Hepatic Arterial Infusion Chemotherapy with Serplulimab and the Bevacizumab Biosimilar HLX04 for Advanced Hepatocellular Carcinoma: A Prospective, Observational Phase II Clinical Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.4. Study Endpoints and Assessments
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Efficacy
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Main Inclusion and Exclusion Criteria
- Willing to participate in the clinical study; fully understands the study, has signed the informed consent form (ICF), and is willing and able to complete all trial procedures.
- Age ≥ 18 years at the time of signing the ICF.
- Diagnosed with hepatocellular carcinoma (HCC) via histopathology or cytology, or clinically diagnosed based on the American Association for the Study of Liver Diseases (AASLD) criteria.
- Barcelona Clinic Liver Cancer (BCLC) stage C, with or without portal vein tumor thrombus (VP3–4 or Cheng’s classification II–IV) confirmed by imaging.
- No prior systemic therapy for HCC, including chemotherapy, sorafenib, regorafenib, lenvatinib, or other small-molecule anti-angiogenic agents.
- At least one measurable target lesion per RECIST v1.1, which has not previously undergone surgery, radiotherapy, or other local therapies (e.g., radiofrequency ablation, percutaneous ethanol or acetic acid injection, cryotherapy, high-intensity focused ultrasound, transarterial chemoembolization, or transarterial embolization). Previously treated lesions may be selected as target lesions if they show clear progression per RECIST v1.1. Local treatments must have been completed at least 4 weeks before baseline screening, with treatment-related adverse events (AEs) recovered to NCI-CTCAE Grade ≤1.
- Child-Pugh liver function classification: Grade A or well-compensated Grade B (≤7 points) within 7 days prior to screening.
- Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 within 7 days prior to screening.
- Expected survival ≥12 weeks.
- For patients with HBsAg (+) or HBcAb (+), HBV-DNA must be <2000 IU/mL to be eligible. Patients negative for HCV antibodies or HCV-RNA are eligible. If HCV-RNA is positive, the patient must agree to receive standard antiviral therapy per local guidelines and have ALT/AST ≤ 3 × ULN.
- Normal major organ function as assessed within 14 days prior to randomization, without prior transfusion, albumin, recombinant human thrombopoietin, or colony-stimulating factor treatment.
| System | Parameter | Criteria |
|---|---|---|
| Hematology | White Blood Cells (WBC) | ≥3.0 × 109/L |
| Neutrophils (ANC) | ≥1.5 × 109/L | |
| Platelets (PLT) | ≥75 × 109/L | |
| Hemoglobin (Hb) | ≥90 g/L | |
| Liver Function | Total Bilirubin (TBIL) | ≤1.5 × Upper Limit of Normal (ULN) |
| Alanine Aminotransferase (ALT) | ≤5 × ULN (except for patients with HCV-RNA positivity) | |
| Aspartate Aminotransferase (AST) | ≤5 × ULN (except for patients with HCV-RNA positivity) | |
| Albumin | ≥28 g/L | |
| Renal Function/Urinalysis | Creatinine (Cr) | ≤1.5 × ULN; if >1.5 × ULN, creatinine clearance rate should be ≥50 mL/min (calculated according to the Cockcroft-Gault formula) |
| Proteinuria | Routine urine protein test ≤1+ or quantitative <1.0 g/24 h | |
| Coagulation Function | Prothrombin Time (PT) | ≤1.5 × ULN |
| Activated Partial Thromboplastin Time (APTT) | ≤1.5 × ULN | |
| International Normalized Ratio (INR) | ≤1.5 × ULN |
- 12.
- Female patients must meet one of the following criteria:
- (1)
- Postmenopausal (no menstruation for ≥1 year without another known cause)
- (2)
- Surgically sterile (bilateral oophorectomy and/or hysterectomy)
- (3)
- If of childbearing potential:
- Negative serum pregnancy test within 7 days before randomization.
- Agreement to use highly effective contraception (failure rate <1% per year) or remain abstinent from the time of consent to at least 150 days after the last dose. Highly effective contraception includes bilateral tubal ligation, vasectomy of male partner, hormonal contraceptives that inhibit ovulation, or intrauterine devices (IUDs). Breastfeeding is not allowed.
- 13.
