Routine Imaging Surveillance After Frontline ABVD Improves Outcome in High-Risk Hodgkin Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatments
2.2. Inclusion and Exclusion Criteria
2.3. Follow-Up Procedures and Diagnosis of Relapse
2.4. Study Endpoints
- Overall EFS, defined as the time from CR following initial therapy to the occurrence of any event (as defined for second EFS) or the last follow-up. This measure was used to evaluate the long-term impact of follow-up strategies on disease control and second relapse-free survival.
- First and second EFS among patients with asymptomatic (clinically silent) relapses, both in the overall study population and within the imaging cohort.
- Response rates to salvage therapy, as assessed by FDG-PET/CT, both prior to and after AHSCT in each cohort.
- The prognostic impact of established HL risk factors on EFS outcomes.
2.5. Statistical Analysis
Propensity-Score-Matched (PSM) Sub-Analysis to Mitigate Baseline Prognostic Differences
3. Results
3.1. Relapse and Surveillance
3.2. Clinical Features at Relapse
3.3. Salvage Therapy and Response
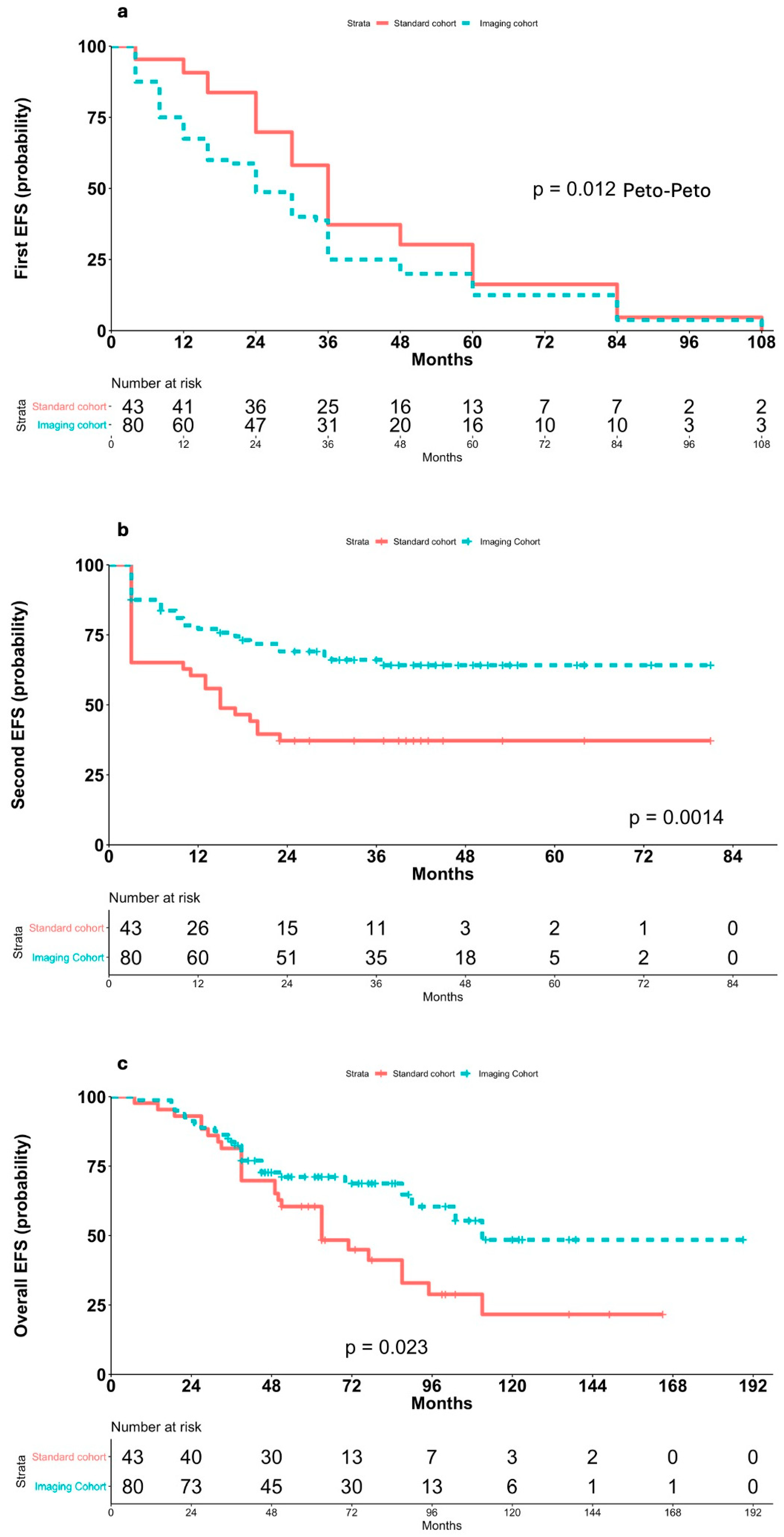
3.4. Event-Free Survival and Outcomes
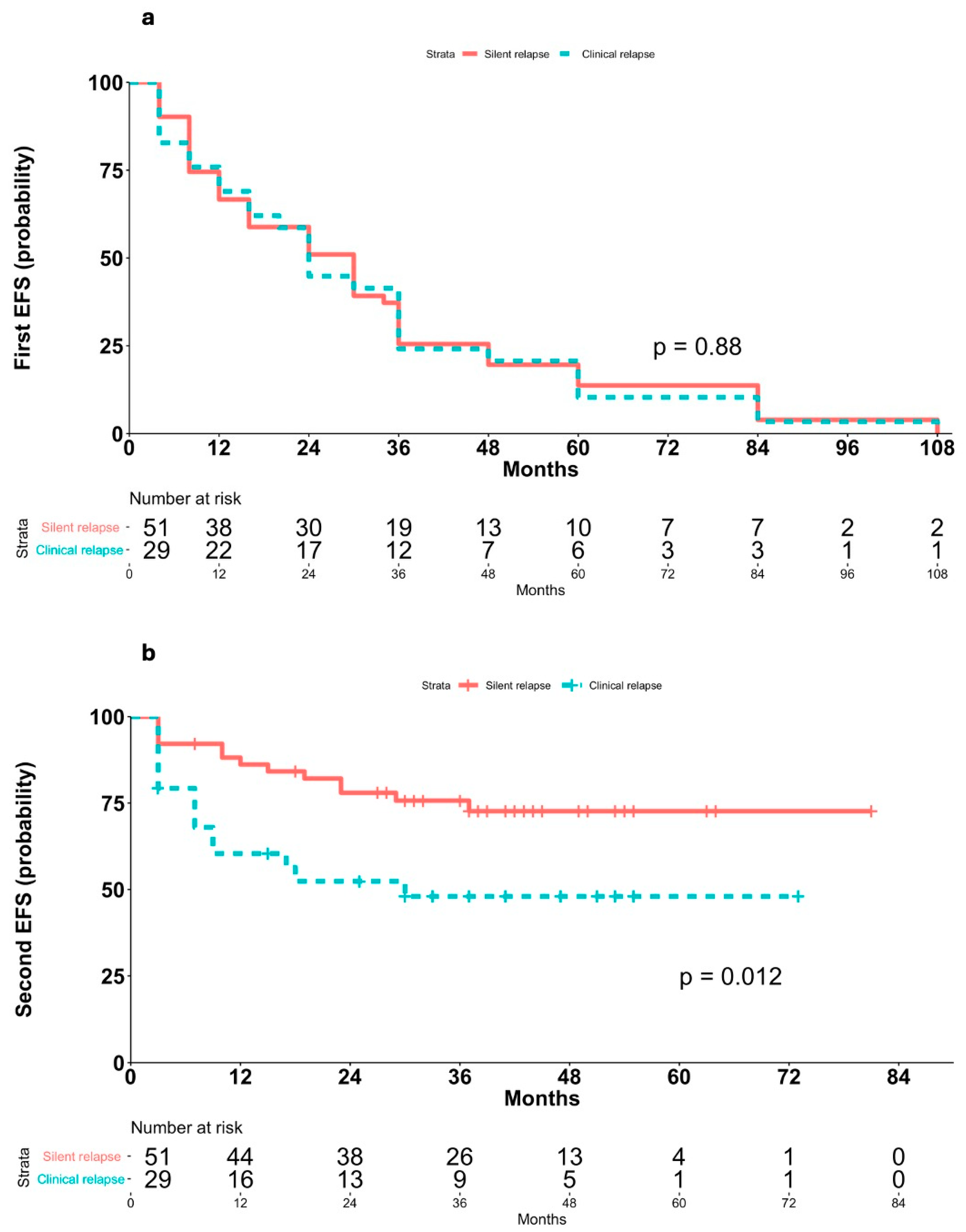
3.5. Impact of Symptomatic vs. Asymptomatic Relapses
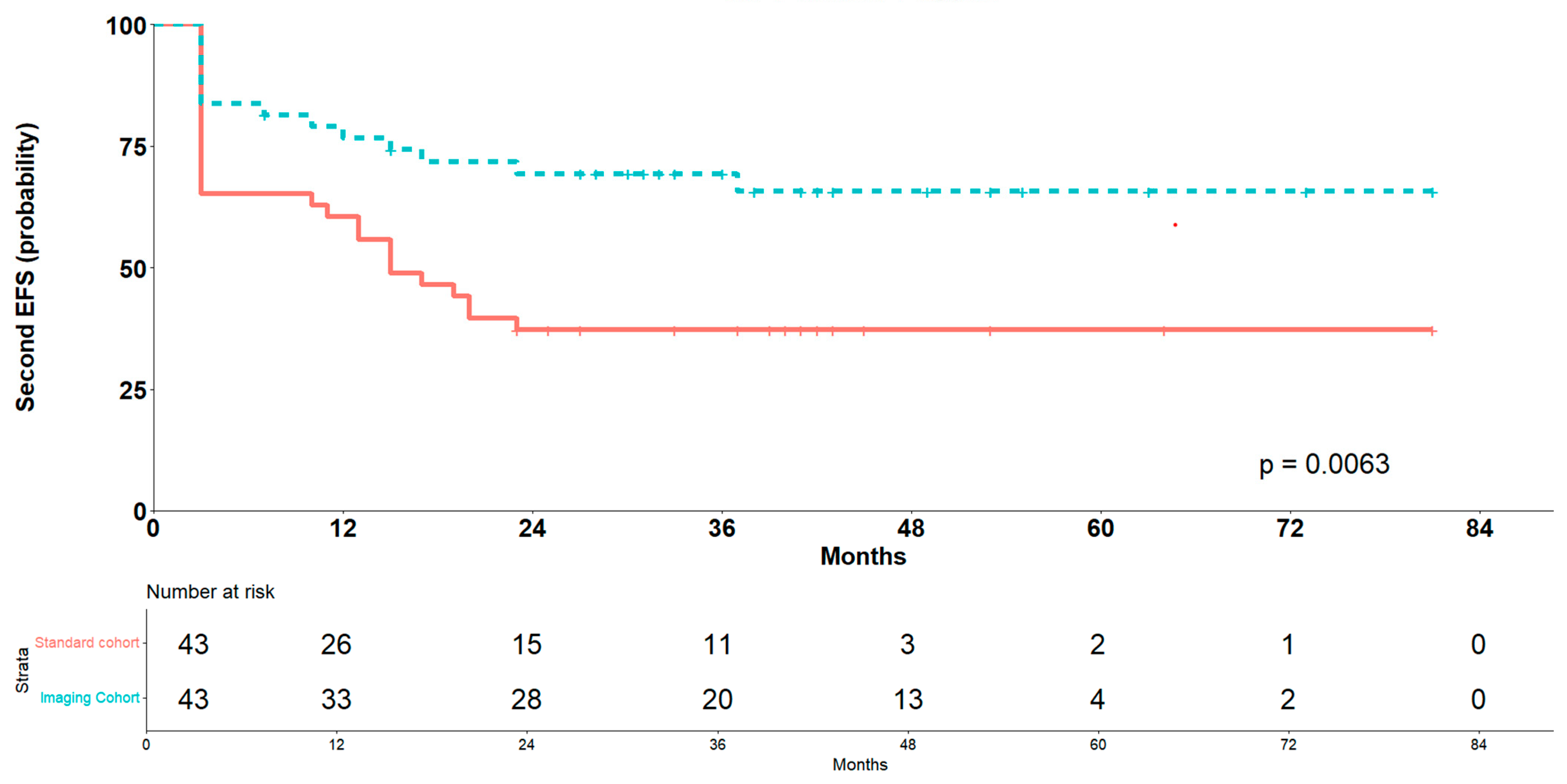
3.6. Outcomes After PSM Sub-Analysis
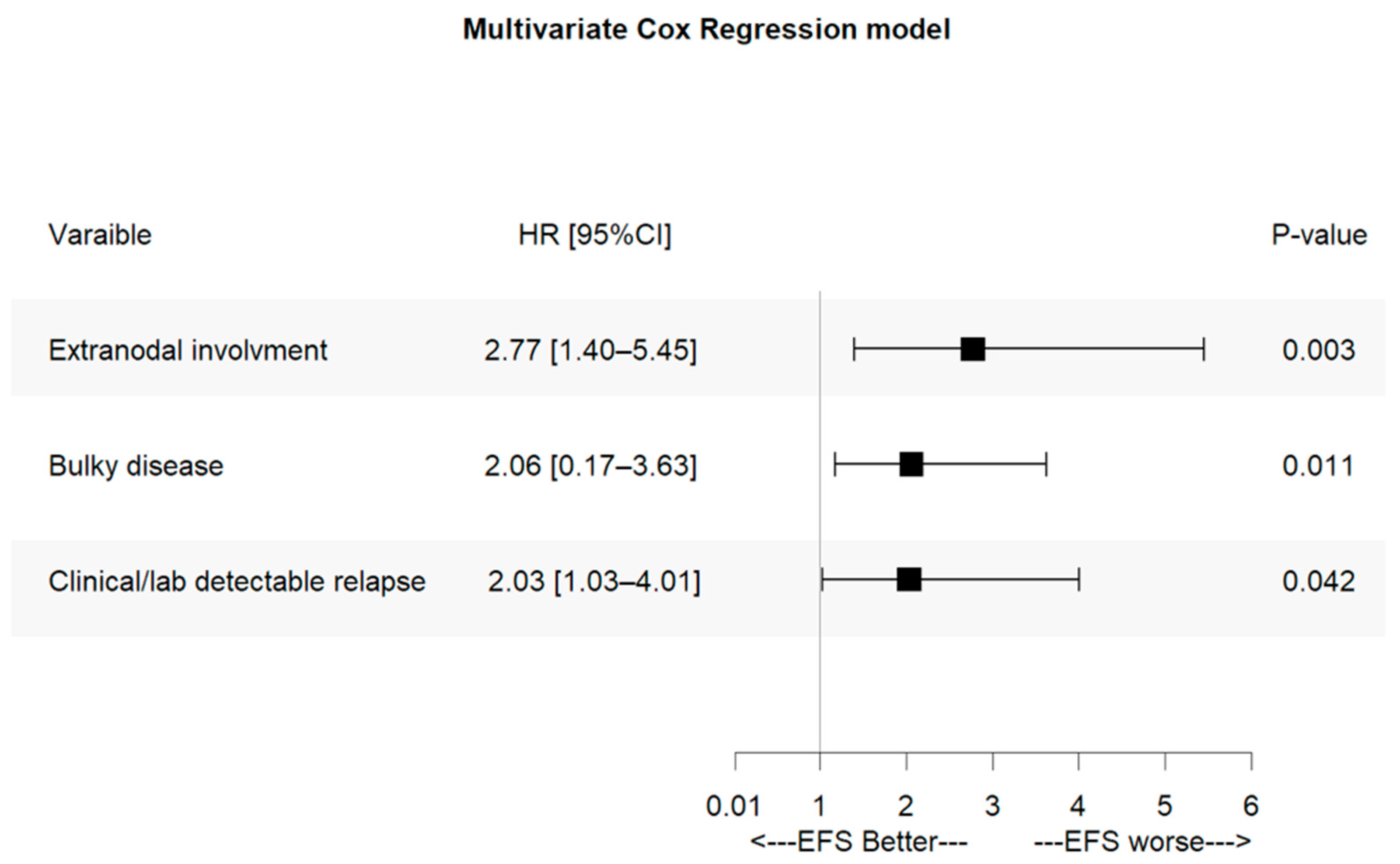
3.7. Prognostic Factors for Event-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CR | Complete response |
| HL | Hodgkin lymphoma |
| AHSCT | Autologous hematopoietic stem-cell transplant |
| ABVD | Adriamycin, bleomycin, vinblastine, and dacarbazine |
| US | Ultrasonography |
| FDG | Fluorodeoxyglucose computed tomography (CT) scans |
| PET | Positron emission tomography |
| CT | Computed tomography |
| ESR | Erythrocyte sedimentation rate |
| PR | Partial response |
| EFS | Event-free survival |
| 95% CI | 95% confidence interval |
| HR | Hazard ratio |
| PH | Proportional hazards |
| PMS | Propensity score matched |
