Immune Checkpoint Inhibitors in Merkel Cell Carcinoma of the Skin: A 2025 Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
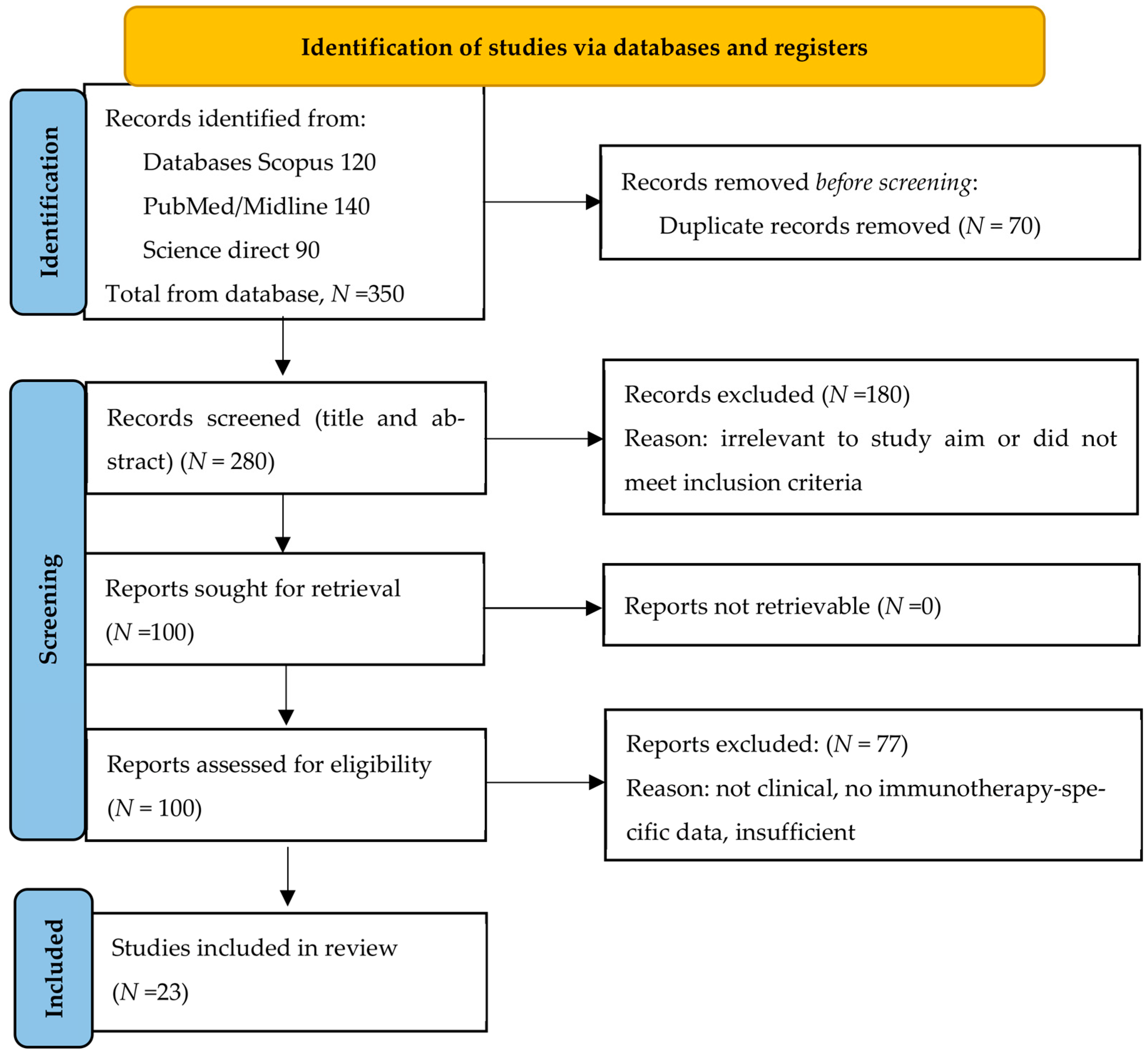
2. Methods
3. Results
3.1. Critical Appraisal (Summary Table S1 in Supplementary Materials)
- Small Sample Sizes—Most trials enrolled only a few dozen patients, reflecting MCC’s scarcity. Small sample size reduces statistical power and precision; for example, the neoadjuvant Checkmate 358 study had 33 patients [13] and KEYNOTE-017 enrolled 50, which constrains subgroup analyses and the detection of modest effects [33].
- Lack of Randomized Controls trials—Several pivotal studies were single-arm or non-randomized (e.g., CITN-09/KEYNOTE-017 and JAVELIN Merkel 200 [33,34]), relying on historical comparisons. The absence of a control arm increases potential selection bias and makes it difficult to attribute outcomes solely to the immunotherapy [13]. Ongoing randomized trials (ADMEC-O [18], ADAM [32], STAMP [35], I-MAT [36]) aim to address this, but their final results are pending [34].
- Limited Blinding—Among the randomized trials, blinding was inconsistently applied. Notably, the phase II ADMEC-O trial was open-label, meaning patients and investigators knew the assigned treatment [18]. Open-label designs can introduce performance and detection bias (e.g., differences in ancillary care or assessment of progression), especially for endpoints like radiographic DFS. By contrast, the ADAM trial’s double-blind design minimizes such bias [32].
- Endpoint and Follow-up Constraints—Many studies reported surrogate or short-term endpoints (objective response rate, 12 or 24 month progression/recurrence-free survival) with immature overall survival data. For example, the interim results of ADMEC-O showed a 9–10% improvement in 1–2-year DFS with adjuvant nivolumab, but this did not reach statistical significance and OS outcomes remain not yet mature [18]. Short follow-up can miss late recurrences and long-term toxicities [31].
- Trial Execution and Reporting—We noted variations in endpoint definitions and possible attrition biases. In ADMEC-O, an imbalance in post-surgery radiation between arms occurred, which could confound DFS results [34]. Additionally, the high dropout or non-evaluable rates in some trials were not always clearly reported, raising concern for attrition bias. Finally, publication bias is possible, as positive trials are more likely to be reported; our comprehensive search and PRISMA-based selection aimed to mitigate this, but the predominance of encouraging results suggests that small negative studies might be underrepresented [9].
