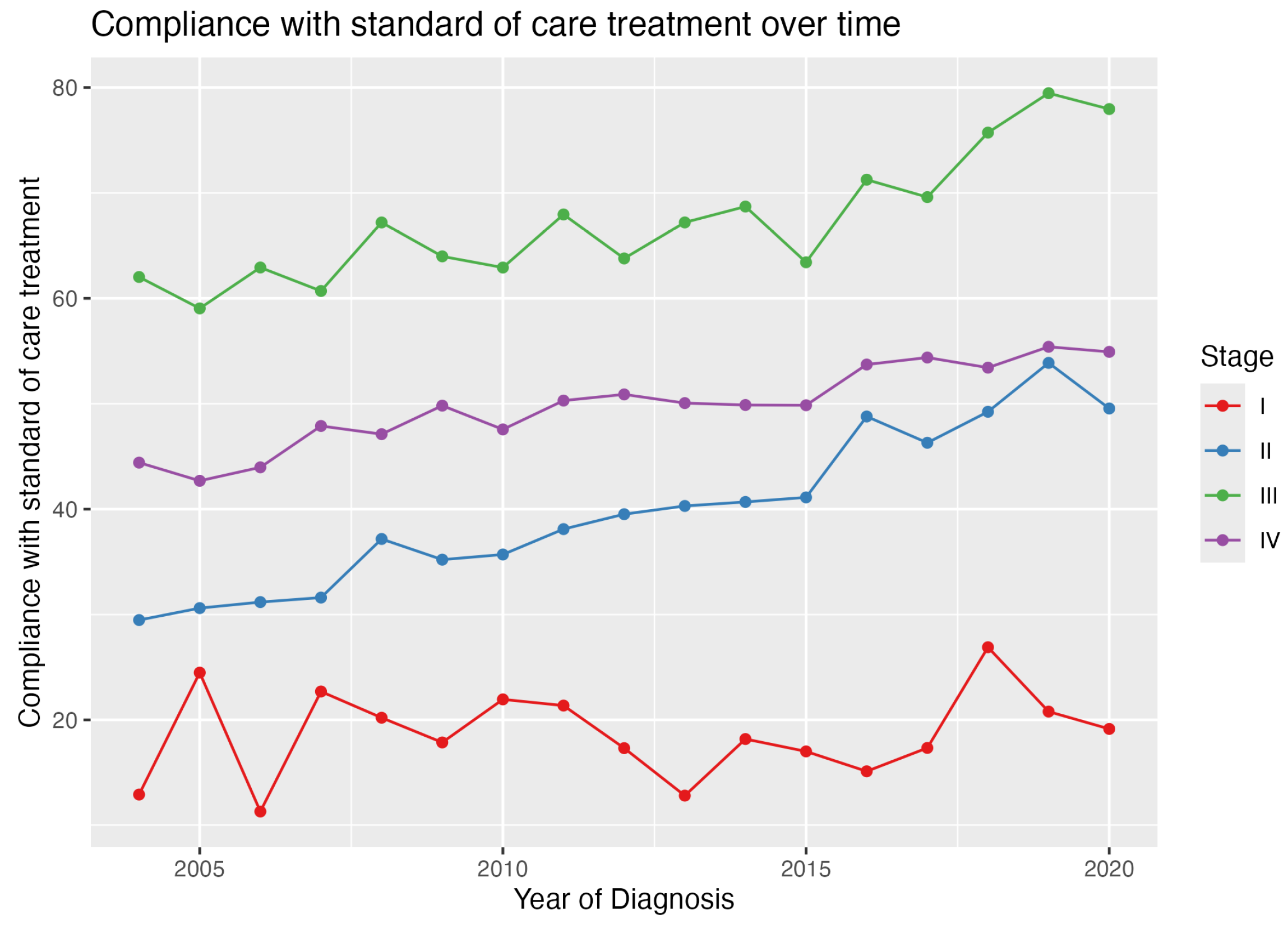
3.2. GCC Rates
3.3. Factors Associated with GCC
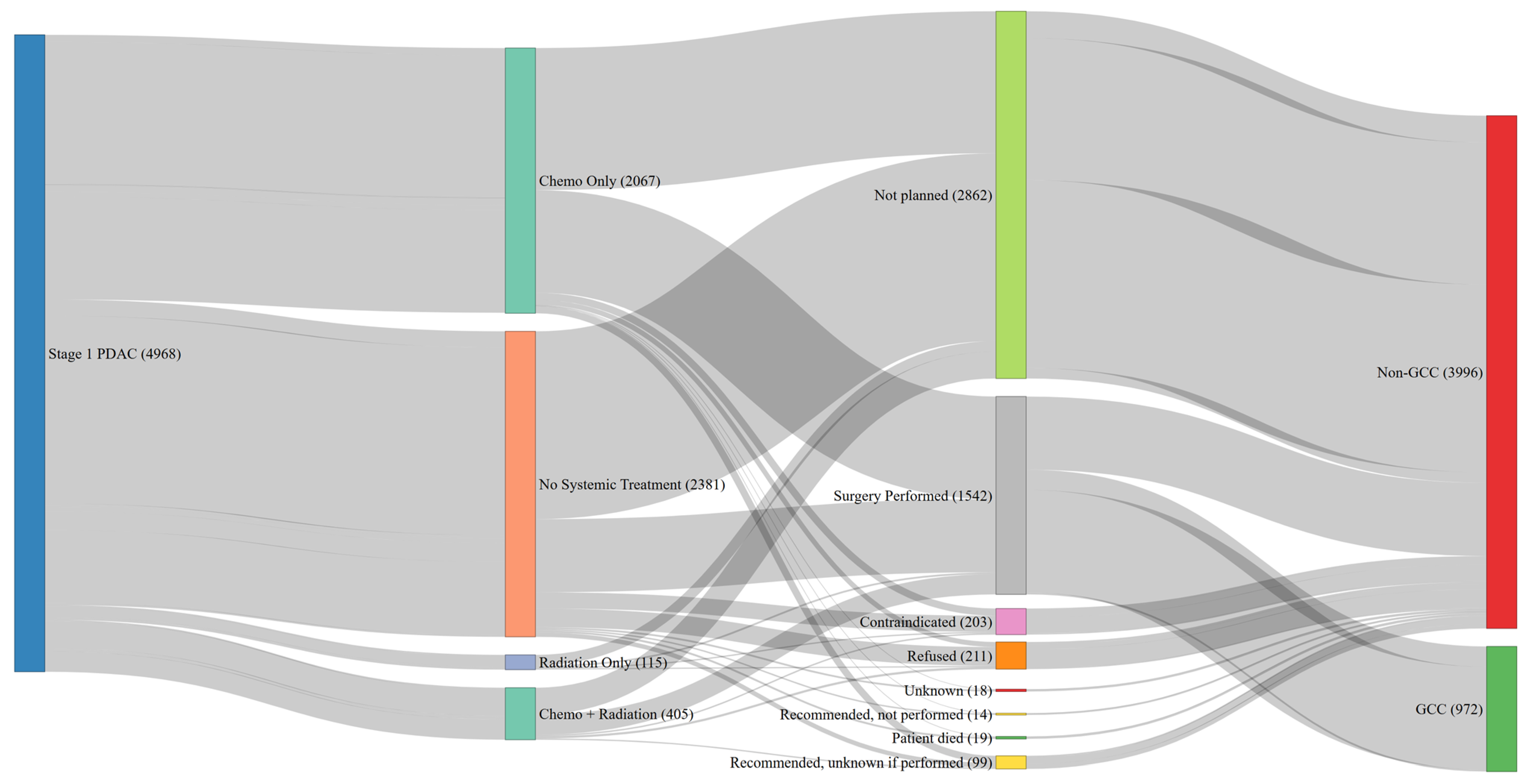
