Understanding the Will Rogers Phenomenon in Cholangiocarcinoma Research and Beyond
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Case Definitions
2.2. Data Source and Population
2.3. Outcome Assessment
2.4. Variable Selection
2.5. Statistical Analysis
2.6. Reclassification Analysis
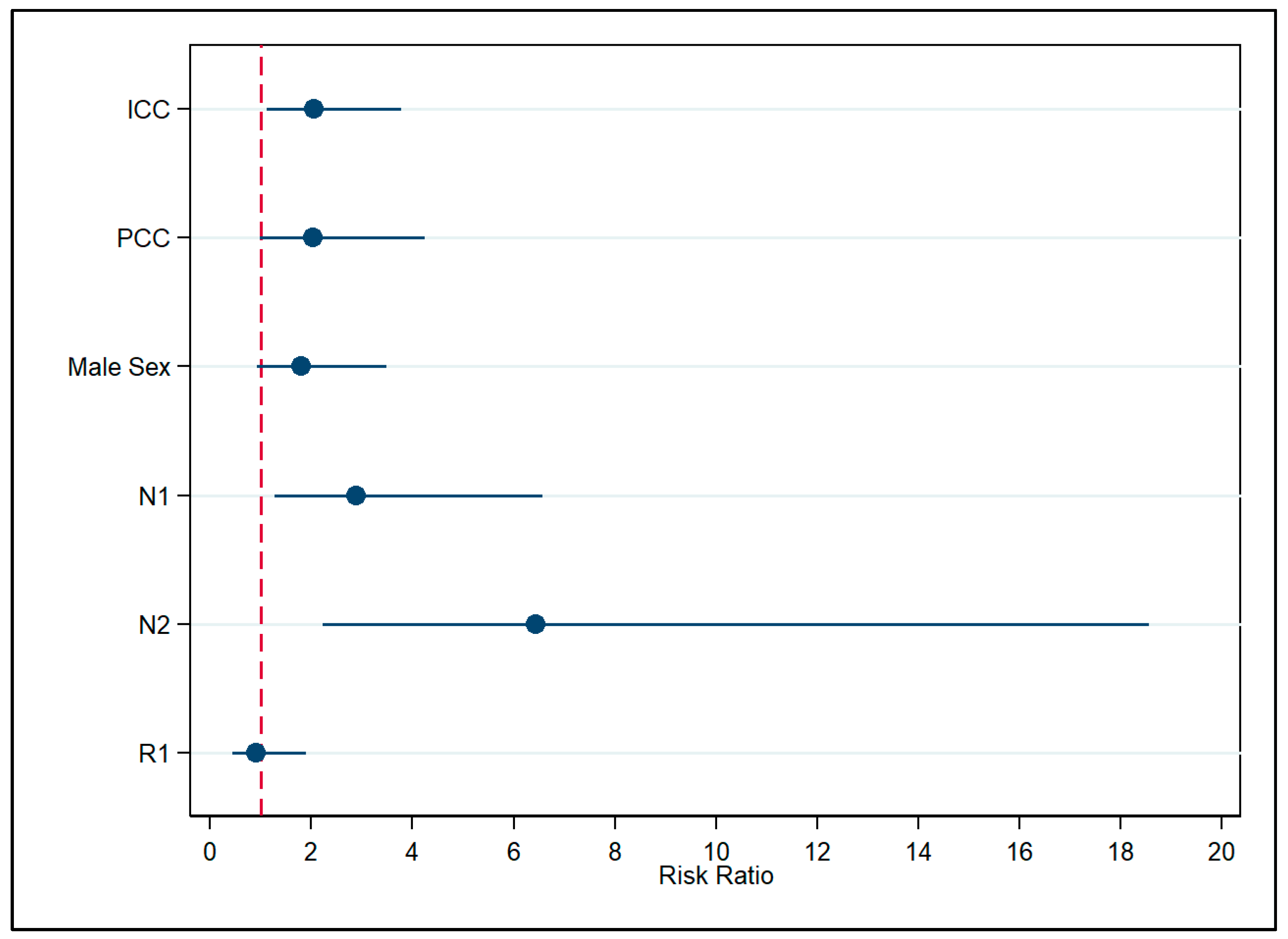
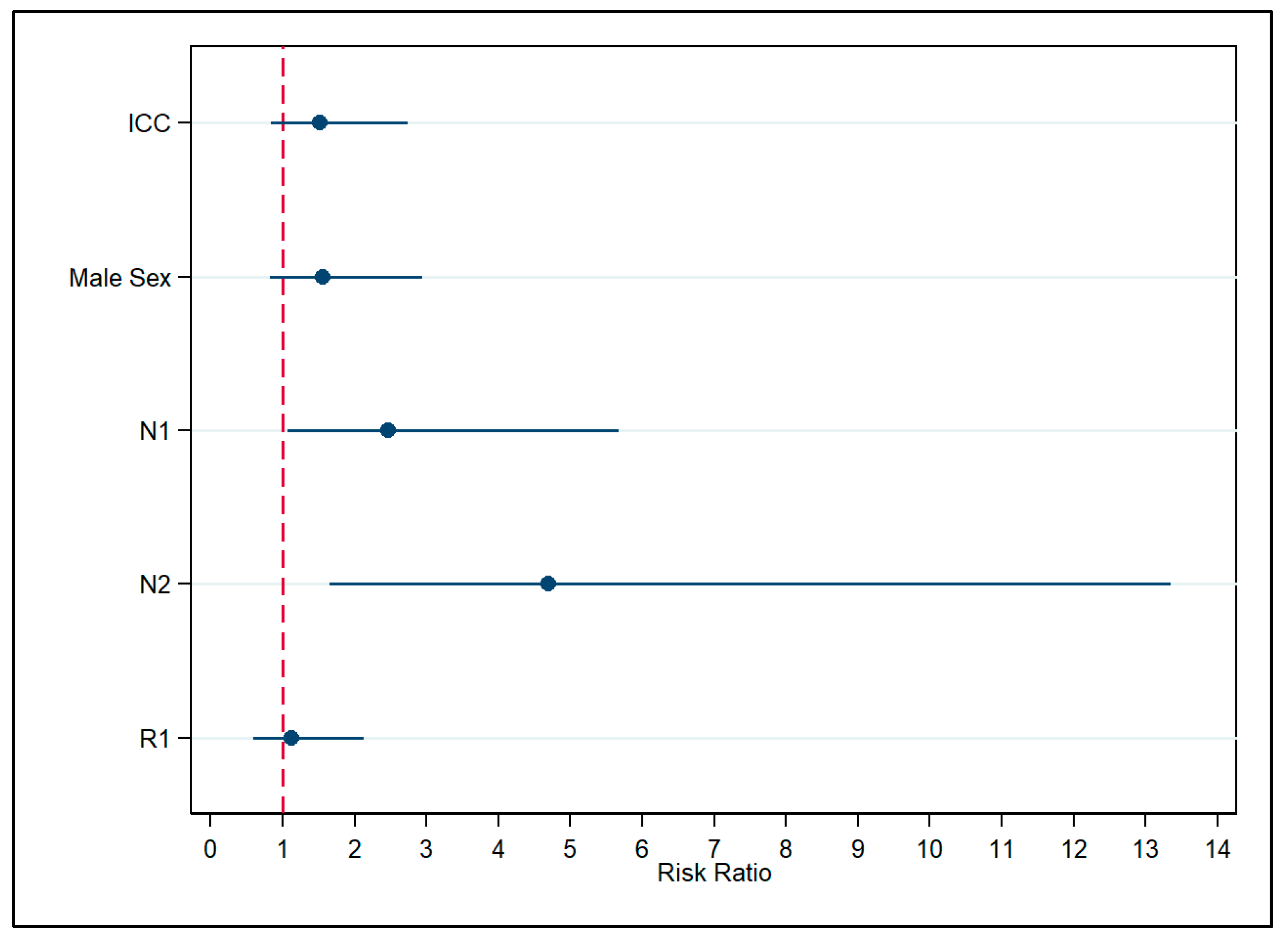
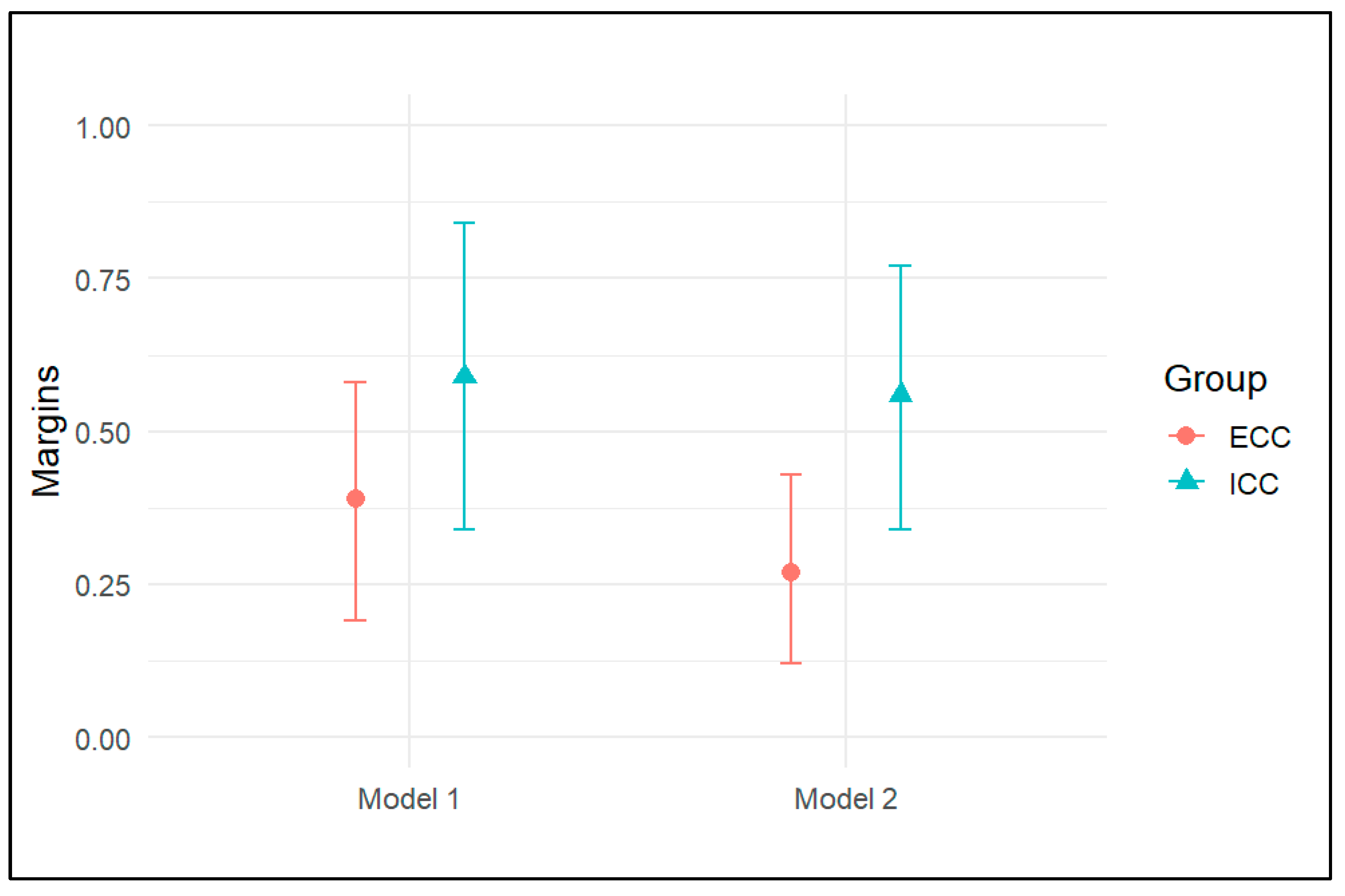
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| AJCC | American Joint Committee on Cancer |
| BIC | Bayesian Information Criterion |
| CC | Cholangiocarcinoma |
| CI | Confidence Interval |
| DCC | Distal Cholangiocarcinoma |
| ECC | Extrahepatic Cholangiocarcinoma |
| EPV | Event-per-variable |
| ICC | Intrahepatic Cholangiocarcinoma |
| IQR | Interquartile Range |
| LNM | Lymph Node Metastasis |
| NROC | National Research Oncology Center |
| PCC | Perihilar Cholangiocarcinoma |
| PTBD | Percutaneous Transhepatic Biliary Drainage |
| RR | Risk Ratio |
| SE | Standard Error |
| TNM | Tumor Nodes Metastases |
| VIF | Variance Inflation Factor |
| WRP | Will Rogers Phenomenon |
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Jiang, K.; Al-Diffhala, S.; Centeno, B.A. Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification. Cancer Control 2018, 25, 1073274817744625. [Google Scholar] [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Klatskin, G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am. J. Med. 1965, 38, 241–256. [Google Scholar] [CrossRef]
- Walter, D.; Ferstl, P.; Waidmann, O.; Trojan, J.; Hartmann, S.; Schnitzbauer, A.A.; Zeuzem, S.; Kraywinkel, K. Cholangiocarcinoma in Germany: Epidemiologic trends and impact of misclassification. Liver Int. 2019, 39, 316–323. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. 1), 19–31. [Google Scholar] [CrossRef]
