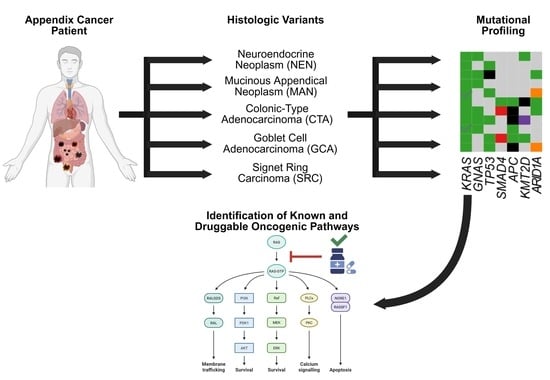
The Genomic Topography of Appendiceal Cancers: Our Current Understanding, Clinical Perspectives, and Future Directions
Simple Summary
Abstract
1. Introduction
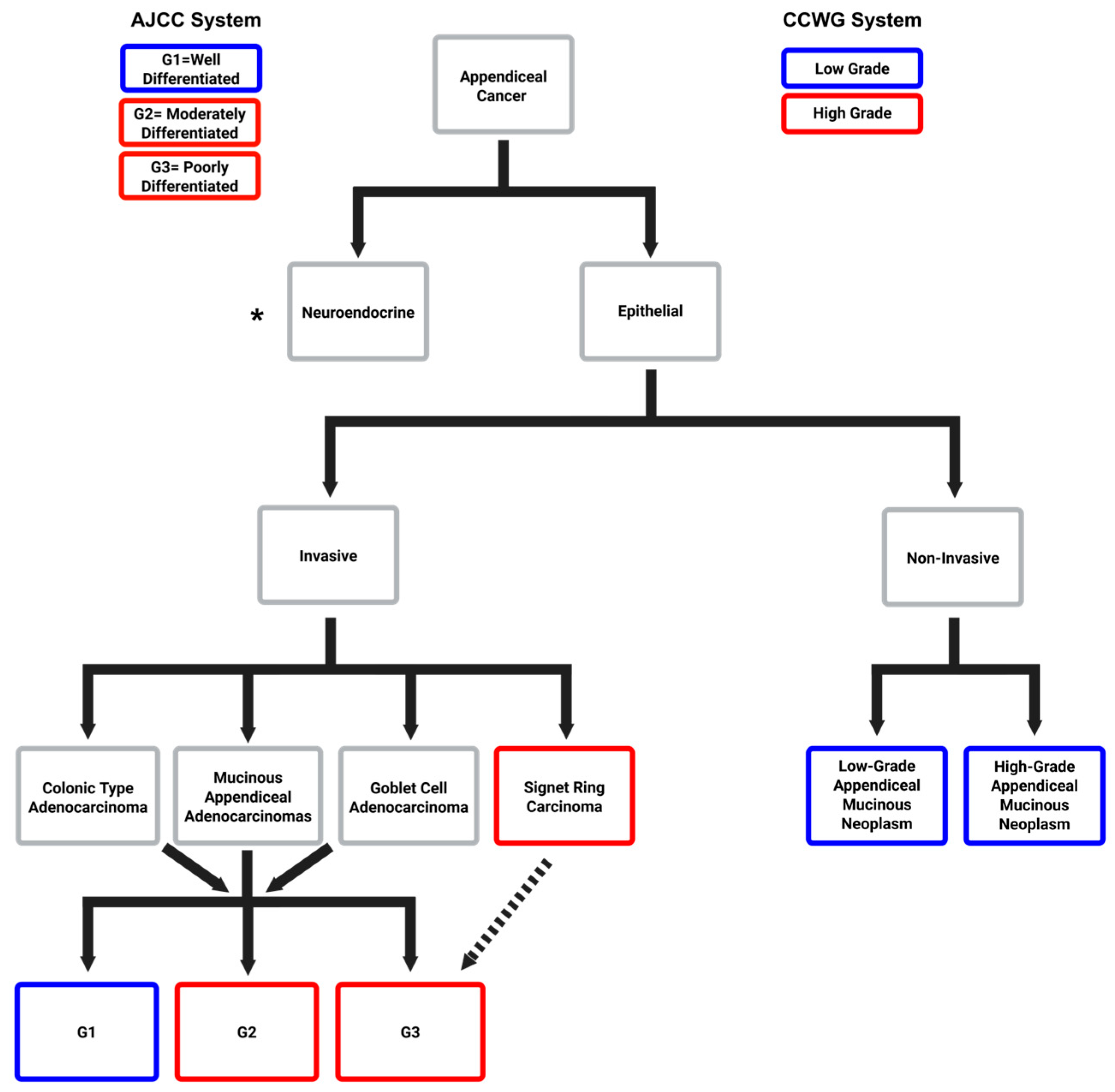
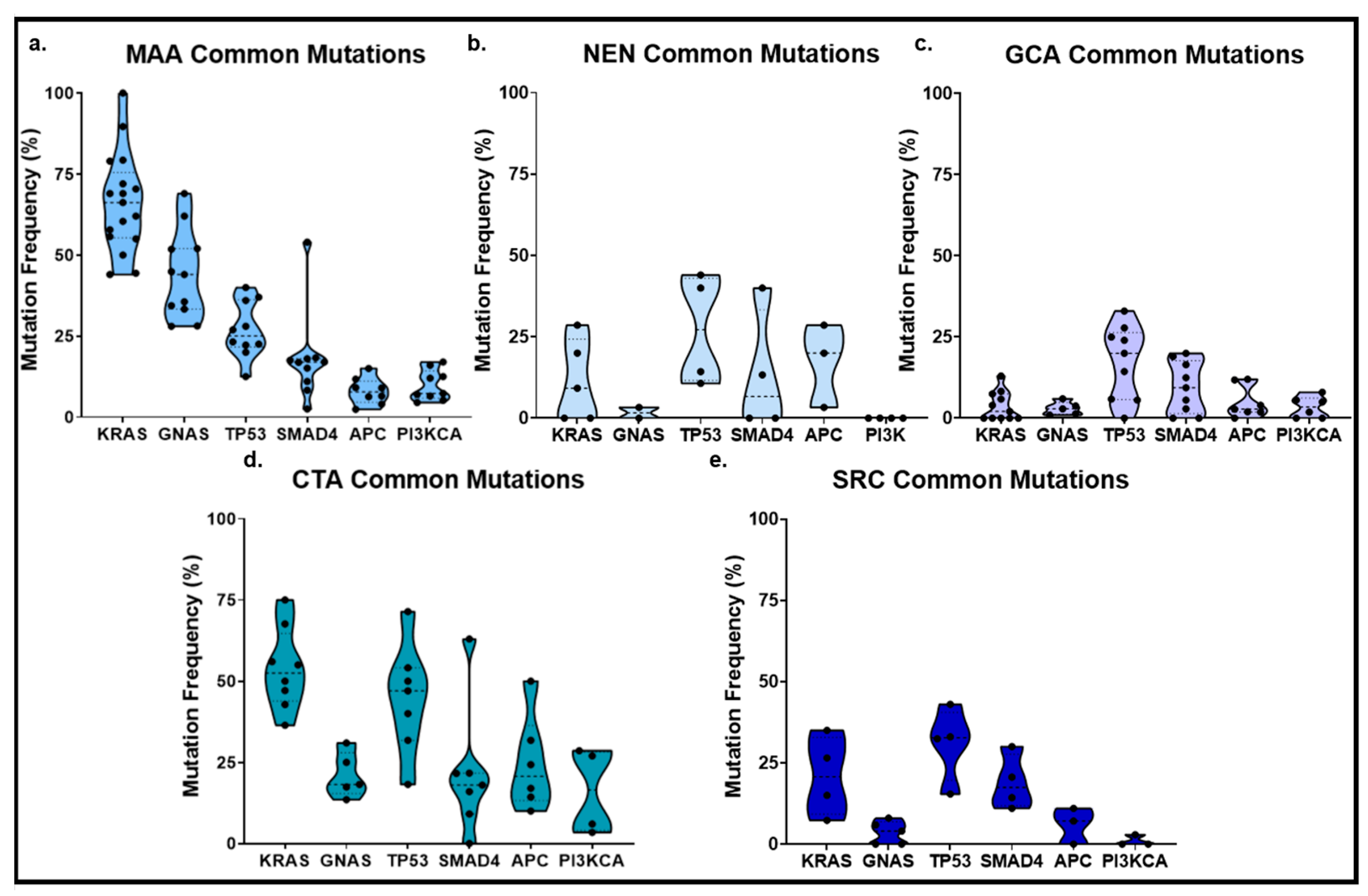
2. Appendiceal Neuroendocrine Neoplasms
3. Mucinous Neoplasms of the Appendix
4. Appendiceal Goblet Cell Adenocarcinoma
5. Appendiceal Colonic-Type Adenocarcinoma
6. Appendiceal Signet Ring Cell Adenocarcinoma
7. Molecular Divergence Between Appendiceal and Colorectal Cancers
8. Mutated Genes with Potential Clinical Actionability in AC
9. New Technologies for Understanding AC Pathophysiology and Therapeutic Response
10. Discussion
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Appendix Cancer |
| CRC | Colorectal Cancer |
| WES | Whole-Exome Sequencing |
| NGS | Next-Generation Sequencing |
| KRAS | Kirsten Rat Sarcoma viral oncogene homolog |
| GNAS | Guanine Nucleotide-binding protein, Alpha Stimulating |
| TP53 | Tumor Protein 53 |
References
- Carr, N.J.; McCarthy, W.F.; Sobin, L.H. Epithelial Noncarcinoid Tumors and Tumor-Like Lesions of the Appendix: A Clinicopathologic Study of 184 Patients with a Multivariate Analysis of Prognostic Factors. Cancer 1995, 75, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Polydorides, A.D.; Wen, X. Clinicopathologic parameters and outcomes of mucinous neoplasms confined to the appendix: A benign entity with excellent prognosis. Mod. Pathol. 2022, 35, 1732–1739. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Shetty, A.V.; Kazmi, S.M.A.; Zhang, N.; Morris, J.; Taggart, M.; Fournier, K.; Royal, R.; Mansfield, P.; Eng, C.; et al. Impact of Molecular Alterations and Targeted Therapy in Appendiceal Adenocarcinomas. Oncologist 2013, 18, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, R.; Rieser, C.; Choudry, M.; Melnitchouk, N.; Hechtman, J.; Bahary, N. Current Management of Appendiceal Neoplasms. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 118–132. [Google Scholar] [CrossRef]
- Shaib, W.L.; Goodman, M.; Chen, Z.; Kim, S.; Brutcher, E.; Bekaii-Saab, T.; El-Rayes, B.F. Incidence and Survival of Appendiceal Mucinous Neoplasms: A SEER Analysis. Am. J. Clin. Oncol. 2017, 40, 569–573. [Google Scholar] [CrossRef]
- Turaga, K.K.; Pappas, S.G.; Gamblin, T.C. Importance of Histologic Subtype in the Staging of Appendiceal Tumors. Ann. Surg. Oncol. 2012, 19, 1379–1385. [Google Scholar] [CrossRef]
- Carr, N.J. Updates in Appendix Pathology. Surg. Pathol. Clin. 2020, 13, 469–484. [Google Scholar] [CrossRef]
- Crona, J.; Skogseid, B. GEP-NETS Update: Genetics of Neuroendocrine Tumors. Eur. Soc. Endocrinol. 2016, 174, 275–290. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Shen, J.; Jácome, A.A.; Guerra, J.L.; Scally, C.P.; Taggart, M.W.; Foo, W.C.; Matamoros, A.; Shaw, K.R.; Fournier, K. Integrated Clinico-Molecular Profiling of Appendiceal Adenocarcinoma Reveals a Unique Grade-Driven Entity Distinct from Colorectal Cancer. Br. J. Cancer 2020, 123, 1262–1270. [Google Scholar] [CrossRef]
- Levine, E.A.; Votonopolous, K.I.; Shen, P.; Russell, G.; Fenstermaker, J.; Mansfield, P.; Bartlett, D.; Stewart, J.H. A Multicenter Randomized Trial to Evaluate Toxicities After Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin or Mitomycin in Patients with Appendiceal Tumors. J. Am. Coll. Surg. 2018, 226, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Nitecki, S.S.; Wolff, B.G.; Schlinkert, R.; Sarr, M.G. The Natural History of Treated Primary Adenocarcinoma of the Appendix. Ann. Surg. 1994, 219, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Shen, J.; Hardy-Abeloos, C.; Huang, J.; Ross, J.; Miller, V.; Jacobs, M.; Chen, I.; Xu, D.; Ali, S.; et al. Genomic Landscape of Appendiceal Neoplasms. JCO Precis. Oncol. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. New Standard of Care for Appendiceal Epithelial Neoplasms and Pseudomyxoma Peritonei Syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef]