3.4. Guideline Discordance in Stage 1 PDAC
3.5. Reasons for No Surgery for Stage 1
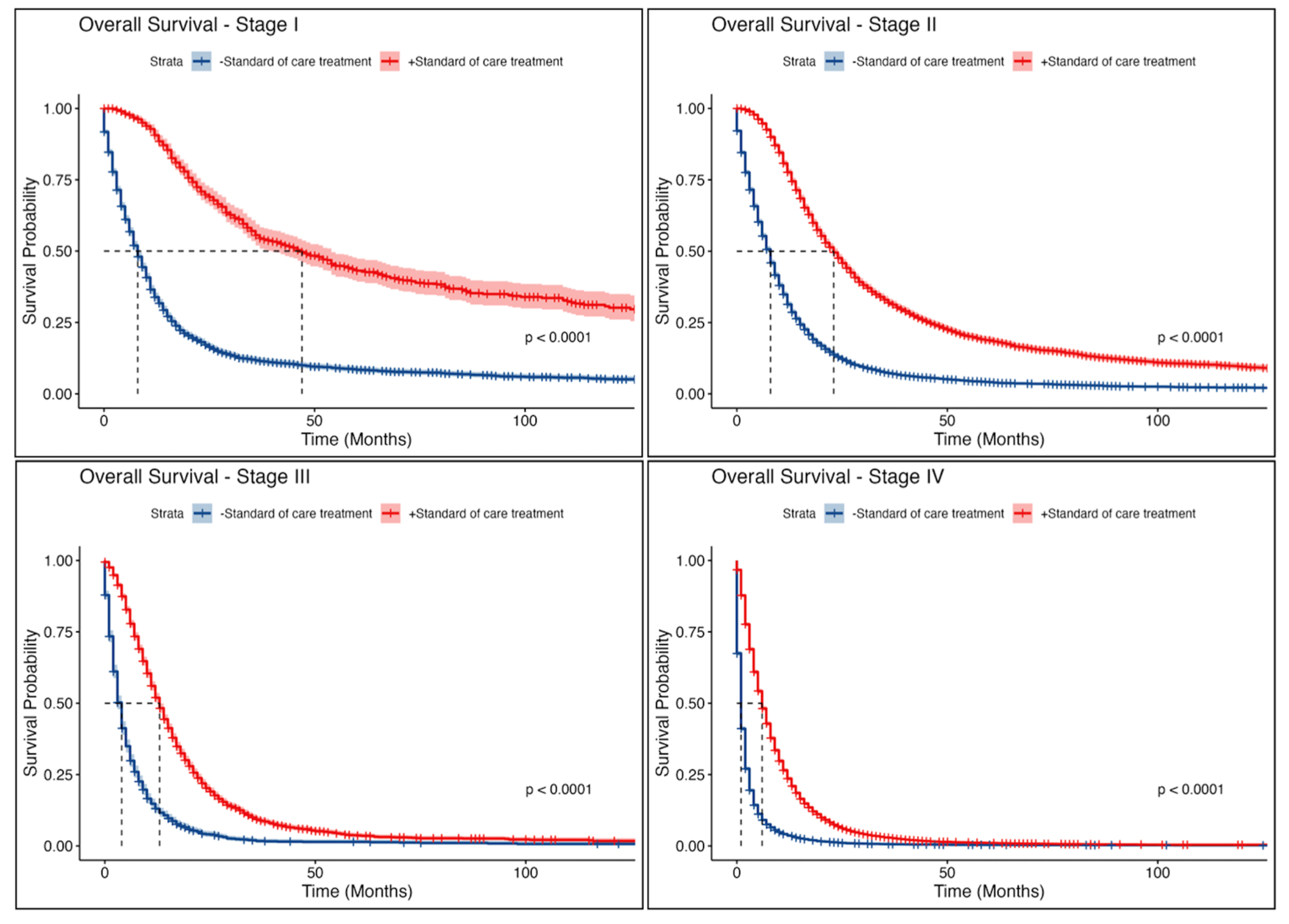
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures. Available online: https://www.cancer.org/research/cancer-facts-statistics.html (accessed on 15 December 2024).
