Chemotherapy Liberates a Broadening Repertoire of Tumor Antigens for TLR7/8/9-Mediated Potent Antitumor Immunity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Cell Lines, and Culture Conditions
2.2. Mice, Husbandry, and Subcutaneous Tumor Implantation
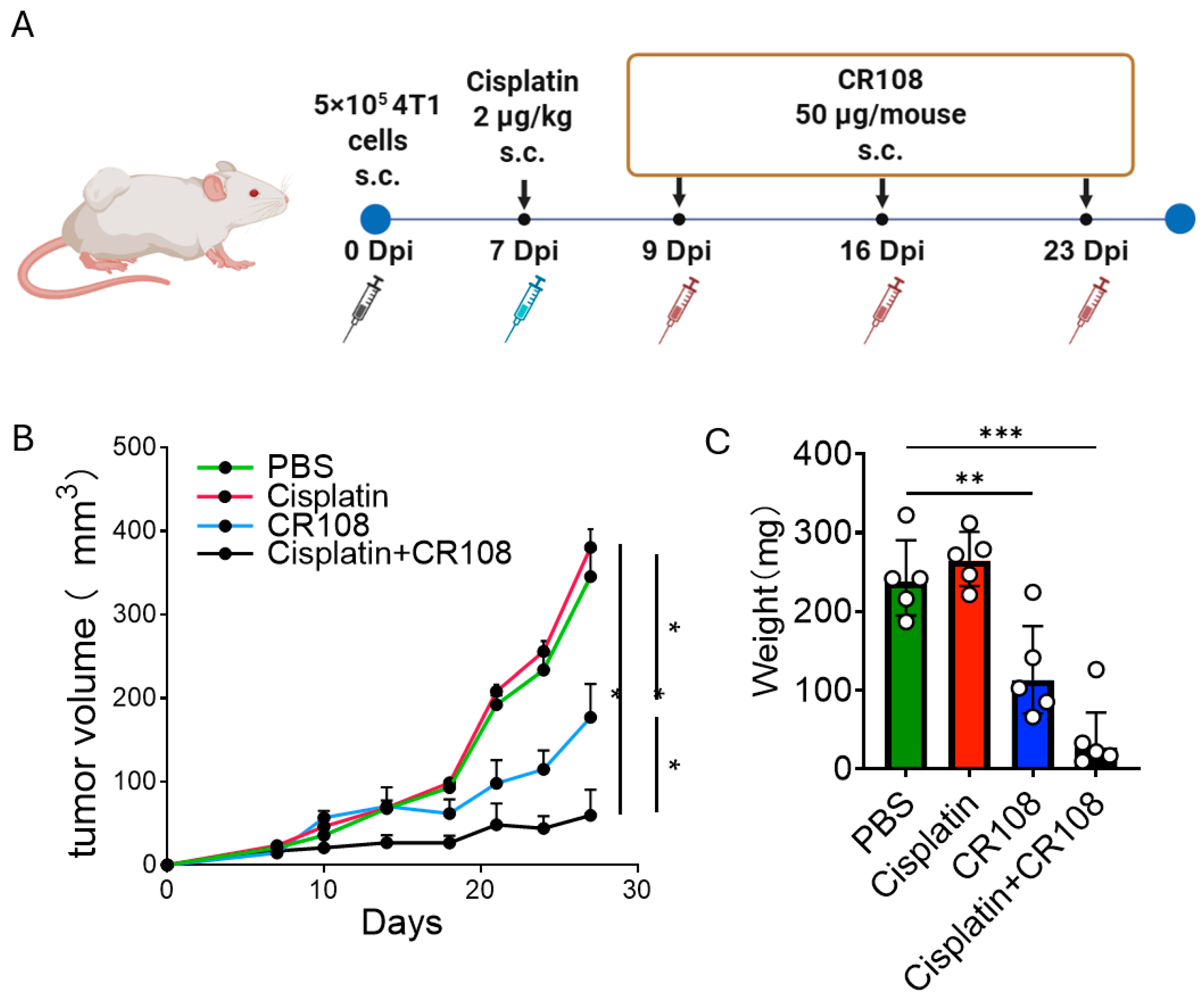
2.3. Chemotherapy and CR108 Regimens
2.4. Secretome Proteomics
2.5. Synthetic Peptides and IFN-γ ELISPOT
2.6. Flow Cytometry Phenotyping
2.7. Immunofluorescence and Immunohistochemistry
2.8. Sample Collection and T-Cell Isolation
2.9. Nucleic Acid Preparation
2.10. Library Construction (Two-Step Multiplex PCR)
2.11. Sequencing Platform and Run Conditions
2.12. Primary Read Processing
2.13. Clonotype Definition and Productive Filters
2.14. Quality Control and Inclusion Criteria
2.15. Repertoire Summaries for Figure Generation
2.16. cBioPortal Analysis
2.17. GEPIA Analysis
2.18. Statistics
2.19. Ethics
3. Results
3.1. Re-Establishing Cisplatin–CR108 Efficacy in 4T1 as a Benchmark for Mechanistic Analyses
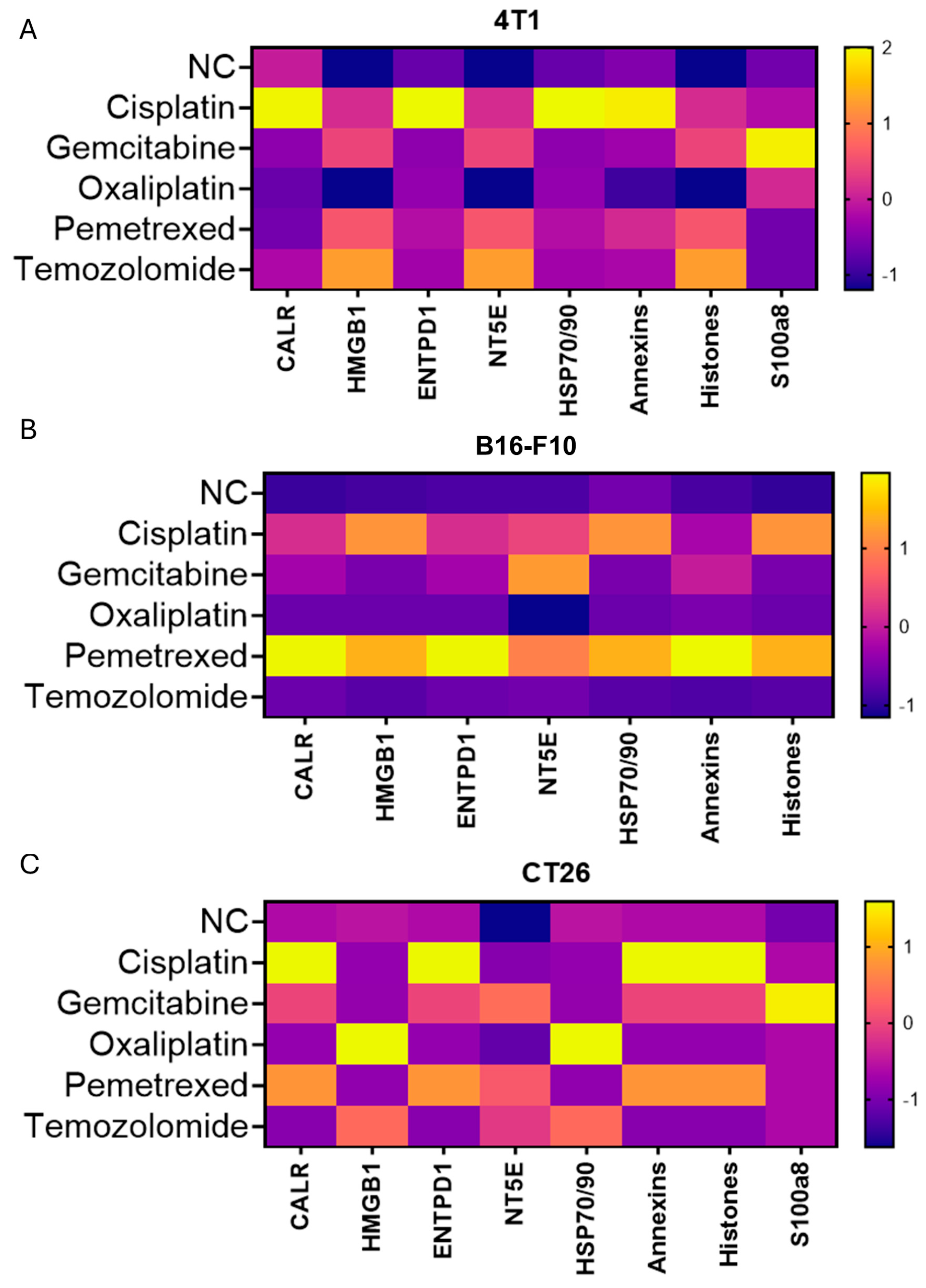