References
- LaCasce, A.S. Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol. Oncol. 2019, 37, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Armand, P.; Bello, C.M.; Benitez, C.M.; Bierman, P.J.; Boughan, K.M.; Dabaja, B.; et al. Hodgkin Lymphoma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 755–781. [Google Scholar] [CrossRef] [PubMed]
- Pingali, S.R.; Jewell, S.W.; Havlat, L.; Bs, M.A.B.; Thompson, J.R.; Eastwood, D.C.; Bartlett, N.L.; Armitage, J.O.; Wagner-Johnston, N.D.; Vose, J.M.; et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer 2014, 120, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.H.; Hutchings, M.; Brown, P.d.N.; Linderoth, J.; Mylam, K.J.; Molin, D.; Johnsen, H.E.; Bøgsted, M.; Jerkeman, M.; El-Galaly, T.C. No survival benefit associated with routine surveillance imaging for Hodgkin lymphoma in first remission: A Danish-Swedish population-based observational study. Br. J. Haematol. 2016, 173, 236–244. [Google Scholar] [CrossRef]
- Dann, E.J.; Berkahn, L.; Mashiach, T.; Frumer, M.; Agur, A.; McDiarmid, B.; Bar-Shalom, R.; Paltiel, O.; Goldschmidt, N. Hodgkin lymphoma patients in first remission: Routine positron emission tomography/computerized tomography imaging is not superior to clinical follow-up for patients with no residual mass. Br. J. Haematol. 2013, 164, 694–700. [Google Scholar] [CrossRef]
- Lee, A.I.; Zuckerman, D.S.; Abbeele, A.D.V.D.; Aquino, S.L.; Crowley, D.; Toomey, C.; Lacasce, A.S.; Feng, Y.; Neuberg, D.S.; Hochberg, E.P. Surveillance imaging of Hodgkin lymphoma patients in first remission. Cancer 2010, 116, 3835–3842. [Google Scholar] [CrossRef]
- Guadagnolo, B.A.; Punglia, R.S.; Kuntz, K.M.; Mauch, P.M.; Ng, A.K. Cost-Effectiveness Analysis of Computerized Tomography in the Routine Follow-Up of Patients After Primary Treatment for Hodgkin’s Disease. J. Clin. Oncol. 2006, 24, 4116–4122. [Google Scholar] [CrossRef]
