Failure to Rescue After Surgery for Pancreatic Cancer: A Systematic Review and Narrative Synthesis of Risk Factors and Safety Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Synthesis
2.3.1. Data Extraction
2.3.2. Definition Framework and Grouping
2.3.3. Data Synthesis
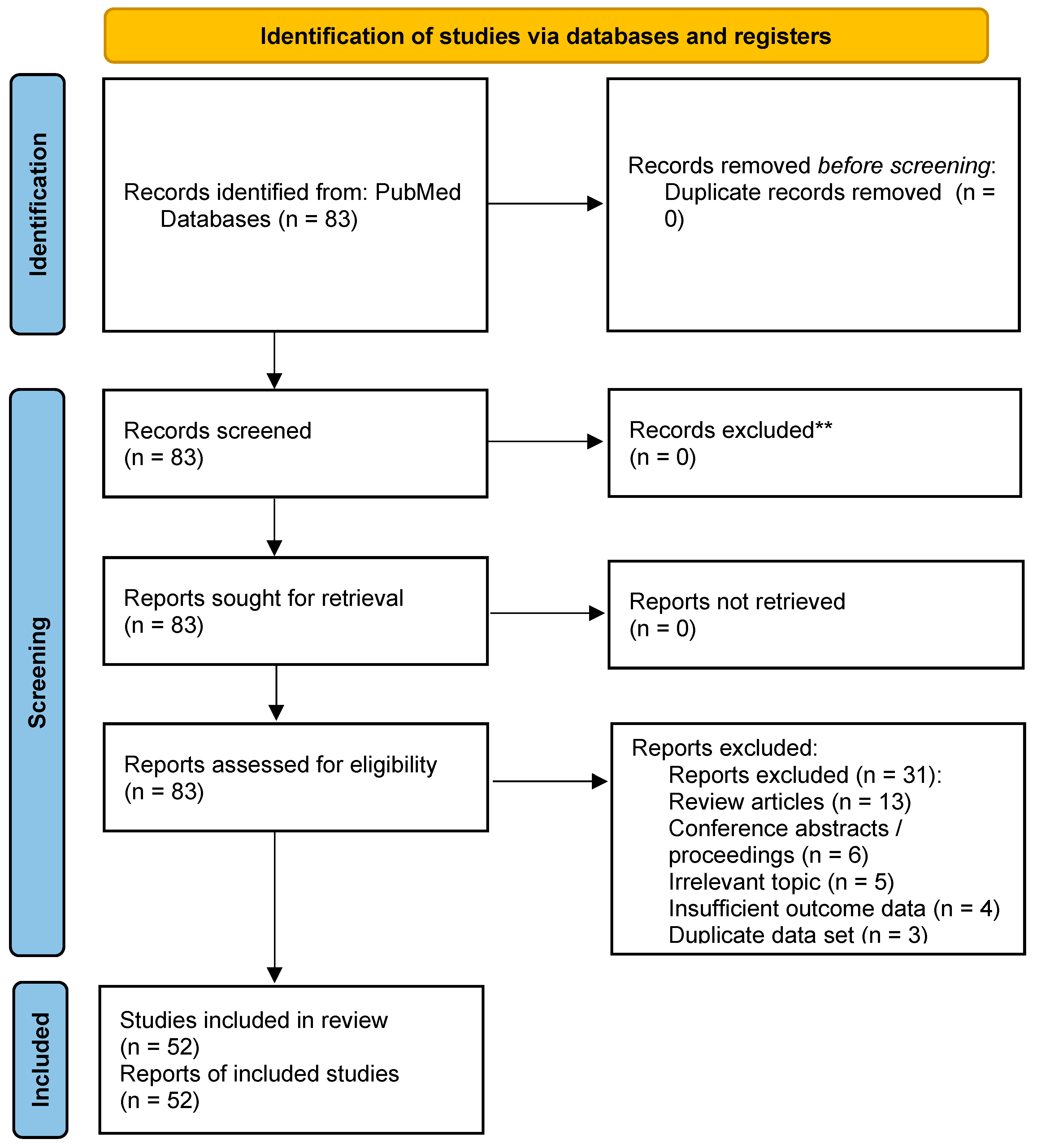
3. Results
3.1. Characteristics of the Included Studies
3.2. Summary of FTR Descriptions
3.2.1. FTR Definition
3.2.2. Time Windows, Severity Thresholds, and Denominators
3.2.3. Reported FTR Outcomes
3.3. Strategies for Reducing FTR in Pancreatic Surgery
3.3.1. Organizational Strategies
3.3.2. Evolution of Surgical Techniques
3.3.3. Improvements in Perioperative Management
3.3.4. Consideration of Patient-Related Factors
3.3.5. Emphasis on Non-Technical Skills (NTSs)
3.4. Role of Non-Technical Skills (NTSs) in Reducing FTR
3.4.1. Decision-Making
3.4.2. Situational Awareness
3.4.3. Communication
3.4.4. Teamwork
3.4.5. Leadership
3.4.6. Stress and Fatigue
4. Discussion
4.1. Statement of Main Findings
4.2. Strengthening Non-Technical Skills (NTS) to Reduce FTR: The Central Insight
4.2.1. Why NTS Matters for Rescue
4.2.2. Evidence Gap in the Current Literature
4.3. Current Landscape and Definition-Driven Limitations of FTR
4.3.1. Definition Heterogeneity and Interpretability
4.3.2. Studies Not Reporting FTR and Why It Matters
4.3.3. The Persistent Blind Spot: Post-Discharge Rescue
4.3.4. A Reference Definition: 90-Day FTR Among CD ≥ III Complications
4.4. Practical Implementation Strategies for Embedding NTS in Surgical Practice
- (a)
- Decision-making
- (b)
- Situational awareness tools
- (c)
- Structured communication frameworks
- (d)
- Team training and simulation
- (e)
- Leadership and role of clarity
- (f)
- Stress and fatigue
4.5. Future Directions for Research and Implementation
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Pancreatoduodenectomy |
| POPF | Postoperative Pancreatic Fistula |
| PPH | Postpancreatectomy Hemorrhage |
| FTR | Failure to Rescue |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| HBP | Hepato-Biliary-Pancreatic |
| NSQIP | National Surgical Quality Improvement Program |
| RN | Retrospective Analyses of National Registries |
| RS | Retrospective Single-Center Studies |
| RM | Retrospective Multicenter Studies |
| RI | Retrospective International Multicenter Studies |
| PN | Prospective National Registry Studies |
| PM | Prospective Multicenter Domestic Studies |
| PI | Prospective International Multicenter Studies |
| NIS | Nationwide Inpatient Sample |
| HCUP | Healthcare Cost and Utilization Project |
| AHRQ | Agency for Healthcare Research and Quality |
| AHA | American Hospital Association (Annual Survey) |
| PUF | Participant-Use File |
| MSQC | Michigan Surgical Quality Collaborative |
| MEDPAR | Medicare Provider Analysis and Review |
| PMSI | Programme de Médicalisation des Systèmes d’Information |
| DRG | Diagnosis-Related Group |
| RDC | Research Data Center |
| DPCA | Dutch Pancreatic Cancer Audit. |
| OSHPD | Office of Statewide Health Planning and Development |
| SNPPCR | Swedish National Pancreatic & Periampullary Cancer Registry |
| StuDoQ | StuDoQ|Pancreas registry |
| DGAV | German Society for General and Visceral Surgery |
| NCD | National Clinical Database |
| JSHBPS | Japanese Society of Hepato-Biliary-Pancreatic Surgery |
| MPOG | Multicenter Perioperative Outcomes Group |
| DICA | Dutch Institute for Clinical Auditing |
| DHBA | Dutch Hepato-Biliary Audit |
| PORSCH | Dutch Stepped-Wedge “Algorithm-Based Care” Program/Trial After PD |
| CRF | Case Report Form |
| EPJ | Electronic Patient Journal |
| ISGPS | International Study Group of Pancreatic Surgery |
| ICD | International Classification of Diseases |
| HV/IV/LV | High/Intermediate/Low Volume |
| PP | Pylorus-Preserving Pancreaticoduodenectomy |
| PRPD | Pylorus-Resecting Pancreaticoduodenectomy |
| DP | Distal Pancreatectomy |
| TP | Total Pancreatectomy |
| CP | Central Pancreatectomy |
| LP | Laparoscopic |
| Rb | Robotic |
| MIS | Minimally Invasive Surgery |
| RAMPS: | Radical Antegrade Modular Pancreatosple-Nectomy |
| AAA | Abdominal Aortic Aneurysm |
| CABG | Coronary Artery Bypass Grafting |
| HPD | hepatopancreatoduodenectomy |
| ICU | Intensive Care Unit |
| EWS | Early Warning Score/System |
| ERAS | Enhanced Recovery After Surgery |
| IR | Interventional Radiology |
| MDT | Multidisciplinary Team |
| EHR | Electronic Health Record |
| SBAR | Situation, Background, Assessment, Recommendation |
| NTS | Non-Technical Skills |
| NOTSS | Non-Technical Skills for Surgeons |
| PROSPERO | International Prospective Register of Systematic Reviews |
| ROBINS-I | Risk of Bias in Non-randomized Studies of Interventions |
| RoB 2 | Risk of Bias 2 |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
References
- Harnoss, J.C.; Ulrich, A.B.; Harnoss, J.M.; Diener, M.K.; Büchler, M.W.; Welsch, T. Use and results of consensus definitions in pancreatic surgery: A systematic review. Surgery 2014, 155, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Hirao, T.; Unno, M.; Fujii, T.; Yoshitomi, H.; Suzuki, S.; Satoi, S.; Takahashi, S.; Kainuma, O.; Suzuki, Y. Postoperative infectious complications after pancreatic resection. Br. J. Surg. 2015, 102, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Pugalenthi, A.; Protic, M.; Gonen, M.; Kingham, T.P.; D’Angelica, M.I.; Dematteo, R.P.; Fong, Y.; Jarnagin, W.R.; Allen, P.J. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2016, 113, 188–193. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Clement, G.; Lenne, X.; Farges, O.; Delpero, J.R.; Theis, D.; Pruvot, F.R.; Truant, S. Failure-to-rescue in patients undergoing pancreatectomy: Is hospital volume a standard for quality improvement programs? Nationwide analysis of 12,333 patients. Ann. Surg. 2018, 268, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, P.; Muller, X.; Malleo, G.; Park, J.S.; Hwang, H.K.; Napoli, N.; Javed, A.A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in pancreatic surgery: A novel tool for unbiased outcome comparisons. Ann. Surg. 2019, 270, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Smits, F.J.; Henry, A.C.; Besselink, M.G.; Busch, O.R.; van Eijck, C.H.; Arntz, M.; Bollen, T.L.; van Delden, O.M.; van den Heuvel, D.; van der Leij, C.; et al. Algorithm-based care versus usual care for the early recognition and management of complications after pancreatic resection in the Netherlands: An open-label, nationwide, stepped-wedge cluster-randomised trial. Lancet 2022, 399, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.C.; Shoucair, S.; Nigam, A.; Park, B.U.; Fishbein, T.M.; Radkani, P.; Winslow, E.R. The utility of axial imaging among selected patients in the early postoperative period after pancreatectomy. Surgery 2024, 176, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Krautz, C.; Nimptsch, U.; Weber, G.F.; Mansky, T.; Grützmann, R. Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann. Surg. 2018, 267, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Uttinger, K.L.; Diers, J.; Baum, P.; Pietryga, S.; Baumann, N.; Hankir, M.; Germer, C.T.; Wiegering, A. Mortality, complications and failure to rescue after surgery for esophageal, gastric, pancreatic and liver cancer patients based on minimum caseloads set by the German Cancer Society. Eur. J. Surg. Oncol. 2022, 48, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Diers, J.; Lichthardt, S.; Kastner, C.; Schlegel, N.; Germer, C.T.; Wiegering, A. Mortality and complications following visceral surgery: A nationwide analysis based on the diagnostic categories used in German hospital invoicing data. Dtsch. Arztebl. Int. 2019, 116, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Müssle, B.; Korn, S.; Sturm, D.; Bork, U.; Distler, M.; Grählert, X.; Klimova, A.; Trebesius, N.; Kleespies, A.; et al. Pancreatoduodenectomy with or without prophylactic falciform ligament wrap around the hepatic artery for prevention of postpancreatectomy haemorrhage: Randomized clinical trial (PANDA trial). Br. J. Surg. 2021, 109, 37–45. Available online: https://academic.oup.com/bjs/article/109/1/37/6422748 (accessed on 1 October 2025). [PubMed]