- Generalizability and Clinical Applicability An expanded discussion is necessary to examine how well clinical trial findings translate to the broader MCC patient population. Because MCC is uncommon, clinical trials often involve highly selective patient cohorts, which may limit external validity. Key factors affecting generalizability include patient selection criteria, demographic representation, and real-world clinical context.
- Patient Selection and Fitness: Trial participants tended to be relatively fit (ECOG 0–1) with controlled comorbidities, whereas real-world MCC patients are often elderly with significant medical issues. The median age in trials (~65–70) is somewhat lower than the general MCC population, and frail patients were underrepresented [37]. Furthermore, strict eligibility criteria (organ function cut-offs, exclusion of recent malignancies, etc.) meant approximately one in five real-world oncology patients would not qualify for typical phase III trials [37]. Physicians also tend to enroll patients with better prognoses (younger, fewer comorbidities) [37]. This selection bias can inflate efficacy and reduce toxicity in trials compared to unselected populations. Clinicians must therefore judiciously extrapolate results to older or frailer patients, assessing whether the survival benefit outweighs risks in those less robust than trial subjects.
- Immunosuppression and Viral Status: A noteworthy proportion of MCC patients are immunosuppressed, for example, organ transplant recipients, human immunodeficiency virus (HIV)-positive, or with chronic lymphocytic leukemia, but such patients were largely excluded from trials [38]. Consequently, data on immunotherapy efficacy in immunosuppressed MCC are sparse. Emerging real-world evidence indicates these patients derive lower benefit—in a retrospective analysis, immunosuppressed MCC patients had an initial ICI response rate of ~50% versus ~62% in immunocompetent patients, along with higher progression and toxicity rates [38]. For example, 52% of immunosuppressed patients experienced serious immune-related side effects, compared to 37% of those with intact immunity. These findings suggest that while checkpoint inhibitors can be effective in immunosuppressed MCC, responses are attenuated and risks heightened, underscoring caution when generalizing trial outcomes to this subgroup. Additionally, the Merkel cell polyomavirus (MCPyV) status of patients might influence disease biology. Most trials enrolled both virus-positive and negative tumors without stratification, and encouragingly, responses have been observed in both groups for instance, pooled analyses show similar objective response rates regardless of MCPyV status [39]. However, since MCPyV prevalence differs by region (e.g., higher in North America/Europe, lower in Australia), the trial populations’ viral status mix may not reflect all settings. Ongoing research into virus-specific immunity may further clarify if virus-negative (UV-driven) MCC requires adapted immunotherapy strategies.
- Geographic and Ethnic Representation: The major immunotherapy trials in MCC have been conducted in North America, Europe, and Australia, with very limited participation from Asia, Africa, or South America [40]. Consequently, the ethnic diversity of trial cohorts was narrow—a large majority of patients were of White/European ancestry, mirroring MCC’s higher incidence in those populations. This homogeneity raises questions about applicability to other ethnic groups. While there is no evidence that tumor responses to PD-1/PD-L1 inhibitors differ by race, disparities in healthcare access and genetic backgrounds could influence real-world outcomes. The lack of diverse representation is an acknowledged challenge in oncology trials [9]. Efforts to broaden eligibility and include international sites are needed to ensure findings are globally relevant.
- Real-World Clinical Settings: Outcomes in routine practice may be less favorable than in trials. Outside the controlled trial environment, patients often have more advanced comorbidities, may receive treatment in non-specialized centers, or face interruptions in therapy. Indeed, studies across oncology had found that real-world survival can be significantly shorter than trial-reported survival—one analysis noted a ~6-month decrement in median overall survival for real-world patients on new cancer therapies [41]. For checkpoint inhibitors specifically, real-world patients appear to experience diminished benefit compared to trial patients in I-MAT trial [36], likely due to the inclusion of higher-risk individuals. In MCC, practical issues such as managing immune-related adverse events in older patients, or continuation of therapy beyond progression, may differ from protocol-driven trials. All these factors mean that the impressive 3-year survival ~60% seen with pembrolizumab in trials could be harder to achieve broadly, in I-MAT trial [36]. Clinicians should incorporate trial data flexibly, tailoring treatment plans to individual patient factors (age, immune status, comorbidities) and discussing the uncertainties of benefit magnitude in each context.
3.2. Recent Reviews, Guidelines and Major Prospective Clinical Trials
3.2.1. Neoadjuvant Immunotherapy
3.2.2. Adjuvant Immunotherapy (Table 2, Table 3 and Table 4)
| Study start: January 2019 Primary completion: completed accrual Study completion finish: 2026–2027 not publicly announced yet |
| Key Study Details |
|
| Study start: 26 October 2020 Primary completion: 1 April 2027 Study completion finish: 1 April 2028 |
| Key Study Details |
|
| Feature | STAMP Trial (NCT03712605) | I-MAT Trial (NCT04291885) |
|---|---|---|
| Sponsor | ECOG-ACRIN/National Cancer Institute (USA) | Melanoma and Skin Cancer Trials (Australia/New Zealand) |
| Start Year | 2019 | 2020 |
| Status | Closed to accrual; in follow-up phase | Active, not recruiting |
| Participants | ~280 patients | 122 patients |
| Eligibility | Stage I–III MCC, completely resected | Stage I–III MCC |
| Intervention | Pembrolizumab 200 mg IV q21d × 17 cycles (~1 yr) | Avelumab 800 mg IV q2w × 6 mos |
| Control Arm | Standard-of-care observation | Placebo |
| Primary Endpoint | Recurrence-free and overall survival (RFS and OS) | RFS |
| Secondary Endpoints | DMFS, toxicity, QoL, impact of radiation | OS, DSS, toxicity, QoL |
| Geographic Scope | United States only | Australia and New Zealand |
| Radiation Therapy | Optional, per standard of care | Allowed, based on clinical indication |
| Follow-up Duration | 5 years | 2 years |
3.2.3. Primary Therapy and Salvage Use
3.3. Mechanisms of Resistance
- (1)
- Primary resistance (upfront progressive or stable disease with subsequent progression, having received at least 6 weeks and up to 6 months of anti-PD-(L)1 therapy).