- Levine, J.M.; Weiner, M.; Kelly, K.M. Routine Use of PET Scans After Completion of Therapy in Pediatric Hodgkin Disease Results in a High False Positive Rate. J. Pediatr. Hematol. 2006, 28, 711–714. [Google Scholar] [CrossRef]
- Picardi, M.; Pugliese, N.; Cirillo, M.; Zeppa, P.; Cozzolino, I.; Ciancia, G.; Pettinato, G.; Salvatore, C.; Quintarelli, C.; Pane, F. Advanced-stage Hodgkin Lymphoma: US/Chest Radiography for Detection of Relapse in Patients in First Complete Remission—A Randomized Trial of Routine Surveillance Imaging Procedures. Radiology 2014, 272, 262–274. [Google Scholar] [CrossRef]
- Picardi, M.; De Renzo, A.; Pane, F.; Nicolai, E.; Pacelli, R.; Salvatore, M.; Rotoli, B. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk. Lymphoma 2007, 48, 1721–1727. [Google Scholar] [CrossRef]
- Picardi, M.; Fonti, R.; Della Pepa, R.; Giordano, C.; Pugliese, N.; Nicolai, E.; Salvatore, M.; Mainolfi, C.; Venetucci, P.; Rascato, M.; et al. 2-deoxy-2[F-18] fluoro-D-glucose positron emission tomography Deauville scale and core-needle biopsy to determine successful management after six doxorubicin, bleomycin, vinblastine and dacarbazine cycles in advanced-stage Hodgkin lymphoma. Eur. J. Cancer 2020, 132, 85–97. [Google Scholar] [CrossRef]
- Josting, A.; Sieniawski, M.; Glossmann, J.-P.; Staak, O.; Nogova, L.; Peters, N.; Mapara, M.; Dörken, B.; Ko, Y.; Metzner, B.; et al. High-dose sequential chemotherapy followed by autologous stem cell transplantation in relapsed and refractory aggressive non-Hodgkin’s lymphoma: Results of a multicenter phase II study. Ann. Oncol. 2005, 16, 1359–1365. [Google Scholar] [CrossRef]
- Santoro, A.; Magagnoli, M.; Spina, M.; Pinotti, G.; Siracusano, L.; Michieli, M.; Nozza, A.; Sarina, B.; Morenghi, E.; Castagna, L.; et al. Ifosfamide, gemcitabine, and vinorelbine: A new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica 2007, 92, 35–41. [Google Scholar] [CrossRef]