- Gupta, S.; Parsa, V.; Adsay, V.; Heilbrun, L.K.; Smith, D.; Shields, A.F.; Weaver, D.; Phillip, P.A.; El-Rayes, B.F. Clinicopathological Analysis of Primary Epithelial Appendiceal Neoplasms. Med. Oncol. 2009, 27, 1073–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruoff, C.; Hanna, L.; Zhi, W.; Shahzad, G.; Gotlieb, V.; Saif, M. Cancers of the Appendix: Review of the Literatures. ISRN Oncol. 2011, 2011, 728579. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, Z.; Song, Y.; Li, W.; Diao, D.; Dang, C.; Zhang, H. The Management and Prognostic Prediction of Adenocarcinoma of Appendix. Sci. Rep. 2016, 6, 39027. [Google Scholar] [CrossRef]
- Connor, S.J.; Hanna, G.B.; Frizelle, F.A. Appendiceal Tumors: Retrospective Clinicopathologic Analysis of Appendiceal Tumors from 7,970 appendectomies. Dis. Colon Rectum 1998, 41, 75–80. [Google Scholar] [CrossRef]
- Schumpelick, V.; Dreuw, B.; Ophoff, K.; Prescher, A. Appendix and cecum. Embryology, anatomy, and surgical applications. Surg. Clin. N. Am. 2000, 80, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Leonards, L.; Pahwa, A.; Patel, M.; Petersen, J.; Nguyen, M.; Jude, C. Neoplasms of the Appendix: Pictorial Review with Clinical and Pathological Correlation. Radiographics 2017, 37, 1059–1083. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Cashin, P.; Sugarbaker, P.H. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Colorectal and Appendiceal Peritoneal Metastases: Lessons Learned from PRODIGE 7. J. Gastrointest. Oncol. 2021, 12 (Suppl. 1), S120–S128. [Google Scholar] [CrossRef]
- Valasek, M.A.; Pai, R.K. An Update on the Diagnosis, Grading, and Staging of Appendiceal Mucinous Neoplasms. Adv. Anat. Pathol. 2018, 25, 38–60. [Google Scholar] [CrossRef] [PubMed]
- Gündoğar, Ö.; Kımıloğlu, E.; Komut, N.; Cin, M.; Bektaş, S.; Gönüllü, D.; İlgün, A.S.; Erdoğan, N. Evaluation of Appendiceal Mucinous Neoplasms with a New Classification System and Literature Review. Turk. J. Gastroenterol. 2018, 29, 533–542. [Google Scholar] [CrossRef]
- Bradley, R.F.; Stewart, J.H., IV; Russel, G.B.; Levine, E.A.; Geisinger, K.R. Pseudomyxoma Peritonei of Appendiceal Origin: A Clinicopathologic Analysis of 101 Patients Uniformly Treated at a Single Institution, with Literature Review. Am. J. Surg. Pathol. 2006, 30, 551–559. [Google Scholar] [CrossRef]
- Davison, J.M.; Choudry, H.A.; Pingpank, J.F.; Ahrendt, S.A.; Holtzman, M.P.; Zureikat, A.H.; Zeh, H.J.; Ramalingam, L.; Zhu, B.; Nikiforova, M.; et al. Clinicopathologic and Molecular Analysis of Disseminated Appendiceal Mucinous Neoplasms: Identification of Factors Prediciting Survival and Proposed Criteria for a Three-Tiered Assessment of Tumor Grade. Mod. Pathol. 2014, 27, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Chicago Consensus Working Group. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Appendiceal Neoplasms. Cancer 2020, 126, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Misdraji, J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod. Pathol. 2014, 28 (Suppl. 1), S67–S79. [Google Scholar] [CrossRef]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and Metastatic Peritoneal Surface Malignancies. Nat. Rev. Dis. Primers 2021, 7, 91. [Google Scholar] [CrossRef]
- Legué, L.M.; Creemers, G.-J.; de Hingh, I.H.J.T.; Lemmens, V.E.P.P.; Huysentruyt, C.J. Review: Pathology and Its Clinical Relevance of Mucinous Appendiceal Neoplasms and Pseudomyxoma Peritonei. Clin. Color. Cancer 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Park, H.Y.; Kwon, M.J.-K.; Ho, S.K.; Kim, Y.J.; Kim, N.Y.; Kim, M.J.; Min, K.-W.; Choi, K.C.; Nam, E.S.; Cho, S.J.; et al. Targeted Next-Generation Sequencing of Well-Differentiated Rectal, Gastric, and Appendiceal Neuroendocrine Tumors to Identify Potential Targets. Hum. Pathol. 2019, 87, 83–94. [Google Scholar] [CrossRef]
- Yanai, Y.; Saito, T.; Hayashi, T.; Akazawa, Y.; Yatagai, N.; Tsuyama, S.; Tomita, S.; Hirai, S.; Ogura, K.; Matsumoto, T.; et al. Molecular and clinicopathological features of appendiceal mucinous neoplasms. Virchows Arch. Int. J. Pathol. 2021, 478, 413–426. [Google Scholar] [CrossRef]
- Tokunaga, R.; Xiu, J.; Johnston, C.; Goldberg, R.M.; Philip, P.A.; Seeber, A.; Naseem, M.; Lo, J.H.; Arai, H.; Battaglin, F.; et al. Molecular Profiling of Appendiceal Adenocarcinoma and Comparison with Right-Sided and Left-Sided Colorectal Cancer. Transl. Cancer Mech. Ther. 2019, 25, 3096–3103. [Google Scholar] [CrossRef]