- Hu, J.-X.; Zhao, C.; WenBiao, C.; Liu, Q.; Li, Q.-W.; Lin, Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.G.; Chiaro, M.D.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Ma, Y.; Wang, X.; Campbell-Flohr, S.A.; Rathouz, P.J.; Ronnekleiv-Kelly, S.M.; Abbott, D.E.; Weber, S.M. National Trends in Centralization of Surgical Care and Multimodality Therapy for Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2019, 24, 2021. [Google Scholar] [CrossRef] [PubMed]
- Fromer, M.W.; Mouw, T.J.; Scoggins, C.R.; Egger, M.E.; Philips, P.; McMasters, K.M.; Martin, R.C. Barriers to resection following neoadjuvant chemotherapy for resectable pancreatic adenocarcinoma: A national and local perspective. J. Surg. Oncol. 2024, 130, 284. [Google Scholar] [CrossRef]
- Badgery, H.E.; Muhlen-Schulte, T.; Zalcberg, J.R.; D’souza, B.; Gerstenmaier, J.F.; Pickett, C.; Samra, J.; Croagh, D.; Pancreatic Cancer Image Biobank Authorship Group. Determination of “borderline resectable” pancreatic cancer–A global assessment of 30 shades of grey. HPB 2023, 25, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau. American Community Survey, 2018 American Community Survey 1-Year Estimates, Table B01003. 2020. Available online: https://data.census.gov/cedsci/ (accessed on 15 December 2024).
- California Cancer Registry. Cancer Incidence and Mortality Data. Available online: https://www.ccrcal.org (accessed on 15 December 2024).
- World Health Organization. International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int (accessed on 15 December 2024).
- NCCN Guidelines for Patients: Pancreatic Cancer. 2024. Available online: https://www.nccn.org/patients/guidelines/content/PDF/pancreatic-patient.pdf (accessed on 15 December 2024).
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Asbun, H.; Bain, A.; Behrman, S.W.; Benson, A.B., III; Binder, E.; Cardin, D.B.; Cha, C.; et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 1028–1061. [Google Scholar] [CrossRef]
- Tempero, M.A.; Arnoletti, J.P.; Behrman, S.W.; Ben-Josef, E.; Benson, A.B., III; Casper, E.S.; Cohen, S.J.; Czito, B.; Ellenhorn JD, I.; Hawkins, W.G.; et al. Pancreatic Adenocarcinoma, Version 2.2012: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2012, 10, 703–713. [Google Scholar] [CrossRef] [PubMed]
- RSTUDIO TEAM. 2020. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL. Available online: http://www.rstudio.com/ (accessed on 15 December 2024).