- Male patients must agree to abstain or use contraception as follows: If the partner is of childbearing potential or pregnant, the patient must use a condom or remain abstinent from the time of consent to at least 150 days after the last dose. The reliability of abstinence should be assessed based on trial duration, patient preference, and lifestyle. Periodic abstinence (calendar, ovulation, basal body temperature, or post-ovulation methods) and withdrawal are not considered reliable contraceptive methods.
- Known intrahepatic cholangiocarcinoma, mixed-cell carcinoma, or fibrolamellar carcinoma.
- History of hepatic encephalopathy.
- Portal hypertension with upper gastrointestinal bleeding within 6 months, or esophageal/gastric varices with red signs (gastroscopy required within 6 months before randomization), or high bleeding risk per investigator assessment.
- Known central nervous system (CNS) or leptomeningeal metastases.
- Presence of distant metastases (hepatic portal lymph node metastases are permitted).
- Co-infection with HBV and HCV (detectable HBV-DNA or HBsAg-positive with anti-HCV antibody-positive or detectable HCV-RNA).
- Other active malignancies within the past 5 years (except cured localized cancers such as basal cell carcinoma, squamous cell carcinoma of the skin, superficial bladder cancer, carcinoma in situ of the prostate, cervix, or breast).
- Prior or planned organ or bone marrow transplantation.
- Uncontrolled pleural effusion, pericardial effusion, or ascites requiring frequent drainage (≥ once per month).
- Stroke, myocardial infarction, unstable angina, or uncontrolled arrhythmia within the past 6 months (including QTc interval ≥450 ms in males or ≥470 ms in females, calculated using the Fridericia formula).
- Heart failure NYHA > II or LVEF < 50% on echocardiogram.
- Uncontrolled hypertension despite optimal medical management (systolic BP ≥ 150 mmHg or diastolic BP ≥ 100 mmHg).
- History of hypertensive crisis or hypertensive encephalopathy.
- Active HIV infection, tuberculosis, or other active infections.
- History or current evidence of pulmonary fibrosis, interstitial lung disease, pneumoconiosis, radiation pneumonitis, drug-related pneumonitis, or severely impaired lung function that may interfere with drug safety evaluation.
- Active or suspected autoimmune disease, except controlled hypothyroidism on replacement therapy or controlled Type 1 diabetes on insulin therapy.
- Receipt of a live vaccine within 28 days.
- Systemic corticosteroid (>10 mg/day prednisone or equivalent) or immunosuppressive therapy within 14 days before screening, unless for acute, short-term use (e.g., contrast allergy prophylaxis). Inhaled or topical steroids are permitted.
- Major surgery within 28 days (defined as requiring ≥3 weeks for recovery before study treatment initiation).
- Prior treatment with T-cell co-stimulation or immune checkpoint inhibitors, including but not limited to CTLA-4, PD-1, or PD-L1/2 inhibitors.
- Prior treatment with bevacizumab or its biosimilars.
- Participation in another clinical trial within 14 days before randomization.
- Known allergy to monoclonal antibodies, study drug excipients, platinum, or fluoropyrimidine-based chemotherapy agents.
- Pregnant or breastfeeding women.
- Evidence of non-gastrointestinal bleeding (e.g., hemoptysis, abnormal vaginal bleeding) at screening or Grade 2 bleeding within 3 months, or Grade ≥3 bleeding within 6 months before consent.
- Full-dose anticoagulation or thrombolytic therapy within 7 days prior to randomization (except prophylactic anticoagulation for an open intravenous line, provided INR ≤ 1.5 × ULN and APTT ≤ 1.5 × ULN within 14 days before randomization). Prophylactic low-molecular-weight heparin (e.g., enoxaparin 40 mg/day) is allowed.
- Chronic daily NSAID use. Occasional NSAID use for symptom relief (e.g., headache, fever) is allowed.
- The following conditions within 6 months:
- (1)
- Esophagotracheal fistula, gastrointestinal perforation, intra-abdominal fistula, or intra-abdominal abscess.
- (2)
- Intestinal obstruction or symptoms requiring parenteral hydration/nutrition or enteral feeding.
- (3)
- Intra-abdominal inflammatory processes, including peptic ulcer, diverticulitis, or colitis.
- (4)
- Major vascular diseases (e.g., aortic aneurysm requiring repair or recent peripheral arterial thrombosis).
- Unhealed wounds, active ulcers, or untreated fractures.
- History of substance abuse or drug dependence.
- Any other condition deemed unsuitable by the investigator.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Global Cancer Observatory; World Health Organization: Lyon, France; Available online: https://gco.iarc.fr/ (accessed on 1 September 2025).