- Augustinus, S.; Mackay, T.M.; Andersson, B.; Beane, J.D.; Busch, O.R.; Gleeson, E.M.; Koerkamp, B.G.; Keck, T.; van Santvoort, H.C.; Tingstedt, B.; et al. Ideal outcome after pancreatoduodenectomy: A transatlantic evaluation of a harmonized composite outcome measure. Ann. Surg. 2023, 278, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.M.; Wellner, U.F.; van Rijssen, L.B.; Stoop, T.F.; Busch, O.R.; Groot Koerkamp, B.; Bausch, D.; Petrova, E.; Besselink, M.G.; Keck, T.; et al. Variation in pancreatoduodenectomy as delivered in two national audits. Br. J. Surg. 2019, 106, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An international study group of pancreatic surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, T.; Marchegiani, G.; Di Gioia, A.; Amadori, B.; Perri, G.; Salvia, R.; Bassi, C. Patterns of mortality after pancreatoduodenectomy: A root cause, day-to-day analysis. Surgery 2022, 172, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Beugniez, C.; Sauvanet, A.; Sulpice, L.; Gaujoux, S.; Turrini, O.; Truant, S.; Schwarz, L.; Piessen, G.; Regimbeau, J.M.; Muscari, F.; et al. Root-cause analysis of mortality after pancreatic resection (CARE study): A multicenter cohort study. Ann. Surg. 2021, 274, 789–796. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Lenne, X.; Clément, G.; Turrini, O.; Theis, D.; Pruvot, F.R.; Bruandet, A.; Truant, S. Referring patients to expert centers after pancreatectomy is too late to improve outcome. Inter-hospital transfer analysis in nationwide study of 19,938 patients. Ann. Surg. 2020, 272, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Williams, S.V.; Krakauer, H.; Schwartz, J.S. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med. Care. 1992, 30, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, E.M.; Pitt, H.A.; Mackay, T.M.; Wellner, U.F.; Williamsson, C.; Busch, O.R.; Koerkamp, B.G.; Keck, T.; van Santvoort, H.C.; Tingstedt, B.; et al. Failure to rescue after pancreatoduodenectomy: A transatlantic analysis. Ann. Surg. 2021, 274, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, A.A.; Dimick, J.B. Variation in mortality after high-risk cancer surgery: Failure to rescue. Surg. Oncol. Clin. N. Am. 2012, 21, 389–395. Available online: https://www.surgonc.theclinics.com/article/S1055-3207(12)00024-5/abstract (accessed on 1 October 2025). [CrossRef] [PubMed]
- Portuondo, J.I.; Shah, S.R.; Singh, H.; Massarweh, N.N. Failure to rescue as a surgical quality indicator: Current concepts and future directions for improving surgical outcomes. Anesthesiology 2019, 131, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, A.A.; Osborne, N.H.; Birkmeyer, J.D.; Dimick, J.B. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J. Am. Coll. Surg. 2010, 211, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Sheetz, K.H.; Krell, R.W.; Englesbe, M.J.; Birkmeyer, J.D.; Campbell, D.A., Jr.; Ghaferi, A.A. The importance of the first complication: Understanding failure to rescue after emergent surgery in the elderly. J. Am. Coll. Surg. 2014, 219, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Tamirisa, N.P.; Parmar, A.D.; Vargas, G.M.; Mehta, H.B.; Kilbane, E.M.; Hall, B.L.; Pitt, H.A.; Riall, T.S. Relative contributions of complications and failure to rescue on mortality in older patients undergoing pancreatectomy. Ann. Surg. 2016, 263, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Capretti, G.; Balzano, G.; Gianotti, L.; Stella, M.; Ferrari, G.; Baccari, P.; Zuliani, W.; Braga, M.; Zerbi, A. Management and outcomes of pancreatic resections performed in high-volume referral and low-volume community hospitals led by surgeons who shared the same mentor: The importance of training. Dig. Surg. 2018, 35, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Smits, F.J.; Molenaar, I.Q.; Besselink, M.G.; Borel Rinkes, I.H.M.; van Eijck, C.H.J.; Busch, O.R.; van Santvoort, H.C.; Dutch Pancreatic Cancer Group. Early recognition of clinically relevant postoperative pancreatic fistula: A systematic review. HPB (Oxford) 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Calcagno, P.; Giani, A.; Maspero, M.; Bertoglio, C.L.; De Martini, P.; Magistro, C.; Sgrazzutti, C.; Vanzulli, A.; Ferrari, G. Is routine CT scan after pancreaticoduodenectomy a useful tool in the early detection of complications? A single center retrospective analysis. Langenbecks Arch. Surg. 2022, 407, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Masui, T.; Nakano, K.; Yogo, A.; Yoh, T.; Nagai, K.; Anazawa, T.; Takaori, K.; Uemoto, S. Combination of postoperative C-reactive protein value and computed tomography imaging can predict severe pancreatic fistula after pancreatoduodenectomy. HPB (Oxford) 2020, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Augustin, T.; Burstein, M.D.; Schneider, E.B.; Morris-Stiff, G.; Wey, J.; Chalikonda, S.; Walsh, R.M. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery 2016, 160, 987–996. Available online: https://www.surgjournal.com/article/S0039-6060(16)30342-7/fulltext (accessed on 1 October 2025). [CrossRef] [PubMed]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J.; et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: Results of a randomized clinical trial. Ann. Surg. 2022, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- van Hilst, J.; de Rooij, T.; Bosscha, K.; Brinkman, D.J.; van Dieren, S.; Dijkgraaf, M.G.; Gerhards, M.F.; de Hingh, I.H.J.T.; Karsten, T.M.; Lips, D.J.; et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zureikat, A.H.; Beane, J.D.; Zenati, M.S.; Al Abbas, M.I.; Boone, B.A.; Moser, A.J.; Bartlett, D.L.; Hogg, M.E.; Zeh, H.J., 3rd. 500 minimally invasive robotic pancreatoduodenectomies: One decade of optimizing performance. Ann. Surg. 2021, 273, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Kinny-Köster, B.; Habib, J.R.; Javed, A.A.; Shoucair, S.; van Oosten, A.F.; Fishman, E.K.; Lafaro, K.J.; Wolfgang, C.L.; Hackert, T.; He, J. Technical progress in robotic pancreatoduodenectomy: TRIANGLE and periadventitial dissection for retropancreatic nerve plexus resection. Langenbecks Arch. Surg. 2021, 406, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haigh, P.I.; Bilimoria, K.Y.; DiFronzo, L.A. Early postoperative outcomes after pancreaticoduodenectomy in the elderly. Arch. Surg. 2011, 146, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Spolverato, G.; Kim, Y.; Pawlik, T.M. Trends in hospital volume and failure to rescue for pancreatic surgery. J. Gastrointest. Surg. 2015, 19, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.A.; Krell, R.W.; Abdelsattar, Z.M.; McCahill, L.E.; Kwon, D.; Frankel, T.L.; Hendren, S.; Campbell, D.A., Jr.; Wong, S.L. Pancreatic resection results in a statewide surgical collaborative. Ann. Surg. Oncol. 