Hormonal Therapy Patterns in Older Men with Prostate Cancer in the United States, 2010–2019
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Subjects
2.3. Indications for HT
2.4. Definitions
2.5. Statistical Analyses
2.6. Data Availability
3. Results
3.1. Patient and Cancer Characteristics
3.2. Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
- HT (NDC and HCPCS) Codes
| Drug Name | NDC Code | HCPCS Code |
| Agonists | ||
| Leuprolide (Lupron, Eligard) | 00074-3642, 00074-3473, 00074-3683, 00185-7400, 00781-4003, 47335-0936, 55150-0478, 62935-0223, 62935-0303, 62935-0453, 62935-0753, 69448-0014, 72664-0611 | J1950, J1952, J9217, J9218, J9219 |
| Goserelin (Zoladex) | 50090-2027, 50090-3466, 70720-0950, 70720-0951 | J9202 |
| Triptorelin (Trelstar) | 00023-5902, 00023-5904, 00023-5906 | J3315 |
| Histrelin | 67979-0501, 67979-0401 | J1675, J9225, J9226 |
| Antagonists | ||
| Degarelix | 55566-8303, 55566-8403 | J9155 |
| Relugolix | 72974-0120 | |
| Antiandrogens | ||
| Old generation drugs | ||
| Flutamide | 69097-0915 | |
| Nilutamide | 59212-0111, 62559-0173, 66993-0212, 82454-0212 | |
| Bicalutamide | 00904-6019, 16714-0571, 16714-0816, 16729-0023, 47335-0485, 54868-4503, 54868-6133, 60429-0177, 60505-2642, 62559-0680, 62559-0890, 63629-5321, 63672-0005, 65841-0613, 67253-0191, 68382-0224 | |
| New generation drugs | ||
| Enzalutamide | 00469-0125 | |
| Apalutamide | 59676-0600 | |
| Darolutamide | 50419-0395 | |
| Abiraterone (CYP17 inhibitor) | 00093-1125, 00143-9597, 00378-6920, 00904-6948, 16714-0963, 42291-0024, 42291-0073, 57894-0150, 57894-0184 |
- ICD 9 and ICD 10 Codes
| Treatment | ICD-9, ICD-10 or HCPCS/CPT Code |
| Orchiectomy | (CPT codes 54520) ICD-9: 62.4, V45.77 ICD-10: Z90.79 |
| Radiation Therapy | External beam radiotherapy (CPT codes 77400–77416, 77420, 77425, 77430), Brachytherapy (CPT codes 77776–77778), ICD-9: 92.21–92.27, 92.3 ICD-10: Z51.0 DVY07ZZ HCPCS/CPT: 77401–77416, 77418, 77520, 77522–77525, 55875, 77373 |
| prostatectomy | ICD-9: 60.3, 60.4, 60.5, V45.77 ICD-10: Z90.79 HCPCS/CPT: 55801, 55810, 55812, 55815, 55840, 55842, 55845, 55860, 55862, 55865, 55866, 52601, 52612, 52614 |
| Demographic characteristics and comorbidities | |
| Age | NAACCR Item Number 230 |
| Race/Ethnicity | Non-Hispanics white, Non-Hispanics Black, Hispanics, others |
| Congestive heart failure | ICD-9 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0–428.9 ICD-10 (I50) |
| Valvular disease | ICD-9 093.20–093.24, 394.0–397.1, 397.9, 424.0–424.99, 746.3–746.6, V42.2, V43.3 ICD-10 (I35) |
| Pulmonary circulation disorders | ICD-9 415.11–415.19, 416.0–416.9, 417.9 (491–496) ICD-10 (I26–I27) |
| Peripheral vascular disorders | ICD-9 440–440.9, 441.00–441.9, 442.0–442.9, 443.1–443.9, 444.21–444.22, 447.1,449, 557.1, 557.9, V43.4 (443) ICD-10 (I73) |
| Lymphoma | ICD-9 200.00–202.38, 202.50–203.01, 203.02–203.82, 203.8–203.81, 238.6, 273.3 ICD-10 (C85) |
| Diabetes mellitus | ICD-9 249.00–249.31, 250.00–250.33, 648.00–648.04, 249.40–249.91, 250.40–250.93, 775.1 (250) ICD-10 (E08–E13) |
| Hypertension | ICD-9 401.1, 401.9, 642.00–642.04, 401.0, 402.00–405.99, 437.2, 642.10–642.24, 642.70–642.94 (401–405) ICD-10 (I10) |