- (2)
- Secondary resistance—upfront partial or complete response with subsequent progressive disease, or stable disease for >6 months prior to progression disease, after at least 6 months of anti-PD-(L)1 therapy, with progression occurring ≤12 weeks of anti-PD-(L)1 therapy cessation.
- (3)
- Late progression—if patient had upfront complete/partial response or stable disease for >6 months prior to progression, with progression occurring >12 weeks following anti-PD-(L)1 therapy discontinuation.
- (1)
- Intra-tumoral STING activation: While STING agonists can enhance immune responses, chronic activation may lead to immune exhaustion or paradoxical suppression [57]. The STING protein was found to be absent in MCC cells themselves, but present in the surrounding immune and stromal cells within the tumor microenvironment [58]. This suggests that STING activators may exert their effects indirectly in MCC, by signaling through these non-tumor cells, rather than acting directly on cancer cells [same as above*]. This observation also suggests that resistance may not be due to chronic activation within cancer cells, but rather to a direct lack of STING expression in the target cells, or that its effect is dose-dependent, as high doses can lead to reduced efficacy or even cause “adverse effects.” [59].
- (2)
- Tumor-associated macrophages (TAMs) can create an immunosuppressive microenvironment, inhibiting T-cell infiltration and function [60], as noted by Professor Ann Silk, a leading expert in Merkel cell carcinoma (MCC).
- (3)
- (4)
- (5)
- (6)
3.4. What Measures Will Have the Greatest Impact on Improving Outcomes in MCC?
3.5. Future Direction in Research
3.5.1. Adoptive T-Cell Transfer
3.5.2. Therapeutic Vaccines:
- (1)
- Peptide-based vaccines: These vaccines consist of short peptide sequences of tumor antigens and require strong adjuvants to enhance the immune response. They are taken up by dendritic cells, which present them to T cells.
- (2)
- mRNA vaccines: These vaccines use synthetic mRNA at the desired antigen concentration and are typically in a lipid-based compound. They have a good safety profile and the ability to rapidly stimulate the immune system.
- (3)
- (4)
- Plasmid/viral vector vaccines: These use a virus to deliver genetic material that elicits an antigen, leading to an immune response. They can improve targeting of treatments to tumor sites and avoid excessive immune activation.
- (5)
- Exosome-based vaccines: These use extracellular vesicles carrying membrane proteins to increase the immune response and can stimulate T cells similarly to dendritic cells.
3.5.3. Combining Different Treatment Modalities
3.5.4. Biomarkers
3.5.5. Improving Quality of Life During and After Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1L | First-line therapy |
| 2L | Second-line therapy |
| ADMEC-O | Adjuvant Merkel Cell Carcinoma Nivolumab Trial |
| ADAM | Adjuvant Avelumab Merkel Cell Carcinoma Trial |
| ATT | Adoptive T-cell Transfer |
| ctDNA | Circulating Tumor DNA |
| DFS | Disease-Free Survival |
| DMFS | Distant Metastasis-Free Survival |
| DNA | Deoxyribonucleic Acid |
| DOR | Duration of Response |
| DSS | Disease-Specific Survival |
| ECOG | Eastern Cooperative Oncology Group |
| EP | Electroporation |
| FDA | Food and Drug Administration of the United States |
| ICI | Immune Checkpoint Inhibitor |
| IO | Immune-Oncology |
| I-MAT | Immunotherapy Merkel Adjuvant Trial |
| JAVELIN | JAVELIN Merkel 200 Avelumab Trial |
| KEYNOTE-017 | Pembrolizumab Phase II Trial in Advanced MCC |
| LVSI | Lymphovascular Space Invasion |
| MCC | Merkel Cell Carcinoma |
| MCPyV | Merkel Cell Polyomavirus |
| metMCC | Metastatic Merkel Cell Carcinoma |
| mRNA | Messenger Ribonucleic Acid |
| NOS | Not otherwise specified |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| pCR | Pathological Complete Response |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PFS | Progression-Free Survival |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| q21d | Every 21 Days |
| q2w | Every 2 Weeks |
| QoL | Quality of Life |
| RFS | Recurrence-Free Survival |
| SBRT | Stereotactic Body Radiation Therapy |
| SOC | Standard of Care |
| STAMP | Surgically Treated Adjuvant Merkel Cell Carcinoma with Pembrolizumab Trial |
| STING | Stimulator of Interferon Genes |
| TAM | Tumor-Associated Macrophages |
| TGF-β | Transforming Growth Factor Beta |
| UV | Ultraviolet Radiation |
| WHO | World Health Organization |
References
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I.; International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef]
- Singh, N.; McClure, E.M.; Akaike, T.; Park, S.Y.; Huynh, E.T.; Goff, P.H.; Nghiem, P. The evolving treatment landscape of Merkel cell carcinoma. Curr. Treat. Options Oncol. 2023, 24, 1231–1258. [Google Scholar] [CrossRef]
- Squibb, B.-M. An Investigational Immuno-Therapy Study to Investigate the Safety and Effectiveness of Nivolumab, and Nivolumab Combination Therapy in Virus-Associated Tumors—Full Text View—Clinicaltrials.Gov. 2018. Available online: https://clinicaltrials.gov/study/NCT02488759 (accessed on 30 January 2021).
- Becker, J.C.; Stang, A.; Schrama, D.; Ugurel, S. Merkel cell carcinoma: Integrating epidemiology, immunology, and therapeutic updates. Am. J. Clin. Dermatol. 2024, 25, 541–557. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov (accessed on 2 July 2025).
- Available online: https://www.cancer.gov (accessed on 2 July 2025).
- Available online: https://www.mypathologyreport.ca/pathology-dictionary/ (accessed on 15 July 2025).
