Optimizing Anti-PD1 Immunotherapy: An Overview of Pharmacokinetics, Biomarkers, and Therapeutic Drug Monitoring
Abstract
Simple Summary
Abstract
1. Introduction
2. Pharmacokinetics of Anti-PD-1
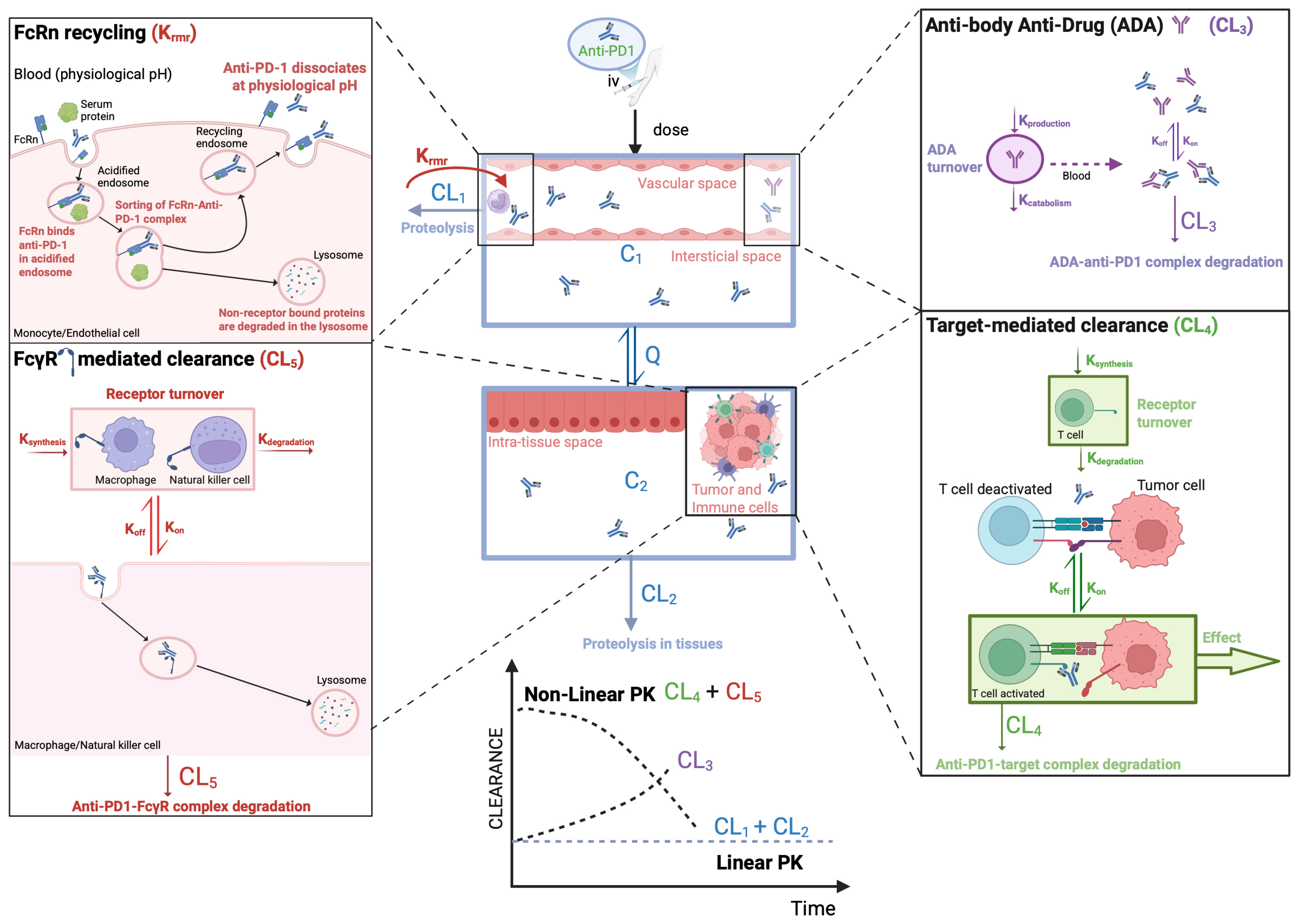
2.1. Disposition Mechanisms of Anti-PD-1 mAbs
2.1.1. Target-Mediated Drug Disposition
2.1.2. Non-Selective Mechanisms
Neonatal Fc Receptor
Gamma Fc Receptor
Tissue Proteolysis
Autoantibodies
2.1.3. Anti-Drug Antibodies
2.2. Pharmacokinetic Models
2.3. Factors Influencing Pharmacokinetics of Anti-PD-1
2.3.1. Albumin and Cachexia
2.3.2. Disease Burden
3. Pharmacokinetic–Pharmacodynamic Relations
3.1. Exposure–Efficacy Relations
3.2. Exposure–Safety Relations
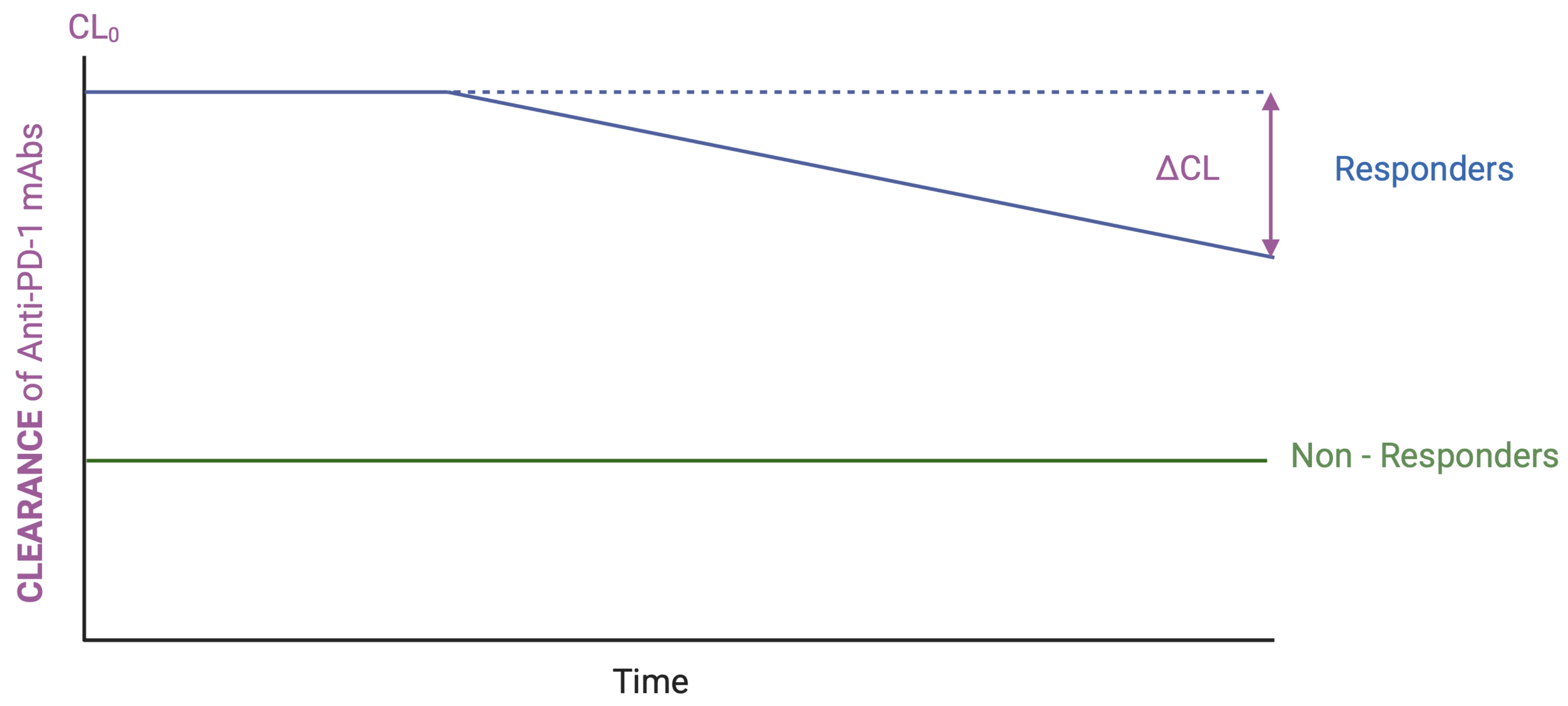
3.3. Clearance–Efficacy and/or Safety Relationships
4. Biomarkers for Anti-PD-1 Therapies
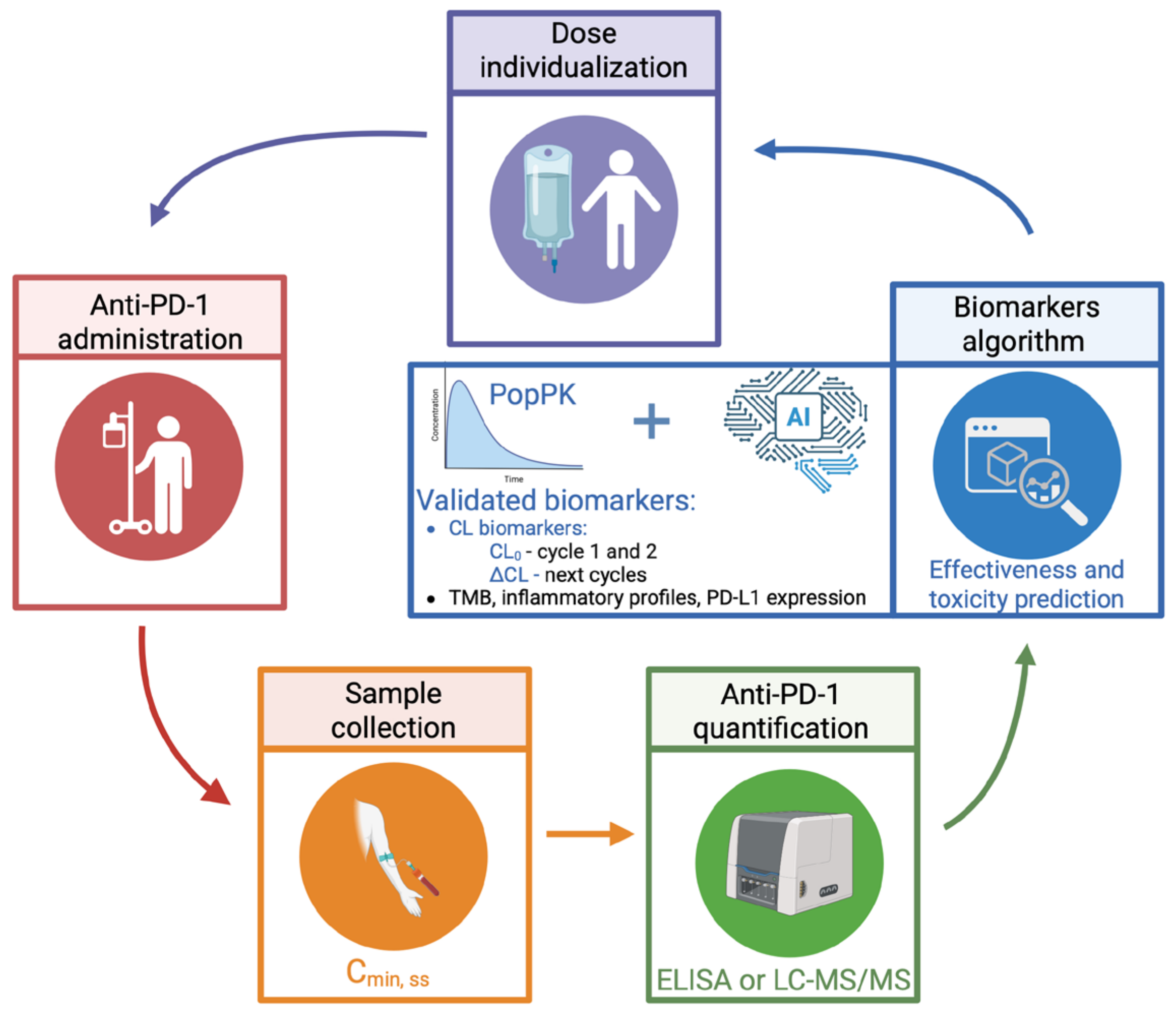
5. Rethinking Dosing Strategies for Anti-PD-1: The Role of TDM
6. Clinical Implications and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADAs | Anti-drug antibodies |
| ADCC | Antibody-dependent cellular citotoxicity |
| ALB | Albumin |
| Anti-TIM3 | Anti-T cell mucin domain 3 |
| Anti-TIGIT | Anti-T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains |
| AUC | Area under plasmatic concentration versus time curve |
| BW | Body weight |
| CL | Clearance |
| CL0 | Baseline clearance |
| CL–R | Clearance–response |
| Cmin, ss | Steady-state trough concentrations |
| CTLA4 | Cytotoxic T- lymphocyte-associated antigen 4 |
| DCs | Dendritic cells |
| ELISA | Enzyme-linked immunosorbent assay |
| ECM | Extracellular matrix |
| E–R | Exposure–Response |
| FcRn | Neonatal Fc receptor |
| FcRs | Fc receptors |
| FcγR | Gamma Fc receptor |
| ICIs | Immune checkpoint inhibitors |
| IFN-γ | Gamma-interferon |
| Ig | Immunoglobulin |
| IL-1 | Interleukin 1 |
| irAEs | Immune-related adverse events |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| mAbs | Monoclonal antibodies |
| MMP | Matrix metalloproteinases |
| NK | Natural killer |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PB | Peripheral blood |
| PBPK | Physiologically based pharmacokinetic |
| PD | Pharmacodynamics |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PK | Pharmacokinetics |
| PK–PD | Pharmacokinetic–Pharmacodynamic |
| PopPK | Population pharmacokinetics |
| PS | Performance status |
| TDM | Therapeutic drug monitoring |
| TMB | Tumor mutational burden |
| TMDD | Target-mediated drug disposition |
| TNF-α | Tumor necrosis factor-alpha |
| Vd | Volume of distribution |
| ΔCL | Change in clearance over time |
References
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Aksoylar, H.-I.; Le Bourgeois, T.; Strauss, L.; Weaver, J.D.; Delcuze, B.; Charest, A.; Patsoukis, N.; Boussiotis, V.A. Publisher Correction: Phosphorylation of PD-1-Y248 is a marker of PD-1-mediated inhibitory function in human T cells. Sci. Rep. 2020, 10, 15905. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572. [Google Scholar] [CrossRef]
- Yao, S.; Wang, S.; Zhu, Y.; Luo, L.; Zhu, G.; Flies, S.; Xu, H.; Ruff, W.; Broadwater, M.; Choi, I.-H.; et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 2009, 113, 5811–5818. [Google Scholar] [CrossRef]
- Huang, X.; Venet, F.; Wang, Y.L.; Lepape, A.; Yuan, Z.; Chen, Y.; Swan, R.; Kherouf, H.; Monneret, G.; Chung, C.-S.; et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 6303–6308. [Google Scholar]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Wei, F.; Zhong, S.; Ma, Z.; Kong, H.; Medvec, A.; Ahmed, R.; Freeman, G.J.; Krogsgaard, M.; Riley, J.L. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc. Natl. Acad. Sci. USA 2013, 110, E2480–E2489. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Mueller, D.L. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010, 11, 21–27. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, S.H.; Heo, Y.S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24, 1190. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Shang, B.; Sun, J.; Liu, X.; Su, L.; Jiang, S. Mechanistic insight of predictive biomarkers for antitumor PD-1/PD-L1 blockade: A paradigm shift towards immunome evaluation (Review). Oncol. Rep. 2020, 44, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, L.; Li, M.; Hou, X.; Zhang, B.; Chen, J.; Li, S.; Chen, L. Clinical efficacy and safety of individualized pembrolizumab administration based on pharmacokinetic in advanced non-small cell lung cancer: A prospective exploratory clinical trial. Lung Cancer 2023, 178, 183–190. [Google Scholar] [CrossRef]
- Desnoyer, A.; Broutin, S.; Delahousse, J.; Maritaz, C.; Blondel, L.; Mir, O.; Chaput, N.; Paci, A. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 2, immune checkpoint inhibitor antibodies. Eur. J. Cancer 2020, 128, 119–128. [Google Scholar] [CrossRef]
- Freshwater, T.; Kondic, A.; Ahamadi, M.; Li, C.H.; de Greef, R.; de Alwis, D.; Stone, J.A. Evaluation of dosing strategy for pembrolizumab for oncology indications. J. Immunother. Cancer 2017, 5, 43. [Google Scholar] [CrossRef]