- Musso, M.; Scalone, R.; Marcacci, G.; Lanza, F.; Di Renzo, N.; Cascavilla, N.; Di Bartolomeo, P.; Crescimanno, A.; Perrone, T.; Pinto, A. Fotemustine plus etoposide, cytarabine and melphalan (FEAM) as a new conditioning regimen for lymphoma patients undergoing auto-SCT: A multicenter feasibility study. Bone Marrow Transplant. 2009, 45, 1147–1153. [Google Scholar] [CrossRef]
- Sellner, L.; Boumendil, A.; Finel, H.; Choquet, S.; de Rosa, G.; Falzetti, F.; Scime, R.; Kobbe, G.; Ferrara, F.; Delmer, A.; et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: A retrospective study from the EBMT. Bone Marrow Transplant. 2015, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised Response Criteria for Malignant Lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 4, ISBN 9789283224310. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Lister, T.A.; Crowther, D.; Sutcliffe, S.B.; Glatstein, E.; Canellos, G.P.; Young, R.C.; Rosenberg, S.A.; Coltman, C.A.; Tubiana, M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J. Clin. Oncol. 1989, 7, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M.; ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Viviani, S.; Vanazzi, A.; Frassoni, S.; Rusconi, C.; Rossi, A.; Romano, A.; Patti, C.; Schiavotto, C.; Sorasio, R.; Marasco, V.; et al. High-dose chemotherapy and autologous stem cell transplant as first salvage treatment for relapsed or refractory Hodgkin Lymphoma in the era of PET-adapted strategies. Leuk. Lymphoma 2024, 65, 460–471. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, M.S.; Elhassan, T.A.M.; Khan, Z.A.; Elshenawy, M.A.; Maghfoor, I. Impact of Risk Factors and Long Term Survival Analysis of Patients with Primary Refractory Hodgkin Lymphoma Who Underwent High Dose Chemotherapy and Autologous Stem Cell Transplant. Biol. Blood Marrow Transplant. 2023, 29, 451.e1–451.e12. [Google Scholar] [CrossRef]