2015, 22, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.A.; Chung, C.W.; Schmidt, C.M.; Jester, A.; Kilbane, M.E.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M.; Ceppa, E.P. Impact of fellow versus resident assistance on outcomes following pancreatoduodenectomy. J. Gastrointest. Surg. 2017, 21, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Gani, F.; Johnston, F.M.; Nelson-Williams, H.; Cerullo, M.; Dillhoff, M.E.; Schmidt, C.R.; Pawlik, T.M. Hospital volume and the costs associated with surgery for pancreatic cancer. J. Gastrointest. Surg. 2017, 21, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Varley, P.R.; Geller, D.A.; Tsung, A. Factors influencing failure to rescue after pancreaticoduodenectomy: A National Surgical Quality Improvement Project perspective. J. Surg. Res. 2017, 214, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Merath, K.; Bagante, F.; Akgul, O.; Dillhoff, M.; Cloyd, J.; Pawlik, T.M. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections among the Medicare population. J. Gastrointest. Surg. 2018, 22, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, N.; Capretti, G.; Sandini, M.; Damascelli, A.; Cristel, G.; De Cobelli, F.; Gianotti, L.; Zerbi, A.; Braga, M. Impact of sarcopenic obesity on failure to rescue from major complications following pancreaticoduodenectomy for cancer: Results from a multicenter study. Ann. Surg. Oncol. 2018, 25, 308–317. [Google Scholar] [CrossRef] [PubMed]
- van Rijssen, L.B.; Zwart, M.J.; van Dieren, S.; de Rooij, T.; Bonsing, B.A.; Bosscha, K.; van Dam, R.M.; van Eijck, C.H.; Gerhards, M.F.; Gerritsen, J.J.; et al. Variation in hospital mortality after pancreatoduodenectomy is related to failure to rescue rather than major complications: A nationwide audit. HPB (Oxford) 2018, 20, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, M.; Gani, F.; Chen, S.Y.; Canner, J.K.; Dillhoff, M.; Cloyd, J.; Pawlik, T.M. Routine intensive care unit admission among patients undergoing major pancreatic surgery for cancer: No effect on failure to rescue. Surgery 2019, 165, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Burns, S.; Paredes, A.Z.; Pawlik, T.M. Accessing surgical care for pancreaticoduodenectomy: Patient variation in travel distance and choice to bypass hospitals to reach higher volume centers. J. Surg. Oncol. 2019, 120, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, E.M.; Clarke, J.R.; Morano, W.F.; Shaikh, M.F.; Bowne, W.B.; Pitt, H.A. Patient-specific predictors of failure to rescue after pancreaticoduodenectomy. HPB (Oxford) 2019, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Merath, K.; Chen, Q.; Bagante, F.; Sun, S.; Akgul, O.; Idrees, J.J.; Dillhoff, M.; Schmidt, C.; Cloyd, J.; Pawlik, T.M. Variation in the cost-of-rescue among Medicare patients with complications following hepatopancreatic surgery. HPB (Oxford) 2019, 21, 310–318. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Mackay, T.M.; Tol, J.A.M.G.; van Delden, O.M.; van Lienden, K.P.; Nio, C.Y.N.; Phoa, S.K.S.; Fockens, P.; van Hooft, J.E.; Verheij, J.; et al. Impact of expanding indications on surgical and oncological outcome in 1434 consecutive pancreatoduodenectomies. HPB (Oxford) 2019, 21, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Wroński, M.; Cebulski, W.; Witkowski, B.; Guzel, T.; Karkocha, D.; Lech, G.; Słodkowski, M. Surgical management of the grade C pancreatic fistula after pancreatoduodenectomy. HPB (Oxford) 2019, 21, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.B.H.; Jafri, R.Z.; Khan, N.A. Best achievable results need territorial familiarity: Impact of living donor liver transplant experience on outcomes after pancreaticoduodenectomy. Ann. Med. Surg. (Lond) 2020, 55, 213–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nymo, L.S.; Kleive, D.; Waardal, K.; Bringeland, E.A.; Søreide, J.A.; Labori, K.J.; Mortensen, K.E.; Søreide, K.; Lassen, K. Centralizing a national pancreatoduodenectomy service: Striking the right balance. BJS Open. 2020, 4, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Hirahara, N.; Miyata, H.; Yamamoto, H.; Matsuyama, R.; Kumamoto, T.; Homma, Y.; Mori, M.; Seto, Y.; Wakabayashi, G.; et al. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: An analysis of patients registered in the National Clinical Database in Japan. J. Hepatobiliary Pancreat. Sci. 2021, 28, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Lequeu, J.B.; Cottenet, J.; Facy, O.; Perrin, T.; Bernard, A.; Quantin, C. Failure to rescue in patients with distal pancreatectomy: A nationwide analysis of 10,632 patients. HPB (Oxford) 2021, 23, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Pastrana Del Valle, J.; Mahvi, D.A.; Fairweather, M.; Wang, J.; Clancy, T.E.; Ashley, S.W.; Urman, R.D.; Whang, E.E.; Gold, J.S. The improvement in post-operative mortality following pancreaticoduodenectomy between 2006 and 2016 is associated with an improvement in the ability to rescue patients after major morbidity, not in the rate of major morbidity. HPB (Oxford) 2021, 23, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Giuliani, T.; Di Gioia, A.; Andrianello, S.; Zingaretti, C.C.; Brentegani, G.; De Pastena, M.; Fontana, M.; Pea, A.; et al. Pancreatoduodenectomy at the Verona Pancreas Institute: The evolution of indications, surgical techniques, and outcomes: A retrospective analysis of 3000 consecutive cases. Ann Surg. 2022, 276, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, A.; Giuliani, T.; Marchegiani, G.; Andrianello, S.; Bonamini, D.; Secchettin, E.; Esposito, A.; Bassi, C.; Salvia, R. Pancreatoduodenectomy in obese patients: Surgery for nonmalignant tumors might be deferred. HPB (Oxford). 2022, 24, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Potter, K.C.; O’Grady, J.; Aziz, M.; Mayo, S.C.; Pommier, R.; Gilbert, E.W.; Rocha, F.; Sheppard, B.C. Intensive Care Unit Observation after Pancreatectomy: Treating the Patient or the Surgeon? J. Surg. Oncol. 2022, 125, 847–855. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jso.26800 (accessed on 1 October 2025). [CrossRef] [PubMed]
- van Beek, D.J.; Takkenkamp, T.J.; Wong-Lun-Hing, E.M.; de Kleine, R.J.; Walenkamp, A.M.E.; Klaase, J.M.; Nijkamp, M.W.; Valk, G.D.; Molenaar, I.Q.; Hagendoorn, J.; et al. Risk factors for complications after surgery for pancreatic neuroendocrine tumors. Surgery 2022, 172, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Fukada, M.; Murase, K.; Higashi, T.; Yasufuku, I.; Sato, Y.; Tajima, J.Y.; Kiyama, S.; Tanaka, Y.; Okumura, N.; Matsuhashi, N. Perioperative predictive factors of failure to rescue following highly advanced hepatobiliary-pancreatic surgery: A single-institution retrospective study. World J. Surg. Oncol. 2023, 21, 365. [Google Scholar] [CrossRef] [PubMed]