- Ahamadi, M.; Freshwater, T.; Prohn, M.; Li, C.; de Alwis, D.; de Greef, R.; Elassaiss-Schaap, J.; Kondic, A.; Stone, J. Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 49–57. [Google Scholar] [CrossRef]
- Peer, C.J.; Goldstein, D.A.; Goodell, J.C.; Nguyen, R.; Figg, W.D.; Ratain, M.J. Opportunities for using in silico—Based extended dosing regimens for monoclonal antibody immune checkpoint inhibitors. Br. J. Clin. Pharmacol. 2020, 86, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Epling, D.; Dolphin, N.; Paccaly, A.; Conrado, D.; Davis, J.D.; Al-Huniti, N. Population pharmacokinetics modeling and exposure—Response analyses of cemiplimab in patients with recurrent or metastatic cervical cancer. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, X.; Guo, X.; Jiang, S.; Chen, T.; Hu, Z.; Liu, H.; Bai, Y.; Xue, M.; Hu, R.; et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 2018, 564, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, E.; Oroya, A.; Anto, N.P.; Bachais, M.; Rudd, C.E. PD-1 endocytosis unleashes the cytolytic potential of checkpoint blockade in tumor immunity. Cell Rep. 2024, 43, 114907. [Google Scholar] [CrossRef]
- Okada, M.; Chikuma, S.; Kondo, T.; Hibino, S.; Machiyama, H.; Yokosuka, T.; Nakano, M.; Yoshimura, A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017, 20, 1017–1028. [Google Scholar] [CrossRef]
- White, D.; Cote-Martin, A.; Bleck, M.; Garaffa, N.; Shaaban, A.; Wu, H.; Liu, D.; Young, D.; Scheer, J.; Lorenz, I.C.; et al. Programmed Cell Death-1 (PD-1) anchoring to the GPI-linked co-receptor CD48 reveals a novel mechanism to modulate PD-1-dependent inhibition of human T cells. Mol. Immunol. 2023, 156, 31–38. [Google Scholar] [CrossRef]
- Chen, N.; Li, Z.; Liu, H.; Jiang, A.; Zhang, L.; Yan, S.; He, W.; Yang, J.; Liu, T. Enhancing PD-1 blockade in NSCLC: Reprogramming tumor immune microenvironment with albumin-bound statins targeting lipid rafts and mitochondrial respiration. Bioact. Mater. 2025, 49, 140–153. [Google Scholar] [CrossRef]
- D’Hooghe, L.; Chalmers, A.D.; Heywood, S.; Whitley, P. Cell surface dynamics and cellular distribution of endogenous FcRn. PLoS ONE 2017, 12, e0182695. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, R.; Kraehenbuhl, J.P. Receptor-mediated transport of IgG. J. Cell Biol. 1984, 99 Pt 2, 159s–164s. [Google Scholar] [CrossRef] [PubMed]
- Medesan, C.; Radu, C.; Kim, J.; Ghetie, V.; Ward, E.S. Localization of the site of the IgG molecule that regulates maternofetal transmission in mice. Eur. J. Immunol. 1996, 26, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Ward, E.S. Transcytosis and catabolism of antibody. Immunol. Res. 2002, 25, 97–114. [Google Scholar] [CrossRef]
- Raghavan, M.; Bonagura, V.R.; Morrison, S.L.; Bjorkman, P.J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995, 34, 14649–14657. [Google Scholar] [CrossRef]
- Ober, R.J.; Martinez, C.; Vaccaro, C.; Zhou, J.; Ward, E.S. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol. 2004, 172, 2021–2029. [Google Scholar] [CrossRef]
- Pyzik, M.; Rath, T.; Lencer, W.I.; Baker, K.; Blumberg, R.S. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J. Immunol. 2015, 194, 4595–4603. [Google Scholar] [CrossRef]
- Pannek, A.; Houghton, F.J.; Verhagen, A.M.; Dower, S.K.; Hinde, E.; Gleeson, P.A. Dynamics of intracellular neonatal Fc receptor-ligand interactions in primary macrophages using biophysical fluorescence techniques. Mol. Biol. Cell 2022, 33, ar6. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Howard, K.A. FcRn expression in cancer: Mechanistic basis and therapeutic opportunities. J. Control. Release 2021, 337, 248–257. [Google Scholar] [CrossRef]
- Claypool, S.M.; Dickinson, B.L.; Wagner, J.S.; Johansen, F.-E.; Venu, N.; Borawski, J.A.; Lencer, W.I.; Blumberg, R.S. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol. Biol. Cell 2004, 15, 1746–1759. [Google Scholar] [CrossRef]
- Latvala, S.; Jacobsen, B.; Otteneder, M.B.; Herrmann, A.; Kronenberg, S. Distribution of FcRn Across Species and Tissues. J. Histochem. Cytochem. 2017, 65, 321–333. [Google Scholar] [CrossRef]
- Chaudhury, C.; Mehnaz, S.; Robinson, J.M.; Hayton, W.L.; Pearl, D.K.; Roopenian, D.C.; Anderson, C.L. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003, 197, 315–322. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef]