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Du, Z.; Lai, Z.; Ouyang, H.; Shen, J.; Wen, D.; Li, Q.; Zhang, Y.; Wei, W.; et al. Lenvatinib, Toripalimab plus FOLFOX Chemotherapy in Hepatocellular Carcinoma Patients with Extrahepatic Metastasis: A Biomolecular Exploratory, Phase II Trial (LTSC). Clin. Cancer Res. 2023, 29, 5104–5115. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, J.; Liu, C.; Chai, Z.; Wang, N.; Zhang, H.; Cheng, S. All-trans-retinoic acid (ATRA) plus oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX) versus FOLFOX alone as palliative chemotherapy in patients with advanced hepatocellular carcinoma and extrahepatic metastasis: Study protocol for a randomized controlled trial. Trials 2019, 20, 245. [Google Scholar] [PubMed]
- Qin, S.; Bai, Y.; Lim, H.Y.; Thongprasert, S.; Chao, Y.; Fan, J.; Yang, T.S.; Bhudhisawasdi, V.; Kang, W.K.; Zhou, Y.; et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013, 31, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Lyu, N.; Kong, Y.; Mu, L.; Lin, Y.; Li, J.; Liu, Y.; Zhang, Z.; Zheng, L.; Deng, H.; Li, S.; et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J. Hepatol. 2018, 69, 60–69. [Google Scholar] [CrossRef]
- Li, Q.J.; He, M.K.; Chen, H.W.; Fang, W.Q.; Zhou, Y.M.; Xu, L.; Wei, W.; Zhang, Y.J.; Guo, Y.; Guo, R.P.; et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J. Clin. Oncol. 2022, 40, 150–160. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Li, Q.; Han, J.; Yang, Y.; Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol. 2022, 13, 1070961. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Li, G. Precision oncology and molecular therapies for hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 882–885. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Lai, Z.; He, M.; Bu, X.; Xu, Y.; Huang, Y.; Wen, D.; Li, Q.; Xu, L.; Zhang, Y.; Wei, W.; et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur. J. Cancer 2022, 174, 68–77. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Gu, S.; Zhang, Y.; Wu, L.; Wu, J.; Shao, G.; Zhang, Y.; Xu, L.; Yin, T.; et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin. Cancer Res. 2021, 27, 1003–1011. [Google Scholar] [CrossRef]
- Gao, W.; Feng, L.; Zhao, X.; Huang, Z.; Chen, D.; Yin, G.; Guo, W.; Zhong, Q.; Chen, X.; Fang, J.; et al. Comparison of the efficacy and safety of neoadjuvant PD-1 inhibitors plus chemotherapy vs targeted therapy plus chemotherapy in locally advanced hypopharyngeal squamous cell carcinoma. Front. Immunol. 2024, 15, 1466310. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Serplulimab: First Approval. Drugs 2022, 82, 1137–1141. [Google Scholar] [CrossRef]