- Li, V.; Serrano, P.E. Prediction of postoperative mortality in patients with organ failure following pancreaticoduodenectomy. Am. Surg. 2023, 89, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, Z.; Lima, H.A.; Alaimo, L.; Endo, Y.; Ejaz, A.; Beane, J.; Dillhoff, M.; Cloyd, J.; Pawlik, T.M. Hepatopancreatic surgeons versus pancreatic surgeons: Does surgical subspecialization impact patient care and outcomes? J. Gastrointest. Surg. 2023, 27, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Suurmeijer, J.A.; Henry, A.C.; Bonsing, B.A.; Bosscha, K.; van Dam, R.M.; van Eijck, C.H.; Gerhards, M.F.; van der Harst, E.; de Hingh, I.H.; Intven, M.P.; et al. Outcome of pancreatic surgery during the first 6 years of a mandatory audit within the Dutch Pancreatic Cancer Group. Ann. Surg. 2023, 278, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Theijse, R.T.; Stoop, T.F.; Hendriks, T.E.; Suurmeijer, J.A.; Smits, F.J.; Bonsing, B.A.; Lips, D.J.; Manusama, E.; van der Harst, E.; Patijn, G.A.; et al. Nationwide outcome after pancreatoduodenectomy in patients at very high risk (ISGPS-D) for postoperative pancreatic fistula. Ann. Surg. 2023, 281, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Vawter, K.; Kuhn, S.; Pitt, H.; Wells, A.; Jensen, H.K.; Mavros, M.N. Complications and failure-to-rescue after pancreatectomy and hospital participation in the targeted American College of Surgeons National Surgical Quality Improvement Program registry. Surgery 2023, 174, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Cannas, S.; Casciani, F.; Vollmer, C.M.; Pancreas Fistula Study Group. Extending quality improvement for pancreatoduodenectomy within the high-volume setting: The experience factor. Ann. Surg. 2024, 279, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, M.R.; Hendriks, T.E.; Wouters, M.; Nielen, M.; de Hingh, I.H.J.T.; Koerkamp, B.G.; van Santvoort, H.C.; Busch, O.R.; den Dulk, M.; Klaase, J.M.; et al. Assessing quality of hepato-pancreato-biliary surgery: Nationwide benchmarking. Br. J. Surg. 2024, 111, znae119. [Google Scholar] [CrossRef] [PubMed]
- Duclos, C.; Durin, T.; Marchese, U.; Sauvanet, A.; Laurent, C.; Ayav, A.; Turrini, O.; Sulpice, L.; Addeo, P.; Souche, F.R.; et al. Management and outcomes of hemorrhage after distal pancreatectomy: A multicenter study at high volume centers. HPB (Oxford) 2024, 26, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.T.; Martinez, A.E.; Keim, R.L.; Mazzaferro, S.E.; Mir, K.S.; Gorman, M.A.; Shah, U.S. Implementation of robotic pancreaticoduodenectomy at a community tertiary care hospital utilizing a comprehensive curriculum. Am. J. Surg. 2024, 228, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.C.; Smits, F.J.; Daamen, L.A.; Busch, O.R.; Bosscha, K.; van Dam, R.M.; van Dam, C.J.L.; van Eijck, C.H.; Festen, S.; van der Harst, E.; et al. Root-cause analysis of mortality after pancreatic resection in a nationwide cohort. HPB (Oxford) 2025, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Pasha, S.A.; Demyan, L.; Standring, O.; King, D.A.; Newman, E.; DePeralta, D.; Gholami, S.; Weiss, M.J.; Melis, M. Evaluating the association of area deprivation index (ADI) on postoperative outcomes in pancreatic adenocarcinoma. J. Surg. Oncol. 2025, 131, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kinny-Köster, B.; Halm, D.; Tran, D.; Kaiser, J.; Heckler, M.; Hank, T.; Hinz, U.; Berchtold, C.; Al-Saeedi, M.; Roth, S.; et al. Who do we fail to rescue after pancreatoduodenectomy? Outcomes among >4000 procedures expose windows of opportunity. Ann. Surg. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Leech, N.; Krige, J.E.J.; Sobnach, S.; Kloppers, J.C.; Bernon, M.M.; Burmeister, S.; Jonas, E.G. Does the textbook outcome in pancreatic surgery score after pancreaticoduodenectomy for ampullary carcinoma have prognostic value? S Afr. J. Surg. 2024, 62, 33–38. Available online: https://journals.co.za/doi/10.36303/SAJS.00414 (accessed on 1 October 2025). [CrossRef] [PubMed]
- PancreasGroup.org Collaborative. Pancreatic surgery outcomes: Multicentre prospective snapshot study in 67 countries. Br. J. Surg. 2024, 111, znad330. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Anyanwu, A.; Gross, C.R.; Adams, D.H.; Varghese, R. The intra-aortic balloon pump as a rescue device: Do we need to shift our strategy for cardiogenic shock rescue after cardiac surgery? J. Thorac. Cardiovasc. Surg. 2025, 170, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, X.; Wang, S.; Zhang, C.S. The impact of body mass index on multiple complications, respiratory complications, failure to rescue and in-hospital mortality after laparoscopic pancreaticoduodenectomy: A single-center retrospective study. J. Laparoendosc. Adv. Surg. Technol. A. 2024, 34, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Capretti, G.; Ricci, C.; Langella, S.; Nicolini, D.; Pacilio, C.A.; Aymerito, F.; Ingaldi, C.; Mocchegiani, F.; Russolillo, N.; Ferrero, F.; et al. Outcomes after pancreatoduodenectomy and total pancreatectomy in patients with a high-risk pancreatic anastomosis: An entropy balance analysis. Surgery 2025, 181, 109277. [Google Scholar] [CrossRef] [PubMed]
- Tschaidse, T.; Hofmann, F.O.; Renz, B.; Hungbauer, M.; Klinger, C.; Buhr, H.J.; Uhl, W.; Mees, S.T.; Keck, T.; Reissfelder, C.; et al. Perioperative outcomes in an age-adapted analysis of the German StuDoQ|Pancreas registry for PDAC. BMC Surg. 2025, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Uttinger, K.; Niezold, A.; Weimann, L.; Plum, P.S.; Baum, P.; Diers, J.; Brunotte, M.; Rademacher, S.; Germer, C.T.; Seehofer, D.; et al. Weekday effect of surgery on in-hospital outcome in pancreatic surgery: A population-based study. Langenbecks Arch. Surg. 2024, 410, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann. Surg. 2009, 250, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, E.M.; Pitt, H.A. Failure to rescue after the Whipple: What do we know? Adv. Surg. 2022, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Sauvanet, A. Ancient and modern history of pancreatic surgery. Pancreas 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Beal, E.W.; Kimbrough, C.W.; Bagante, F.; Merath, K.; Dillhoff, M.; Schmidt, C.; White, S.; Cloyd, J.; Pawlik, T.M. Perioperative complications and the cost of rescue or failure to rescue in hepato-pancreato-biliary surgery. HPB (Oxford). 2018, 20, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Cassin, B.R.; Barach, P.R. Making sense of root cause analysis investigations of surgery-related adverse events. Surg. Clin. North. Am. 2012, 92, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Ramasubramanian, S.; Jeyaraman, M.; Hartl, R.; Tavakoli, J.; Cho, S.K.; Scaramuzzo, L.; Singh, H.; Louie, P.K.; Demetriades, A.K.; et al. Framework for adoption of enabling technologies for improved outcomes in spine surgery. Global Spine, J. 2025, 15, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Jerath, A.; Perez d’Empaire, P.; Carrier, F.M.; Turgeon, A.F.; McIsaac, D.I.; Idestrup, C.; Lorello, G.; Flexman, A.; Kidane, B.; et al. Familiarity of the Surgeon-Anesthesiologist Dyad and Major Morbidity After High-Risk Elective Surgery. JAMA Surg. 2025, 160, 772–781. Available online: https://jamanetwork.com/journals/jamasurgery/fullarticle/2834597 (accessed on 1 October 2025). [CrossRef] [PubMed]
- De Bie, A.J.R.; Subbe, C.P.; Bezemer, R.; Cooksley, T.; Kellett, J.G.; Holland, M.; Bouwman, R.A.; Bindels, A.J.G.H.; Korsten, H.H.M. Differences in identification of patients’ deterioration may hamper the success of clinical escalation protocols. QJM 2019, 112, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.; Barach, P.R. Toward a learning system for ERAS: Embedding implementation and learning evaluation. In Enhanced Recovery After Surgery: A Complete Guide to Optimizing Outcomes; Ljungvist, O., Urman, R.D., Francis, N.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 361–372. [Google Scholar] [CrossRef]
- Hanson, C.C.; Barach, P. Improving cardiac care quality and safety through partnerships with patients and their families. Prog. Pediatr. Cardiol. 2012, 33, 73–79. [Google Scholar] [CrossRef]