3.2. Cisplatin Triggers Immunogenic Cell Death (ICD) in 4T1 Cells and Promotes the Release of Damage-Associated Molecular Patterns (DAMPs) and Intracellular Cargo
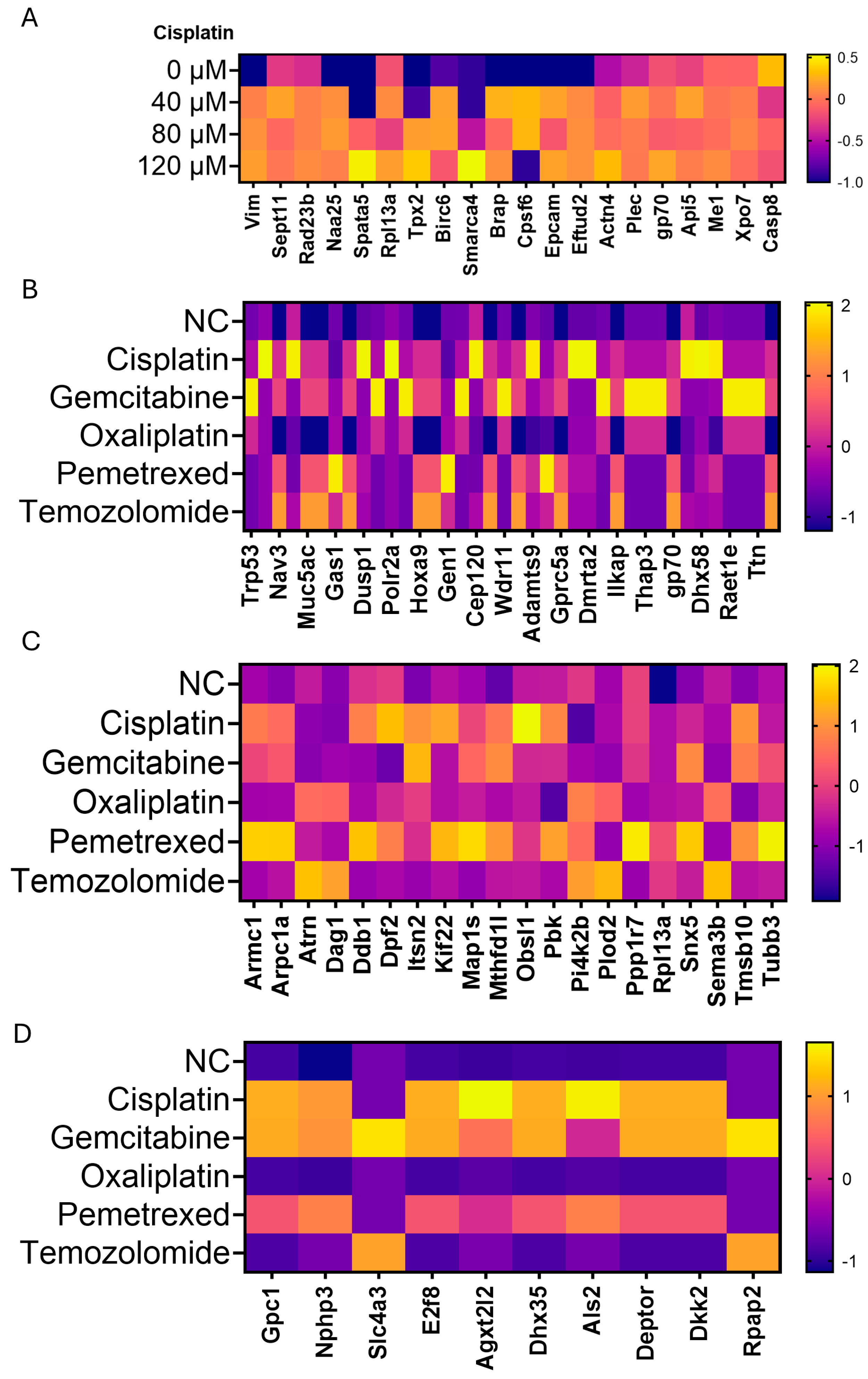
3.3. Broadening of the Tumor Antigen (TA) Landscape by Diverse Chemotherapeutics in Three Murine Cancer Models
3.4. Cisplatin + CR108 Increase T-Cell Recognition of Candidate TA Peptides in Tumor-Draining LNs
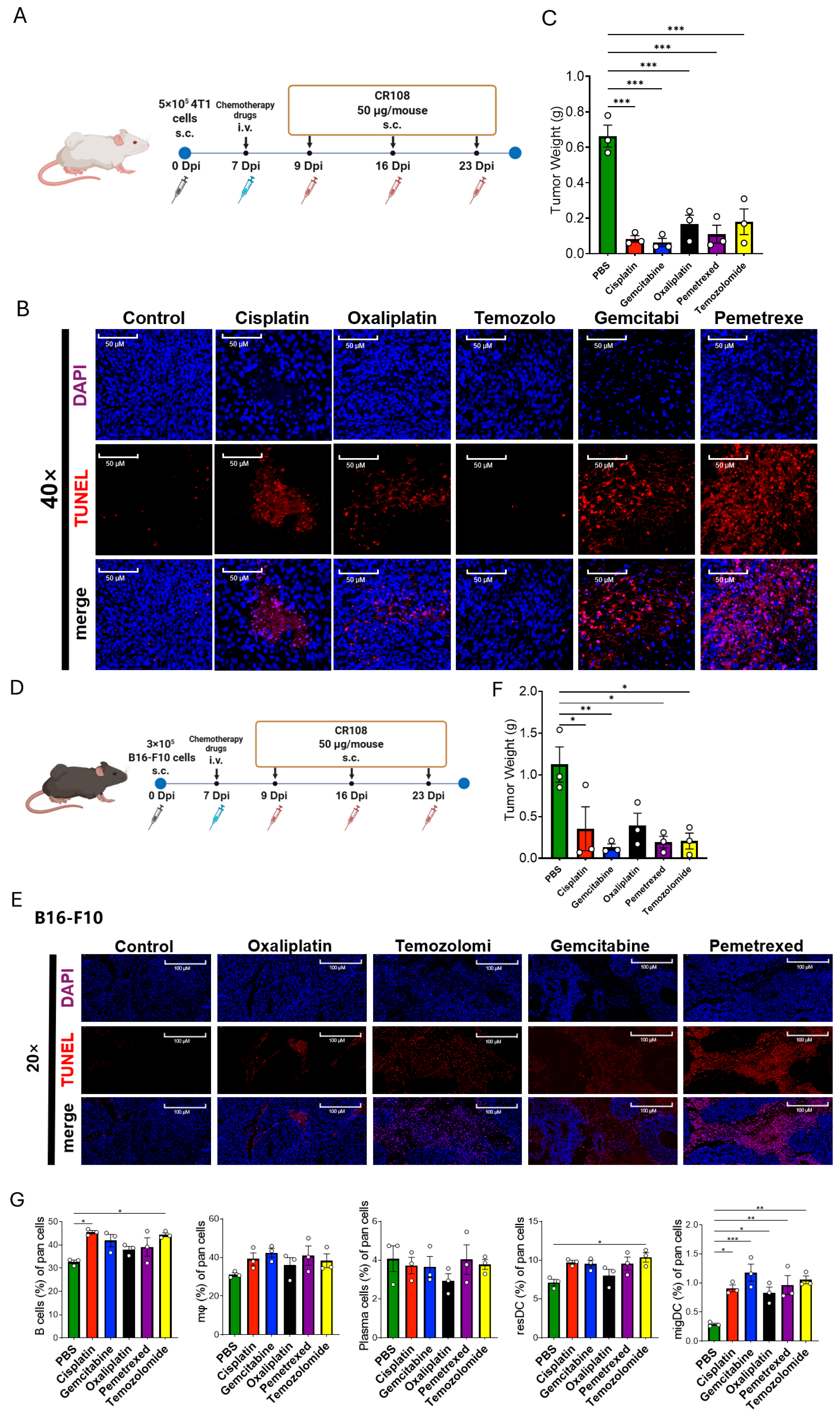
3.5. Diverse Chemotherapeutics Synergize with CR108 to Suppress 4T1 and B16-F10 Tumors and Remodel the Immune Microenvironment
3.6. Chemotherapeutic Mechanism and Tumor Lineage Shape TAs Profiles; DAMP Fingerprints Vary but Do Not Predict Benefit
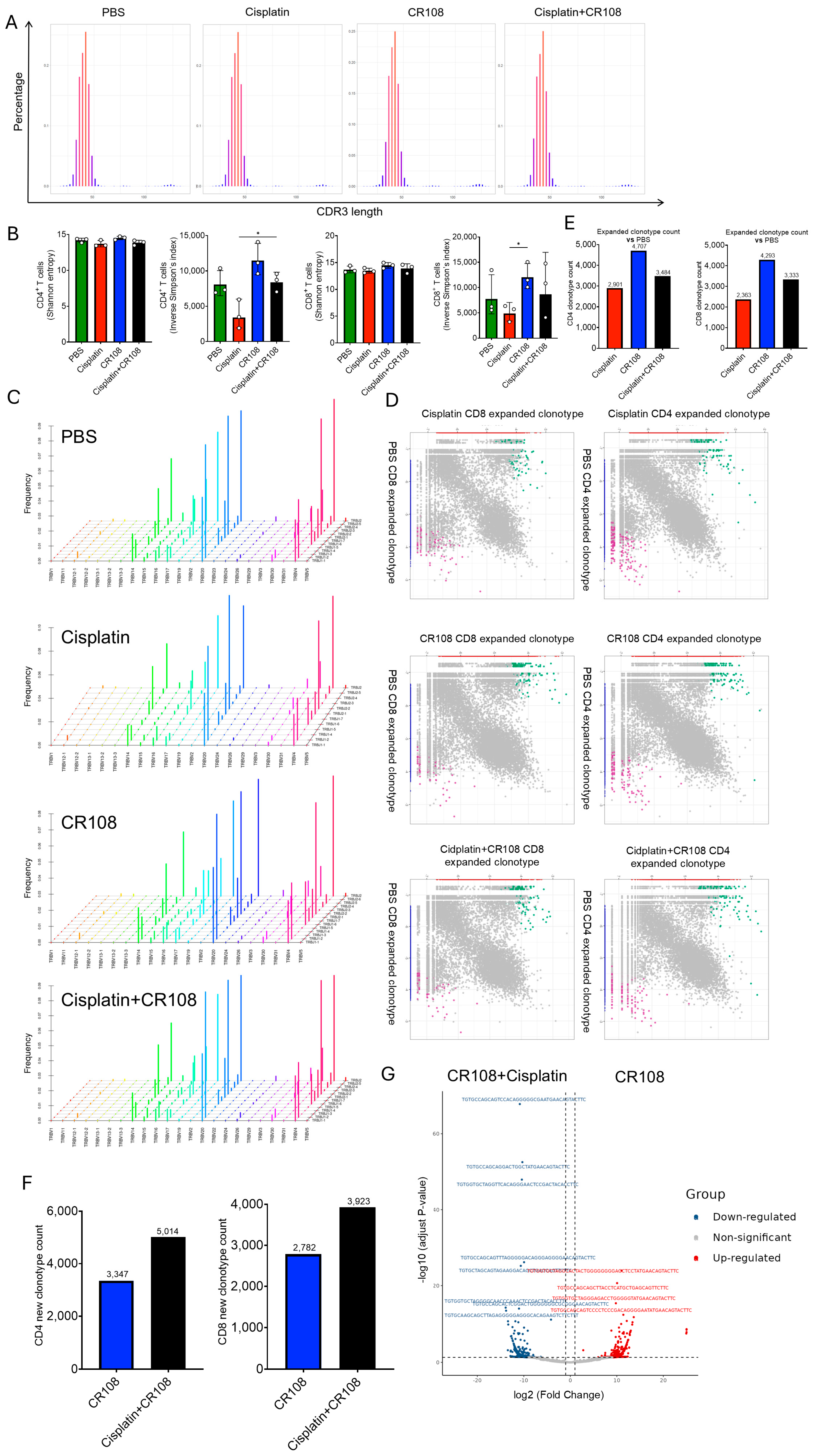
3.7. Synergistic Cisplatin–CR108 Interplay Remodels TCR Abundance While Preserving Repertoire Architecture
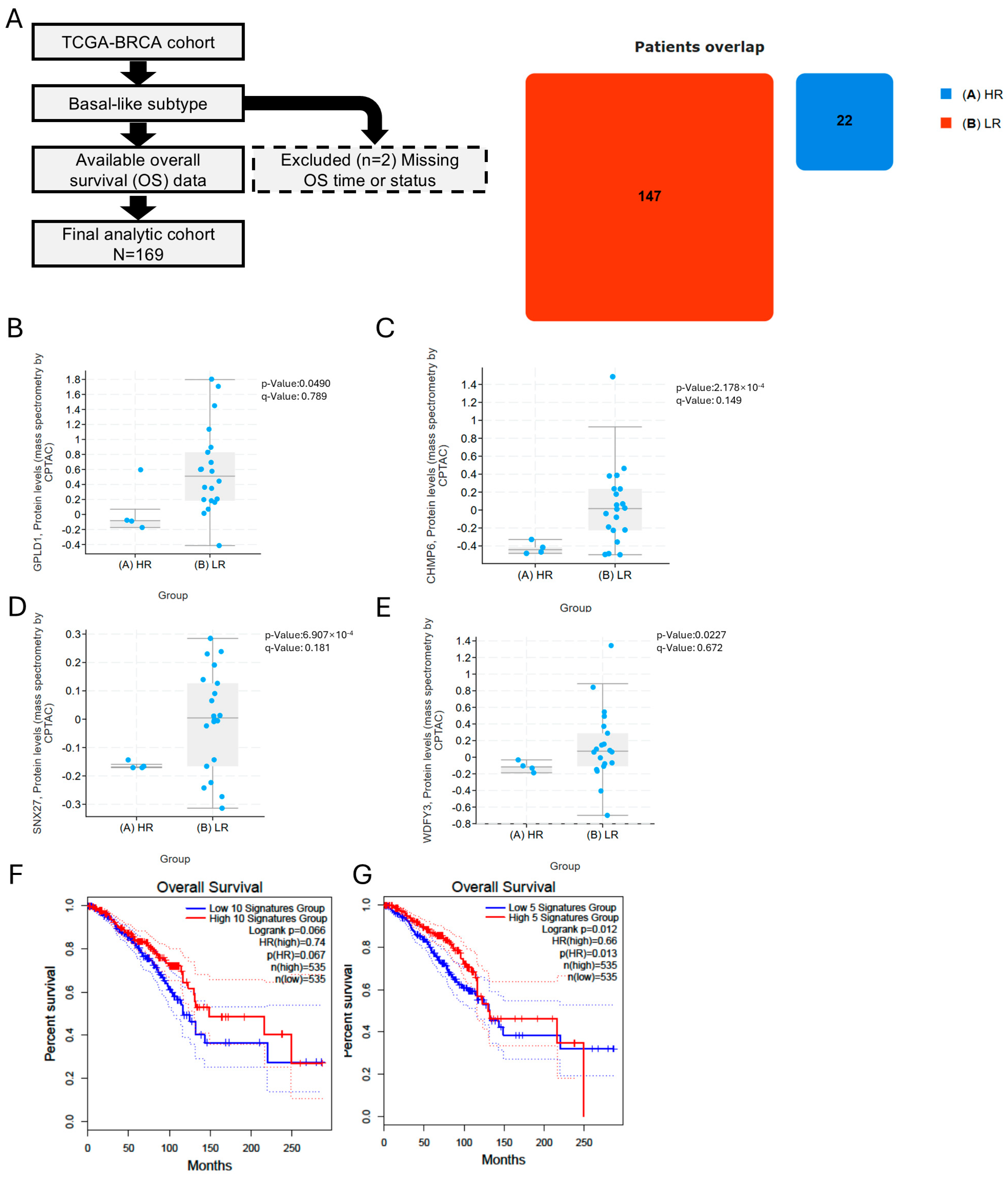
3.8. Soluble TA Generation/Export and Downstream Processing Associate with Favorable Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | antigen-presenting cell |
| BRCA | breast invasive carcinoma (TCGA code) |