- Castillo, A.M.M.; Vu, T.T.; Liva, S.G.; Chen, M.; Xie, Z.; Thomas, J.; Remaily, B.; Guo, Y.; Subrayan, U.L.; Costa, T.; et al. Murine cancer cachexia models replicate elevated catabolic pembrolizumab clearance in humans. JCSM Rapid Commun. 2021, 4, 232–244. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Ruan, Z.; Lou, H.; Jiang, B. Translate Pharmacokinetics of PD-1/PD-L1 Monoclonal Antibodies from Cynomolgus Monkey to Human: Comparison of Different Approaches. J. Pharm. Sci. 2024, 113, 2915–2921. [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, X.; Li, K.; Zhang, T. FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front. Immunol. 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G.C.; Clatworthy, M.R. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, L.P.; Kim, J.; Wu, Y.; Mohanty, S.; Phillips, G.S.; Birmingham, D.J.; Robinson, J.M.; Anderson, C.L. FcγRIIb on liver sinusoidal endothelium clears small immune complexes. J. Immunol. 2012, 189, 4981–4988. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.M.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Moro, M.; Sommariva, M.; Cancila, V.; Boeri, M.; Centonze, G.; Ferro, S.; Ganzinelli, M.; Gasparini, P.; Huber, V.; et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin. Cancer Res. 2019, 25, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef]
- Tan, C.L.; Kuchroo, J.R.; Sage, P.T.; Liang, D.; Francisco, L.M.; Buck, J.; Thaker, Y.R.; Zhang, Q.; McArdel, S.L.; Juneja, V.R.; et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 2021, 218, e20182232. [Google Scholar] [CrossRef]
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Golay, J.; Semenzato, G.; Rambaldi, A.; Foà, R.; Gaidano, G.; Gamba, E.; Pane, F.; Pinto, A.; Specchia, G.; Zaja, F.; et al. Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. mAbs 2013, 5, 826–837. [Google Scholar] [CrossRef]
- Gearing, A.J.; Thorpe, S.J.; Miller, K.; Mangan, M.; Varley, P.G.; Dudgeon, T.; Ward, G.; Turner, C.; Thorpe, R. Selective cleavage of human IgG by the matrix metalloproteinases, matrilysin and stromelysin. Immunol. Lett. 2002, 81, 41–48. [Google Scholar] [CrossRef]
- Joo, V.; Petrovas, C.; de Leval, L.; Noto, A.; Obeid, M.; Fenwick, C.; Pantaleo, G. A CD64/FcγRI-mediated mechanism hijacks PD-1 from PD-L1/2 interaction and enhances anti-PD-1 functional recovery of exhausted T cells. Front. Immunol. 2023, 14, 1213375. [Google Scholar] [CrossRef]
- Hao, L.Y.; Lerrer, S.; Paiola, M.; Moore, E.K.; Gartshteyn, Y.; Song, R.; Goeckeritz, M.; Black, M.J.; Bukhari, S.; Hu, X.; et al. Exclusion of PD-1 from the immune synapse: A novel strategy to modulate T cell function. Mol. Ther. Oncol. 2024, 32, 200839. [Google Scholar] [CrossRef] [PubMed]
- Brezski, R.J.; Vafa, O.; Petrone, D.; Tam, S.H.; Powers, G.; Ryan, M.H.; Luongo, J.L.; Oberholtzer, A.; Knight, D.M.; Jordan, R.E. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc. Natl. Acad. Sci. USA 2009, 106, 17864–17869. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.A.; Wang, J.; Strome, S.E. Strome, Engineering of Fc Multimers as a Protein Therapy for Autoimmune Disease. Front. Immunol. 2020, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef]
- Balbín, M.; Pendás, A.M.; Uría, J.A.; Jiménez, M.G.; Freije, J.P.; López-Otín, C. Expression and regulation of collagenase-3 (MMP-13) in human malignant tumors. APMIS 1999, 107, 45–53. [Google Scholar] [CrossRef]
- Azzam, H.S.; Arand, G.; Lippman, M.E.; Thompson, E.W. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J. Natl. Cancer Inst. 1993, 85, 1758–1764. [Google Scholar] [CrossRef]
- Naylor, M.S.; Stamp, G.W.; Davies, B.D.; Balkwill, F.R. Expression and activity of MMPS and their regulators in ovarian cancer. Int. J. Cancer 1994, 58, 50–56. [Google Scholar] [CrossRef]
- Kusukawa, J.; Sasaguri, Y.; Shima, I.; Kameyama, T.; Morimatsu, M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am. J. Clin. Pathol. 1993, 99, 18–23. [Google Scholar] [CrossRef]
- Powell, W.C.; Knox, J.D.; Navre, M.; Grogan, T.M.; Kittelson, J.; Nagle, R.B.; Bowden, G.T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993, 53, 417–422. [Google Scholar]
- Davies, B.; Waxman, J.; Wasan, H.; Abel, P.; Williams, G.; Krausz, T.; Neal, D.; Thomas, D.; Hanby, A.; Balkwill, F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993, 53, 5365–5369. [Google Scholar]
- Yoshimoto, M.; Itoh, F.; Yamamoto, H.; Hinoda, Y.; Imai, K.; Yachi, A. Expression of MMP-7(PUMP-1) mRNA in human colorectal cancers. Int. J. Cancer 1993, 54, 614–618. [Google Scholar] [CrossRef]
- Jones, B.; Moshyedi, P.; Gallo, S.; Tombran-Tink, J.; Arand, G.; Reid, D.A.; Thompson, E.W.; Chader, G.J.; Waldbillig, R.J. Characterization and novel activation of 72-kDa metalloproteinase in retinal interphotoreceptor matrix and Y-79 cell culture medium. Exp. Eye Res. 1994, 59, 257–269. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Z.; Wu, Q.; Hou, J.; Chen, Y. Overexpression of MMP-2 in laryngeal squamous cell carcinoma: A potential indicator for poor prognosis. Otolaryngol. Head Neck Surg. 2005, 132, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, D.; Subashini, V.; Nanthanalaxmi, M.; Saranya, I.; Selvamurugan, N. Regulation of matrix metalloproteinase-13 in cancer: Signaling pathways and non-coding RNAs in tumor progression and therapeutic targeting. World J. Clin. Oncol. 2025, 16, 105996. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.A.; Varshini, M.S.; Kumar, R.S. Matrix Metalloproteinase-2 (MMP-2): As an Essential Factor in Cancer Progression. Recent Pat. Anti-Cancer Drug Discov. 2025, 20, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.P.; Pinto, M.L.; Pinto, A.T.; Pinto, M.T.; Monteiro, C.; Oliveira, M.I.; Santos, S.G.; Relvas, J.B.; Seruca, R.; Mantovani, A.; et al. Matrix metalloproteases as maestros for the dual role of LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC Cancer 2015, 15, 456. [Google Scholar] [CrossRef]