- Baker, D.P.; Salas, E.; King, H.; Battles, J.; Barach, P. The role of teamwork in the professional education of physicians: Current status and assessment recommendations. Jt. Comm. J. Qual. Patient Saf. 2005, 31, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Flin, R.; Martin, L.; Goeters, K.M.; Hörmann, H.J.; Amalberti, R.; Valot, C.; Nijhuis, H. Development of the NOTECHS (non-technical skills) system for assessing pilots’ CRM skills. Hum. Factors Aerosp. Saf. 2003, 3, 95–117. [Google Scholar]
- Barach, P.; Weinger, M.B. Trauma team performance. In Trauma: Critical Care; Wilson, W.C., Grande, C.M., Hoyt, D.B., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 101–113. [Google Scholar] [CrossRef]
- Barach, P.; Johnson, J.K. Understanding the complexity of redesigning care around the clinical microsystem. Qual. Saf. Health Care. 2006, 15, i10–i16. [Google Scholar] [CrossRef] [PubMed]
- Bognár, A.; Barach, P.; Johnson, J.K.; Duncan, R.C.; Birnbach, D.; Woods, D.; Holl, J.L.; Bacha, E.A. Errors and the burden of errors: Attitudes, perceptions, and the culture of safety in pediatric cardiac surgical teams. Ann. Thorac. Surg. 2008, 85, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Rattray, N.A.; Flanagan, M.E.; Militello, L.G.; Barach, P.; Franks, Z.; Ebright, P.; Rehman, S.U.; Gordon, H.S.; Frankel, R.M. “Do you know what I know?”: How communication norms and recipient design shape the content and effectiveness of patient handoffs. J. Gen. Intern. Med. 2019, 34, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Uramatsu, M.; Fujisawa, Y.; Mizuno, S.; Souma, T.; Komatsubara, A.; Miki, T. Do failures in non-technical skills contribute to fatal medical accidents in Japan? A review of the 2010–2013 national accident reports. BMJ Open. 2017, 7, e013678. [Google Scholar] [CrossRef] [PubMed]
- Flin, R.; O’Connor, P. Safety at the Sharp End: A Guide to Non-Technical Skills; CRC Press: London, UK, 2008; p. 330. [Google Scholar] [CrossRef]
- Reason, J. Patient Safety; Wiley-Blackwell: Chichester, UK, 2010. [Google Scholar]
- Yule, S.; Flin, R.; Paterson-Brown, S.; Maran, N.; Rowley, D. Development of a rating system for surgeons’ non-technical skills. Med. Educ. 2006, 40, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Moorthy, K.; Sarker, S.K.; Chang, A.; Darzi, A. Systems approaches to surgical quality and safety: From concept to measurement. Ann. Surg. 2004, 239, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Arulampalam, T.; Barach, P. Human factors in surgery: Optimal surgical team proficiency and decision making. Bull. R. Coll. Surg. Engl. 2023, 105, 128–133. Available online: https://publishing.rcseng.ac.uk/doi/10.1308/rcsbull.2023.45 (accessed on 1 October 2025). [CrossRef]
- Edmondson, A.C. Psychological Safety and Learning Behavior in Work Teams. Adm. Sci. Q. 1999, 44, 350–383. [Google Scholar] [CrossRef]
- Sutcliffe, K.M.; Lewton, E.; Rosenthal, M.M. Communication failures: An insidious contributor to medical mishaps. Acad. Med. 2004, 79, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Leape, L.L. Error in medicine. JAMA. 1994, 272, 1851–1857. Available online: https://jamanetwork.com/journals/jama/article-abstract/384554 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Reason, J. Managing the Risks of Organizational Accidents; Routledge: London, UK, 1997; p. 272. [Google Scholar] [CrossRef]
- Vaughan, D. The Challenger Launch Decision: Risky Technology, Culture, and Deviance at NASA, Enlarged ed.; University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- Wong, L.R.; Flynn-Evans, E.; Ruskin, K. Fatigue Risk Management: The Impact of Anesthesiology Residents’ Work Schedules on Job Performance and a Review of Potential Countermeasures. Anesth. Analg. 2018, 126, 1340–1348. Available online: https://journals.lww.com/anesthesia-analgesia/fulltext/2018/04000/fatigue_risk_management__the_impact_of.38.aspx (accessed on 1 October 2025). [CrossRef] [PubMed]
- Pouw, M.E.; Peelen, L.M.; Moons, K.G.M.; Kalkman, C.J.; Lingsma, H.F. Including post-discharge mortality in calculation of hospital standardised mortality ratios: Retrospective analysis of hospital episode statistics. BMJ 2013, 347, f5913. Available online: https://www.bmj.com/content/347/bmj.f5913 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. Available online: https://journals.lww.com/annalsofsurgery/fulltext/2004/08000/classification_of_surgical_complications__a_new.3.aspx (accessed on 1 October 2025). [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Hilal, M.A.; Adham, M.; PAllen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. Available online: https://www.surgjournal.com/article/S0039-6060(16)30757-7/fulltext (accessed on 1 October 2025). [CrossRef] [PubMed]
- Strasberg, S.M.; Linehan, D.C.; Hawkins, W.G. The accordion severity grading system of surgical complications. Ann. Surg. 2009, 250, 177–186. Available online: https://journals.lww.com/annalsofsurgery/fulltext/2009/08000/the_accordion_severity_grading_system_of_surgical.1.aspx (accessed on 1 October 2025). [CrossRef] [PubMed]
- Swanson, R.S.; Pezzi, C.M.; Mallin, K.; Loomis, A.M.; Winchester, D.P. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: More than 20,000 resections from the national cancer data base. Ann. Surg. Oncol. 2014, 21, 4059–4067. Available online: https://link.springer.com/article/10.1245/s10434-014-4036-4 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Ellis, D.I.; Altan, D.; Chang, D.C. Failure and Rescue in Surgery—Surgical Covenant, Palliative Care, and Reimagining Quality. JAMA Surg. 2022. Online ahead of print. Available online: https://jamanetwork.com/journals/jamasurgery/fullarticle/2796288 (accessed on 1 October 2025). [PubMed]
- Agha, R.A.; Fowler, A.J.; Sevdalis, N. The role of non-technical skills in surgery. Ann. Med. Surg. (Lond) 2015, 4, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.H.; Raemer, D.B.; Carroll, J.S.; Sunder, N.; Felstein, D.M.; Cooper, J.B. Crisis resource management training for an anaesthesia faculty: A new approach to continuing education. Med. Educ. 2004, 38, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; Graham, S.; Bonacum, D. The human factor: The critical importance of effective teamwork and communication in providing safe care. Qual. Saf. Health Care. 2004, 13, i85–i90. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.C.; Regenbogen, S.E.; Studdert, D.M.; Lipsitz, S.R.; Rogers, S.O.; Zinner, M.J.; Gawande, A.A. Patterns of communication breakdowns resulting in injury to surgical patients. J. Am. Coll. Surg. 2007, 204, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Lingard, L.; Espin, S.; Whyte, S.; Regehr, G.; Baker, G.R.; Reznick, R.; Bohnen, J.; Orser, B.; Doran, D.; Grober, E. Communication failures in the operating room: An observational classification of recurrent types and effects. Qual. Saf. Health Care. 2004, 13, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. TeamSTEPPS®: National Implementation. Available online: https://www.ahrq.gov/teamstepps/index.html (accessed on 9 August 2025).
- King, H.B.; Battles, J.; Baker, D.P.; Alonso, A.; Salas, E.; Webster, J.; Toomey, L.; Salisbury, M. TeamSTEPPS™: Team Strategies and Tools to Enhance Performance and Patient Safety. In Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 3: Performance and Tools); Henriksen, K., Battles, J.B., Keyes, M.A., Grady, M.L., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK43686/ (accessed on 9 August 2025). [PubMed]