| Hypothyroidism | ICD-9 243–244.2, 244.8, 244.9 ICD-10 E03 |
| Hyperlipidemia | ICD-9 (272) ICD-10 (E78) |
| Chronic kidney disease | ICD-9 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 585.3, 585.4, 585.5, 585.6, 585.9, 586, V42.0, V45.1, V45.11, V45.12, V56.0-V56.32, V56.8 ICD-10 N18, N17, N19 |
| Chronic liver disease | ICD-9 070.22, 070.23, 070.32, 070.33, 070.44, 070.54, 456.0, 456.1, 456.20, 456.21, 571.0, 571.2, 571.3, 571.40–571.49, 571.5, 571.6, 571.8, 571.9, 572.3, 572.8, 573.5, V42.7 ICD-10 (K76, K75) |
| Chronic pulmonary disease | ICD-9 490–492.8, 493.00–493.92, 494–494.1, 495.0–505, 506.4, ICD-10 (J41–J44, I28) |
| Chronic peptic ulcer | ICD-9 531.41, 531.51, 531.61, 531.70, 531.71, 531.91, 532.41, 532.51, 532.61, 532.70, 532.71, 532.91, 533.41, 533.51, 533.61, 533.70, 533.71, 533.91, 534.41, 534.51, 534.61, 534.70, 534.71, 534.91 ICD-10 K27 |
| Cerebral vascular accident | ICD-9 (430–438) ICD-10 (I60–I67) |
| Neurological disorders | ICD-9 330.1–331.9, 332.0, 333.4, 333.5, 333.71, 333.72, 333.79, 333.85, 333.94, 334.0–335.9, 338.0, 340, 341.1–341.9, 345.00–345.11, 345.2–345.3, 345.40–345.91, 347.00–347.01, 347.10–347.11, 649.40–649.44, 768.7, 768.70, 768.71, 768.72, 780.3, 780.31, 780.32, 780.33, 780.39, 780.97, 784.3 ICD-10 G20, G35, G40–G41, G60–G64, G80–G83 |
| HIV and Aids | ICD-9 042–044.9 ICD-10 B20, Z21, Z83 |
| Metastatic cancer | ICD-9 196.0–199.1, 209.70, 209.71, 209.72, 209.73, 209.74, 209.75, 209.79, 789.51 ICD-10 C77, C80, C7B |
| Solid tumor without metastasis | ICD-9 140.0–172.9, 174.0–175.9, 179–195.8, 209.00–209.24, 209.25–209.3, 209.30–209.36, 258.01–258.03 ICD-10 C00, C43, C50, C55, C76 |
| Rheumatoid arthritis/collagen vascular diseases | ICD-9 701.0, 710.0–710.9, 714.0–714.9, 720.0–720.9, 725 ICD-10 M05–M06, M32, M34, M35.3 |
| Coagulation deficiency | ICD-9 286.0–286.9, 287.1, 287.3–287.5, 289.84, 649.30–649.34 ICD-10 D68 |
| Obesity | ICD-9 278.0, 278.00, 278.01, 278.03, 649.10–649.14, 793.91, V85.30-V85.39, V85.41-V85.45, V85.54 ICD-10 E66 |
| Weight loss | ICD-9 260–263.9, 783.21, 783.22 ICD-10 R63 |
| Fluid and electrolyte disorders | ICD-9 276.0–276.9 ICD-10 E87 |
| Blood loss anemia | ICD-9 280.0, 648.20–648.24 ICD-10 D50 |
| Deficiency anemias | ICD-9 280.1–281.9, 285.21–285.29, 285.9 ICD-10 D51, D52, D55, D53 |
| Alcohol abuse | ICD-9 291.0–291.3, 291.5, 291.8, 291.81, 291.82, 291.89, 291.9, 303.00–303.93, 305.00–305.03 ICD-10 F10 |
| Drug abuse | ICD-9 292.0, 292.82–292.89, 292.9, 304.00–304.93, 305.20–305.93, 648.30–648.34 ICD-10 Z71, F19, F11, F55, F15 |
| Psychoses | ICD-9 295.00–298.9, 299.10, 299.11 ICD-10 F29, F23, F53 |
| Depression | ICD-9 300.4, 301.12, 309.0, 309.1, 311 ICD-10 F32 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Mohler, J.L.; Lee, R.J.; Antonarakis, E.S. NCCN Guidelines Insights: Prostate cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: II. The effect of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 43, 209–223. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Alibhai, S.M.H.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2.2020. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 5 March 2020).