- Xu, F.; Wei, Y.; Tang, Z.; Liu, B.; Dong, J. Tumor-associated macrophages in lung cancer: Friend or foe? (Review). Mol. Med. Rep. 2020, 22, 4107–4115. [Google Scholar]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Egeblad, M.; Rank, F.; Askautrud, H.A.; Pennington, C.J.; Pedersen, T.X.; Christensen, I.J.; Edwards, D.R.; Werb, Z.; Lund, L.R. Matrix metalloproteinase 13 is induced in fibroblasts in polyomavirus middle T antigen-driven mammary carcinoma without influencing tumor progression. PLoS ONE 2008, 3, e2959. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Heesom, K.; Parkinson, E.K.; Prime, S.S. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br. J. Cancer 2014, 111, 1230–1237. [Google Scholar] [CrossRef]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pr. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J. Clin. Investig. 2015, 125, 2194–2202. [Google Scholar] [CrossRef]
- Sesarman, A.; Vidarsson, G.; Sitaru, C. The neonatal Fc receptor as therapeutic target in IgG-mediated autoimmune diseases. Cell. Mol. Life Sci. 2010, 67, 2533–2550. [Google Scholar] [CrossRef]
- Hajjar, S.; Zhou, X. pH sensing at the intersection of tissue homeostasis and inflammation. Trends Immunol. 2023, 44, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Tardy, M.P.; Gastaud, L.; Boscagli, A.; Peyrade, F.; Gallamini, A.; Thyss, A. Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol. Oncol. 2016, 35, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, J.; Vicino, A.; Michielin, O.; Coukos, G.; Kuntzer, T.; Obeid, M. A severe case of neuro-Sjögren’s syndrome induced by pembrolizumab. J. Immunother. Cancer 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Langan, E.A.; Grätz, V.; Zillikens, D.; Terheyden, P. Checkpoint Inhibition May Trigger the Rare Variant of Anti-LAD-1 IgG-Positive, Anti-BP180 NC16A IgG-Negative Bullous Pemphigoid. Front. Immunol. 2019, 10, 1934. [Google Scholar] [CrossRef]
- Pollack, R.; Ashash, A.; Cahn, A.; Rottenberg, Y.; Stern, H.; Dresner-Pollak, R. Immune Checkpoint Inhibitor-induced Thyroid Dysfunction Is Associated with Higher Body Mass Index. J. Clin. Endocrinol. Metab. 2020, 105, e3620–e3627. [Google Scholar] [CrossRef]
- Genta, S.; Lajkosz, K.; Yee, N.R.; Spiliopoulou, P.; Heirali, A.; Hansen, A.R.; Siu, L.L.; Saibil, S.; Stayner, L.-A.; Yanekina, M.; et al. Autoimmune PaneLs as PrEdictors of Toxicity in Patients TReated with Immune Checkpoint InhibiTors (ALERT). J. Exp. Clin. Cancer Res. 2023, 42, 276. [Google Scholar] [CrossRef]
- Swanson, S.J. What are clinically significant anti-drug antibodies and why is it important to identify them. Front. Immunol. 2024, 15, 1401178. [Google Scholar] [CrossRef]
- Galle, P.; Finn, R.S.; Mitchell, C.R.; Ndirangu, K.; Ramji, Z.; Redhead, G.S.; Pinato, D.J. Treatment-emergent antidrug antibodies related to PD-1, PD-L1, or CTLA-4 inhibitors across tumor types: A systematic review. J. Immunother. Cancer 2024, 12, e008266. [Google Scholar] [CrossRef]
- Agrawal, S.; Statkevich, P.; Bajaj, G.; Feng, Y.; Saeger, S.; Desai, D.D.; Park, J.; Waxman, I.M.; Roy, A.; Gupta, M. Evaluation of Immunogenicity of Nivolumab Monotherapy and Its Clinical Relevance in Patients with Metastatic Solid Tumors. J. Clin. Pharmacol. 2017, 57, 394–400. [Google Scholar] [CrossRef]
- de Vries, F.; Smit, A.A.; Wolbink, G.; de Vries, A.; Loeff, F.C.; Franssen, E.J. Case report: Pharmacokinetics of pembrolizumab in a patient with stage IV non-small cell lung cancer after a single 200 mg administration. Front. Oncol. 2022, 12, 960116. [Google Scholar] [CrossRef] [PubMed]
- Leven, C.; Padelli, M.; Carré, J.L.; Bellissant, E.; Misery, L. Immune Checkpoint Inhibitors in Melanoma: A Review of Pharmacokinetics and Exposure-Response Relationships. Clin. Pharmacokinet. 2019, 58, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Cowles, S.C.; Sheen, A.; Santollani, L.; Lutz, E.A.; Lax, B.M.; Palmeri, J.R.; Freeman, G.J.; Wittrup, K.D. An affinity threshold for maximum efficacy in anti-PD-1 immunotherapy. mAbs 2022, 14, 2088454. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Sriram, J.; Pluim, D.; Maas, R.; van Groningen, H.; Piet, B.; ter Heine, R. Therapeutic Exposure and Successful Response to Pembrolizumab in a Patient with Non–Small-Cell Lung Cancer Despite Significant Renal Loss Due to Paraneoplastic Nephrotic Syndrome. Clin. Lung Cancer 2021, 22, e220–e223. [Google Scholar] [CrossRef]