- Fletcher, G.C.L.; McGeorge, P.; Flin, R.H.; Glavin, R.J.; Maran, N.J. The role of non-technical skills in anaesthesia: A review of current literature. Br. J. Anaesth. 2002, 88, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Chejfec-Ciociano, J.M.; Barach, P.; Gelb, A.W. Operationalising perioperative safety: Learning to walk the talk. Br. J. Anaesth. 2025, 135, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Taha-Mehlitz, S.; Ortlieb, N.; Ochs, V.; Honaker, M.D.; Rosenberg, R.; Lock, J.F.; Bolli, M.; Cattin, P.C.C. Machine learning in pancreas surgery, what is new? literature review. Front. Surg. 2023, 10, 1142585. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. Available online: https://www.bmj.com/content/366/bmj.l4898 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care. 2012, 28, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hunt, R.H. Systematic reviews: The good, the bad, and the ugly. Am. J. Gastroenterol. 2009, 104, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Portuondo, J.; Shah, S.R.; Raval, M.V.; Pan, I.W.; Zhu, H.; Fallon, S.C.; Harris, A.H.; Singh, H.; Massarweh, N.N. Complications and failure to rescue after inpatient pediatric surgery. Ann. Surg. 2022, 276, e239–e246. [Google Scholar] [CrossRef] [PubMed]
- Biccard, B.M.; Madiba, T.E.; South African Surgical Outcomes Study Investigators. The South African Surgical Outcomes Study: A 7-day prospective observational cohort study. S Afr. Med, J. 2015, 105, 465–475. Available online: https://www.sajaa.co.za/index.php/sajaa/article/view/1590 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Glance, L.G.; Osler, T.M.; Mukamel, D.B.; Dick, A.W. Effect of complications on mortality after coronary artery bypass grafting surgery: Evidence from New York State. J. Thorac. Cardiovasc. Surg. 2007, 134, 53–58. Available online: https://www.jtcvs.org/article/S0022-5223(07)00543-0/fulltext (accessed on 1 October 2025). [CrossRef] [PubMed]
- Hull, L.; Arora, S.; Aggarwal, R.; Darzi, A.; Vincent, C.; Sevdalis, N. The impact of nontechnical skills on technical performance in surgery: A systematic review. J. Am. Coll. Surg. 2012, 214, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Neily, J.; Mills, P.D.; Young-Xu, Y.; Carney, B.T.; West, P.; Berger, D.H.; Mazzia, L.; Paull, D.E.; Bagian, J.P. Association between implementation of a medical team training program and surgical mortality. JAMA 2010, 304, 1693–1700. Available online: https://jamanetwork.com/journals/jama/fullarticle/186748 (accessed on 1 October 2025). [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Design | Duration | No. of Institutions | No. of Patients | Data Source | Procedure Mix (PD/DP/TP) & Approach |
|---|---|---|---|---|---|---|---|
| Ghaferi et al. [23] | 2010 | RN | 2000–2006 | 672 | 8862 | NIS (HCUP-AHRQ) + AHA Annual Survey | NR (identified by pancreatectomy ICD-9-CM codes; PD/DP/TP breakdown not reported) |
| Haigh et al. [36] | 2011 | RN | 2005–2007 | 183 | 2610 | ACS NSQIP participant-use files | PD 100% (classic or pylorus-preserving) |
| Amini et al. [37] | 2015 | RN | 2000–2011 | 1802 | 35,986 | HCUP Nationwide Inpatient Sample | PD 51.7%; DP 33.3%; TP 5.6% (Others 9.4%) (Open 94.9%; MIS 5.1%) |
| Healy et al. [38] | 2015 | RM | 2008–2013 | 19 | 1007 | Michigan Surgical Quality Collaborative (MSQC) | PD 62.1% (Whipple 48.1% + PP-Whipple 14.0%); DP 34.1%; TP 1.8% (Open 100%) |
| Tamirisa et al. [25] | 2016 | RM | 2011–2012 | 37 | 2694 | ACS NSQIP Pancreatectomy Demonstration Project | PD 64.4%; DP 30.7%; TP 3.0% |
| Carr et al. [39] | 2017 | RS | 2013–2015 | 1 | 254 | ACS NSQIP (QITI) + institutional PD database | PD 100% |
| Gani et al. [40] | 2017 | RN | 2002–2011 | ~1834 | 11,081 | HCUP Nationwide Inpatient Sample (AHRQ) | PD 65.8%; DP 24.6%; TP 3.7% (Open 95%; MIS 5.0%) |
| Varley et al. [41] | 2017 | RN | 2005–2012 | NR | 4514 | ACS NSQIP PUF | PD 100% |
| Capretti et al. [26] | 2018 | RM | 2010–2013 | 7 | 856 | Prospectively collected hospital databases, centrally merged at Humanitas | PD 61.8%; DP 28.2%; TP 10.0% (LP 10.7%) |
| Chen et al. [42] | 2018 | RN | 2013–2015 | NR | 15,140 | MEDPAR Inpatient Files + Denominator File | PD/DP/TP breakdown NR (Open 90.6%; MIS 9.4%) |
| El Amrani et al. [4] | 2018 | RN | 2012–2015 | NR (exact count not specified) | 12,333 | PMSI (ICD-10 + French procedure classification; linked administrative data) | PD 68.9%; DP 26.9%; TP 3.2%; central 1.0% |
| Krautz et al. [8] | 2018 | RN | 2009–2014 | ~654 | 60,858 | Nationwide DRG statistics (Federal Statistical Office & Länder RDC) | PD (proximal) 60.3%; DP 26.5%; TP 8.0% (remaining: segmental 2.8%; other partial 2.4%) |
| Pecorelli et al. [43] | 2018 | RM | 2008–2015 | 3 | 120 | Institutional prospective databases at three university-affiliated high-volume centers; preoperative CT assessed centrally | PD 100%; DP 0%; TP 0% |
| van Rijssen et al. [44] | 2018 | PN | 2014–2015 | 18 | 1342 | Dutch Pancreatic Cancer Audit (DPCA) | PD 100% |
| Cerullo et al. [45] | 2019 | RN | 2010–2014 | NR | 3280 | Truven Health Analytics MarketScan Commercial Claims & Encounters | PD 93.3%; TP 6.7% |
| Diaz et al. [46] | 2019 | RM | 2005–2016 | 189 | 23,014 | California OSHPD hospital discharge database | PD 100% |
| Gleeson et al. [47] | 2019 | RN | 2005–2012 | NR | 5027 | ACS NSQIP PUF | PD 100%; DP 0%; TP 0% |
| Merath et al. [48] | 2019 | RN | 2013–2015 | 737 | 13,873 | MEDPAR Inpatient Files linked with Denominator File; AHA Survey; Medicare cost reports (wage index) | NR |
| Sánchez-Velázquez et al. [5] | 2019 | RI | 2012–2015 | 23 | 2375 | Prospective databases from each center centrally pooled (whipplebenchmarks.org) | PD 100% (Open 100%) |
| van Roessel et al. [49] | 2019 | RS | 1992–2017 | 1 | 1434 | Institutional prospective database; survival from National Cancer Registry | PD 100% (PPPD 81.9%; Whipple 18.1%) (Open 96.4%; LP 3.6%) |
| Wroński et al. [50] | 2019 | RS | 2003–2017 | 1 | 43 | Single institution (Medical University of Warsaw) | PD 100%; DP 0% |
| Bhatti et al. [51] | 2020 | RS | 2011–2018 | 1 | 116 | Single-institution hospital records | PD 100% (standard PD 70%; PPPD 19%; PD + organ resection 11%; vascular resection 12%) |
| El Amrani et al. [18] | 2020 | RN | 2012–2018 | NR (nationwide; all hospitals) | 19,938 | PMSI (ICD-10 diagnoses + CCAM procedures) | PD 75.0%; DP 23.9%; CP 0.7%; TP 0.4% |
| Nymo et al. [52] | 2020 | RN | 2015–2016 | 5 | 394 | NoRGast national quality registry + EPJ cross-check; deaths via National Registry linkage | PD 100% |
| Endo et al. [53] | 2021 | RN | 2011–2014 | ≈4000 | 422 | National Clinical Database (NCD) | HPD (which includes PD as a component) 100%; Major HPD (60%); Minor HPD (40%) |
| Gleeson et al. [20] | 2021 | RI | 2014–2017 | ≈224 | 22,983 | ACS NSQIP; DPCA; SNPPCR; DGAV StuDoQ Pancreas | PD 100% (MIS 6.0%) |
| Lequeu et al. [54] | 2021 | RN | 2009–2018 | 631 | 10,632 | PMSI (Programme de Médicalisation des Systèmes d’Information) | DP 100% (Open 77.0%; LP 23.0%) |
| Pastrana et al. [55] | 2021 | RN | 2006–2016 | ~121–680 | 32,165 | ACS NSQIP PUF | PD 100% |
| Bassi et al. [56] | 2022 | RS | 2000–2019 | 1 | 2989 | Institutional database (prospectively collected; retrospectively analyzed) | PD 100% (PPPD 86.0%; Whipple 14.0%) |
| Di Gioia et al. [57] | 2022 | RS | 2010–2019 | 1 | 1865 | University of Verona Hospital Trust (Pancreas Institute) prospective database; retrospectively analyzed | PD 100% |