- Tanda, E.T.; d’Amato, A.L.; Rossi, G.; Croce, E.; Boutros, A.; Cecchi, F.; Spagnolo, F.; Queirolo, P. Merkel cell carcinoma: An immunotherapy fairy-tale? Front. Oncol. 2021, 11, 739006. [Google Scholar] [CrossRef] [PubMed]
- Aquino de Moraes, F.C.; Kreuz, M.; Amaral de Lara, I.C.; Macena Lobo, A.d.O.; Rodríguez Burbano, R.M. Efficacy and safety of PD-1/PD-L1 inhibitors in patients with Merkel Cell Carcinoma: A systematic review and Meta-analysis. BMC Cancer 2024, 24, 1357. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Joseph, K.; Prajapati, V.H.; Thachuthara, A.J.; Lian, J.; Assouline, A.; Yu, E.; Veness, M. Merkel cell carcinoma of the skin: Deducing the pattern of spread from an international aggregated database of 949 patients. Curr. Oncol 2025, 32, 211. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.-P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatia, S.; Longino, N.V.; Miller, N.J.; Kulikauskas, R.; Iyer, J.G.; Ibrani, D.; Blom, A.; Byrd, D.R.; Parvathaneni, U.; Twitty, C.G.; et al. Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 598–608. [Google Scholar] [CrossRef]
- Kuchimanchi, N.; Gajula, S.; Gaughan, E.M.; Witt, R.G. Immune checkpoint inhibitor-related dysautonomia in Merkel cell carcinoma: A case report. Cancer Rep. 2025, 8, e70274. [Google Scholar] [CrossRef] [PubMed]
- Correa Roa, C.; Laxague, F.; Fernández Vila, J.M. An unusual complete response to immunotherapy in a locoregional recurrent Merkel cell carcinoma. Cureus 2025, 17, e77152. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Ugurel, S.; Leiter, U.; Meier, F.; Gutzmer, R.; Haferkamp, S.; Zimmer, L.; Livingstone, E.; Eigentler, T.K.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): Disease-free survival results from a randomised, open-label, phase 2 trial. Lancet 2023, 402, 798–808. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. ImmunoTher. Cancer 2023, 11, e006789. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.-J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-line avelumab treatment in patients with metastatic Merkel cell carcinoma: 4-year follow-up from part B of the JAVELIN Merkel 200 study. ESMO Open 2024, 9, 103461. Available online: https://www.esmoopen.com/article/S2059-7029(21)00290-0/fulltext (accessed on 2 July 2025). [CrossRef]
- Gaiser, M.R.; Bongiorno, M.; Brownell, I. PD-L1 Inhibition with Avelumab for Metastatic Merkel Cell Carcinoma. Expert Rev. Clin. Pharmacol. 2018, 11, 345–359. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.-J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Primary and biomarker analyses of a phase II study. J. ImmunoTher. Cancer 2021, 9, e002646. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.S.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Milella, M.; Brownell, I.; et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer. 2018, 6, 7. [Google Scholar] [CrossRef]
- Shirley, M. Avelumab: A Review in Metastatic Merkel Cell Carcinoma. Target. Oncol. 2018, 13, 395–406. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. ImmunoTher. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Russell, J.; Lebbé, C.; Chmielowski, B.; Gambichler, T.; Grob, J.-J.; Kiecker, F.; Rabinowits, G.; Terheyden, P.; Zwiener, I.; et al. Efficacy and Safety of First-line Avelumab Treatment in Patients with Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018, 4, e180077. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Mo, J.; Zaremba, A.; Inderjeeth, A.J.; El Zeinaty, P.; Li, A.; Wicky, A.; Della Marta, N.; Marqueste, C.G.; Bohne, A.S.; Matias, M.; et al. Clinical outcomes and management following progressive disease with anti-PD-(L)1 therapy in patients with advanced Merkel Cell Carcinoma. Eur. J. Cancer 2025, 217, 115254. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Eng. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Pardoll, D.M. Neoadjuvant anti–PD-1-based immunotherapy: Evolving a new standard of care. J. Immunother. Cancer 2025, 13, e010833. [Google Scholar] [CrossRef] [PubMed]
- Lebbé, C.; Becker, J.C.; Grob, J.-J.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Saiag, P.; Middleton, M.R.; Bastholt, L.; et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 2396–2403. [Google Scholar]
- Available online: https://ichgcp.net/clinical-trials-registry/NCT03271372 (accessed on 15 July 2025).
- Bradford, D.; Demko, S.; Jin, S.; Mishra-Kalyani, P.; Beckles, A.R.; Goldberg, K.B.; Lemery, S.; Ward, A.; Keegan, P.; Pazdur, R. FDA Accelerated Approval of Pembrolizumab for Recurrent Locally Advanced or Metastatic Merkel Cell Carcinoma. Oncologist 2020, 25, e1077–e1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angelo, S.P.; Lebbé, C.; Nghiem, P.; Brohl, A.S.; Mrowiec, T.; Leslie, T.; Georges, S.; Güzel, G.; Shah, P. Biomarker Analyses Investigating Disease Biology and Associations with Outcomes in the JAVELIN Merkel 200 Trial of Avelumab in Metastatic Merkel Cell Carcinoma. Clin. Cancer Res. 2024, 30, 4352–4362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.dana-farber.org/clinical-trials/19-712 (accessed on 14 July 2025).
- Available online: https://ichgcp.net/clinical-trials-registry/NCT04291885 (accessed on 14 July 2025).