- Zhu, X.; Qian, H.; Sun, J.; Wu, M.; Yu, C.; Ding, Y.; Zhang, X.; Chai, K.; Li, X. A phase 1 randomized study compare the pharmacokinetics, safety and immunogenicity of HLX04 to reference bevacizumab sourced from the United States, the European Union, and China in healthy Chinese male volunteers. Cancer Chemother Pharmacol. 2021, 8, 465–474. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Bai, Y.; Shu, Y.; Li, W.; Yin, X.; Cheng, Y.; Sun, G.; Deng, Y.; Zhong, H.; et al. HLX04-mCRC03 Investigators. Efficacy, Safety, and Immunogenicity of HLX04 Versus Reference Bevacizumab in Combination with XELOX or mFOLFOX6 as First-Line Treatment for Metastatic Colorectal Cancer: Results of a Randomized, Double-Blind Phase III Study. BioDrugs 2021, 35, 445–458. [Google Scholar] [CrossRef]
- Ren, Z.; Shao, G.; Shen, J.; Zhang, L.; Zhu, X.; Fang, W.; Sun, G.; Bai, Y.; Wu, J.; Liu, L.; et al. Phase 2 Study of the PD-1 Inhibitor Serplulimab plus the Bevacizumab Biosimilar HLX04 in Patients with Previously Treated Advanced Hepatocellular Carcinoma. Liver Cancer 2023, 12, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Shao, G.; Shen, J.; Zhang, L.; Zhu, X.; Fang, W.; Sun, G.; Bai, Y.; Wu, J.; Liu, L.; et al. Phase 2 study of serplulimab with the bevacizumab biosimilar HLX04 in the first-line treatment of advanced hepatocellular carcinoma. Cancer Immunol. Immunother. 2025, 74, 69. [Google Scholar] [CrossRef]
- Tang, J.; Shalabi, A.; Hubbard-Lucey, V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.V. Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin. Immunopathol. 2019, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Galle, P.R.; Zhu, A.X.; Ducreux, M.; Cheng, A.L.; Ikeda, M.; Tsuchiya, K.; Aoki, K.I.; Jia, J.; et al. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer 2022, 12, 238–250. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address eee, European association for the study of the L, EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Hartke, J.; Johnson, M.; Ghabril, M. The diagnosis and treatment of hepatocellular carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159. [Google Scholar] [CrossRef]
- Zhong, J.H.; Peng, N.F.; You, X.M.; Ma, L.; Xiang, X.; Wang, Y.Y.; Gong, W.F.; Wu, F.X.; Xiang, B.D.; Li, L.Q. Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: A real-world study. Oncotarget 2017, 8, 18296–18302. [Google Scholar] [CrossRef]