| Sutton et al. [58] | 2022 | RS | 2013–2020 | 1 | 637 | Institutional NSQIP (100% capture of pancreatectomies); MPOG (intra-op); hospital cost administrative data (USD) | Whipple/Total pancreatectomy 63%; DP/RAMPS 37% (Open 81.5%; MIS 18.5% by back-calculatin) |
| van Beek et al. [59] | 2022 | RM | 2008–2019 | 2 | 123 | Hospital surgical databases & histopathology archives; electronic patient records | PD/PPPD 41.5%; DP 40.7%; TP 4.9% (enucleation 9.8%; combined 3.3%) (Open 66.7%; LP 13.0%; Rb 20.3%) |
| Fukada et al. [60] | 2023 | RS | 2010–2022 | 1 | 177 | Single-institution hospital dataset (JSHBPS-certified training institution) | NR (mixed HPB procedures; PD/DP/TP distribution unclear) |
| Li et al. [61] | 2023 | RS | 2011–2020 | 1 | 58 | Single-center HPB prospective registry + retrospective chart completion | PD 100% |
| Moazzam et al. [62] | 2023 | RN | 2013–2017 | 677 | 19,625 | 100% Medicare Standard Analytic Files (SAFs) | NR |
| Suurmeijer et al. [63] | 2023 | PN | 2014–2019 | 18 → 16 | 5345 | Dutch Pancreatic Cancer Audit (DPCA; DPCG) | PD 79%; DP 21% |
| Theijse et al. [64] | 2023 | RN | 2014–2021 | NR (all national DPCG centers) | 1402 | Dutch Pancreatic Cancer Audit (DPCA) | PD 100% (PPPD 48.2%; PRPD 12.6%; Classic Whipple 39.2%) (Open 74.6%; Rb 20.3%; LP 5.11%) |
| Vawter et al. [65] | 2023 | RN | 2014–2019 | NR | 45,157 | ACS NSQIP standard & pancreas-targeted registries | PD 67%; DP 33% |
| Cannas et al. [66] | 2024 | RI | 2003–2023 | 18 | 8189 | Pancreas Fistula Study Group dataset | PD 100% (MIS 3.8%) |
| de Graaff et al. [67] | 2024 | RN | 2014–2021 | 24 | 7365 | DHBA, DPCA (managed by DICA) | PD 78.9%; DP 21.1% (Open 68.8%; LP 26.8%; 4.4%, minor variable-level missingness remains) |
| Duclos et al. [68] | 2024 | RM | 2014–2018 | 21 | 1188 | 21 high-volume centers (data collected at each site) | DP 100% (Open 52.8%; LP 41.4%; Rb 5.8%) |
| Heckman et al. [69] | 2024 | RS | 2016–2022 | 1 | 65 | Prospectively maintained institutional database + ACS NSQIP | PD 100% (Rb 100%) |
| Henry et al. [70] | 2024 | RN | 2014–2019 | 17 | 149 | Dutch Pancreatic Cancer Audit (DPCA); PORSCH | PD 82%; DP 11%; TP 7% (Open 88%; LP 7%; Rb 6%) |
| Khalid et al. [71] | 2024 | RM | 2014–2023 | NR (multicenter health-system EHR registry) | 314 | Northwell Health multicenter pancreatic cancer database (EHR abstracted to REDCap) | PD (classical; PPPD; LP; Rb;); DP (open; LP; Rb); TP |
| Kinny-Köster et al. [72] | 2024 | RS | 2003–2021 | 1 | 156 | Prospective institutional pancreatectomy registry | PD 100% (Open 96.8%; Rb 3.2%) |
| Leech et al. [73] | 2024 | RS | 1999–2023 | 1 | 79 | Pancreatic resection registry (UCT/Groote Schuur) | PD 100% (PPPD 98.7%; Classical 1.3%) |
| PancreasGroup.org Collaborative [74] | 2024 | PI | 2021 | 354 | 4223 | PancreasGroup.org electronic CRF (mandatory outcome fields) | PD 59.3%; DP 24.5%; TP 6.9%; Enucleation 1.5%; Other 5.2% (percentages may not sum to 100%) (Open 83.9%; LP 11.6%; Rb 4.5%) |
| Patel et al. [75] | 2024 | RN | 2014–2020 | NR (multicenter NSQIP) | 15,790 | ACS NSQIP Pancreatectomy Targeted | PD/DP/TP (PD/DP/TP distribution unclear) |
| Wang et al. [76] | 2024 | RS | 2015–2022 | 1 | 995 | Single hospital database (The First Hospital of Jilin University) | PD 100% (LP 100%) |
| Capretti et al. [77] | 2025 | PM | 2016–2022 | 5 | 277 | Prospectively collected data from participating centers | PD 72.9%; TP 27.1% |
| Tschaidse et al. [78] | 2023 | RN | 2014–2019 | ~60 | 3011 | StuDoQ|Pancreas (DGAV) registry | PD 80.1%; DP 19.9% (Open 94%; LP 5.7%) |
| Uttinger et al. [79] | 2025 | RN | 2010–2020 | 939 | 94,661 | German DRG billing data (Federal Statistical Office) | PD 61.2%; DP 26.6%; TP 9.9% |
| Authors | Year | Definition | Time Window | Severity Threshold | Denominator | Reported FTR (n/N, %) | Post-Discharge Capture |
|---|---|---|---|---|---|---|---|
| Capretti et al. (PD) [77] | 2025 | 90-CD3 [G1] | 90 days | CD ≥ III (≥IIIa) | CD ≥ III complication | 15/73 (20.5%) | Yes (90-day follow-up; method NR) |
| Capretti et al. (TP) [77] | 2025 | 90-CD3 [G1] | 90 days | CD ≥ III (≥IIIa) | CD ≥ III complication | 3/19 (15.8%) | Yes (90-day follow-up; method NR) |
| Lequeu et al. [54] | 2021 | 90-CD3 [G1] | 90 days | CD ≥ III | CD ≥ III complication | 355/3153 (11.2%) | Unclear (national in/outpatient linkage, external death ascertainment NR) |
| Nymo et al. [52] | 2020 | 90-Acc3 [G1] | 90 days | Accordion ≥ 3 (re-anchored to CD ≥ III) | Major complication (Accordion ≥ 3) | 17/125 (13.6%) | Yes (national registry linkage incl. cross-regional EPJ) |
| PancreasGroup.org Collaborative (PD) [74] | 2024 | 90-CD3a [G1] | 90 days | CD ≥ IIIa | Major-complication (CD ≥ IIIa) | 157/717 (21.9%) | Yes (prospective 90-day follow-up via CRF; ascertainment method NR) |
| PancreasGroup.org Collaborative (DP) [74] | 2024 | 90-CD3a [G1] | 90 days | CD ≥ IIIa | Major-complication (CD ≥ IIIa) | 20/203 (10.0%) | Yes (prospective 90-day follow-up via CRF; ascertainment method NR) |
| Pecorelli et al. [43] | 2018 | 90-CD3 [G1] | 90 days | CD ≥ III (III–IV) | CD ≥ III complication | 23/120 (19.2%) | Yes (90-day mortality; method NR) |
| van Beek et al. [59] | 2022 | 90-CD3 [G1] | 90 days | CD ≥ III | CD ≥ III complication | 1/51 (2.0%) | Unclear (90-day mortality adopted; ascertainment NR) |
| Bassi et al. [56] | 2022 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 70/597 (11.7%) | No (in-hospital only) |
| de Graaff et al. (PD) [67] | 2024 | H/30-CD3a [G3] | In-hospital/30 days | CD ≥ IIIa | Major complications (CD ≥ IIIa) | 7.5% (1.6–28.5%) | No (in-hospital/30 d) |
| de Graaff et al. (DP) [67] | 2024 | H/30-CD3a [G3] | In-hospital/30 days | CD ≥ IIIa | Major complications (CD ≥ IIIa) | 3.1% (0–14.9%) | No (in-hospital/30 d) |
| Di Gioia et al. [57] | 2022 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 57/404 (14.1%) | No (in-hospital only) |
| Fukada et al. [60] | 2023 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 9/177 (5.1%) | No (in-hospital only) |
| Leech et al. [73] | 2024 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 3/21 (14.3%) | No (in-hospital only) |
| Suurmeijer et al. [63] | 2023 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | PD 54/404 > 46/426 > 34/462 (13.4 > 10.8 > 7.4%); DP 5/57 > 6/84 > 5/85 (8.8 > 7.1 > 5.9%) | No (in-hospital/30 d; 90 d not obtained) |
| Theijse et al. [64] | 2023 | H-CD3a [G3] | In-hospital | CD ≥ IIIa | Major complications (CD ≥ IIIa) | 57/642 (8.9%) | No (DPCA −30 d only) |
| van Rijssen et al. [44] | 2018 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 56/391 (14.3%) | No (in-hospital only) |
| van Roessel et al. [49] | 2019 | H-CD3 [G3] | In-hospital | CD ≥ III | Major complications (CD ≥ III) | 31/463 (6.7%) | No (in-hospital only) |
| Gleeson et al. [20] | 2021 | H-CD3-Mix [G3] | In-hospital | CD ≥ III or ISGPS POPF B/C (mixed) | CD ≥ III or POPF B/C (mixed) | 8.20% | No (in-hospital only) |
| Chen et al. [42] | 2018 | 90-Any-Admin [G4] | 90 days | Any (administrative) | Any complication | Pancreas Open 19.4%, MIS 13.4% | Yes (Medicare denominator file) |
| Carr et al. [39] | 2017 | H/30-Any [G5] | 30 d/In-hospital | Any (non-CD) | Any complication | Fellow 6%, Resident 4% | Unclear (30 d and in-hospital mixed) |
| Haigh et al. [36] | 2011 | 30-Any-NSQIP [G5] | 30 days | NSQIP “≥1 morbidity” | “≥1 morbidity” | older 10.1% vs. younger 4.1% | Yes (NSQIP 30 d) |