- Munari, G.; Businello, G.; Mattiolo, P.; Pennelli, G.; Sbaraglia, M.; Borga, C.; Pucciarelli, S.; Spolverato, G.; Mescoli, C.; Galuppini, F.; et al. Molecular Profiling of Appendiceal Serrated Lesions, Polyps and Mucinous Neoplasms: A Single-Centre Experience. J. Cancer Res. Clin. Oncol. 2021, 147, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Alakus, H.; Babicky, M.L.; Ghosh, P.; Yost, S.; Jepsen, K.; Dai, Y.; Arias, A.; Samuels, M.L.; Mose, E.S.; Schwab, R.B.; et al. Genome-Wide Mutational Landscape of Mucinous Carcinomatosis Peritonei of Appendiceal Origin. Genome Med. 2014, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Baca, Y.; Battaglin, F.; Kawanishi, N.; Wang, J.; Soni, S.; Zhang, W.; Millsteain, J.; Johnston, C.; Goldberg, R.M.; et al. Molecular Characterization of Appendiceal Goblet Cell Carcinoid. Mol. Cancer Ther. 2020, 19, 2634–2640. [Google Scholar] [CrossRef]
- Wen, K.W.; Grenert, J.P.; Joseph, N.M.; Shafizadeh, N.; Huang, A.; Hosseini, M.; Kakar, S. Genomic Profile of Appendiceal Goblet Cell Carcinoid is Distinct Compared to Appendiceal Neuroendocrine Tumor and Conventional Adenocarcinoma. Hum. Pathol. 2017, 77, 164–174. [Google Scholar] [CrossRef]
- Johncilla, M.; Stachler, M.; Misdraji, J.; Lisovsky, M.; Yozu, M.; Lindeman, N.; Lauwers, G.Y.; Odze, R.D.; Srivastava, A. Mutational Landscape of Goblet Cell Carcinoids and Adenocarcinoma Ex Goblet Cell Carcinoids of the Appendix is Distinct from Typical Carcinoids and Colorectal Adenocarcinomas. Mod. Pathol. 2018, 31, 989–996. [Google Scholar] [CrossRef]
- Foote, M.B.; Walch, H.; Chatila, W.; Vakiani, E.; Chandler, C.; Steinrucke, F.; Nash, G.M.; Stadler, Z.; Chung, S.; Yaeger, R.; et al. Molecular Classification of Appendiceal Adenocarcinoma. J. Clin. Oncol. 2023, 41, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- The AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Borazanci, E.; Millis, S.Z.; Kimbrough, J.; Doll Von Hoff, D.; Ramanathan, R.K. Potential Actionable Targets in Appendiceal Cancer Detected by Immunohistochemistry, Fluorescent In Situ Hybridization, and Mutational Analysis. J. Gastrointest. Oncol. 2017, 8, 164–172. [Google Scholar] [CrossRef]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Van de Moorele, M.; De Hertogh, M.; Sagaert, X.; Van Cutsem, E. Appendiceal Cancer: A Review of the Literature. Acta Gastro-Enteroligica Belg. 2020, 83, 441–448. [Google Scholar]
- Wang, D.; Ge, H.; Lu, Y.; Gong, X. Incidence trends and survival analysis of appendiceal tumors in the United States: Primarily changes in appendiceal neuroendocrine tumors. PLoS ONE 2023, 18, e0294153. [Google Scholar] [CrossRef] [PubMed]
- Onyempka Davis, A.; McLeod, M.; Oysaiji, T. Typical carcinoids, goblet cell carcinoids, mixed adenoneuroendocrine carcinomas, neuroendocrine carcinomas and adenocarcinomas of the appendix: A comparative analysis of survival profile and predictors. J. Gastrointest. Oncol. 2019, 10, 300–306. [Google Scholar] [CrossRef]
- Griniatsos, J.; Michail, O. Appendiceal Neuroendocrine Tumors: Recent Insights and Clinical Implications. World J. Gastrointest. Oncol. 2010, 2, 192–196. [Google Scholar] [CrossRef]
- Deschamps, L.; Couvelard, A. Endocrine Tumors of the Appendix: A Pathologic Review. Arch. Pathol. Lab. Med. 2010, 134, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Grillo, F.; Massa, F.; Maletta, F.; Mastracci, L.; Campora, M.; Ferro, J.; Vanoli, A.; Papotti, M. Neuroendocrine Neoplasms of the Appendix, Colon and Rectum. Pathologica 2021, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Twito, O.; Paran, H.; Avital, S.; Kravtsov, V.; Rosenblum, R.C.; Rotman-Pikielny, P.; Klein, N. Temporal Trends in Incidence, Evaluation and Management of Neuroendocrine Neoplasms of the Appendix: 14 Years’ Experience. Am. J. Surg. 2021, 221, 1000–1004. [Google Scholar] [CrossRef]
- Moris, D.; Tsilimigras, D.I.; Vagios, S.; Ntanasis-Stathopoulos, I.; Karachaliou, G.-S.; Papalampros, A.; Alexandrou, A.; Blazer, D.G., III; Felekouras, E. Neuroendocrine Neoplasms of the Appendix: A Review of the Literature. Anticancer Res. 2018, 38, 601–611. [Google Scholar]
- Nadler, A.; Cukier, M.; Rowsell, C.; Kamali, S.; Feinberg, Y.; Singh, S.; Lawm, C.H.L. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch. Int. J. Pathol. 2013, 462, 501–505. [Google Scholar] [CrossRef]