- Orcutt, X.; Chen, K.; Mamtani, R.; Long, Q.; Parikh, R.B. Evaluating generalizability of oncology trial results to real-world patients using machine learning-based trial emulations. Nat. Med. 2025, 31, 457–465. [Google Scholar] [CrossRef]
- Gong, E.; Zawacki, L.; Fan, X.; Hippe, D.S.; Menon, A.A.; Remington, A.J.; Lachance, K.; Akaike, T.; Tachiki, L.; Park, S.Y.; et al. Immunotherapy response in immunosuppressed patients with Merkel cell carcinoma: Analysis of 183 patients. BMJ Oncol. 2025, 4, e000654. [Google Scholar] [CrossRef]
- Garza-Davila, V.F.; Valdespino-Valdes, J.; Barrera, F.J.; Ocampo-Candiani, J.; Garza-Rodríguez, V. Clinical impact of immunotherapy in Merkel cell carcinoma patients: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2022, 87, 121–130. [Google Scholar] [CrossRef]
- Bhatia, S.; Brohl, A.S.; Brownell, I.; Chandra, S.; Dakhil, S.; Ernstoff, M.S.; Fecher, L.A.; Gooley, T.; Hanna, G.J.; Hibbert, R.; et al. ADAM trial: A multicenter, randomized, double-blinded, placebo-controlled, phase 3 trial of adjuvant avelumab (anti-PD-L1 antibody) in Merkel cell carcinoma patients with clinically detected lymph node metastases; NCT03271372. J. Clin. Oncol. 2018, 36, TPS9605. [Google Scholar] [CrossRef]
- ECOG-ACRIN Cancer Research Group. Home. Available online: https://ecog-acrin.org/ (accessed on 14 July 2025).
- Patel, T.; Butz, R.; Boulmay, B.; Vaitaitis, V. Current trends in clinical trials for Merkel cell carcinoma (MCC). Cancers 2025, 17, 2340. [Google Scholar] [CrossRef]
- Available online: https://www.euracan.eu (accessed on 2 July 2025).
- Grignani, G.; Rutkowski, P.; Lebbé, C.; Guida, M.; Gaudy-Marqueste, C.; Spagnolo, F.; Burgess, M.; Morano, F.; Montaudié, H.; Depenni, R.; et al. Phase II study of retifanlimab in patients with recurrent locally advanced or metastatic Merkel cell carcinoma (POD1UM-201). J. Immunother. Cancer 2025, 13, e012478. [Google Scholar] [CrossRef]
- Bhatia, S.; Topalian, S.L.; Sharfman, W.H.; Meyer, T.; Steven, N.; Lao, C.D.; Ferris, R.L.; Kudchadkar, R.R.; Nghiem, P.; Ferris, R.L.; et al. Nivolumab with or without ipilimumab in patients with recurrent or metastatic Merkel cell carcinoma: A nonrandomized, open-label, international, multicenter phase I/II study. J. Clin. Oncol. 2025, 43, 1137–1147. Available online: https://ascopubs.org/doi/pdf/10.1200/JCO-24-02138 (accessed on 2 July 2025). [CrossRef] [PubMed]
- Available online: https://seer.cancer.gov (accessed on 2 July 2025).
- Tai, P.; Veness, M.; Prajapati, V.; Thachuthara, A.J.; Lian, J.; Assouline, A.; Yu, E.; Joseph, K. Merkel cell carcinoma: Local recurrence rate versus radiation dose study from a 949-patient database. Curr. Oncol. 2025, 32, 202. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.W.; Lebbé, C.; Grignani, G.; Nathan, P.; Dirix, L.; Fenig, E.; Ascierto, P.A.; Sandhu, S.; Munhoz, R.; Benincasa, E.; et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: Experience from a global expanded access program. J. Immunother. Cancer 2020, 8, e000313. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Updated overall survival data after >5 years of follow-up. ESMO Open 2021, 6, 100290. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J. Immunother. Cancer 2021, 9, e002478. [Google Scholar] [CrossRef]
- Farooq, M.S.; Ertmann, E.; Vargas, G.; Shafique, N.; Mitchell, T.C.; Schuchter, L.M.; Amaravadi, R.K.; Karakousis, G.C.; Miura, J. Combination ipilimumab/nivolumab for refractory Merkel cell carcinoma: A single-institution experience. J. Clin. Oncol. 2025, 43, e21528. [Google Scholar] [CrossRef]
- Kim, S.; Wuthrick, E.; Blakaj, D.; Eroglu, Z.; Verschraegen, C.; Thapa, R.; Mills, M.; Dibs, K.; Liveringhouse, C.; Russell, J.; et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2022, 400, 1008–1019. [Google Scholar] [CrossRef]
- Available online: https://www.moffitt.org/newsroom/news-releases/dual-checkpoint-inhibitor-blockade-shows-promise-as-first-line-and-salvage-therapy-for-merkel-cell-carcinoma-patients/ (accessed on 2 July 2025).
- Sakaida, E.; Ebata, T.; Iwasawa, S.; Kurimoto, R.; Yonemori, S.; Ota, S.; Nakatani, Y.; Sekine, I.; Takiguchi, Y. Potential activity of amrubicin as a salvage therapy for Merkel cell carcinoma. Intern. Med. 2017, 56, 567–570. [Google Scholar] [CrossRef]
- Oldani, S.; Prinzi, N.; Morano, F.; Cingarlini, S.; Di Giacomo, A.M.; Niger, M.; Prisciandaro, M.; Raimondi, A.; Randon, G.; Pircher, C.C.; et al. Design and rationale of the phase II PANDORA trial: First line chemo-immunotherapy in advanced Merkel cell carcinoma. Future Oncol. 2025, 21, 2127–2133. [Google Scholar] [CrossRef]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tang, Y.; Liang, X. Decoding STING’s roles in cancer: Immunity, pain, dormancy, and autophagy. Mol. Cell Biochem. 2025, 480, 4697–4723. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, T.; Jani, S.; Goff, P.H.; Bhakuni, R.; Tabachnick-Cherny, S.; Smythe, K.; Seaton, B.W.; Tachiki, L.; Kulikauskas, R.; Church, C.; et al. Intratumoral STING agonist reverses immune evasion in PD-(L)1-refractory Merkel cell carcinoma: Mechanistic insights from detailed biomarker analyses. J. Immunother. Cancer 2024, 12, e009803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, X. Targeting cGAS-STING pathway for reprogramming tumor-associated macrophages to enhance anti-tumor immunotherapy. Biomark. Res. 2025, 13, 43. [Google Scholar] [CrossRef]
- Silk, A.W.; Davar, D. Tumor-Associated Macrophages in Merkel Cell Carcinoma: Old Balances, New Checks. Clin. Cancer Res. 2024, 30, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Cianciotti, B.C.; Magnani, Z.I.; Ugolini, A.; Camisa, B.; Merelli, I.; Vavassori, V.; Potenza, A.; Imparato, A.; Manfredi, F.; Abbati, D.; et al. TIM-3, LAG-3, or 2B4 gene disruptions increase the anti-tumor response of engineered T cells. Front. Immunol. 2024, 15, 1315283. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Zhou, Y. The immunosuppressive landscape in the tumor microenvironment. Immunol. Res. 2024, 72, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.-Q.; Wei, X.-W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef]
- Wang, F.; Hao, Y. Therapeutic vaccine strategies in cancer: Advances, challenges, and future directions. Clin. Cancer Bull. 2025, 2, 63–79. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Hamzat, Y.; Alqudah, A.; Alzoubi, L. Neoantigen vaccines: Advancing personalized cancer immunotherapy. Explor. Immunol. 2025, 5, 1003190. [Google Scholar] [CrossRef]
- American Cancer Society. Immunotherapy for Merkel Cell Carcinoma (MCC). Cancer.org. Revised 10 January 2025. Available online: https://www.cancer.org/cancer/types/merkel-cell-skin-cancer/treating/immunotherapy.html (accessed on 27 July 2025).