- Calomino, N.; Carbone, L.; Kelmendi, E.; Piccioni, S.A.; Poto, G.E.; Bagnacci, G.; Resca, L.; Guarracino, A.; Tripodi, S.; Barbato, B.; et al. Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center. Medicina 2025, 61, 860. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Ferreccio, C.; Adsay, V.; Soerjomataram, I.; Bray, F.; Koshiol, J. Gallbladder and extrahepatic bile duct cancers in the Americas: Incidence and mortality patterns and trends. Int. J. Cancer 2020, 147, 978–989. [Google Scholar] [CrossRef]
- Abioye, O.F.; Kaufman, R.; Greten, T.F.; Monge, C. Disparities in Cholangiocarcinoma Research and Trials: Challenges and Opportunities in the United States. JCO Glob. Oncol. 2025, 11, e2400537. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Ribero, D.; Pinna, A.D.; Guglielmi, A.; Ponti, A.; Nuzzo, G.; Giulini, S.M.; Aldrighetti, L.; Calise, F.; Gerunda, G.E.; Tomatis, M.; et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch. Surg. 2012, 147, 1107–1113. [Google Scholar] [CrossRef]
- Zhang, X.F.; Beal, E.W.; Bagante, F.; Chakedis, J.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br. J. Surg. 2018, 105, 848–856. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [CrossRef]
- Clark, C.J.; Wood-Wentz, C.M.; Reid-Lombardo, K.M.; Kendrick, M.L.; Huebner, M.; Que, F.G. Lymphadenectomy in the staging and treatment of intrahepatic cholangiocarcinoma: A population-based study using the National Cancer Institute SEER database. HPB 2011, 13, 612–620. [Google Scholar] [CrossRef]