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Pilepich, M.V.; Caplan, R.; Byhardt, R.W.; Lawton, C.A.; Gallagher, M.J.; Mesic, J.B.; Hanks, G.E.; Coughlin, C.T.; Porter, A.T.; Shipley, W.U.; et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: Report of Radiation Therapy Oncology Group Protocol 85-31. J. Clin. Oncol. 1997, 15, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- American Urological Association. Prostate Cancer: Clinically Localized Disease. AUA Guideline. Available online: https://www.auanet.org/guidelines-and-quality/guidelines/clinically-localized-prostate-cancer-aua/astro-guideline-2022 (accessed on 5 March 2020).
- Bekelman, J.E.; Rumble, R.B.; Chen, R.C.; Pisansky, T.M.; Finelli, A.; Feifer, A.; Nguyen, P.L.; Loblaw, D.A.; Zietman, A.L.; Taplin, M.E.; et al. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology guideline. J. Clin. Oncol. 2018, 36, 3251–3258. [Google Scholar] [CrossRef]
- Hussain, M.; Tangen, C.M.; Berry, D.L.; Higano, C.S.; Crawford, E.D.; Liu, G.; Wilding, G.; Prescott, S.; Sundaram, S.K.; Small, E.J.; et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 2013, 368, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S. NCCN Guidelines Updates: Management of prostate cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 583–586. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Hershman, D.L.; Kushi, L.H.; Shao, T.; Buono, D.; Kershenbaum, A.; Tsai, W.Y.; Fehrenbacher, L.; Lin Gomez, S.; Miles, S.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J. Clin. Oncol. 2010, 28, 4120–4128. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Grossfeld, G.D.; Lubeck, D.P.; Carroll, P.R. National practice patterns and time trends in androgen ablation for localized prostate cancer. J. Natl. Cancer Inst. 2003, 95, 981–989. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Medicare Payment Advisory Commission. Report to the Congress: Effects of Medicare Payment Changes on Oncology Services; Medicare Payment Advisory Commission: Washington, DC, USA, 2007.
- Scardino, P. Update: NCCN prostate cancer clinical practice guidelines. J. Natl. Compr. Cancer Netw. 2005, 3, S29–S33. [Google Scholar]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Orihuela, E.; Goodwin, J.S. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2007, 25, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER-Medicare Linked Database. Available online: https://healthcaredelivery.cancer.gov/seermedicare/ (accessed on 5 March 2020).
- Centers for Medicare & Medicaid Services. Medicare Coverage. Available online: https://www.medicare.gov/what-medicare-covers (accessed on 5 March 2020).
- Garcia-Albeniz, X.; Chan, J.; Paciorek, A.; Logan, R.; Kenfield, S.; Cooperberg, M.; Carroll, P.; Hernán, M. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur. J. Cancer 2015, 51, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Rumble, R.B.; Loblaw, D.A.; Finelli, A.; Ehdaie, B.; Cooperberg, M.R.; Morgan, S.C.; Tyldesley, S.; Haluschak, J.J.; Tan, W.; et al. Active surveillance for the management of localized prostate cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 2016, 34, 2182–2190. [Google Scholar] [CrossRef]
- Warren, J.L.; Klabunde, C.N.; Schrag, D.; Bach, P.B.; Riley, G.F. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med. Care 2002, 40, IV-3–IV-18. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Potosky, A.L.; Legler, J.M.; Warren, J.L. Development of a comorbidity index using physician claims data. J. Clin. Epidemiol. 2000, 53, 1258–1267. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- University of Texas Medical Branch Institutional Review Board. Available online: https://www.utmb.edu/provost/resources/research-regulations-and-compliance/irb/home (accessed on 24 September 2025).