- Taggart, M.W.; Abraham, S.C.; Overman, M.J.; Mansfield, P.F.; Rashid, A. Goblet Cell Carcinoid Tumor, Mixed Goblet Cell Carcinoid-Adenocarcinoma, and Adenocarcinoma of the Appendix: Comparison of Clinicopathologic Features and Prognosis. Arch. Pathol. Lab. Med. 2015, 139, 782–790. [Google Scholar] [CrossRef]
- Popa, O.; Taban, S.M.; Pantea, S.; Plopeanu, A.D.; Barna, R.A.; Cornianu, M.; Pascu, A.-A.; Dema, A.L.C. The New WHO Classification of Gastrointestinal Neuroendocrine Tumors and Immunohistochemical Expression of Somatostatin Receptor 2 and 5. Exp. Ther. Med. 2021, 22, 1179. [Google Scholar] [CrossRef]
- van Riet, J.; van de Werken, A.J.G.; Cuppen, E.; Eskens, F.A.L.M.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.-J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef]
- Ramnani, D.M.; Wistuba, I.I.; Behrens, C.; Gazdar, A.F.; Sobin, L.H.; Albores-Saavedra, J. K-Ras and p53 Mutations in the Pathogenesis of Classical and Goblet Cell Carcinoids of the Appendix. Cancer 2000, 86, 14–21. [Google Scholar] [CrossRef]
- Polyak, K.; Lee, M.H.; Erdjument-Bromage, H.; Koff, A.; Roberts, J.M.; Tempst, P.; Massagué, J. Cloning of p27Kip1, a Cyclin-Dependent Kinase Inhibitor and a Potential Mediator of Exracellular Antimitogenic Signals. Cell 1994, 78, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Carpizo, D.R.; Harris, C.R. Genetic Drivers of Ileal Neuroendocrine Tumors. Cancers 2021, 13, 5070. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. The Genetics of Neuroendocrine Tumors. Semin. Oncol. 2013, 40, 37–44. [Google Scholar] [CrossRef]
- Cunningham, J.L.; de Ståhl, T.D.; Sjöblom, T.; Westin, G.; Dumanski, J.P.; Janson, E.T. Common pathogenetic mechanism involving human chromosome 18 in familial and sporadic ileal carcinoid tumors. Genes Chromosomes Cancer 2010, 50, 82–94. [Google Scholar] [CrossRef]
- Carr, N.J.; Emory, T.S.; Sobin, L.H. Epithelial Neoplasms of the Appendix and Colorectum: An Analysis of Cell Proliferation, Apoptosis, and Expression of p53, CD44, and bcl-2. Arch. Pathol. Lab. Med. 2002, 126, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, R.M.; van Velthuysen, M.L.F.; Verwaal, V.J.; Zoetmulder, F.A.N. Appendiceal Neoplasms and Pseudomyxoma Peritonei: A Population Based Study. Eur. J. Surg. Oncol. (EJSO) 2008, 34, 196–201. [Google Scholar] [CrossRef]
- Szych, C.; Steabler, A.; Connolly, D.C.; Wu, R.; Cho, K.R.; Ronnett, B.M. Molecular Genetic Evidence Supporting the Clonality and Appendiceal Origin of Pseudomyxoma Peritonei in Women. Am. J. Pathol. 1999, 154, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Morris, D.L. Secreted mucins in pseudomyxoma peritonei: Pathophysiological significance and potential therapeutic prospects. Orphanet J. Rare Dis. 2014, 9, 71. [Google Scholar] [CrossRef]
- Benedix, F.; Reimer, A.; Gastinger, I.; Mroczkowski, P.; Lippert, H.; Kube, R. Primary appendiceal carcinoma- Epidemiology, surgery, and survival: Results of a German multi-center study. Eur. J. Surg. Oncol. 2010, 36, 763–771. [Google Scholar] [CrossRef]
- Bradley, R.F.; Carr, N.J. Pseudomyxoma Peritonei: Pathology, a Historical Overview, and Proposal for Unified Nomenclature and Updated Grading. Am. J. Surg. Pathol. Rev. Rep. 2019, 24, 88–93. [Google Scholar] [CrossRef]
- Kabbani, W.; Houlihan, P.S.; Rajayalaksh, L.; Hamilton, S.R.; Rashid, A. Mucinous and Nonmucinous Appendiceal Adenocarcinomas: Different Clinicopathological Features but Similar Genetic Alterations. Mod. Pathol. 2002, 15, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Grotz, T.E.; Royal, R.E.; Mansfield, P.F.; Overman, M.J.; Mann, G.N.; Robinson, K.A.; Beaty, K.A.; Rafeeq, S.; Matamoros, A.; Taggart, M.W.; et al. Stratification of Outcomes for Mucinous Appendiceal Adenocarcinoma with Peritoneal Metastasis by Histological Grade. World J. Gastrointest. Oncol. 2017, 9, 354–362. [Google Scholar] [CrossRef]