- Hurkmans, D.P.; Sassen, S.D.; de Joode, K.; Putter, L.; Basak, E.A.; Wijkhuijs, A.J.; Joerger, M.; Debets, R.; Koch, B.C.; Van der Leest, C.H.; et al. Prospective real-world study on the pharmacokinetics of pembrolizumab in patients with solid tumors. J. Immunother. Cancer 2021, 9, e002344. [Google Scholar] [CrossRef]
- Bajaj, G.; Wang, X.; Agrawal, S.; Gupta, M.; Roy, A.; Feng, Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients with Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 58–66. [Google Scholar] [CrossRef]
- Chaudhury, C.; Brooks, C.L.; Carter, D.C.; Robinson, J.M.; Anderson, C.L. Albumin binding to FcRn: Distinct from the FcRn-IgG interaction. Biochemistry 2006, 45, 4983–4990. [Google Scholar] [CrossRef]
- Oganesyan, V.; Damschroder, M.M.; Cook, K.E.; Li, Q.; Gao, C.; Wu, H.; Dall’ACqua, W.F. Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem. 2014, 289, 7812–7824. [Google Scholar] [CrossRef]
- Mezo, A.R.; McDonnell, K.A.; Hehir, C.A.T.; Low, S.C.; Palombella, V.J.; Stattel, J.M.; Kamphaus, G.D.; Fraley, C.; Zhang, Y.; Dumont, J.A.; et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc. Natl. Acad. Sci. USA 2008, 105, 2337–2342. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front. Immunol. 2014, 5, 682. [Google Scholar] [CrossRef]
- Roch, B.; Coffy, A.; Jean-Baptiste, S.; Palaysi, E.; Daures, J.-P.; Pujol, J.-L.; Bommart, S. Cachexia—Sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020, 143, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, M.; Yagishita, S.; Jo, H.; Akagi, K.; Higashiyama, R.I.; Masuda, K.; Shinno, Y.; Okuma, Y.; Yoshida, T.; Goto, Y.; et al. Early change in the clearance of pembrolizumab reflects the survival and therapeutic response: A population pharmacokinetic analysis in real-world non-small cell lung cancer patients. Lung Cancer 2022, 173, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tohi, M.; Irie, K.; Mizuno, T.; Okuyoshi, H.; Hirabatake, M.; Ikesue, H.; Muroi, N.; Eto, M.; Fukushima, S.; Tomii, K.; et al. Population Pharmacokinetics of Nivolumab in Japanese Patients with Nonsmall Cell Lung Cancer. Ther. Drug Monit. 2023, 45, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Kim, K.; Manna, M.; Thomas, J.; Remaily, B.C.; Montgomery, E.J.; Costa, T.; Granchie, L.; Xie, Z.; Guo, Y.; et al. Decoupling FcRn and tumor contributions to elevated immune checkpoint inhibitor clearance in cancer cachexia. Pharmacol. Res. 2024, 199, 107048. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, J.; Shao, X.; Ishii, Y.; Roy, A.; Bello, A.; Lee, R.; Zhang, J.; Wind-Rotolo, M.; Feng, Y. Development of a prognostic composite cytokine signature based on the correlation with nivolumab clearance: Translational PK/PD analysis in patients with renal cell carcinoma. J. Immunother. Cancer 2019, 7, 348. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, J.; Bello, A.; Roy, A.; Sheng, J. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Chinese Patients with Previously Treated Advanced Solid Tumors, Including Non-Small Cell Lung Cancer. J. Clin. Pharmacol. 2019, 59, 1415–1424. [Google Scholar] [CrossRef]
- Higashiyama, R.I.; Yoshida, T.; Yagishita, S.; Ohuchi, M.; Sakiyama, N.; Torasawa, M.; Shirasawa, M.; Masuda, K.; Shinno, Y.; Matsumoto, Y.; et al. Safety Implications of Switching Pembrolizumab Dosage from 200 mg Every 3 Weeks to 400 mg Every 6 Weeks in Patients with Advanced NSCLC. J. Thorac. Oncol. 2022, 17, 1227–1232. [Google Scholar] [CrossRef]
- Bellesoeur, A.; Ollier, E.; Allard, M.; Hirsch, L.; Boudou-Rouquette, P.; Arrondeau, J.; Thomas-Schoemann, A.; Tiako, M.; Khoudour, N.; Chapron, J.; et al. Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients? Cancers 2019, 11, 1784. [Google Scholar] [CrossRef]
- Hamuro, L.; Statkevich, P.; Bello, A.; Roy, A.; Bajaj, G. Nivolumab Clearance Is Stationary in Patients With Resected Melanoma on Adjuvant Therapy: Implications of Disease Status on Time-Varying Clearance. Clin. Pharmacol. Ther. 2019, 106, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, J.; Li, H.; Liu, J.; Xu, Y.; Song, P.; Liu, Q.; Zhao, H.; Xu, J.; Maher, V.; et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 2017, 101, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Shchelokov, D.; Demin, O., Jr. Receptor occupancy assessment and interpretation in terms of quantitative systems pharmacology: Nivolumab case study. mAbs 2023, 15, 2156317. [Google Scholar] [CrossRef] [PubMed]
- Hamimed, M.; Devillier, R.; Weiller, P.-J.; Marin, C.; Schiano, J.-M.; Belmecheri, N.; Etienne-Grimaldi, M.-C.; Ciccolini, J.; Harbi, S. Life-threatening toxicities upon Pembrolizumab intake: Could pharmacokinetics be the bad guy? Cancer Chemother. Pharmacol. 2024, 93, 627–632. [Google Scholar] [CrossRef]