- Tanner, E.J.; Chi, D.S.; Eisenhauer, E.L.; Diaz-Montes, T.P.; Santillan, A.; Bristow, R.E. Surveillance for the detection of recurrent ovarian cancer: Survival impact or lead-time bias? Gynecol. Oncol. 2010, 117, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.G.; Malmgren, J.A.; Atwood, M.K. Breast cancer distant recurrence lead time interval by detection method in an institutional cohort. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Evens, A.M. Newly Diagnosed Classical Hodgkin Lymphoma: Optimizing Outcomes in a New Therapeutic Era. Hematol. Oncol. 2025, 43, e70066. [Google Scholar] [CrossRef]
- Yılmaz, U.; Ateşoğlu, E.B.; Ferhanoğlu, B. Tight competition for the first-line treatment of Hodgkin’s lymphoma: A comprehensive review of practice-changing developments in recent years. Expert Opin. Biol. Ther. 2025, 25, 731–747. [Google Scholar] [CrossRef]
- Biasoli, I.; Spector, N. New agents in relapsed/refractory Hodgkin’s lymphoma. Rev. Bras. de Hematol. e Hemoter. 2017, 39, 193–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Mi, L.; Li, Z.; Zhu, J.; Wei, T.; Wu, W. Novel Agents for Relapsed and Refractory Classical Hodgkin Lymphoma: A Review. Front. Oncol. 2022, 12, 929012. [Google Scholar] [CrossRef] [PubMed]
- Crocchiolo, R.; Fallanca, F.; Giovacchini, G.; Ferreri, A.J.M.; Assanelli, A.; Verona, C.; Pescarollo, A.; Bregni, M.; Ponzoni, M.; Gianolli, L.; et al. Role of 18FDG-PET/CT in detecting relapse during follow-up of patients with Hodgkin’s lymphoma. Ann. Hematol. 2009, 88, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- El-Galaly, T.C.; Mylam, K.J.; Brown, P.; Specht, L.; Christiansen, I.; Munksgaard, L.; Johnsen, H.E.; Loft, A.; Bukh, A.; Iyer, V.; et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica 2011, 97, 931–936. [Google Scholar] [CrossRef]
- Goldschmidt, N.; Or, O.; Klein, M.; Savitsky, B.; Paltiel, O. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann. Hematol. 2010, 90, 165–171. [Google Scholar] [CrossRef]
- Duggan, D.B.; Petroni, G.R.; Johnson, J.L.; Glick, J.H.; Fisher, R.I.; Connors, J.M.; Canellos, G.P.; Peterson, B.A. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J. Clin. Oncol. 2003, 21, 607–614. [Google Scholar] [CrossRef]