- Chapuis, A.G.; Afanasiev, O.K.; Iyer, J.G.; Paulson, K.G.; Parvathaneni, U.; Hwang, J.H.; Lai, I.; Roberts, I.M.; Sloan, H.L.; Bhatia, S.; et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol. Res. 2014, 2, 27–36. [Google Scholar] [CrossRef]
- Saeed, A.F. Tumor-Associated Macrophages: Polarization, Immunoregulation, and Immunotherapy. Cells 2025, 14, 741. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. Available online: https://www.sciencedirect.com/science/article/pii/S0952791515001739 (accessed on 2 July 2025). [CrossRef]
- Xiang, S.; Li, S.; Xu, J. Unravelling T cell exhaustion through co-inhibitory receptors and its transformative role in cancer immunotherapy. Clin. Transl. Med. 2025, 15, e70345. [Google Scholar] [CrossRef]
- Gambichler, T.; Schrama, D.; Käpynen, R.; Weyer-Fahlbusch, S.S.; Becker, J.C.; Susok, L.; Kreppel, F.; Abu Rached, N. Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update. Vaccines 2024, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Milhem, M.M.; Sacco, J.J.; Michels, J.; In, G.K.; Muñoz Couselo, E.; Schadendorf, D.; Beasley, G.M.; Niu, J.; Chmielowski, B.; et al. RP1 Combined with Nivolumab in Advanced Anti-PD-1-Failed Melanoma (IGNYTE). J. Clin. Oncol. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Zeitouni, N.; Orloff, M. Biosafety of RP1: Considerations from a nursing perspective. Front. Mol. Biosci. 2023, 10, 1178382. [Google Scholar]
- Zheng, H.; Dai, W.; Antonia, S.J.; Cantwell, M.J.; Beg, A.A. Development of MEM-288, a dual-transgene armed and conditionally replication-enhanced oncolytic adenovirus with potent systemic antitumor immunity. Cancer Res. 2020, 80 (Suppl. S16), 4578. [Google Scholar] [CrossRef]
- Porter, D.L.; Maloney, D.G. Cytokine Release Syndrome (CRS). UpToDate. Updated 2 April 2024. Literature Review Current Through Jun 2025. Available online: https://www.uptodate.com/contents/cytokine-release-syndrome-crs (accessed on 2 July 2025).
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Mc Neil, V.; Lee, S.W. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers 2025, 17, 1408. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). A randomized Phase II Study of Anti–PD-1 Antibody [MK-3475 (Pembrolizumab)] Alone Versus Anti–PD-1 Antibody Plus Stereotactic Body Radiation Therapy in Advanced Merkel Cell Carcinoma. ClinicalTrials.gov. Updated 20 April 2025. Available online: https://clinicaltrials.gov/study/NCT03304639 (accessed on 2 July 2025).
- Chemo-Immunotherapy in Patients with Resectable Merkel Cell Carcinoma Prior to Surgery (MERCURY). ClinicalTrials.gov. Updated 11 June 2025. Available online: https://clinicaltrials.gov/study/NCT05594290 (accessed on 2 July 2025).
- Available online: https://clinicaltrials.gov/study/NCT04261855 (accessed on 2 July 2025).
- Everolimus and Lutetium Lu 177 Dotatate in Metastatic Midgut Neuroendocrine Tumors (NET RETREAT/iPRRT). ClinicalTrials.gov. Updated 9 July 2025. Available online: https://clinicaltrials.gov/study/NCT05773274 (accessed on 2 July 2025).
- Starrett, G.J.; Thakuria, M.; Chen, T.; Marcelus, C.; Cheng, J.; Nomburg, J.; Thorner, A.R.; Slevin, M.K.; Powers, W.; Burns, R.T.; et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med. 2020, 12, 30. [Google Scholar] [CrossRef]
- Akaike, T.; Thakuria, M.; Silk, A.W.; Hippe, D.S.; Park, S.Y.; So, N.A.; Maloney, N.J.; Gunnell, L.; Eschholz, A.; Kim, E.Y.; et al. Circulating tumor DNA assay detects Merkel cell carcinoma recurrence, disease progression, and minimal residual disease: Surveillance and prognostic implications. J. Clin. Oncol. 2024, 42, 3151–3161. [Google Scholar] [CrossRef]
- Alnaeem, M.M.; Al Qadire, M.; Nashwan, A.J. Unmet needs, burden, and quality of life among family caregivers of patients with advanced hematological malignancy. Psychol. Health Med. 2025, 30, 1573–1588. [Google Scholar] [CrossRef]
- Al-Ghabeesh, S.H.; Al-Kalaldah, M.; Rayyan, A.; Al-Rifai, A.; Al-Halaiqa, F. Psychological distress and quality of life among Jordanian women diagnosed with breast cancer: The role of trait mindfulness. Eur. J. Cancer Care 2019, 28, e13082. [Google Scholar] [CrossRef] [PubMed]
- Fazio, L.; Brock, G. Erectile dysfunction: Management update. CMAJ 2004, 170, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clinical Data Summary for Eroxon® Gel. Updated 2025. Available online: https://eroxon.com/healthcare-professionals/clinical-data (accessed on 2 July 2025).