- Sun, G.W.; Shook, T.L.; Kay, G.L. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J. Clin. Epidemiol. 1996, 49, 907–916. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef]
- Knol, M.J.; Vandenbroucke, J.P.; Scott, P.; Egger, M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am. J. Epidemiol. 2008, 168, 1073–1081. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Optimal approximate conversions of odds ratios and hazard ratios to risk ratios. Biometrics 2020, 76, 746–752. [Google Scholar] [CrossRef]
- Mitani, A.A.; Haneuse, S. Small Data Challenges of Studying Rare Diseases. JAMA Netw. Open 2020, 3, e201965. [Google Scholar] [CrossRef]
- Barros, A.J.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to obtain the P value from a confidence interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef]
- Khan, S.A.; Emadossadaty, S.; Ladep, N.G.; Thomas, H.C.; Elliott, P.; Taylor-Robinson, S.D.; Toledano, M.B. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J. Hepatol. 2012, 56, 848–854. [Google Scholar] [CrossRef]
- Hang, H.; Jeong, S.; Sha, M.; Kong, D.; Xi, Z.; Tong, Y.; Xia, Q. Cholangiocarcinoma: Anatomical location-dependent clinical, prognostic, and genetic disparities. Ann. Transl. Med. 2019, 7, 744. [Google Scholar] [CrossRef]
- Shimada, K.; Sano, T.; Sakamoto, Y.; Esaki, M.; Kosuge, T.; Ojima, H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: A comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J. Surg. 2007, 31, 2016–2022. [Google Scholar] [CrossRef]
- Hong, S.M.; Pawlik, T.M.; Cho, H.; Aggarwal, B.; Goggins, M.; Hruban, R.H.; Anders, R.A. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 2009, 146, 250–257. [Google Scholar] [CrossRef]