- Torrey, M.J.; Poen, J.C.; Hoppe, R.T. Detection of relapse in early-stage Hodgkin’s disease: Role of routine follow-up studies. J. Clin. Oncol. 1997, 15, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- El-Galaly, T.; Mylam, K.J.; Bøgsted, M.; Brown, P.; Rossing, M.; Gang, A.O.; Haglund, A.; Arboe, B.; Clausen, M.R.; Jensen, P.; et al. Role of routine imaging in detecting recurrent lymphoma: A review of 258 patients with relapsed aggressive non-Hodgkin and Hodgkin lymphoma. Am. J. Hematol. 2014, 89, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Petrausch, U.; Samaras, P.; Veit-Haibach, P.; Tschopp, A.; Soyka, J.D.; Knuth, A.; Hany, T.F.; Mischo, A.; Renner, C.; Schaefer, N.G. Hodgkin’s lymphoma in remission after first-line therapy: Which patients need FDG–PET/CT for follow-up? Ann. Oncol. 2009, 21, 1053–1057. [Google Scholar] [CrossRef] [PubMed]

| Standard Cohort (n = 43) | Imaging Cohort (n = 80) | p-Value | |
|---|---|---|---|
| Sex | |||
| Female | 22 (51.2) | 44 (55) | 0.78 |
| Male | 21 (48.8) | 36 (45) | |
| Age, years | |||
| Median, (range) | 30 (19–68) | 31 (20–66) | 0.96 |
| Elderly patients (>60 years old) | 2 (4.7) | 5 (6.2) | 0.13 |
| Histological type | |||
| Nodular sclerosis | 27 (62.8) | 50 (62.5) | 0.92 |
| Mixed cellularity | 12 (27.9) | 22 (29.7) | |
| Nodular lymphocyte predominant | 2 (4.7) | 2 (2.5) | |
| Lymphocyte-rich | 1 (2.3) | 4 (5) | |
| Lymphocyte-depleted | 1 (2.3) | 2 (2.5) | |
| Ann Arbor stage | |||
| IIB | 8 (18.6) | 18 (22.5) | 0.40 |
| IIIA | 11 (25.6) | 18 (22.5) | |
| IIIB | 12 (27.9) | 28 (35) | |
| IVA | 4 (9.3) | 10 (12.5) | |
| IVB | 8 (18.6) | 6 (7.5) | |
| Risk factors | |||
| Involvement of ≥ 3 nodal areas | 37 (86) | 70 (87.5) | 0.82 |
| Bulky disease † | 26 (60.5) | 54 (67.5) | 0.43 |
| High erythrocyte sedimentation rate ‡ | 27 (62.8) | 54 (67.5) | 0.59 |
| Extranodal involvement (in stages < IV) | 14 (32.6) | 31 (38.8) | 0.46 |
| International Prognostic Score (grouped) | |||
| 0–1 | 13 (30.2%) | 26 (32.5) | 0.96 |
| 2–3 | 17 (39.5%) | 30 (37.5) | |