| CDR3 | complementarity-determining region 3 |
| CR108 | TLR7/8/9 agonist used in this study |
| DAMP | damage-associated molecular pattern |
| DC | dendritic cell |
| dLN | tumor-draining lymph node |
| ELISPOT | enzyme-linked immunospot |
| ER | endoplasmic reticulum |
| ERAP1/2 | endoplasmic reticulum aminopeptidase 1/2 |
| ESCRT | endosomal sorting complexes required for transport |
| GEPIA | Gene Expression Profiling Interactive Analysis |
| GO | Gene Ontology |
| HSP | heat-shock protein |
| ICD | immunogenic cell death |
| ICS | intracellular cytokine staining |
| IFN-γ | interferon-gamma |
| IHC | immunohistochemistry |
| IQR | inter-quartile range |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LN | lymph node |
| mDC | myeloid dendritic cell |
| OS | overall survival |
| PBS | phosphate-buffered saline |
| PDX | patient-derived xenograft |
| pDC | plasmacytoid dendritic cell |
| q7d | once every 7 days |
| TAA | tumor-associated antigen |
| TA(s) | tumor antigen(s) |
| TAP | transporter associated with antigen processing |
| TCGA | The Cancer Genome Atlas |
| TCR | T-cell receptor |
| TCRβ | T-cell receptor beta chain |
| TDATTAs | tumor-derived antigenic targets |
| TIME | tumor immune microenvironment |
| TLR | Toll-like receptor |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| TRBJ | TCR beta joining gene |
| TRBV | TCR beta variable gene |
| i.v. | intravenous |
| s.c. | subcutaneous |
References
- Subbiah, V.; Gouda, M.A.; Ryll, B.; Burris, H.A., 3rd; Kurzrock, R. The evolving landscape of tissue-agnostic therapies in precision oncology. CA Cancer J. Clin. 2024, 74, 433–452. [Google Scholar] [CrossRef]
- Pail, O.; Lin, M.J.; Anagnostou, T.; Brown, B.D.; Brody, J.D. Cancer vaccines and the future of immunotherapy. Lancet 2025, 406, 189–202. [Google Scholar] [CrossRef]
- Yang, K.; Halima, A.; Chan, T.A. Antigen presentation in cancer-mechanisms and clinical implications for immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 604–623. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, J.; Ji, W.; Guan, X. Therapeutic landscape in mutational triple negative breast cancer. Mol. Cancer 2018, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, H.; Yang, W.; Zhang, L. The next generation of immunotherapies for lung cancers. Nat. Rev. Clin. Oncol. 2025, 22, 592–616. [Google Scholar] [CrossRef]