- Hussain, M.M.; Wang, J.M.; Zhai, A.Q.; Li, F.Y.; Hu, H.J. Comparison of prognostic factors and their differences in intrahepatic, hilar, and distal cholangiocarcinoma: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2025, 17, 107995. [Google Scholar] [CrossRef]
- Waseem, D.; Tushar, P. Intrahepatic, perihilar and distal cholangiocarcinoma: Management and outcomes. Ann. Hepatol. 2017, 16, 133–139. [Google Scholar] [CrossRef]
- Jiang, J.H.; Fang, D.Z.; Hu, Y.T. Influence of surgical margin width on survival rate after resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. BMJ Open 2023, 13, e067222. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Matsuyama, Y.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Nakashima, O.; Kudo, M. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: A nationwide survey of the Liver Cancer Study Group of Japan. Surgery 2020, 167, 793–802. [Google Scholar] [CrossRef]
- Ercolani, G.; Dazzi, A.; Giovinazzo, F.; Ruzzenente, A.; Bassi, C.; Guglielmi, A.; Scarpa, A.; D’Errico, A.; Pinna, A.D. Intrahepatic, peri-hilar and distal cholangiocarcinoma: Three different locations of the same tumor or three different tumors? Eur. J. Surg. Oncol. 2015, 41, 1162–1169. [Google Scholar] [CrossRef]
- Liu, W.W.; Tu, J.F.; Ying, X.H.; Chen, Z.J.; Wang, Y.B. Postoperative survival of extrahepatic and intrahepatic cholangiocarcinoma after surgery: A population-based cohort. BMJ Open 2022, 12, e049789. [Google Scholar] [CrossRef]
- Mukkamalla, S.K.R.; Naseri, H.M.; Kim, B.M.; Katz, S.C.; Armenio, V.A. Trends in Incidence and Factors Affecting Survival of Patients With Cholangiocarcinoma in the United States. J. Natl. Compr. Canc Netw. 2018, 16, 370–376. [Google Scholar] [CrossRef]