- Ralph, D.J.; Eardley, I.; Taubel, J.; Terrill, P.; Holland, T. Efficacy and Safety of MED2005, a Topical Glyceryl Trinitrate Formulation, in the Treatment of Erectile Dysfunction: A Randomized Crossover Study. J. Sex. Med. 2018, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, W.J.G.; Brockhors, G.B.; Burnett, A.L.; Holland, T.J.; James, K.W. PD52-07: Efficacy and safety of MED3000, a novel topical therapy for the treatment of erectile dysfunction. J. Urol. 2024, 211 (Suppl. S4), e1234. [Google Scholar] [CrossRef]
- Ahmad, M.; Esmeiran, H.; Alafafsheh, A.; Al-Ghabeesh, S.; Al-Hamdan, Z. Beliefs and preferred communication channels toward patients with cancer in Jordan. World Cancer Res. J. 2016, 3, e753. [Google Scholar]
- Rayan, A.; Al-Ghabeesh, S.; Qarallah, I. Critical Care Nurses’ Attitudes, Roles, and Barriers Regarding Breaking Bad News. SAGE Open Nurs. 2022, 8, 23779608221089999. [Google Scholar] [CrossRef]
- Saifan, A.; Bashayreh, I.; Al-Ghabeesh, S.; Batiha, A.; Alrimawi, I.; Al-Saraireh, M.; Al-Momani, M. Exploring Factors that inhibit effective pain management in cancer patients among healthcare professionals. Cent. Eur. J. Nurs. Midwifery 2019, 10, 967–976. [Google Scholar] [CrossRef]
- Al-Ghabeesh, S.H.; Abu-Moghli, F.; Salsali, M.; Saleh, M. Exploring sources of knowledge utilized by Jordanian registered nurses. J. Eval. Clin. Pract. 2013, 19, 889–894. [Google Scholar] [CrossRef]
| References | Setting | Intervention | Outcome/Results | Keypoints |
|---|---|---|---|---|
| Neoadjuvant | ||||
| Topalian S.L. (2020) [13] | Neoadjuvant | Nivolumab | 50–60% pCR in resectable MCC. Significant tumor downsizing | Neoadjuvant IO can achieve high rates of pCR, potentially simplify surgery and improve outcomes in localized disease |
| Bhatia S. (2020) [14] | Neoadjuvant (cohort A) | Intratumoral 1L-12 plasmids DNA via electroporation (tavo-EP) | Objective response in injected and non-injected tumors. Demonstrates initial safety/efficacy in early-stage MCC | Intratumoral IO is a promising approach for inducing local and systemic anti-tumor responses, relevant for neoadjuvant strategies |
| Kuchimanchi N. (2025) [15] | Neoadjuvant | Preoperative nivolumab induced rare dysautonomia treated with IVIG | 57-year-old patient developed dysautonomia (orthostatic hypotension, urinary retention, hearing loss, diplopia) after 2 cycles. IVIG markedly improved neurological symptoms. It highlights both the efficacy of neoadjuvant ICI and the need to manage uncommon severe side effects. | A very rare toxicity of ICI CC. Importance of vigilant monitoring for irAEs and rapid intervention. Early IVIG treatment in this case reversed the dysautonomia, suggesting that timely diagnosis and treatment of irAEs can allow patients to continue benefiting from ICI without lasting harm. |
| Correa Roa C. (2025) [16] | Neoadjuvant for loco-regional recurrence | Preoperative nivolumab for 6 cycles | 80-year-old patient with arm lesion and axillary nodes. Surgery found only scar tissue: pCR in tumor bed or nodes, and no further recurrence after resection | High immunologic sensitivity of MCC: even aggressive recurrence achieves pCR; “revolutionizing” treatment for advanced and recurrent MCC. |
| Adjuvant | ||||
| Becker J.C. (2023) [17,18] | Adjuvant (ADMEC-O) | Nivolumab vs. observation | Improved DFS after complete resection of MCC, reduces recurrence. OS results not mature yet | Significantly improves DFS in resected MCC, establishing a new SOC for high-risk patients. Support further adjuvant trials. |
| Primary and salvage therapy in advMCC: see the text for more details. | ||||
| D’Angelo S.P. (2021) [19] | Primary/salvage | Avelumab | Updated OS data > 5 years: durable responses, with many long-term responders | Avelumab: long term survival benefits in mMCC, including previously treated patients |
| Nghiem P.T. (2016) [10] | Primary/salvage | Pembrolizumab | 56% ORR with durable responses. First evidence for PD-1 blockade in advMCC | Pembrolizumab is highly effective in advMCC, as new treatment option |
| Gaiser M.R. (2018) [20] | Primary/salvage | Avelumab (review) | ~33% ORR in refractory disease, <60% in treatment-naïve cases; avelumab is safe | Avelumab is cornerstone of metastatic MCC treatment; effective in both 1L and refractory settings |
| D’Angelo S.P. (2021) [21] | Primary | Avelumab (1L) | 4-year FU: sustained responses and long-term OS in 1L treatment of mMCC | Avelumab as 1L therapy: durable responses and prolonged survival |
| Kaufman H.L. (2016, 2018) [22,23] | Salvage | Avelumab (previously treated) | Updated efficacy results after ≥1 year FU up confirmed durable responses in chemo-refractory patients | Avelumab offers durable responses for progression after prior chemotherapy |
| Shirley M. (2018) [24] | Primary/salvage | Avelumab | Avelumab approval, efficacy and safety profile in metMCC | Reaffirms its role as the first approved IO drug for MCC, effective across treatment lines |
| D’Angelo S.P. (2020) [25] | Salvage | Avelumab (previously treated) | Long-term data and biomarker analyses: durable responses and insights into response predictors | Data supports avelumab benefits, with potential for biomarker-guided therapy in salvage settings |
| D’Angelo S.P. (2018) [26] | Primary | Avelumab (1L) | Interim analysis: ~62% ORR and manageable safety profile in 1L setting. | Avelumab is an effective and safe 1L option for metMCC |