- Xiang, X.; Zhong, J.H.; Wang, Y.Y.; You, X.M.; Ma, L.; Xiang, B.D.; Li, L.Q. Distribution of tumor stage and initial treatment modality in patients with primary hepatocellular carcinoma. Clin. Transl. Oncol. 2017, 19, 891–897. [Google Scholar] [CrossRef]
- Cappuyns, S.; Corbett, V.; Yarchoan, M.; Finn, R.S.; Llovet, J.M. Critical Appraisal of Guideline Recommendations on Systemic Therapies for Advanced Hepatocellular Carcinoma: A Review. JAMA Oncol. 2024, 10, 395–404. [Google Scholar] [CrossRef]
- Xiang, J.; Si, J.; Hao, Y.; Wei, J.; Wang, W.; Guan, Y.; Xu, C.; Song, Z. Efficacy and safety of immune checkpoint inhibitors (ICIs) combined with antiangiogenic therapy for thymic epithelial tumors (TETs): A retrospective study. Transl. Cancer Res. 2023, 12, 550–557. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, X.; Wu, Z.; Wei, Q.; Cai, Y.; Zheng, Y.; Hu, X.; Hu, H.; Zhang, X.; Pan, H.; et al. Efficacy and safety of PD-1 inhibitor combined with antiangiogenic therapy for unresectable hepatocellular carcinoma: A multicenter retrospective study. Cancer Med. 2022, 11, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, S.; Jiang, T.; Zhu, B.; Li, X.; Zhao, C.; Jia, Y.; Shi, J.; Zhang, L.; Liu, X.; et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019, 7, 630–643. [Google Scholar] [CrossRef]
- Cai, X.; Wei, B.; Li, L.; Chen, X.; Liu, W.; Cui, J.; Lin, Y.; Sun, Y.; Xu, Q.; Guo, W.; et al. Apatinib enhanced anti-PD-1 therapy for colon cancer in mice via promoting PD-L1 expression. Int. Immunopharmacol. 2020, 88, 106858. [Google Scholar] [CrossRef]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9, eaak9679. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, L.; Jian, H.; Chen, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Sun, M.; Han, L.; et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): First interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1167–1179. [Google Scholar] [PubMed]
- Zhang, T.Q.; Geng, Z.J.; Zuo, M.X.; Li, J.B.; Huang, J.H.; Huang, Z.L.; Wu, P.H.; Gu, Y.K. Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (TRIPLET): A phase II study. Signal Transduct. Target. Ther. 2023, 8, 413. [Google Scholar] [CrossRef]
- Chen, S.; Xu, B.; Wu, Z.; Wang, P.; Yu, W.; Liu, Z.; Huang, X.; Wu, Y.; Li, T.; Guo, W. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: A multicenter retrospective study. BMC Cancer 2021, 21, 1126. [Google Scholar] [CrossRef]