- McCusker, M.E.; Coté, T.R.; Clegg, L.X.; Sobin, L.H. Primary Malignant Neoplasms of the Appendix: A Population-Based Study from the Surveillance, Epidemiology, and End-Results Program, 1973–1998. Cancer 2002, 94, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Fournier, K.; Hu, C.-Y.; Eng, C.; Tagart, M.; Royal, R.; Mansfield, P.; Chang, G.J. Improving the AJCC/TNM Staging for Adenocarcinomas of the Appendix The Prognostic Impact of Histological Grade. Ann. Surg. 2013, 257, 1072–1078. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Perrone, F.; Mennitto, A.; Gleeson, E.M.; Milione, M.; Tamborini, E.; Busico, A.; Settanni, G.; Berenato, R.; Caporale, M.; et al. Toward the Molecular Dissection of Peritoneal Pseudomyxoma. Ann. Oncol. 2016, 27, 2097–2103. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Loree, J.M.; Fournier, K.F.; Shaw, K.R.; Taggart, M.W.; Foo, W.C.; Matamoros, A.; Mehdizadeh, A.; Ahmed, S.U.; Guerra, J.L.; et al. Comprehensive Genomic Profiling of Appendiceal Adenocarcinoma. J. Clin. Oncol. 2018, 36, S298. [Google Scholar] [CrossRef]
- Hara, L.; Saito, T.; Hayashi, T.; Yimit, A.; Takahashi, M.; Keiko, M.; Takahashi, M.; Yao, T. A Mutation Spectrum that Includes GNAS, KRAS, and TP53 May be Shared by Mucinous Neoplasms of the Appendix. Pathol. Res. Pract. 2015, 211, 657–664. [Google Scholar] [CrossRef]
- Matson, D.R.; Xu, J.; Huffman, L.; Barroilhet, L.; Accola, M.; Rehrauer, W.M.; Weisman, P. KRAS and GNAS Co-Mutation in Metastatic Low-Grade Appendiceal Mucinous Neoplasm (LAMN) to the Ovaries: A Practical Role for Next-Generation Sequencing. Am. J. Case Rep. 2017, 18, 558–562. [Google Scholar] [CrossRef]
- Shetty, S.; Thomas, P.; Ramanan, B.; Sharma, P.; Govindarajan, V.; Loggie, B. KRAS mutations and p53 overexpression in pseudomyxoma peritonei: Association with phenotype and prognosis. J. Surg. Res. 2013, 180, 97–103. [Google Scholar] [CrossRef]
- Nishikawa, G.; Sekine, S.; Ogawa, R.; Matsubara, A.; Mori, T.; Taniguchi, H.; Kushima, R.; Hiraoka, N.; Tsuta, K.; Tsuda, H.; et al. Frequent GNAS Mutations in Low-Grade Appendiceal Mucinous Neoplasms. Br. J. Cancer 2013, 108, 951–958. [Google Scholar] [CrossRef]
- Holowatyj, A.; Eng, C.; Wen, W.; Idrees, K.; Guo, X. Spectrum of Somatic Cancer Gene Variations Among Adults with Appendiceal Cancer by Age. JAMA Netw. Open 2020, 3, e2028644. [Google Scholar] [CrossRef]
- Liu, X.; Mody, K.; de Abreu, F.B.; Pipas, J.M.; Peterson, J.D.; Gallagher, T.L.; Suriawinata, A.A.; Ripple, G.H.; Hourdequin, K.C.; Smith, K.D.; et al. Molecular Profiling of Appendiceal Epithelial Tumors Using Massively Parallel Sequencing to Identify Somatic Mutations. Clin. Chem. 2014, 60, 1004–1011. [Google Scholar] [CrossRef]
- Zhu, X.; Salhab, M.; Tomaszewicz, K.; Meng, X.; Mathew, C.; Bathini, V.; Switzer, B.; Walter, O.; Cosar, E.F.; Wang, X.; et al. Heterogenous Mutational Profile and Prognosis Conferred by TP53 Mutations in Appendiceal Mucinous Neoplasms. Hum. Pathol. 2019, 85, 260–269. [Google Scholar] [CrossRef]
- Davison, J.M.; Hartman, D.A.; Singhi, A.D.; Choudry, H.A.; Ahrendt, S.A.; Zureikat, A.H.; Ramalingam, L.; Nikiforova, M.; Pai, R.K. Loss of SMAD4 Protein Expression is Associated with High Tumor Grade and Poor Prognosis in Disseminated Appendiceal Mucinous Neoplasms. Am. J. Surg. Pathol. 2014, 38, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Taggart, M.W.; Galbincea, J.; Mansfield, P.J.; Fournier, K.F.; Royal, R.E.; Overman, M.J.; Rashid, A.; Abraham, S.C. High-Level Microsatellite Instability in Appendiceal Carcinomas. Am. J. Surg. Pathol. 2014, 37, 1192–2000. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. Goblet cell carcinoids of the appendix: Tumor biology, mutations and management strategies. World J. Gastrointest. Surg. 2016, 8, 660–669. [Google Scholar] [CrossRef]
- Isaacson, P. Crypt Cell Carcinoma of the Appendix (So-Called Adenocarcinoid Tumor). Am. J. Surg. Pathol. 1981, 5, 213–224. [Google Scholar] [CrossRef]
- Carr, N.J.; Sobin, L.H. Neuroendocrine Tumors of the Appendix. Semin. Diagn. Pathol. 2004, 21, 108–119. [Google Scholar] [CrossRef]
- Pham, T.H.; Wolff, B.; Abraham, S.C.; Drelichman, E. Surgical and Chemotherapy Treatment Outcomes of Goblet Cell Carcinoid: A Tertiary Cancer Center Experience. Ann. Surg. Oncol. 2006, 13, 370–376. [Google Scholar] [CrossRef]
- Roy, P.; Chetty, R. Goblet Cell Carcinoid Tumors of the Appendix: An Overview. World J. Gastrointest. Oncol. 2010, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. NCI Thesaurus Version 18.11d. 2018. Available online: https://ncit.nci.nih.gov/ncitbrowser/ (accessed on 24 October 2022).