- Hamad, A.; DePuccio, M.J.; Reames, B.N.; Dave, A.; Kurien, N.; Cloyd, J.M.; Shen, C.; Pawlik, T.M.; Tsung, A.; McAlearney, A.S.; et al. Disparities in Stage-Specific Guideline-Concordant Cancer-Directed Treatment for Patients with Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2021, 25, 2889. [Google Scholar] [CrossRef]
- Tohme, S.; Kaltenmeier, C.; Bou-Samra, P.; Varley, P.R.; Tsung, A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann. Surg. Oncol. 2018, 25, 3427. [Google Scholar] [CrossRef] [PubMed]
- Salami, A.; Alvarez, N.H.; Joshi, A.R. Geographic disparities in surgical treatment recommendation patterns and survival for pancreatic adenocarcinoma. HPB 2017, 19, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kasumova, G.G.; Eskander, M.F.; de Geus, S.W.; Neto, M.M.; Tabatabaie, O.; Ng, S.C.; Miksad, R.A.; Mahadevan, A.; Rodrigue, J.R.; Tseng, J.F. Regional variation in the treatment of pancreatic adenocarcinoma: Decreasing disparities with multimodality therapy. Surgery 2017, 162, 275–284. [Google Scholar] [CrossRef]
- Dimou, F.; Sineshaw, H.; Parmar, A.D.; Tamirisa, N.P.; Jemal, A.; Riall, T.S. Trends in Receipt and Timing of Multimodality Therapy in Early-Stage Pancreatic Cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2016, 20, 93–103. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Finks, J.F.; Osborne, N.H.; Birkmeyer, J.D. Trends in hospital volume and operative mortality for high-risk surgery. N. Engl. J. Med. 2011, 364, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. National Failure to Operate on Early Stage Pancreatic Cancer. Ann. Surg. 2007, 246, 173. [Google Scholar] [CrossRef]
- Visser, B.C.; Ma, Y.; Zak, Y.; Poultsides, G.A.; Norton, J.A.; Rhoads, K.F. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB 2012, 14, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Woodmass, J.; Lipschitz, J.; McKay, A. Physician attitudes and treatment patterns for pancreatic cancer. World J. Surg. Oncol. 2011, 9, 21. [Google Scholar] [CrossRef]
- Fan, Q.; Hussaini SM, Q.; Barrow, L.C.; Feliciano, J.; Pollack, C.E.; Yabroff, K.R.; Nogueira, L. Association of area-level mortgage denial and guideline-concordant non-small-cell lung cancer care and outcomes in the United States. Cancer Med. 2024, 13, e6921. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.D.; Blackard, B.; Carpenter, W.R.; Mishel, M.H.; Chen, R.C.; Godley, P.A.; Mohler, J.L.; Bensen, J.T. Receipt of National Comprehensive Cancer Network guideline-concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer 2013, 119, 2282. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.A.; Woldesenbet, S.; Moazzam, Z.; Endo, Y.; Munir, M.M.; Shaikh, C.; Rueda, B.O.; Alaimo, L.; Resende, V.; Pawlik, T.M. Association of Minority-Serving Hospital Status with Post-Discharge Care Utilization and Expenditures in Gastrointestinal Cancer. Ann. Surg. Oncol. 2023, 30, 7217–7225. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; Kaiser, K.; Lark, M.; Ruedinger, B.; Robb, B.W.; Morgan, T.; Park, S.; Schleyer, T.K.L.; Haggstrom, D.A.; Mohanty, S. NCCN guideline concordance in colon and rectal cancer patients within a comprehensive health system. Am. J. Surg. 2024, 240, 116114. [Google Scholar] [CrossRef] [PubMed]