- Lopez de Rodas, M.; Villalba-Esparza, M.; Sanmamed, M.F.; Chen, L.; Rimm, D.L.; Schalper, K.A. Biological and clinical significance of tumour-infiltrating lymphocytes in the era of immunotherapy: A multidimensional approach. Nat. Rev. Clin. Oncol. 2025, 22, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Anyaegbu, C.C.; Lake, R.A.; Heel, K.; Robinson, B.W.; Fisher, S.A. Chemotherapy enhances cross-presentation of nuclear tumor antigens. PLoS ONE 2014, 9, e107894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, R.; Zhang, Y.; Guo, Y.; Hui, Z.; Wang, P.; Sun, Y. Galectin-9 expression clinically associated with mature dendritic cells infiltration and T cell immune response in colorectal cancer. BMC Cancer 2022, 22, 1319. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Martin, K.; Schreiner, J.; Zippelius, A. Modulation of APC Function and Anti-Tumor Immunity by Anti-Cancer Drugs. Front. Immunol. 2015, 6, 501. [Google Scholar]
- Wu, Q.; Zhang, H.; Sun, S.; Wang, L.; Sun, S. Extracellular vesicles and immunogenic stress in cancer. Cell Death Dis. 2021, 12, 894. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 2024, 625, 593–602. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Gong, N.; Alameh, M.G.; El-Mayta, R.; Xue, L.; Weissman, D.; Mitchell, M.J. Enhancing in situ cancer vaccines using delivery technologies. Nat. Rev. Drug Discov. 2024, 23, 607–625. [Google Scholar] [CrossRef]
- Haen, S.P.; Loffler, M.W.; Rammensee, H.G.; Brossart, P. Towards new horizons: Characterization, classification and implications of the tumour antigenic repertoire. Nat. Rev. Clin. Oncol. 2020, 17, 595–610. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nikolos, F.; Lee, Y.C.; Jain, A.; Tsouko, E.; Gao, H.; Kasabyan, A.; Leung, H.E.; Osipov, A.; Jung, S.Y.; et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat. Commun. 2020, 11, 6299. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440, 808–812. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, A.; Amoscato, A.; Angelini, R.; et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat. Commun. 