- An, C.; Fu, Y.; Li, W.; Zuo, M.; Wu, P. Postprogression treatment of lenvatinib plus PD-1 inhibitor in advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy. Cancer 2023, 129, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Peng, W.; Zhang, W.; Yang, Z.; Hu, Z.; Pang, Y.; Hu, D.; Chen, J.; Wang, J.; Zhou, Z.; et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and PD-1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J. Gastroenterol. 2023, 58, 413–424. [Google Scholar] [CrossRef]
- Mei, J.; Li, S.H.; Li, Q.J.; Sun, X.Q.; Lu, L.H.; Lin, W.P.; Zheng, L.; Chen, M.S.; Shi, M.; Wei, W.; et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J. Hepatocell. Carcinoma 2021, 8, 167–176. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Zheng, G.L.; Zhang, T.Q.; Shao, H.Y.; Pan, J.Y.; Huang, Z.L.; Zuo, M.X. Hepatic arterial infusion chemotherapy with anti-angiogenesis agents and immune checkpoint inhibitors for unresectable hepatocellular carcinoma and meta-analysis. World J. Gastroenterol. 2024, 30, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef] [PubMed]
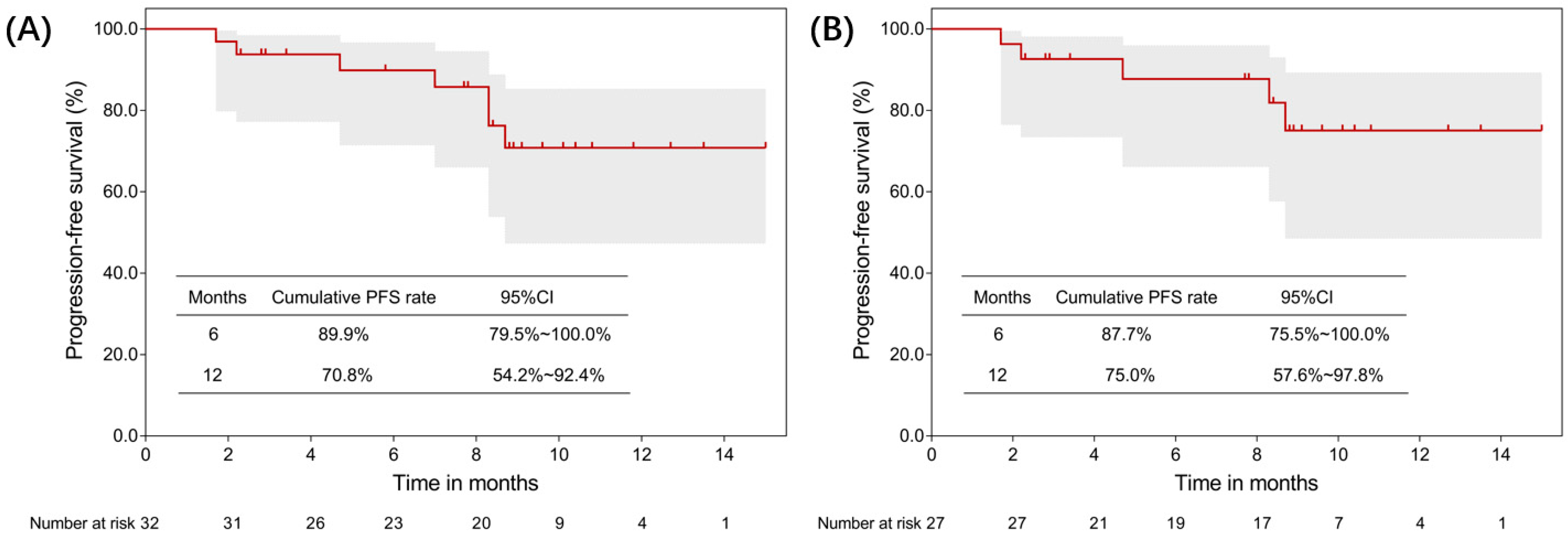

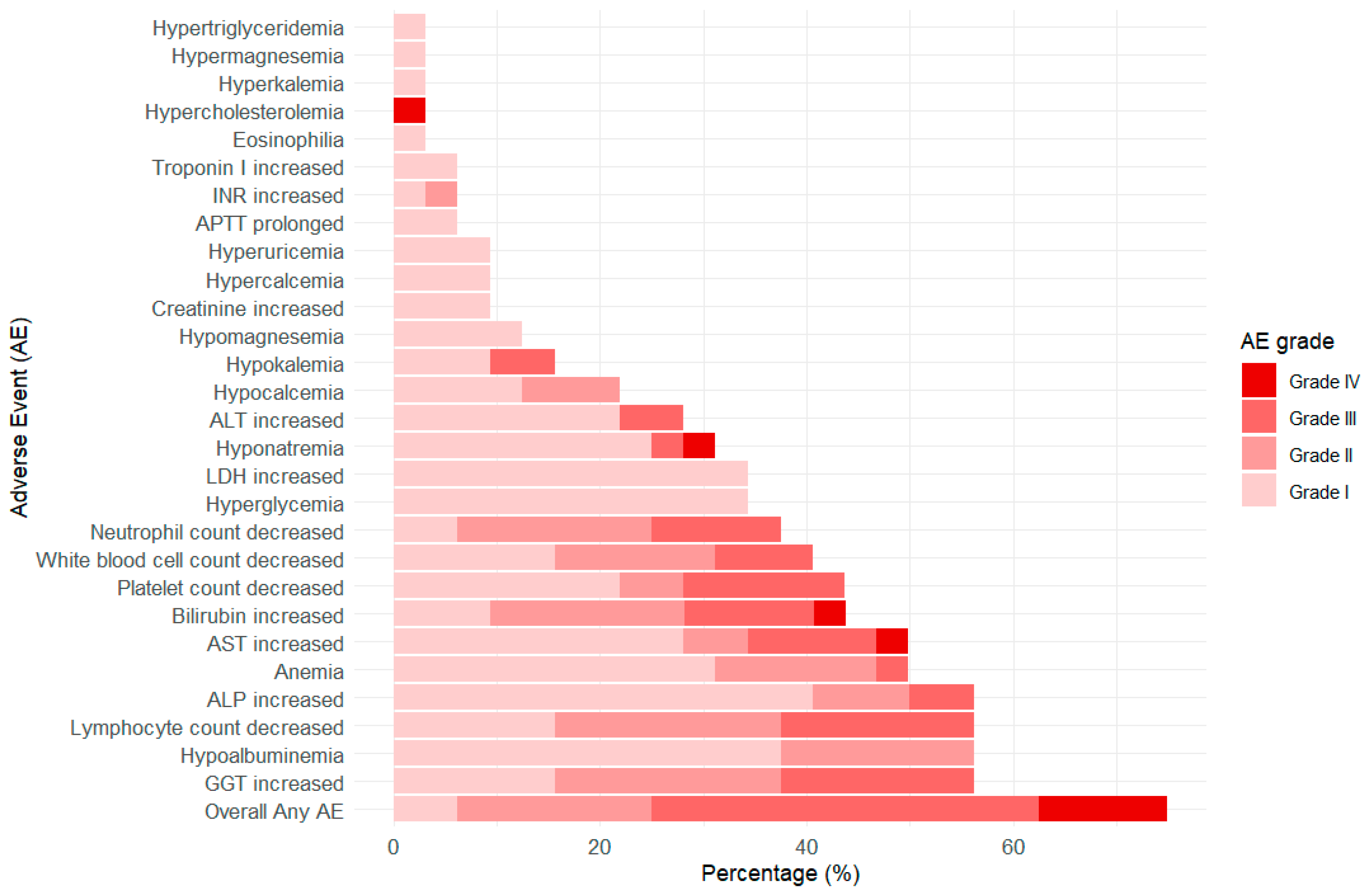
| Characteristics | Patients (N = 32) |
|---|---|
| Age (year), median (IQR) | 62.0 (53.0~66.8) |
| Sex n (%) | |
| Male | 26 (81.2) |
| Female | 6 (18.8) |
| Drinking history n (%) | |
| No | 18 (56.2) |
| Yes | 14 (43.8) |
| Smoking history n (%) | |
| No | 15 (46.9) |
| Yes | 17 (53.1) |
| Family history of liver cancer n (%) | |
| No | 29 (90.6) |
| Yes | 3 (9.4) |
| HBV infection n (%) | |
| No | 10 (31.2) |
| Yes | 22 (68.8) |
| HCV infection n (%) | |
| No | 30 (93.8) |
| Yes | 2 (6.2) |
| Hypertension n (%) | |
| No | 22 (68.8) |
| Yes | 10 (31.2) |
| Diabetes n (%) | |
| No | 25 (78.1) |
| Yes | 7 (21.9) |
| Cardiovascular and cerebrovascular diseases n (%) | |
| No | 29 (90.6) |
| Yes | 3 (9.4) |
| Cirrhosis n (%) | |
| No | 12 (37.5) |
| Yes | 20 (62.5) |
| Portal vein cancer thrombus n (%) | |
| No | 16 (50.0) |
| Yes | 16 (50.0) |
| Number of lesions n (%) | |
| Single lesion | 5 (15.6) |
| Multiple lesions | 27 (84.4) |
| Lymph node metastasis n (%) | |
| No | 29 (90.6) |
| Yes | 3 (9.4) |
| Extrahepatic metastasis n (%) | |
| No | 27 (84.4) |
| Yes | 5 (15.6) |
| CNLC stage n (%) | |
| Ib | 2 (6.3) |
| IIa | 2 (6.3) |
| IIb | 5 (15.6) |
| IIIa | 14 (43.8) |
| IIIb | 9 (28.1) |
| AJCC stage n (%) | |
| II | 1 (3.1) |
| IIIA | 8 (25.0) |
| IIIB | 16 (50.0) |
| IVA | 2 (6.3) |
| IVB | 5 (15.6) |