- Atallah-Yunes, S.A.; Kadado, A.J.; Soe, M.H. Pericardial effusion due to pembrolizumab-induced immunotoxicity: A case report and literature review. Curr. Probl. Cancer 2019, 43, 504–510. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, Y.; Sun, W.; Li, Z.; Wang, C. Clinical features, diagnosis, and management of pembrolizumab-induced myasthenia gravis. Clin. Exp. Immunol. 2023, 211, 85–92. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Y.; Lin, G.; Chen, C. Dosing Regimens of Immune Checkpoint Inhibitors: Attempts at Lower Dose, Less Frequency, Shorter Course. Front. Oncol. 2022, 12, 906251. [Google Scholar] [CrossRef]
- Colard-Thomas, J.; Manceron, C.; Duflos, C.; Herman, F.; Simon, M.; Maria, A.; Faillie, J.-L.; Viala, M.; Palassin, P. Comparison of clinical safety between standard versus extended interval dosing of immune checkpoint inhibitors: A real-world retrospective cohort study. ESMO Open 2023, 8, 102070. [Google Scholar] [CrossRef]
- Zhou, H.; Li, N.; Tang, H.; Chen, H.; Chen, X.; Zhang, L.; Tao, D. Delayed thrombocytopenia as a rare but serious adverse event secondary to immune checkpoint inhibitor: A case report. Ann. Palliat. Med. 2021, 10, 5881–5886. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Bajaj, G.; Agrawal, S.; Bello, A.; Lestini, B.; Finckenstein, F.G.; Park, J.-S.; Roy, A. Nivolumab Exposure-Response Analyses of Efficacy and Safety in Previously Treated Squamous or Nonsquamous Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 5394–5405. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, J.; Ivaturi, V.; Gopalakrishnan, M.; Feng, Y.; Schmidt, B.; Statkevich, P.; Goodman, V.; Gobburu, J.; Bello, A.; et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann. Oncol. 2020, 31, 302–309. [Google Scholar] [CrossRef]
- Long, G.; Tykodi, S.; Schneider, J.; Garbe, C.; Gravis, G.; Rashford, M.; Agrawal, S.; Grigoryeva, E.; Bello, A.; Roy, A.; et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann. Oncol. 2018, 29, 2208–2213. [Google Scholar] [CrossRef]
- Guo, Y.; Remaily, B.C.; Thomas, J.; Kim, K.; Kulp, S.K.; Mace, T.A.; Ganesan, L.P.; Owen, D.H.; Coss, C.C.; Phelps, M.A. Antibody Drug Clearance: An Underexplored Marker of Outcomes with Checkpoint Inhibitors. Clin. Cancer Res. 2024, 30, 942–958. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.C.; Kondic, A.G.; Anderson, K.M.; Robinson, A.G.; Garon, E.B.; Riess, J.W.; Jain, L.; Mayawala, K.; Kang, J.; Ebbinghaus, S.W.; et al. Pembrolizumab Exposure–Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin. Cancer Res. 2018, 24, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Turner, D.C.; Felip, E.; Lena, H.; Cappuzzo, F.; Horn, L.; Garon, E.B.; Hui, R.; Arkenau, H.-T.; Gubens, M.A.; et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2016, 27, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Bajaj, G.; Gupta, M.; Agrawal, S.; Yang, A.; Park, J.-S.; Lestini, B.; Roy, A. Quantitative Characterization of the Exposure-Response Relationship for Cancer Immunotherapy: A Case Study of Nivolumab in Patients with Advanced Melanoma. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 40–48. [Google Scholar] [CrossRef]
- Badawi, M.; Coss, C.C.; Phelps, M.A. Letter to the Editor: Exposure–response or clearance–response relationship in immune checkpoint therapy?—A comment on ‘correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer’ by Basak et al. Eur. J. Cancer 2019, 114, 25–26. [Google Scholar] [CrossRef]
- Dai, H.I.; Vugmeyster, Y.; Mangal, N. Characterizing Exposure–Response Relationship for Therapeutic Monoclonal Antibodies in Immuno-Oncology and Beyond: Challenges, Perspectives, and Prospects. Clin. Pharmacol. Ther. 2020, 108, 1156–1170. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Liu, C.; Liu, J.; Subramaniam, S.; Zhao, H.; Blumenthal, G.M.; Turner, D.C.; Li, C.; Ahamadi, M.; et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J. Pharmacokinet. Pharmacodyn. 2017, 44, 403–414. [Google Scholar] [CrossRef]
- Yang, F.; Paccaly, A.J.; Rippley, R.K.; Davis, J.D.; DiCioccio, A.T. Population pharmacokinetic characteristics of cemiplimab in patients with advanced malignancies. J. Pharmacokinet. Pharmacodyn. 2021, 48, 479–494. [Google Scholar] [CrossRef]
- Hurkmans, D.P.; Basak, E.A.; van Dijk, T.; Mercieca, D.; Schreurs, M.W.J.; Wijkhuijs, A.J.M.; Bins, S.; Hoop, E.O.-D.; Debets, R.; Joerger, M.; et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J. Immunother. Cancer 2019, 7, 192. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Mariam, A.; Kamath, S.; Schveder, K.; Mc Leod, H.; Rotroff, D. Biomarkers for Response to Anti-PD-1/Anti-PD-L1 Immune Checkpoint Inhibitors: A Large Meta-Analysis. Oncology 2023, 37, 210–219. [Google Scholar] [PubMed]
- Monette, A.; Warren, S.; Barrett, J.C.; Garnett-Benson, C.; Schalper, K.A.; Taube, J.M.; Topp, B.; Snyder, A. Biomarker development for PD-(L)1 axis inhibition: A consensus view from the SITC Biomarkers Committee. J. Immunother. Cancer 2024, 12, e009427-13. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar] [CrossRef] [PubMed]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I—Pre-analytical and analytical validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef]