- Guglielmi, A.; Ruzzenente, A.; Campagnaro, T.; Pachera, S.; Valdegamberi, A.; Capelli, P.; Pedica, F.; Nicoli, P.; Conci, S.; Iacono, C. Does intrahepatic cholangiocarcinoma have better prognosis compared to perihilar cholangiocarcinoma? J. Surg. Oncol. 2010, 101, 111–115. [Google Scholar] [CrossRef]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.R.; Sosin, D.M.; Wells, C.K. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N. Engl. J. Med. 1985, 312, 1604–1608. [Google Scholar] [CrossRef]
- Albertsen, P.C.; Hanley, J.A.; Barrows, G.H.; Penson, D.F.; Kowalczyk, P.D.; Sanders, M.M.; Fine, J. Prostate cancer and the Will Rogers phenomenon. J. Natl. Cancer Inst. 2005, 97, 1248–1253. [Google Scholar] [CrossRef]
- Nittala, M.R.; Mundra, E.K.; Packianathan, S.; Mehta, D.; Smith, M.L.; Woods, W.C.; McKinney, S.; Craft, B.S.; Vijayakumar, S. The Will Rogers phenomenon, breast cancer and race. BMC Cancer 2021, 21, 554. [Google Scholar] [CrossRef] [PubMed]

| Covariate | Total (n = 39, 100.0%) | ICC (n = 13, 33.3%) | DCC (n = 14, 35.9%) | PCC (n = 12, 30.8%) | p-Value |
|---|---|---|---|---|---|
| Age | 63 (IQR: 54–69) | 62 (IQR: 59–66) | 60 (IQR: 51–70) | 61 (IQR: 51–65) | 0.84 |
| Sex | 1.0 | ||||
| Female | 16 (41.1%) | 5 (38.6%) | 6 (42.6%) | 5 (41.7%) | |
| Male | 23 (58.9%) | 8 (61.4%) | 8 (57.4%) | 7 (58.3%) | |
| LNM | 0.21 | ||||
| N0 | 17 (43.6%) | 8 (61.5%) | 3 (21.4%) | 6 (50.0%) | |
| N1 | 19 (48.7%) | 5 (38.5%) | 9 (64.3%) | 5 (41.7%) | |
| N2 | 3 (7.7%) | 0 (0.00%) | 2 (14.3%) | 1 (8.3%) | |
| Surgical margin | 0.04 | ||||
| R0 | 24 (61.4%) | 11 (84.6%) | 9 (64.3%) | 4 (33.3%) | |
| R1 | 15 (38.6%) | 2 (15.4%) | 5 (35.7%) | 8 (66.7%) | |