2017, 8, 2122. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Yadav, M.; Jhunjhunwala, S.; Phung, Q.T.; Lupardus, P.; Tanguay, J.; Bumbaca, S.; Franci, C.; Cheung, T.K.; Fritsche, J.; Weinschenk, T.; et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014, 515, 572–576. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Marty, R.; Kaabinejadian, S.; Rossell, D.; Slifker, M.J.; van de Haar, J.; Engin, H.B.; de Prisco, N.; Ideker, T.; Hildebrand, W.H.; Font-Burgada, J.; et al. MHC-I Genotype Restricts the Oncogenic Mutational Landscape. Cell 2017, 171, 1272–1283.e15. [Google Scholar] [CrossRef]
- Wu, S.; Xiang, R.; Zhong, Y.; Zhao, S.; Zhang, Z.; Kou, Z.; Zhang, S.; Zhao, Y.; Zu, C.; Zhao, G.; et al. TLR7/8/9 agonists and low-dose cisplatin synergistically promotes tertiary lymphatic structure formation and antitumor immunity. NPJ Vaccines 2025, 10, 13. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, Q.; Yan, Z.; Chen, R.; Zeh Iii, H.J.; Kang, R.; Lotze, M.T.; Tang, D. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 2013, 4, e966. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remedios, C.; et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, C.; Zhong, Y.; Wu, S.; Wang, B. Chemotherapy Liberates a Broadening Repertoire of Tumor Antigens for TLR7/8/9-Mediated Potent Antitumor Immunity. Cancers 2025, 17, 3277. https://doi.org/10.3390/cancers17193277
Zu C, Zhong Y, Wu S, Wang B. Chemotherapy Liberates a Broadening Repertoire of Tumor Antigens for TLR7/8/9-Mediated Potent Antitumor Immunity. Cancers. 2025; 17(19):3277. https://doi.org/10.3390/cancers17193277
Chicago/Turabian StyleZu, Cheng, Yiwei Zhong, Shuting Wu, and Bin Wang. 2025. "Chemotherapy Liberates a Broadening Repertoire of Tumor Antigens for TLR7/8/9-Mediated Potent Antitumor Immunity" Cancers 17, no. 19: 3277. https://doi.org/10.3390/cancers17193277
APA StyleZu, C., Zhong, Y., Wu, S., & Wang, B. (2025). Chemotherapy Liberates a Broadening Repertoire of Tumor Antigens for TLR7/8/9-Mediated Potent Antitumor Immunity. Cancers, 17(19), 3277. https://doi.org/10.3390/cancers17193277