| Amini et al. [37] | 2015 | H-Any-Admin [G6] | In-hospital | Other (ICD-9 “major”) | Any complication (ICD-9 “major”) | All 9.0%; LV 12.0%; IV 8.5%; HV 6.4% | No (in-hospital only; NIS) |
| Cerullo et al. [45] | 2019 | H-Any-Admin [G6] | In-hospital | Other (claims “major”) | Major complications (claims) | 27/920 (2.9%) | No (in-hospital only) |
| El Amrani et al. [18] | 2020 | H-Any-Admin [G6] | In-hospital | Other (administrative “major”) | ≥1 major complications | 940/10,758 (8.7%) | No (in-hospital only) |
| Gani et al. [40] | 2017 | H-Any-Admin [G6] | In-hospital | Any (AHRQ) | Any complication | LV 11.1%, IV 7.1%, HV5.4% | No (in-hospital only) |
| Ghaferi et al. [23] | 2010 | H-Any-Admin [G6] | In-hospital | Other (ICD-9 “major”) | Major complications (ICD-9) | Quintile of hospital mortality (6.4–40.0) | No (in-hospital only) |
| Tamirisa et al. [25] | 2016 | H-Any-NSQIP [G6] | In-hospital | Any (NSQIP events) | Any complication | 34/1111 (3.1%) | No (in-hospital only) |
| Uttinger et al. [79] | 2025 | H-Any-Admin [G6] | In-hospital | Any (administrative/registry) | Any complication | 8040/64,029 (12.6%) | No (in-hospital only) |
| Varley et al. [41] | 2017 | H-Any-NSQIP [G6] | In-hospital | Any (NSQIP major/minor) | Any complication | 312/4514 (6.9%) | No (in-hospital only) |
| Duclos et al. [68] | 2024 | 90-Spec [G7] | 90 days | ISGPS CR-PPH B/C and/or CR-POPF B/C | Specific complications (CR-PPH n = 65; CR-POPF n = 202) | CR-PPH 9/65 (13.8%); CR-POPF 1.3% (n NR) | Yes (90-day outcome capture; method NR) |
| Cannas et al. [66] | 2024 | 90-Acc3-Alt [G8] | 90 days | Accordion ≥3 | Severe complications (Accordion ≥ 3) | 182/1533 (11.9%) | Yes (90-day follow-up) |
| Gleeson et al. [47] | 2019 | 30-NSser-NSQIP [G8] | 30 days | NSQIP serious/major | Serious/major morbidity | 361/5027 (7.2%) | Yes (NSQIP 30 d) |
| Healy et al. [38] | 2015 | 30-Alt [G8] | 30 days | MSQC major (non-Clavien) | Major complications (MSQC) | LV 21.8%, HV 14.9% | Yes (registry 30 d) |
| Li et al. [61] | 2023 | H + 90-CD4 [G8] | In-hospital + 90 days | CD IV | CD IV patients | 19/58 (33.0%) | Unclear (90 d capture; method NR) |
| Pastrana Del Valle et al. [55] | 2021 | 30-NSmaj-NSQIP [G8] | 30 days | NSQIP major morbidity | Major morbidity | Yearly % 9.8→4.1 (2006→2016) | Yes (NSQIP 30 d) |
| Patel et al. [75] | 2024 | 30-CD3-NSQIP [G8] | 30 days | CD ≥ III (NSQIP mapped) | Major complications (CD ≥ III) | 245/4623 (5.3%) | Yes (NSQIP 30 d) |
| Vawter et al. [65] | 2023 | 30-NSser-NSQIP [G8] | 30 days | NSQIP serious morbidity | Serious morbidity | Standard NSQIP: PD 184/1720 (10.7%); DP 47/578 (8.1%); Pancreas-targeted NSQIP: PD 400/5871 (6.8%); DP 94/1681 (5.6%) | Yes (NSQIP 30 d) |
| Wang et al. [76] | 2024 | H-Any-Alt [G8] | In-hospital | Other (enumerated “major”) | “Major complications” (denominator NR) | 24 (2.4%) | No (in-hospital only) |
| Endo et al. [53] | 2021 | Comp-Any [G8] | Composite (in-hospital ≤90 days + 30 days post-discharge) | Any (non-CD) | Any complication | 33.3%/17.0%/9.3% (22/66; 8/47; 18/193) | Partial (composite window) |
| Authors | Organizational/Institutional Factors | Surgical Technique | Perioperative Management | Patient- Related Factors | Non-Technical Skills (NTS) |
|---|---|---|---|---|---|
| Ghaferi et al. [23] | ✔ | ✔ | ✔ | ||
| Haigh et al. [36] | ✔ | ✔ | ✔ | ✔ | ✔ |
| Amini et al. [37] | ✔ | ✔ | |||
| Healy et al. [38] | ✔ | ✔ | ✔ | ||
| Tamirisa et al. [25] | ✔ | ✔ | ✔ | ✔ | ✔ |
| Carr et al. [39] | ✔ | ✔ | ✔ | ||
| Gani et al. [40] | ✔ | ✔ | |||
| Varley et al. [41] | ✔ | ✔ | ✔ | ✔ | |
| Capretti et al. [26] | ✔ | ✔ | ✔ | ||
| Chen et al. [42] | ✔ | ✔ | |||
| El Amrani et al. [4] | ✔ | ✔ | ✔ | ||
| Krautz et al. [8] | ✔ | ||||
| Pecorelli et al. [43] | ✔ | ✔ | |||
| van Rijssen et al. [44] | ✔ | ✔ | ✔ | ✔ | |
| Cerullo et al. [45] | ✔ | ✔ | ✔ | ||
| Diaz et al. [46] | ✔ | ✔ | |||
| Gleeson et al. [47] | ✔ | ✔ | ✔ | ✔ | |
| Merath et al. [48] | ✔ | ||||
| Sánchez-Velázquez et al. [5] | ✔ | ✔ | |||
| van Roessel et al. [49] | ✔ | ✔ | |||
| Wroński et al. [50] | ✔ | ✔ | ✔ | ✔ | |
| Bhatti et al. [51] | ✔ | ✔ | ✔ | ||
| El Amrani et al. [68] | ✔ | ✔ | ✔ | ||
| Nymo et al. [52] | ✔ | ✔ | |||
| Endo et al. [53] | ✔ | ✔ | ✔ | ✔ | |
| Gleeson et al. [20] | ✔ | ✔ | |||
| Lequeu et al. [54] | ✔ | ✔ | ✔ | ||
| Pastrana et al. [55] | ✔ | ✔ | |||
| Bassi et al. [56] | ✔ | ✔ | ✔ | ✔ | ✔ |
| Di Gioia et al. [57] | ✔ | ✔ | ✔ | ✔ | |
| Sutton et al. [58] | ✔ | ✔ | ✔ | ✔ | |
| van Beek et al. [59] | ✔ | ✔ | ✔ | ✔ | ✔ |
| Fukada et al. [60] | ✔ | ✔ | ✔ | ✔ | |
| Li et al. [61] | ✔ | ✔ | ✔ | ||
| Moazzam et al. [62] | ✔ | ||||
| Suurmeijer et al. [63] | ✔ | ✔ | ✔ | ✔ | |
| Theijse et al. [64] | ✔ | ✔ | ✔ | ✔ | |
| Vawter et al. [65] | ✔ | ✔ | ✔ | ||
| Cannas et al. [66] | ✔ | ✔ | ✔ | ✔ | |
| de Graaff et al. [67] | ✔ | ✔ | |||
| Duclos et al. [68] | ✔ | ✔ | ✔ | ||
| Heckman et al. [69] | ✔ | ✔ | ✔ | ||
| Henry et al. [70] | ✔ | ✔ | ✔ | ✔ | ✔ |
| Khalid et al. [71] | ✔ | ✔ | |||
| Kinny-Köster et al. [72] | ✔ | ✔ | ✔ | ✔ | |
| Leech et al. [73] | ✔ | ✔ | ✔ | ||
| Patel et al. [75] | ✔ | ||||
| Uttinger et al. [79] | ✔ | ✔ | |||
| Wang et al. [76] | ✔ | ||||
| PancreasGroup.org Collaborative [74] | ✔ | ✔ | |||
| Capretti et al. [77] | ✔ | ✔ | ✔ | ||
| Tschaidse et al. [78] | ✔ | ✔ |
| Category | Elements |
|---|---|
| Situation awareness | Gathering information |
| Interpreting information | |
| Anticipating future states | |
| Decision-making | Defining the problem |
| Considering options | |
| Selecting and implementing an option | |
| Outcome review | |
| Communication | Sending information clearly and concisely |
| Including context and intent during information exchange | |
| Receiving information, especially by listening | |
| Identifying and addressing barriers to communication | |
| Team working | Supporting others |
| Solving conflicts | |
| Exchanging information | |
| Coordinating activities | |
| Leadership | Using authority |
| Maintaining standards | |
| Planning and prioritizing | |
| Managing workload and resources | |
| Managing stress | Identifying the symptoms of stress |
| Recognizing the effects of stress | |
| Implementing coping strategies | |
| Coping with fatigue | Identifying the symptoms of fatigue |
| Recognizing the effects of fatigue | |
| Implementing coping strategies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uramatsu, M.; Fujisawa, Y.; Barach, P.; Osakabe, H.; Matsumoto, M.; Nagakawa, Y. Failure to Rescue After Surgery for Pancreatic Cancer: A Systematic Review and Narrative Synthesis of Risk Factors and Safety Strategies. Cancers 2025, 17, 3259. https://doi.org/10.3390/cancers17193259
Uramatsu M, Fujisawa Y, Barach P, Osakabe H, Matsumoto M, Nagakawa Y. Failure to Rescue After Surgery for Pancreatic Cancer: A Systematic Review and Narrative Synthesis of Risk Factors and Safety Strategies. Cancers. 2025; 17(19):3259. https://doi.org/10.3390/cancers17193259
Chicago/Turabian StyleUramatsu, Masashi, Yoshikazu Fujisawa, Paul Barach, Hiroaki Osakabe, Moe Matsumoto, and Yuichi Nagakawa. 2025. "Failure to Rescue After Surgery for Pancreatic Cancer: A Systematic Review and Narrative Synthesis of Risk Factors and Safety Strategies" Cancers 17, no. 19: 3259. https://doi.org/10.3390/cancers17193259
APA StyleUramatsu, M., Fujisawa, Y., Barach, P., Osakabe, H., Matsumoto, M., & Nagakawa, Y. (2025). Failure to Rescue After Surgery for Pancreatic Cancer: A Systematic Review and Narrative Synthesis of Risk Factors and Safety Strategies. Cancers, 17(19), 3259. https://doi.org/10.3390/cancers17193259