- Bedognetti, D.; Balwit, J.M.; Wang, E.; Disis, M.L.; Britten, C.M.; Delogu, L.G.; Tomei, S.; Fox, B.A.; Gajewski, T.F.; Marincola, F.M.; et al. SITC/iSBTc Cancer Immunotherapy Biomarkers Resource Document: Online resources and useful tools—A compass in the land of biomarker discovery. J. Transl. Med. 2011, 9, 155. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Bhaumik, S.; Dhodapkar, K.; Grivel, J.-C.J.B.; Gupta, S.; Hanks, B.A.; Janetzki, S.; Kleen, T.O.; Koguchi, Y.; Lund, A.W.; et al. SITC cancer immunotherapy resource document: A compass in the land of biomarker discovery. J. Immunother. Cancer 2020, 8, e000705. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Disis, M.L.; Fox, B.A.; Kaufman, D.R.; Khleif, S.N.; Wang, E. SITC 2018 workshop report: Immuno-Oncology Biomarkers: State of the Art. J. Immunother. Cancer 2018, 6, 138. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Disis, M.L.; Khleif, S.N.; Balwit, J.M.; Marincola, F.M. Immuno-oncology biomarkers 2010 and beyond: Perspectives from the iSBTc/SITC biomarker task force. J. Transl. Med. 2010, 8, 130. [Google Scholar] [CrossRef]
- Yuan, J.; Hegde, P.S.; Clynes, R.; Foukas, P.G.; Harari, A.; Kleen, T.O.; Kvistborg, P.; Maccalli, C.; Maecker, H.T.; Page, D.B.; et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J. Immunother. Cancer 2016, 4, 3. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Nguyen, P.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Esandrio, J.; Xu, H.; Ogurtsova, A.; Bleich, K.B.; Cornish, T.C.; et al. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin. Cancer Res. 2017, 23, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Ritterhouse, L.L. Tumor mutational burden. Cancer Cytopathol. 2019, 127, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. TMB or not TMB as a biomarker: That is the question. Crit. Rev. Oncol. Hematol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Ambrosini, M.; Manca, P.; Nasca, V.; Sciortino, C.; Ghelardi, F.; Seligmann, J.F.; Taieb, J.; Pietrantonio, F. Epidemiology, pathogenesis, biology and evolving management of MSI-H/dMMR cancers. Nat. Rev. Clin. Oncol. 2025, 22, 385–407. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Subbiah, V. Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends Cancer 2023, 9, 828–839. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Wang, Z.; Han, J.; Cui, Y.; Fan, K.; Zhou, X. Circulating microRNA-21 as noninvasive predictive biomarker for response in cancer immunotherapy. Med Hypotheses 2013, 81, 41–43. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Canzoniero, J.V.; Rosner, S.; Zhang, G.; White, J.R.; Belcaid, Z.; Cherry, C.; Balan, A.; Pereira, G.; Curry, A.; et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J. Immunother. Cancer 2022, 10, e004688. [Google Scholar] [CrossRef] [PubMed]
- Dharmapuri, S.; Özbek, U.; Jethra, H.; Jun, T.; Marron, T.U.; Saeed, A.; Huang, Y.-H.; Muzaffar, M.; Pinter, M.; Balcar, L.; et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio appear predictive of immune treatment related toxicity in hepatocellular carcinoma. World J. Gastrointest. Oncol. 2023, 15, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Vareki, S.M.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Chen, J.; Hua, Y.; Zhong, X.; Tang, C.; Gong, S.; Yang, L. The significance of inflammatory markers in prognosticating the effectiveness and safety of immunotherapy in conjunction with chemotherapy during the primary intervention of advanced non-small cell lung carcinoma. Lung Cancer 2024, 192, 107817. [Google Scholar] [CrossRef]
- Catalano, M.; Iannone, L.F.; Nesi, G.; Nobili, S.; Mini, E.; Roviello, G. Immunotherapy-related biomarkers: Confirmations and uncertainties. Crit. Rev. Oncol. Hematol. 2023, 192, 104135. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Lee, L.; Gupta, M.; Sahasranaman, S. Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J. Clin. Pharmacol. 2016, 56, 157–169. [Google Scholar] [CrossRef]
- Stroh, M.; Lum, B.L. Should Therapeutic Drug Monitoring for Monoclonal Antibodies Remain the Exception or Become the Norm? Clin. Pharmacol. Ther. 2016, 100, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Mould, D.R. Why therapeutic drug monitoring is needed for monoclonal antibodies and how do we implement this? Clin. Pharmacol. Ther. 2016, 99, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Imamura, C.K. Therapeutic drug monitoring of monoclonal antibodies: Applicability based on their pharmacokinetic properties. Drug Metab. Pharmacokinet. 2019, 34, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Basak, E.A.; Koolen, S.L.; Hurkmans, D.P.; Schreurs, M.W.; Bins, S.; de Hoop, E.O.; Wijkhuijs, A.J.; Besten, I.D.; Sleijfer, S.; Debets, R.; et al. Correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer. Eur. J. Cancer 2019, 109, 12–20. [Google Scholar] [CrossRef]