- European Association of Urology. EAU Guidelines for Prostate Cancer. 2021. Available online: https://uroweb.org/guideline/prostate-cancer/ (accessed on 5 March 2020).
- Mohler, J.L.; Kantoff, P.W.; Armstrong, A.J.; Bahnson, R.R.; Cohen, M.; D’Amico, A.V.; Eastham, J.A.; Enke, C.A.; Farrington, T.; Higano, C.S.; et al. Prostate Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef]
- Caram, M.E.V.; Borza, T.; Min, H.S.; Griggs, J.J.; Miller, D.C.; Hollenbeck, B.K.; Mukherjee, B.; Skolarus, T.A. Early National Dissemination of Abiraterone and Enzalutamide for Advanced Prostate Cancer in Medicare Part D. J. Oncol. Pract. 2017, 13, e694–e702. [Google Scholar] [CrossRef]
- Nørgaard, M.; Jensen, A.Ø.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sørensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population-based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef]
- Hougen, H.Y.; Swami, N.; Dee, E.C.; Alshalalfa, M.; Meiyappan, K.; Florez, N.; Penedo, F.J.; Nguyen, P.L.; Punnen, S.; Mahal, B.A. Disparities in diagnosis, treatment access, and time to treatment among Hispanic men with metastatic prostate cancer. JCO Oncol. Pract. 2023, 19, 645–653. [Google Scholar] [CrossRef]
- Lowder, D.; Rizwan, K.; McColl, C.; Paparella, A.; Ittmann, M.; Mitsiades, N.; Kaochar, S. Racial disparities in prostate cancer: A complex interplay between socioeconomic inequities and genomics. Cancer Lett. 2022, 531, 71–82. [Google Scholar] [CrossRef]
- Du, X.L.; Fang, S.; Coker, A.L.; Sanderson, M.; Aragaki, C.; Cormier, J.N.; Xing, Y. Racial Disparity and Socioeconomic Status in Association with Survival in Older Men with Localized Prostate Carcinoma. Cancer 2006, 106, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Shahinian, V.B.; Kuo, Y.F.; Freeman, J.L.; Orihuela, E.; Goodwin, J.S. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 2005, 103, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Ma, X.; Hu, J.C.; Barbieri, C.E.; Nagar, H. Trends in androgen deprivation use in men with intermediate-risk prostate cancer who underwent radiation therapy. Adv. Radiat. Oncol. 2022, 7, 100904. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Je, Y.; Schutz, F.A.; Hoffman, K.E.; Hu, J.C.; Parekh, A.; Beckman, J.A.; Choueiri, T.K. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: A meta-analysis of randomized trials. JAMA 2011, 306, 2359–2366. [Google Scholar] [CrossRef]