| PTBD | 0.01 | ||||
| No | 21 (53.8%) | 12 (92.3%) | 5 (35.7%) | 4 (33.3%) | |
| Yes | 18 (46.2%) | 1 (7.7%) | 9 (64.3%) | 8 (66.7%) | |
| Survival state | 0.78 | ||||
| Alive | 19 (48.7%) | 6 (46.2%) | 8 (57.1%) | 5 (41.7%) | |
| Dead | 20 (51.3%) | 7 (53.8%) | 6 (42.9%) | 7 (58.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmedullin, R.; Burkitbayev, Z.; Koishibayev, T.; Spatayev, Z.; Sharmenov, A.; Shatkovskaya, O.; Zharlyganova, D.; Manatova, A.; Kuanysh, Z.; Shalekenov, S.; et al. Understanding the Will Rogers Phenomenon in Cholangiocarcinoma Research and Beyond. Cancers 2025, 17, 3263. https://doi.org/10.3390/cancers17193263
Akhmedullin R, Burkitbayev Z, Koishibayev T, Spatayev Z, Sharmenov A, Shatkovskaya O, Zharlyganova D, Manatova A, Kuanysh Z, Shalekenov S, et al. Understanding the Will Rogers Phenomenon in Cholangiocarcinoma Research and Beyond. Cancers. 2025; 17(19):3263. https://doi.org/10.3390/cancers17193263
Chicago/Turabian StyleAkhmedullin, Ruslan, Zhandos Burkitbayev, Tair Koishibayev, Zhanat Spatayev, Abylaikhan Sharmenov, Oxana Shatkovskaya, Dinara Zharlyganova, Almira Manatova, Zhuldyz Kuanysh, Sanzhar Shalekenov, and et al. 2025. "Understanding the Will Rogers Phenomenon in Cholangiocarcinoma Research and Beyond" Cancers 17, no. 19: 3263. https://doi.org/10.3390/cancers17193263
APA StyleAkhmedullin, R., Burkitbayev, Z., Koishibayev, T., Spatayev, Z., Sharmenov, A., Shatkovskaya, O., Zharlyganova, D., Manatova, A., Kuanysh, Z., Shalekenov, S., & Gaipov, A. (2025). Understanding the Will Rogers Phenomenon in Cholangiocarcinoma Research and Beyond. Cancers, 17(19), 3263. https://doi.org/10.3390/cancers17193263