| Serplulimab/Bevacizumab + TACE/HAIC cycles n (%) | |
| ≥3 cycles | 28 (87.5) |
| <3 cycles | 4 (12.5) |
| Indicator | N = 32 |
|---|---|
| Number of assessable cases n (%) | 32 (100) |
| CR n (%) | 0 (0.0) |
| PR n (%) | 17(53.1) 1 |
| SD n (%) | 12 (37.5) |
| PD n (%) | 3 (9.4) 2 |
| ORR | 17 (53.1, 95%CI: 34.7–70.9) |
| DCR | 29 (90.6, 95%CI: 75.0–97.5) |
| No. | Age | Gender | HBV Infection | Portal Vein Tumor Thrombus | CNLC Stage | Treatment Cycles (Preoperative) | Preoperative Tumor Assessment | Surgical Method |
|---|---|---|---|---|---|---|---|---|
| 1 | 57 | Male | Yes | No | Ib | 4 | SD | Hepatic segments IV, V, VII resection + Cholecystectomy |
| 2 | 72 | Female | Yes | No | IIb | 3 | PR | Left hepatectomy + Cholecystectomy |
| 3 | 66 | Male | No | Yes | IIIa | 3 | PR | Right hepatectomy + Right caudate lobectomy + Cholecystectomy |
| 4 | 50 | Male | No | Yes | IIIa | 3 | PR | Hepatectomy |
| 5 | 54 | Male | Yes | Yes | IIIa | 4 | PR | Right hepatectomy + Cholecystectomy + Lymph node dissection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Si, T.; Li, R.; Xie, X.; Liu, Y.; Fu, L.; Bai, Y.; Yao, J.; Zhang, X.; Yang, M.; et al. Hepatic Arterial Infusion Chemotherapy with Serplulimab and the Bevacizumab Biosimilar HLX04 for Advanced Hepatocellular Carcinoma: A Prospective, Observational Phase II Clinical Trial. Cancers 2025, 17, 3235. https://doi.org/10.3390/cancers17193235
Li H, Si T, Li R, Xie X, Liu Y, Fu L, Bai Y, Yao J, Zhang X, Yang M, et al. Hepatic Arterial Infusion Chemotherapy with Serplulimab and the Bevacizumab Biosimilar HLX04 for Advanced Hepatocellular Carcinoma: A Prospective, Observational Phase II Clinical Trial. Cancers. 2025; 17(19):3235. https://doi.org/10.3390/cancers17193235
Chicago/Turabian StyleLi, Huikai, Tongguo Si, Rentao Li, Xiaojing Xie, Yang Liu, Linlin Fu, Yu Bai, Junchao Yao, Xihao Zhang, Mao Yang, and et al. 2025. "Hepatic Arterial Infusion Chemotherapy with Serplulimab and the Bevacizumab Biosimilar HLX04 for Advanced Hepatocellular Carcinoma: A Prospective, Observational Phase II Clinical Trial" Cancers 17, no. 19: 3235. https://doi.org/10.3390/cancers17193235
APA StyleLi, H., Si, T., Li, R., Xie, X., Liu, Y., Fu, L., Bai, Y., Yao, J., Zhang, X., Yang, M., & Mu, X. (2025). Hepatic Arterial Infusion Chemotherapy with Serplulimab and the Bevacizumab Biosimilar HLX04 for Advanced Hepatocellular Carcinoma: A Prospective, Observational Phase II Clinical Trial. Cancers, 17(19), 3235. https://doi.org/10.3390/cancers17193235