- Potosky, A.L.; Haque, R.; Cassidy-Bushrow, A.E.; Ulcickas Yood, M.; Jiang, M.; Tsai, H.T.; Luta, G.; Keating, N.L. Effectiveness of primary androgen-deprivation therapy for clinically localized prostate cancer. J. Clin. Oncol. 2014, 32, 1324–1330. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Elkin, E.B.; Yee, D.S.; Feifer, A.; Ehdaie, B.; Jacks, L.M.; Atoria, C.L.; Zelefsky, M.J.; Scher, H.I.; Scardino, P.T.; et al. Locally advanced prostate cancer: A population-based study of treatment patterns. BJU Int. 2012, 109, 1309–1314. [Google Scholar] [CrossRef]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Segundo, C.G.S.; Rodriguez, M.A.C.; Macias, V.; Olive, A.P.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Widmark, A.; Klepp, O.; Solberg, A.; Damber, J.E.; Angelsen, A.; Fransson, P.; Lund, J.A.; Tasdemir, I.; Hoyer, M.; Wiklund, F.; et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 2009, 373, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen deprivation therapy plus docetaxel versus ADT alone in metastatic non-castrate prostate cancer: Impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.F.; Gilbert, S.M. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2010, 363, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G.; et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; de Reijke, T.M.; Van Tienhoven, G.; Van den Bergh, A.C.M.; Oddens, J.; Poortmans, P.M.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Chen, M.H.; Renshaw, A.A.; Loffredo, M.; Kantoff, P.W. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA 2008, 299, 289–295. [Google Scholar] [CrossRef] [PubMed]
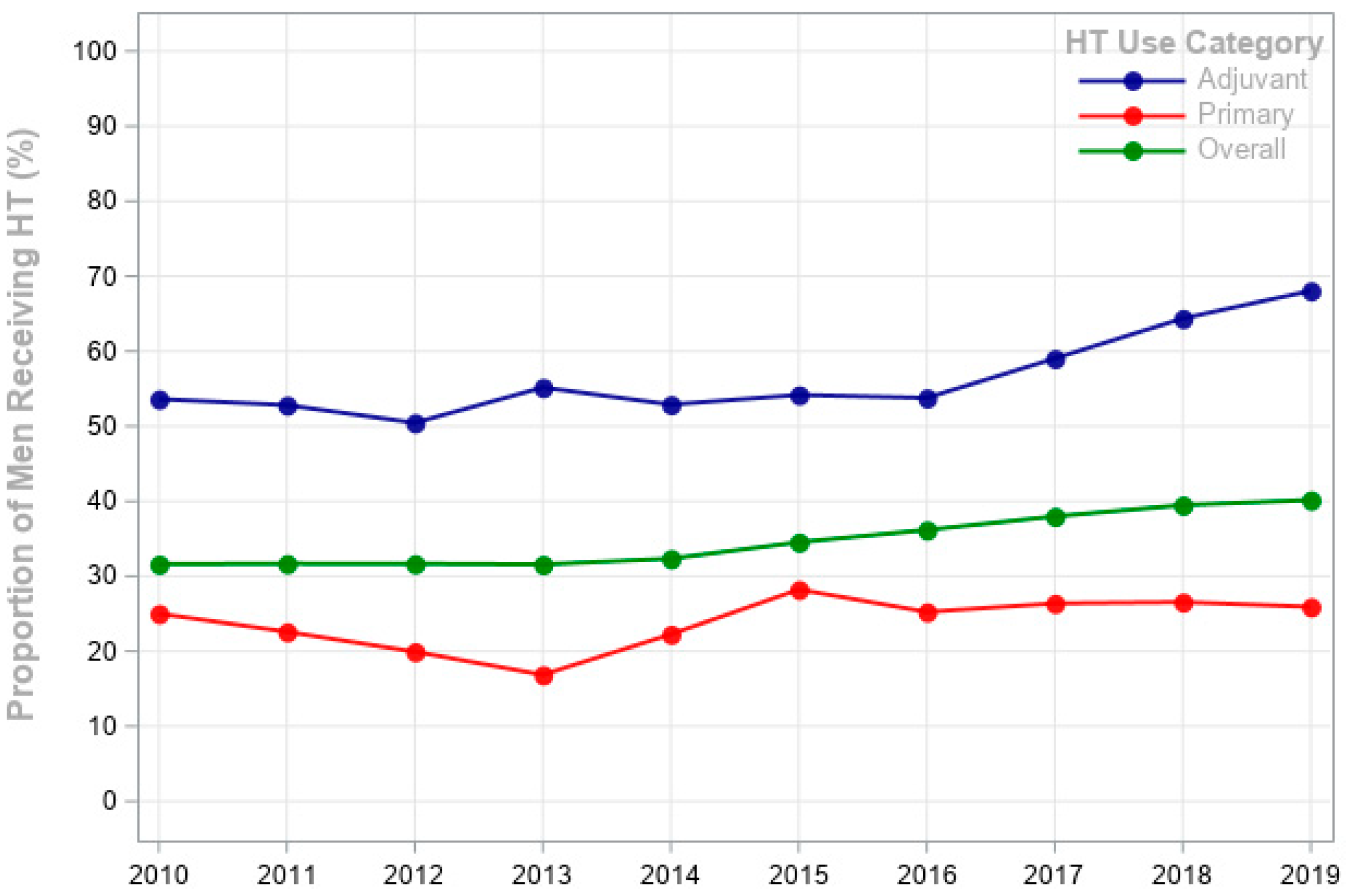
| Variable | No. of Subjects | Any HT † | Primary HT in Patients with Lower-Risk PCa † n = 73,840 | Adjuvant HT in Patients with Higher-Risk PCa † n = 6769 | p Value |
|---|---|---|---|---|---|
| Men receiving HT | 35.1% | 27.3% | 58.6% | ||
| Year of diagnosis | <0.0001 | ||||
| 2010 | 12,931 | 31.6% | 4261 (25%) | 511 (53.6%) | |
| 2011 | 13,227 | 31.7% | 4382 (22.6%) | 462 (52.8%) | |