| 4–7 | 13 (30.2%) | 24 (30) |
| Standard Cohort (n = 43) | Imaging Cohort (n = 80) | p-Value | Imaging Cohort | p-Value | ||
|---|---|---|---|---|---|---|
| Clinical Relapses (n = 29) | Clinically Silent Relapses (n = 51) | |||||
| Median time to relapse, months (range) | 36 (4–108) | 24 (4–108) | 0.012 | 34 (4–84) | 24 (4–108) | 0.02 |
| Ann Arbor stage | ||||||
| ≤IIA | 14 (32.6) | 46 (57.5) | 0.008 | 9 (31.0) | 37 (72.5) | 0.0003 |
| ≥IIB | 29 (67.4) | 34 (42.5) | 20 (69.0) | 14 (27.5) | ||
| Nodal regions involved at restaging as detected by FDG-PET scans after relapse | ||||||
| Superficial | 24 (55.8) | 35 (43.8) | 0.201 | 15 (51.7) | 20 (39.2) | 0.278 |
| Mediastinal compartment | 25 (58.1) | 35 (43.8) | 0.128 | 18 (62.1) | 17 (33.3) | 0.013 |
| Abdominal compartment | 26 (60.5) | 45 (56.3) | 0.652 | 19 (65.5) | 26 (51.0) | 0.207 |
| Unfavorable prognostic factors | ||||||
| Involvement of ≥3 nodal areas | 20 (46.5) | 33 (41.3) | 0.574 | 14 (48.3) | 19 (37.3) | 0.336 |
| Bulky disease † | 25 (58.1) | 19 (23.8) | 0.0003 | 12 (41.4) | 7 (13.7) | 0.005 |
| Extranodal involvement (in stages < IV) | 9 (20.9) | 7 (8.8) | 0.055 | 5 (17.2) | 2 (3.9) | 0.043 |
| Residual mass sites involved at relapse | 10 (23.3) | 16 (20.0) | 0.673 | 6 (20.7) | 10 (19.6) | 0.907 |
| Involvement of extranodal regions at relapse | 14 (32.6) | 15 (18.8) | 0.085 | 8 (27.6) | 7 (13.7) | 0.123 |
| Salvage Therapy | Last Follow-Up After AHSCT | ||||
|---|---|---|---|---|---|
| Overall Response Rate | Complete Remission | n. AHSCT | Complete Remission | Relapse | |
| Standard cohort (n = 43) | 28 (65.1) | 18 (41.9) | 28 (65.1) | 16 (57.1) | 12 (42.9) |
| Imaging cohort (n = 80) | 68 (85) | 55 (68.8) | 68 (26.3) | 53 (77.9) | 15 (22.1) |
| Clinical relapses (n = 29) | 21 (72.4) | 12 (41.4) | 21 (72.4) | 13 (61.9) | 8 (38.1) |
| Clinically silent relapses (n = 51) | 47 (92.2) | 43 (84.3) | 47 (92.2) | 40 (85.1) | 7 (14.9) |
| Relapse Characteristics | Univariate Analysis | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Relapse detection method | ||