- Tang, L.H.; Shi, J.; Soslow, R.A.; Dhall, D.; Wong, W.D.; O’Reilly, E.; Qin, J.; Paty, P.; Weiser, M.R.; Guillem, J.; et al. Pathologic Classification and Clinical Behavior of the Spectrum of Goblet Cell Carcinoid Tumors of the Appendix. Am. J. Surg. Pathol. 2008, 32, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Sinno, S.A.J.; Jurdi, N.M.H. Goblet Cell Tumors of the Appendix: A Review. Ann. Diagn. Pathol. 2019, 43, 151401. [Google Scholar] [CrossRef]
- Reid, M.D.; Basturk, O.; Shaib, W.L.; Xue, Y.; Balci, S.; Choi, H.-J.; Akkas, G.; Memis, B.; Robinson, B.S.; El-Rayes, B.F.; et al. Adenocarcinoma Ex-Goblet Carcinoid (Appendiceal-Type Crypt Cell Adenocarcinoma) is a Morphologically Distinct Entity with Highly Aggressive Behavior and Frequent Association with Peritoneal/Intra-Abdominal Dissemination: An Analysis of 77 Cases. Mod. Pathol. 2017, 29, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Rashid, A.; Xing, Y.; Chiang, Y.-J.; Chagbar, R.B.; Fournier, K.F.; Chang, G.J.; You, N.; Feig, B.W. Varying Malignant Potential of Appendiceal Neuroendocrine Tumors: Importance of Histologic Subtypes. J. Surg. Oncol. 2012, 107, 136–143. [Google Scholar] [CrossRef]
- Clift, A.K.; Kornasiewicz, O.; Drymousis, P.; Faiz, O.; Wasan, H.S.; Kinross, J.M.; Cecil, T.; Frilling, A. Goblet cell carcinomas of the appendix: Rare but aggressive neoplasms with challenging management. Endocr. Connect. 2018, 7, 268–277. [Google Scholar] [CrossRef]
- Zambrano-Vera, K.; Sardi, A.; Munoz-Zuluaga, C.; Studeman, K.; Nieroda, C.; Sittig, M.; King, M.C.; Sipok, A.; Gushchin, V. Outcomes in Peritoneal Carcinomatosis from Appendiceal Goblet Cell Carcinoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC). Ann. Surg. Oncol. 2020, 27, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.H.; Holt, N.; Langer, S.W.; Hasselby, J.P.; Grønbæk, H.; Hillingsø, H.; Mahmoud, M.; Ladekarl, M.; Iversen, L.H.; Kjær, A.; et al. Goblet Cell Carcinoids: Characteristics of a Danish Cohort of 83 Patients. PLoS ONE 2015, 10, e0117627. [Google Scholar] [CrossRef]
- Pai, R.K.; Hartman, D.J.; Gonzalo, D.H.; Lai, K.K.; Downs-Kelly, E.; Goldblum, J.R.; Liu, X.; Patil, D.T.; Bennett, A.E.; Plesec, T.P.; et al. Serrated Lesions of the Appendix Frequently Harbor KRAS Mutations and not BRAF Mutations Indicating a Distinctly Different Serrated Neoplastic Pathway in the Appendix. Hum. Pathol. 2014, 45, 227–235. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Konukiewitz, B.; Foersch, S.; Stenzinger, A.; Steiger, K.; Muckenhuber, A.; Groß, C.; Mollenhauer, M.; Roth, W.; Detlefsen, S.; et al. Appendiceal goblet cell carcinoids and adenocarcinomas ex-goblet cell carcinoid are genetically distincit from primary colorectal-type adenocarcinoma of the appendix. Mod. Pathol. 2018, 31, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Dimmler, A.; Geddert, H.; Faller, G. EGFR, KRAS, BRAF-Mutations and Microsatellite Instability are Absent in Goblet Cell Carcinoids of the Appendix. Pathol.-Res. Pract. 2014, 210, 274–278. [Google Scholar] [CrossRef]
- Danussi, C.; Akavia, U.D.; Niola, F.; Jovic, A.; Lasorella, A.; Pe’er, D.; Lavarone, A. RHPN2 Drives Mesenchymal Transformation in Malignant Glioma by Triggering RhoA Activation. Cancer Res. 2013, 73, 5140–5150. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Tak, Y.G.; Berman, B.P.; Farnham, P.J. Functional Annotation of Colon Cancer Risk SNPs. Nat. Commun. 2014, 5, 5114. [Google Scholar] [CrossRef]
- Stancu, M.; Wu, T.-T.; Wallace, C.; Houlihan, P.S.; Hamilton, S.R.; Rashid, A. Genetic Alterations in Goblet Cell Carcinoids of the Veriform Appendix and Comparison with Gastrointestinal Carcinoid Tumors. Mod. Pathol. 2003, 16, 1189–1198. [Google Scholar] [CrossRef]
- LaFramboise, W.A.; Pai, R.K.; Petrosko, P.; Belsky, M.A.; Dhir, A.; Howard, P.G.; Becich, M.J.; Holtzman, M.P.; Ahrendt, S.A.; Pingpank, J.F.; et al. Discrimination of Low- and High-Grade Appendiceal Mucinous Neoplasms by Targeted Sequencing of Cancer-Related Variants. Mod. Pathol. 2019, 32, 1197–1209. [Google Scholar] [CrossRef]
- Chandana, S.R.; Shah, C. Appendiceal Adenocarcinoma: Analysis of SEER Database. J. Clin. Oncol. 2017, 35, e18097. [Google Scholar] [CrossRef]
- McGory, M.L.; Maggard, M.A.; Kang, H.; O’Connell, J.B.; Ko, C.Y. Malignancies of the Appendix: Beyond Case Series Reports. Dis. Colon Rectum 2005, 48, 2264–2271. [Google Scholar] [CrossRef]
- Yajima, N.; Wada, R.; Yamagishi, S.-I.; Mizumaki, H.; Itabashi, C.; Yagihashi, S. Immunohistochemical Expressions of Cytokeratins, Mucin Core Proteins, p53, and Neuroendocrine Cell Markers in Epithelial Neoplasm of Appendix. Hum. Pathol. 2005, 36, 1217–1225. [Google Scholar] [CrossRef]
- Maru, D.; Wu, T.-T.; Canada, A.; Houlihan, P.S.; Hamilton, S.R.; Rashid, A. Loss of Chromosome 18q and DPC4 (Smad4) Mutations in Appendiceal Adenocarcinomas. Oncogene 2004, 23, 859–864. [Google Scholar] [CrossRef][Green Version]