| 2012 | 11,648 | 31.7% | 3852 (19.9%) | 438 (50.5%) | |
| 2013 | 12,576 | 31.6% | 4187 (16.9%) | 620 (55.2%) | |
| 2014 | 14,311 | 32.4% | 5028 (22.3%) | 520 (52.9%) | |
| 2015 | 15,517 | 34.5% | 6372 (28.2%) | 443 (54.2%) | |
| 2016 | 16,860 | 36.2% | 6272 (25.2%) | 729 (53.8%) | |
| 2017 | 17,592 | 38.0% | 6683 (26.4%) | 664 (59.0%) | |
| 2018 | 17,520 | 39.5% | 6428 (26.6%) | 710 (64.4%) | |
| 2019 | 17,333 | 40.2% | 6496 (25.9%) | 720 (68.1%) | |
| Age | <0.0001 | ||||
| 66–70 | 45,654 | 28.0% | 15,572 (16.8%) | 1455 (54.6%) | |
| 71–75 | 45,817 | 33.8% | 16,398 (22.2%) | 1758 (59.4%) | |
| 76–80 | 30,747 | 40.6% | 11,965 (29.6%) | 1434 (57.8%) | |
| >80 | 27,297 | 43.4% | 10,026 (33.8%) | 1170 (56.2%) | |
| Race/Ethnicity § | <0.0001 | ||||
| White | 118,613 | 40.5% | 43,136 (23.0%) | 4490 (56.7%) | |
| Black | 11,663 | 34.2% | 4426 (30.4%) | 418 (55.3%) | |
| Hispanic | 10,876 | 38.2% | 3379 (31.6%) | 494 (58.1%) | |
| Other | 8363 | 37.9% | 3020 (27.6%) | 415 (63.4%) | |
| Marital status | 0.7538 | ||||
| Married | 61,040 | 35.0% | 21,008 (24.5%) | 1975 (56.6%) | |
| Not Married | 88,475 | 35.3% | 32,953 (24.4%) | 3842 (58.4%) | |
| Clinical stage | <0.0001 | ||||
| Stage I | 5877 | 11.5% | 5272 (6.0%) | - | |
| Stage II | 96,066 | 32.8% | 48,689 (26.4%) | - | |
| Stage III | 18,244 | 39.2% | - | 2298 (59.1%) | |
| Stage IV | 29,328 | 45.0% | - | 3519 (55.9%) | |
| Tumor grade | <0.0001 | ||||
| Well differentiated | 21,078 | 8.8% | 12,684 (4.9%) | - | |
| Moderately differentiated | 43,856 | 26.4% | 17,212 (23.0%) | - | |
| Poorly differentiated/undifferentiated | 48,264 | 55.5% | - | 2301 (73.8%) | |
| Comorbidity Score | <0.0001 | ||||
| 0 | 45,406 | 31.4% | 16,782 (20.4%) | 1492 (61.7%) | |
| 1 | 42,793 | 35.3% | 15,476 (24.4%) | 1641 (59.4%) | |
| 2 | 27,812 | 37.1% | 10,182 (26.4%) | 1155 (57.8%) | |
| ≥3 | 33,504 | 38.5% | 11,521 (28.6%) | 1529 (49.9%) | |
| Radiation | <0.0001 | ||||
| Yes | 34,872 | 51.3% | - | 5817 (57.2%) | |
| No | 114,643 | 30.3% | 53,961 (24.4%) | - |
| Year of Diagnosis | Adjuvant HT in Patients with Higher-Risk PCa | Primary HT in Patients with Lower-Risk PCa § | ||||
|---|---|---|---|---|---|---|
| GnRH Agonist | GnRH Antagonist | Anti-Androgen | GnRH Agonist | GnRH Antagonist | Anti-Androgen | |