| Clinical/lab positive vs. imaging only | 3.07 (1.64–5.75) | 0.00044 |
| Standard vs. imaging follow-up | 0.42 (0.25–0.75) | 0.0015 |
| Ann Arbor stage at relapse | ||
| ≥IIB vs. ≤IIA | 2.19 (1.26–3.81) | 0.006 |
| B-symptoms vs. no B-symptoms | 1.80 (1.05–30.8) | 0.031 |
| Unfavorable prognostic factors | ||
| Involvement of ≥3 vs. <3 nodal areas | 1.16 (0.68–1.99) | 0.575 |
| Bulky disease † vs. no bulky disease | 2.59 (1.51–4.42) | 0.0005 |
| Extranodal involvement vs. no extranodal disease | 3.65 (1.90–7.08) | 0.0006 |
| ESR > 50 mm/s vs. ESR ≤ 50 mm/s | 2.28 (1.31–3.97) | 0.003 |
| IPS 4–7 vs. 2–3 vs. 0–1 | 1.29 (0.92–1.81 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, N.; Picardi, M.; Vincenzi, A.; Giordano, C.; Lucania, A.; Severino, A.; Salvatore, C.; Mascolo, M.; Della Cioppa, P.; Pane, F. Routine Imaging Surveillance After Frontline ABVD Improves Outcome in High-Risk Hodgkin Lymphoma. Cancers 2025, 17, 3242. https://doi.org/10.3390/cancers17193242
Pugliese N, Picardi M, Vincenzi A, Giordano C, Lucania A, Severino A, Salvatore C, Mascolo M, Della Cioppa P, Pane F. Routine Imaging Surveillance After Frontline ABVD Improves Outcome in High-Risk Hodgkin Lymphoma. Cancers. 2025; 17(19):3242. https://doi.org/10.3390/cancers17193242
Chicago/Turabian StylePugliese, Novella, Marco Picardi, Annamaria Vincenzi, Claudia Giordano, Anna Lucania, Alessandro Severino, Claudia Salvatore, Massimo Mascolo, Paola Della Cioppa, and Fabrizio Pane. 2025. "Routine Imaging Surveillance After Frontline ABVD Improves Outcome in High-Risk Hodgkin Lymphoma" Cancers 17, no. 19: 3242. https://doi.org/10.3390/cancers17193242
APA StylePugliese, N., Picardi, M., Vincenzi, A., Giordano, C., Lucania, A., Severino, A., Salvatore, C., Mascolo, M., Della Cioppa, P., & Pane, F. (2025). Routine Imaging Surveillance After Frontline ABVD Improves Outcome in High-Risk Hodgkin Lymphoma. Cancers, 17(19), 3242. https://doi.org/10.3390/cancers17193242