| Nghiem P. (2019) [27] | Primary | Pembrolizumab (1L) | Durable tumor regression, improved OS as 1L therapy in advMCC | Pembrolizumab offers durable benefits as 1L treatment for adv MCC. |
| D’Angelo S.P. (2021) [26] | Primary ICI metastatic disease | Avelumab (1L) | 39 patients, primary and biomarker analyses of 1L avelumab, showing high ORR/DOR | Detailed insight into 1L avelumab efficacy and potential biomarkers for responses |
| D’Angelo S.P. (2025) [28] | Salvage (progress post IO) | Management strategies post-PD-L1 progression | Discuss clinical outcomes and management for disease progression after initial IO | Crucial for understanding next steps and “salvage use” after primary IO failure |
| Mo J. (2025) [28] | Same as above | Same as above | Same as above | Same as above |
| General/contextual reviews: Topalian S.L. (2012 [29], 2015 [30]) were excluded after critical appraisal since they are not specifically for MCC. | ||||
| Lebbé C. et al. (2015) [31] | General MCC treatment | European consensus guideline | Covers diagnosis, treatment, and evolving role of systemic therapy | Includes the integration of IO into overall treatment regimen |
| Aquino de Moraes F. (2024) [9] | Same as above | ICI systemic review and metaanalysis | Efficacy and safety of ICI | Confirms the overall efficacy and safety of ICI |
| References | Settings | Intervention | Outcome/Results | Key Points |
|---|---|---|---|---|
| Nyhiem P. (2021) [50] | Multicenter phase II trial (CITN-09/KEYNOTE-017), US; 50 patients with advanced unresectable/metastatic MCC. Median follow-up 31.8 months. | First line pembrolizumab (2 mg/kg IV every 3 weeks, up to 2 years) | ORR: 58% (30% CR, 28% PR). Median PFS: 16.8 months, 3-years PFS: 39.1%, 3-years OS: 59.4% overall; 89.5% in responders. Median OS not reached. Salvage therapies (chemo/ICI) extended survival in some resistant/relapsed patients. | Longest follow-up for first-line PD-1 blockade. High/durable response rates; most responses persisted ≥3 years. ECOG 0, greater tumor shrinkage, completion of 2 years, and low NLR linked to better survival. Pembrolizumab markedly outperforms historical chemotherapy outcomes. |
| Oldani S. (2025) [55] | Italy (multicenter- open-label phase II PANDORA trial led by National Cancer Institute, Milan) (NCT06086288) | First line combination chemo-immunotherapy (pembrolizumab platinum [cisplatin/carboplatin] +etoposide) in advanced MCC | In progress, evaluating whether adding chemotherapy to upfront immunotherapy improves outcomes in metastatic MCC. Primary endpoint is objective response rate; secondary endpoint includes OS, PFS, DOR. Plan to enroll 35 patients. Rationale: a significant subset of patients develop resistance to single-agent PD-1/PD-L1 therapy, so combining it with chemo (for its quick tumor shrinkage effect) may yield higher response rates and longer control. | Addresses a key unmet need in MCC treatment: improving first line efficacy for those not responding to IO alone. By leveraging the potential synergy of chemo-immunotherapy, PANDAORA aims to enhancing initial tumor response and possibly survival, compared to either modality alone. If successful, this combo approach could redefine the therapeutic standard for advMCC, offering new hopes for patients who historically had limited options. |
| Grignani G. (2025) [45] | Multiregional, open label single-arm phase II trial; 34 sites across USA, Canada, and Europe. Patients ≥ 18 y with recurrent locally advanced or metastatic MCC, chemotherapy—naïve, ECOG 0–1. | Retifanlimab 500 mg IV every 4 weeks (Q4W), for up to 2 years. | 101 patients enrolled (2019–2021). ORR 54.5% (CR 17.8%, PR 36.6%); DCR 60.4%. median DOR: not reached (CR)/25.3 months (PR). Median PFS: 16.0 months; median OS: not reached, 63% alive at 3 years. Safety: grade ≥ 3 immune-related AEs in 10.9%. | Retifanlimab produced frequent, durable responses in advanced MCC, with survival benefit and manageable safety. Represents a new PD-1 inhibitor option for chemotherapy—naïve MCC patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, P.; Alqaisi, O.; Al-Ghabeesh, S.; Sijarina, L.; Yu, E.; Thachuthara, A.J.; Assouline, A.; Souied, O.; Hagel, K.; Joseph, K. Immune Checkpoint Inhibitors in Merkel Cell Carcinoma of the Skin: A 2025 Comprehensive Review. Cancers 2025, 17, 3272. https://doi.org/10.3390/cancers17193272
Tai P, Alqaisi O, Al-Ghabeesh S, Sijarina L, Yu E, Thachuthara AJ, Assouline A, Souied O, Hagel K, Joseph K. Immune Checkpoint Inhibitors in Merkel Cell Carcinoma of the Skin: A 2025 Comprehensive Review. Cancers. 2025; 17(19):3272. https://doi.org/10.3390/cancers17193272
Chicago/Turabian StyleTai, Patricia, Omar Alqaisi, Suhair Al-Ghabeesh, Lorent Sijarina, Edward Yu, Aoife Jones Thachuthara, Avi Assouline, Osama Souied, Kimberly Hagel, and Kurian Joseph. 2025. "Immune Checkpoint Inhibitors in Merkel Cell Carcinoma of the Skin: A 2025 Comprehensive Review" Cancers 17, no. 19: 3272. https://doi.org/10.3390/cancers17193272
APA StyleTai, P., Alqaisi, O., Al-Ghabeesh, S., Sijarina, L., Yu, E., Thachuthara, A. J., Assouline, A., Souied, O., Hagel, K., & Joseph, K. (2025). Immune Checkpoint Inhibitors in Merkel Cell Carcinoma of the Skin: A 2025 Comprehensive Review. Cancers, 17(19), 3272. https://doi.org/10.3390/cancers17193272