- Zornosa, C.; Vandergrift, J.L.; Kalemkerian, G.P.; Ettinger, D.S.; Rabin, M.S.; Reid, M.; Otterson, G.A.; Koczywas, M.; D’Amico, T.A.; Niland, J.C.; et al. First-line systemic therapy practice patterns and concordance with NCCN guidelines for patients diagnosed with metastatic NSCLC treated at NCCN institutions. J. Natl. Compr. Cancer Netw. 2012, 10, 847–856. [Google Scholar] [CrossRef][Green Version]
- Denu, R.A.; Hampton, J.M.; Currey, A.; Anderson, R.T.; Cress, R.D.; Fleming, S.T.; Lipscomb, J.; Sabatino, S.A.; Wu, X.-C.; Wilson, J.F.; et al. Influence of patient, physician, and hospital characteristics on the receipt of guideline-concordant care for inflammatory breast cancer. Cancer Epidemiol. 2016, 40, 7–14. [Google Scholar] [CrossRef]
- LeMasters, T.; Madhavan, S.S.; Sambamoorthi, U.; Hazard-Jenkins, H.W.; Kelly, K.M.; Long, D. Receipt of guideline-concordant care among older women with stage I–III breast cancer: A population-based study. J. Natl. Compr. Cancer Netw. 2018, 16, 703–710. [Google Scholar] [CrossRef] [PubMed]
| Variable | GCC, N = 23,505 1 | No GCC, N = 26,841 1 | All Patients, N = 50,346 1 | p-Value 2 |
|---|---|---|---|---|
| Age, years | 66 (59, 74) | 73 (64, 81) | 70 (61, 78) | <0.001 |
| Sex (Female) | 11,044 (47%) | 13,297 (50%) | 24,341 (48%) | <0.001 |
| Race | <0.001 | |||
| White | 14,379 (61%) | 16,013 (60%) | 30,392 (60%) | |
| Asian | 2938 (12%) | 3230 (12%) | 6168 (12%) | |
| Black | 1677 (7.1%) | 2132 (7.9%) | 3809 (7.6%) | |
| Hispanic | 4339 (18%) | 5263 (20%) | 9602 (19%) | |
| Other | 172 (0.7%) | 203 (0.8%) | 375 (0.7%) | |
| Treatment Center | <0.001 | |||
| Non-NCI | 38,377 (76%) | 21,606 (80%) | 16,771 (71%) | |
| NCI | 11,969 (24%) | 5235 (20%) | 6734 (29%) | |
| Insurance | <0.001 | |||
| Private | 9806 (43%) | 8507 (33%) | 18,313 (37%) | |
| Medicare | 11,781 (51%) | 16,048 (62%) | 27,829 (57%) | |
| No Insurance | 319 (1.4%) | 526 (2.0%) | 845 (1.7%) | |
| Other | 998 (4.4%) | 986 (3.8%) | 1984 (4.1%) | |
| Location of Residence | 0.63 | |||
| Metropolitan | 22,019 (94%) | 25,186 (94%) | 47,205 (94%) | |
| Micropolitan | 1010 (4.3%) | 1094 (4.1%) | 2104 (4.2%) | |
| Small Town | 219 (0.9%) | 256 (1.0%) | 475 (0.9%) | |
| Rural Areas | 255 (1.1%) | 301 (1.1%) | 556 (1.1%) | |
| CD Index | <0.001 | |||
| 0 | 8754 (42%) | 795 (33%) | 16,549 (37%) | |
| 1 | 6226 (30%) | 9592 (41%) | 15,818 (36%) | |
| >2 | 5989 (29%) | 6180 (26%) | 12,169 (27%) | |
| Clinical Stage | <0.001 | |||
| I | 972 (4.1%) | 3996 (15%) | 4968 (9.9%) | |
| II | 5000 (21%) | 7598 (28%) | 12,598 (25%) | |
| III | 3760 (16%) | 1690 (6.3%) | 5450 (11%) | |
| IV | 13,773 (59%) | 13,557 (51%) | 27,330 (54%) |
| OR (95% CI) | p-Value | |
|---|---|---|
| Age | 0.95 (0.95–0.95) | <0.0001 |
| Sex (Female) | 0.98 (0.95–1.03) | 0.46 |
| Race | ||
| White | — | |
| Asian | 0.96 (0.90–1.02) | 0.22 |
| Black | 0.74 (0.68–0.80) | <0.0001 |
| Hispanic | 0.78 (0.74–0.82) | <0.0001 |