- Sureda, M.; Calvo, E.; Mata, J.J.; Escudero-Ortiz, V.; Martinez-Navarro, E.; Catalán, A.; Rebollo, J. Dosage of anti-PD-1 monoclonal antibodies: A cardinal open question. Clin. Transl. Oncol. 2021, 23, 1511–1519. [Google Scholar] [CrossRef]
- Rodallec, A.; Fanciullino, R.; Benzekry, S.; Ciccolini, J.; on Behalf of the EORTC PAMM Group. Is There Any Room for Pharmacometrics with Immuno-Oncology Drugs? Input from the EORTC-PAMM Course on Preclinical and Early-phase Clinical Pharmacology. Anticancer. Res. 2019, 39, 3419–3422. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Casteele, N.V.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668.e3. [Google Scholar] [CrossRef]
- van der Kleij, M.B.; Guchelaar, N.A.; Mathijssen, R.H.; Versluis, J.; Huitema, A.D.; Koolen, S.L.; Steeghs, N. Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin. Pharmacokinet. 2023, 62, 1333–1364. [Google Scholar] [CrossRef]
- Sureda, M.; Jose Mata, J.; Catalan, A.; Escudero, V.; Martinez-Navarro, E.; Rebollo, J. Therapeutic drug monitoring of nivolumab in routine clinical practice. A pilot study. Farm. Hosp. 2020, 44, 81–86. [Google Scholar]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. OncoImmunology 2018, 7, e1466769. [Google Scholar] [CrossRef] [PubMed]
- Limagne, E.; Richard, C.; Thibaudin, M.; Fumet, J.-D.; Truntzer, C.; Lagrange, A.; Favier, L.; Coudert, B.; Ghiringhelli, F. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. OncoImmunology 2019, 8, e1564505. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. CD137 as an Attractive T Cell Co-Stimulatory Target in the TNFRSF for Immuno-Oncology Drug Development. Cancers 2021, 13, 2288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, Y.; Zang, A.; Cheng, Y.; Zhang, Y.; Wang, X.; Chen, Z.; Qu, S.; He, J.; Chen, C.; et al. First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors. J. Hematol. Oncol. 2023, 16, 50. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Kaur, P.; Hammett, T.; Di Conza, G.; Lahn, M. Targeting T regulatory (Treg) cells in immunotherapy-resistant cancers. Cancer Drug Resist. 2024, 7, 2. [Google Scholar] [CrossRef]
| Pharmacokinetic | Pembrolizumab [21,23] | Nivolumab [21,24] | Cemiplimab [25] |
|---|---|---|---|
| Model | Two-compartment model with first-order elimination, zero-order iv infusion rate, and time-varying change in CL | ||
| Linear | Doses range: 2–10 mg/kg t1/2β = 22–27.3 days CL = 0.2–0.22 L/day Vdss = 6 L | Dose range: 0.1 to 20 mg/kg t1/2α = 32.5 h, t1/2β = 25 days CL = 0.226 L/day Vc = 3.63 L; Vp = 2.78 L Tmáx = 1–4 h (Normalized to BW of 80 kg) | Dose range: 1 to 10 mg/kg CL = 0.246 L/day t1/2β = 19.7 days Vdss = 5.89 L |
| Non-linear | Time-varying CL = 16–19 weeks | Time-varying CL = 12–16 weeks | |
| Biomarker | Category | Status | References |
|---|---|---|---|
| Effectiveness | |||
| PD-L1 expression | Protein (tumor/immune) | Approved | [142,143,144] |
| TMB | Genomic | Approved (TMB ≥10 mut/1 Mb) | [144,145] |
| dMMR/MSI-H | Genomic | Approved | [146,147,148] |
| Tumor-infiltrating lymphocytes | Histological | Investigational | [149] |
| Gene expression signatures | Transcriptomic | Investigational | [135] |
| Antibiotic use | Clinical factors | Observational | [150] |
| miRNA signatures (germline) | Epigenetic | Exploratory | [151] |
| Effectiveness and/or Safety | |||
| Lymphocyte counts/NLR | Hematologic | Investigational | [152,153,154] |
| Circulating cytokines and other inflammatory markers | Soluble proteins | Investigational | [155,156,157] |
| Clearance monitoring (CL0 and ΔCL) | Pharmacokinetics | Investigational | This work * [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, J.F.; Fernandes, A.; Tato, D.G.; Moreira, E.; Ribeiro, R.; Reguengo, H.; Gonçalves, J.; Fresco, P. Optimizing Anti-PD1 Immunotherapy: An Overview of Pharmacokinetics, Biomarkers, and Therapeutic Drug Monitoring. Cancers 2025, 17, 3262. https://doi.org/10.3390/cancers17193262
Monteiro JF, Fernandes A, Tato DG, Moreira E, Ribeiro R, Reguengo H, Gonçalves J, Fresco P. Optimizing Anti-PD1 Immunotherapy: An Overview of Pharmacokinetics, Biomarkers, and Therapeutic Drug Monitoring. Cancers. 2025; 17(19):3262. https://doi.org/10.3390/cancers17193262
Chicago/Turabian StyleMonteiro, Joaquim Faria, Alexandrina Fernandes, Diogo Gavina Tato, Elias Moreira, Ricardo Ribeiro, Henrique Reguengo, Jorge Gonçalves, and Paula Fresco. 2025. "Optimizing Anti-PD1 Immunotherapy: An Overview of Pharmacokinetics, Biomarkers, and Therapeutic Drug Monitoring" Cancers 17, no. 19: 3262. https://doi.org/10.3390/cancers17193262
APA StyleMonteiro, J. F., Fernandes, A., Tato, D. G., Moreira, E., Ribeiro, R., Reguengo, H., Gonçalves, J., & Fresco, P. (2025). Optimizing Anti-PD1 Immunotherapy: An Overview of Pharmacokinetics, Biomarkers, and Therapeutic Drug Monitoring. Cancers, 17(19), 3262. https://doi.org/10.3390/cancers17193262