- Font, A.; Abad, A.; Monzó, M.; Sanchez, J.J.; Guillot, M.; Manzano, J.L.; Piñol, M.; Ojanguren, I.; Rosell, R. Prognostic value of K-ras mutations and allelic imbalance on chromosome 18q in patients with resected colorectal cancer. Dis. Colon Rectum 2001, 44, 549–557. [Google Scholar] [CrossRef]
- Andjelkovic, B.; Stojanovic, B.; Stojanovic, M.D.; Milosevic, B.; Cvetkovic, A.; Spasic, M.; Jakovljevic, S.; Cvetkovic, D.; Stojanovic, B.S.; Milosev, D.; et al. Appendiceal Signet Ring Cell Carcinoma: An Atypical Cause of Acute Appendicitis—A Case Study and Review of Current Knowledge. Diagnostics 2023, 13, 2359. [Google Scholar] [CrossRef]
- Burke, A.P.; Sobin, L.H.; Federspiel, B.H.; Shekitka, K.M.; Helwig, E.B. Goblet Cell Carcinoids and Related Tumors of the Veriform Appendix. Am. J. Clin. Pathol. 1990, 94, 27–35. [Google Scholar] [CrossRef]
- Singhi, A.D.; Davison, J.M.; Choudry, H.A.; Pingpank, J.F.; Ahrendt, S.A.; Holtzman, M.P.; Zureikat, A.H.; Zeh, H.J.; Ramalingam, L.; Mantha, G.; et al. GNAS is Frequently Mutated in Both Low-Grade and High-Grade Disseminated Appendiceal Mucinous Neoplasms by Does Not Affect Survival. Hum. Pathol. 2014, 45, 1737–1743. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, S.; Wu, J.; Lin, J.; Pan, C.; Ying, X.; Pan, Z.; Lin, Q.; Liu, R.; Geng, R.; et al. PI3K/AKT- Mediated Upregulation of WDR5 Promotes Colorectal Cancer Metastasis by Directly Targeting ZNF407. Cell Death Dis. 2017, 8, e2686. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.C. The Genetic Basis of Colorectal Cancer: Insights into Critical Pathways of Tumorigenesis. Gastroenterology 2000, 119, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, J.; Prenen, H. Molecular Genetics of Colorectal Cancer. Ann. Gastroenterol. 2014, 27, 9–14. [Google Scholar]
- Chowdhury, S.; Ito, I.; Pattalachinti, V.K.; Shen, J.P. Abstract 880: Activation of the Wnt pathway is associated with GNASR201 mutant appendiceal cancer in the peritoneum. Cancer Res. 2023, 83 (Suppl. 7), 880. [Google Scholar] [CrossRef]
- More, A.; Ito, I.; Haridas, V.; Chowdhury, S.; Gu, Y.; Dickson, P.; Fowlkes, N.; Shen, J.-P. Oncogene Addiction to GNAS in GNASR201 Mutant Tumors. Oncogene 2022, 41, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Li, H.; Wu, W.K.K.; Wong, S.H.; Yu, J. Genomics and Metagenomics of Colorectal Cancer. J. Gastrointest. Oncol. 2019, 6, 1164–1170. [Google Scholar] [CrossRef]
- Pattalachinti, V.K.; Haque, E.; Yousef, M.; Yousef, A.; Chowdhury, S.; Overman, M.; Parseghian, C.M.; Morris, V.K.; Huey, R.W.; Raghav, K.; et al. BRAF Mutant Appendiceal Adenocarcinoma Differs from Colorectal Cancer but Responds to BRAF-Target Therapy. npj Precis. Oncol. 2025, 9, 38. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Su, J.; Jin, G.; Votanopoulos, K.I.; Craddock, L.; Shen, P.; Chou, J.W.; Qasem, S.; O’Neill, S.S.; Perry, K.C.; Miller, L.D.; et al. Prognostic Molecular Classification of Appendiceal Mucinous Neoplasms Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2020, 27, 1439–1447. [Google Scholar] [CrossRef]
- Moaven, O.; Su, J.S.; Jin, G.; Votanopoulos, K.I.; Shen, P.; Mangieri, C.; O’Neill, S.S.; Perry, K.S.; Levine, E.A.; Miller, L.D. Clinical Implications of Genetic Signatures in Appendiceal Cancer Patients with Incomplete Cytoreduction/HIPEC. Ann. Surg. Oncol. 2020, 27, 5016–5023. [Google Scholar] [CrossRef] [PubMed]
- Ihemelandu, C.; Mavros, M.N.; Sugarbaker, P. Adverse Events Postoperatively Had No Impact of Long-Term Survival of Patients Treated with Cytoreductive Surgery with Heated Intraperitoneal Chemotherapy for Appendiceal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2016, 23, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Levine, E.A.; Blazer, D.G., III; Kim, M.K.; Shen, P.; Stewart, J.H., IV; Guy, C.; Hsu, D.S. Gene Expression Profiling of Peritoneal Metastases from Appendiceal and Colon Cancer Demonstrates Unique Biologic Signatures and Predicts Patient Outcomes. J. Am. Coll. Surg. 2012, 214, 599–606. [Google Scholar] [CrossRef]
- Levine, E.A.; Votanopoulos, K.I.; Qasem, S.A.; Philip, J.; Cummins, K.A.; Chou, J.W.; Ruiz, J.; D’Agostino, R.; Shen, P.; Miller, L.D. Prognostic Molecular Subtypes of Low-Grade Cancer of the Appendix. J. Am. Coll. Surg. 2016, 222, 493–503. [Google Scholar] [CrossRef]
- Indini, A.; Rijavec, E.; Ghidini, M.; Corellini, A.; Grossi, F. Targeting KRAS in Solid Tumors: Current Challenges and Future Opportunities of Novel KRAS Inhibitors. Pharmaceutics 2021, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Qvortrup, C. KRASG12C Inhibition in Colorectal Cancer. Lancet Oncol. 2022, 23, 10–11. [Google Scholar] [CrossRef]
- Weller, C.; Burnett, G.L.; Jiang, L.; Chakraborty, S.; Zhang, D.; Vita, N.A.; Dilly, J.; Kim, E.; Maldonato, B.; Seamon, K.; et al. A Neomorphic Protein Interface Catalyzes Covalent Inhibition of RAS-G12D Aspartic Acid in Tumors. Science 2025, 389, eads029. [Google Scholar] [CrossRef]