| Other | 0.79 (0.62–1.01) | 0.058 |
| Insurance | ||
| Private | — | |
| Medicare | 0.95 (0.91–1.00) | 0.042 |
| No Insurance | 0.40 (0.34–0.47) | <0.0001 |
| Other | 0.79 (0.71–0.88) | <0.0001 |
| CD Index | ||
| 0 | — | |
| 1 | 0.93 (0.88–0.98) | 0.0048 |
| >2 | 0.68 (0.65–0.72) | <0.0001 |
| Treatment Center | ||
| Non-NCI | — | |
| NCI | 1.64 (1.56–1.72) | <0.0001 |
| Clinical Stage | ||
| I | — | |
| II | 2.30 (2.10–2.51) | <0.0001 |
| III | 8.49 (7.67–9.40) | <0.0001 |
| IV | 3.76 (3.47–4.09) | <0.0001 |
| Location of Residence | ||
| Metropolitan | — | |
| Micropolitan | 0.97 (0.88–1.07) | 0.55 |
| Small Town | 0.94 (0.76–1.15) | 0.54 |
| Rural Areas | 0.94 (0.77–1.13) | 0.51 |
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.01 | 1.01–1.01 | <0.001 |
| Sex (Female) | 0.95 | 0.94–0.97 | <0.001 |
| GCC | |||
| No | — | — | |
| Yes | 0.39 | 0.38–0.40 | <0.001 |
| Race | |||
| White | — | — | |
| Asian | 0.94 | 0.91–0.97 | <0.001 |
| Black | 1.04 | 1.00–1.08 | 0.035 |
| Hispanic | 1.00 | 0.98–1.03 | 0.76 |
| Other | 0.99 | 0.88–1.11 | 0.84 |
| Insurance | |||
| Private | — | — | |
| Medicare | 0.96 | 0.94–0.98 | <0.001 |
| No Insurance | 1.16 | 1.07–1.26 | <0.001 |
| Other | 1.04 | 0.99–1.10 | 0.11 |
| Treatment Center | 0.0001 | ||
| Non-NCI | — | — | |
| NCI | 0.80 | 0.78–0.82 | |
| CD Index | |||
| 0 | — | — | |
| 1 | 1.09 | 1.06–1.11 | <0.001 |
| >2 | 1.33 | 1.30–1.36 | <0.001 |
| Clinical Stage | |||
| I | — | — | |
| II | 1.33 | 1.28–1.39 | <0.001 |
| III | 2.41 | 2.29–2.52 | <0.001 |
| IV | 4.65 | 4.47–4.83 | <0.001 |
| Location of Residence | |||
| Metropolitan | — | — | |
| Micropolitan | 1.03 | 0.98–1.08 | 0.30 |
| Small Town | 1.04 | 0.94–1.15 | 0.44 |
| Rural Areas | 1.01 | 0.92–1.10 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejie, J.; Ashraf Ganjouei, A.; Hernandez, S.; Wang, J.J.; Romero-Hernandez, F.; Foroutani, L.; Hirose, K.; Nakakura, E.; Corvera, C.U.; Alseidi, A.; et al. Ongoing Failure to Deliver Guideline-Concordant Care for Patients with Pancreatic Cancer. Cancers 2025, 17, 170. https://doi.org/10.3390/cancers17020170
Ejie J, Ashraf Ganjouei A, Hernandez S, Wang JJ, Romero-Hernandez F, Foroutani L, Hirose K, Nakakura E, Corvera CU, Alseidi A, et al. Ongoing Failure to Deliver Guideline-Concordant Care for Patients with Pancreatic Cancer. Cancers. 2025; 17(2):170. https://doi.org/10.3390/cancers17020170
Chicago/Turabian StyleEjie, Jonathan, Amir Ashraf Ganjouei, Sophia Hernandez, Jaeyun Jane Wang, Fernanda Romero-Hernandez, Laleh Foroutani, Kenzo Hirose, Eric Nakakura, Carlos Uriel Corvera, Adnan Alseidi, and et al. 2025. "Ongoing Failure to Deliver Guideline-Concordant Care for Patients with Pancreatic Cancer" Cancers 17, no. 2: 170. https://doi.org/10.3390/cancers17020170
APA StyleEjie, J., Ashraf Ganjouei, A., Hernandez, S., Wang, J. J., Romero-Hernandez, F., Foroutani, L., Hirose, K., Nakakura, E., Corvera, C. U., Alseidi, A., & Adam, M. A. (2025). Ongoing Failure to Deliver Guideline-Concordant Care for Patients with Pancreatic Cancer. Cancers, 17(2), 170. https://doi.org/10.3390/cancers17020170