- Cregg, J.; Edwards, A.V.; Chang, S.; Lee, B.J.; Knox, J.E.; Tomlinson, A.C.A.; Marquez, A.; Liu, Y.; Aay, B.; Wang, Y.; et al. Discovery of Daraxonrasib (RMC-6236), a Potent and Orally Bioavailable RAS(ON) Multi-selective, Noncovalent Tri-complex Inhibitor for the Treatment of Patients with Multiple RAS-Addicted Cancers. J. Med. Chem. 2025, 68, 6064–6083. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovsszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K Pathway in Cancer: Are We Making Headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef]
- Ayala, C.; Sathe, A.; Bai, X.; Grimes, S.M.; Shen, J.; Poultsides, G.A.; Lee, B.; Ji, H.P. Distinct Gene Signatures Define the Epithelial Cell Features of Mucinous Appendiceal Neoplasms and Pseudomyxoma Metastastases. Front. Genet. 2025, 16, 1536982. [Google Scholar] [CrossRef]
- Forsythe, S.D.; Erali, R.A.; Sasikumar, S.; Laney, P.; Shelkey, E.; D’Agostino, R., Jr.; Miller, L.D.; Shen, P.; Levine, E.A.; Soker, S.; et al. Organoid Platform in Preclinical Investigation of Personalized Immunotherapy Efficacy in Appendiceal Cancer: Feasibility Study. Clin. Cancer Res. 2021, 27, 5141–5150. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Wu, C.; O’Rourke, K.P.; Szeglin, B.C.; Zheng, Y.; Sauvé, C.-E.G.; Adileh, M.; Wasserman, I.; Marco, M.R.; Kim, A.S.; et al. A Rectal Cancer Organoid Platform to Study Individual Responses to Chemoradiation. Nat. Med. 2019, 25, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.-S.; et al. Loss of p53 Triggers Wnt-Dependent Systemic Inflammation to Drive Breast Cancer Metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Isella, C.; Vaira, M.; Robella Bellomo, S.E.; Picco, G.; Borsano, A.; Mignone, A.; Petti, C.; Porporato, R.; Ulla, A.A.; Pisacane, A.; et al. Improved Outcome Prediction for Appendiceal Pseudomyxoma Peritonei by Integration of Cancer Cell and Stromal Transcriptional Profiles. Cancers 2020, 12, 1495. [Google Scholar] [CrossRef]
- Votanopoulos, K.; Mazzochi, A.; Sivakumar, H.; Forsythe, S.; Aleman, J.; Levine, E.; Skardal, A. Appendiceal Cancer Patient-Specific Tumor Organoid Model for Predicting Chemotherapy Efficacy Prior to Initiation of Treatment: A Feasibility Study. Ann. Surg. Oncol. 2019, 26, 139–147. [Google Scholar] [CrossRef]
- Mavanur, A.A.; Parimi, V.; O’Malley, M.; Nikiforova, M.; Bartlett, D.L.; Davison, J.M. Establishment and characterization of a murine xenograft model of appendiceal mucinous adenocarcinoma. Int. J. Exp. Pathol. 2010, 91, 357–367. [Google Scholar] [CrossRef] [PubMed]

| Altered Gene | AC Subtype | Percent of Cases per Study (No Pts) in Reference Order | Reference(s) |
|---|---|---|---|
| EGFR | NEN | 40% (5) | [30] |
| RN43 | MAA | 44% (9), 7% (44), 33% (9) | [31,32,33] |
| SMAD2 | MAA | 26% (29) | [34] |
| SMAD3 | MAA | 10% (10) | [34] |
| TGFBR1 | MAA | 10% (10) | [34] |
| TGFBR2 | MAA | 10% (10) | [34] |
| ARID1A | GCA | 15% (84), 15.4% (13), 33% (9), 11% (18) | [12,35,36,37] |
| FAT3 | GCA | 17% (84) | [12] |
| MSH2 | GCA | 17% (18) | [37] |
| SOX9 | GCA | 22% (9), 10% (72), 9.2% (141), 11% (18) | [36,37,38,39] |
| ARID1A | CTA | 11% (208) | [12] |
| ARID1A | SRC | 7% (37), 11% (27) | [12,32] |
| CDH1 | SRC | 7.1% (14) | [40] |
| LRP1B | SRC | 16.7% (24) | [39] |
| PRKDC | SRC | 12.0% (50) | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gironda, D.J.; Erali, R.A.; Forsythe, S.D.; Pullikuth, A.K.; Zheng-Pywell, R.; Cummins, K.A.; Soker, S.; Gui, X.; Levine, E.A.; Votanopoulos, K.I.; et al. The Genomic Topography of Appendiceal Cancers: Our Current Understanding, Clinical Perspectives, and Future Directions. Cancers 2025, 17, 3275. https://doi.org/10.3390/cancers17193275
Gironda DJ, Erali RA, Forsythe SD, Pullikuth AK, Zheng-Pywell R, Cummins KA, Soker S, Gui X, Levine EA, Votanopoulos KI, et al. The Genomic Topography of Appendiceal Cancers: Our Current Understanding, Clinical Perspectives, and Future Directions. Cancers. 2025; 17(19):3275. https://doi.org/10.3390/cancers17193275
Chicago/Turabian StyleGironda, Daniel J., Richard A. Erali, Steven D. Forsythe, Ashok K. Pullikuth, Rui Zheng-Pywell, Kathleen A. Cummins, Shay Soker, Xianyong Gui, Edward A. Levine, Konstantinos I. Votanopoulos, and et al. 2025. "The Genomic Topography of Appendiceal Cancers: Our Current Understanding, Clinical Perspectives, and Future Directions" Cancers 17, no. 19: 3275. https://doi.org/10.3390/cancers17193275
APA StyleGironda, D. J., Erali, R. A., Forsythe, S. D., Pullikuth, A. K., Zheng-Pywell, R., Cummins, K. A., Soker, S., Gui, X., Levine, E. A., Votanopoulos, K. I., & Miller, L. D. (2025). The Genomic Topography of Appendiceal Cancers: Our Current Understanding, Clinical Perspectives, and Future Directions. Cancers, 17(19), 3275. https://doi.org/10.3390/cancers17193275