| 2010 | 281 (8.9%) | 13 (2.5%) | 173 (8.0%) | 1084 (8.6%) | 66 (3.6%) | 569 (7.9%) |
| 2011 | 243 (7.8%) | 22 (4.2%) | 151 (7.0%) | 1026 (8.2%) | 90 (4.9%) | 552 (7.7%) |
| 2012 | 223 (7.1%) | 22 (4.2%) | 125 (5.8%) | 785 (6.3%) | 68 (3.7%) | 445 (6.2%) |
| 2013 | 319 (10.1%) | 44 (8.5%) | 184 (8.5%) | 702 (5.6%) | 90 (4.9%) | 427 (5.9%) |
| 2014 | 256 (8.2%) | 47 (9.0%) | 175 (8.1%) | 1095 (8.7%) | 179 (9.8%) | 655 (9.2%) |
| 2015 | 240 (7.7%) | 38 (7.3%) | 175 (8.1%) | 1664 (13.3%) | 288 (15.8%) | 942 (13.2%) |
| 2016 | 368 (11.8%) | 65 (12.5%) | 284 (13.1%) | 1446 (11.5%) | 248 (13.6%) | 785 (10.9%) |
| 2017 | 365 (11.7%) | 80 (15.4%) | 272 (12.6%) | 1600 (12.8%) | 282 (15.5%) | 908 (12.7%) |
| 2018 | 411 (13.1%) | 85 (16.3%) | 285 (13.2%) | 1596 (12.7%) | 254 (13.9%) | 931 (13.0%) |
| 2019 | 424 (13.6%) | 105 (20.2%) | 330 (15.3%) | 1555 (12.4%) | 259 (14.2%) | 933 (13.1%) |
| Variables | Any HT n = 149,925 Odds Ratio | Adjuvant HT in Patients with Higher-Risk PCa (n = 6769) * Odds Ratio | Primary HT in Patients with Lower-Risk (n = 73,840) Odds Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | ||||
| Year of Diagnosis | |||||||||
| 2011 | 31.7% | 1.01 | 0.95–1.05 | 52.8% | 0.87 (990) | 0.79–0.96 | 22.6% | 0.97 (244) | 0.75–1.25 |
| 2012 | 31.7% | 1.00 | 0.95–1.06 | 0.74 (768) | 0.67–0.82 | 0.88 (221) | 0.68–1.14 | ||
| 2013 | 31.6% | 0.99 | 0.94–1.05 | 50.5% | 0.61 (706) | 0.54–0.67 | 19.9% | 1.06 (342) | 0.84–1.35 |
| 2014 | 32.4% | 1.04 | 0.98–1.08 | 0.86 (1119) | 0.77–0.94 | 0.97 (275) | 0.76–1.24 | ||
| 2015 | 34.5% | 1.14 | 1.08–1.20 | 55.2% | 1.17 (1799) | 1.08–1.28 | 16.9% | 1.02 (240) | 0.79–1.32 |
| 2016 | 36.2% | 1.23 | 1.16–1.28 | 1.01 (1583) | 0.92–1.11 | 1.01 (392) | 0.80–1.26 | ||
| 2017 | 38.0% | 1.33 | 1.26–1.39 | 52.9% | 1.07 (1763) | 0.98–1.17 | 22.3% | 1.25 (392) | 0.98–1.57 |
| 2018 | 39.5% | 1.41 | 1.34–1.48 | 1.08 (1707) | 0.99–1.18 | 1.56 (457) | 1.24–1.97 | ||
| 2019 | 40.2% | 1.45 | 1.38–1.52 | 54.2% | 1.05 (1684) | 0.96–1.15 | 28.2% | 1.84 (490) | 1.46–2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayyaa, M.; Kuo, Y.-F.; Shahinian, V.; Lopez, D.S.; Digbeu, B.; Urban, R.; Baillargeon, J. Hormonal Therapy Patterns in Older Men with Prostate Cancer in the United States, 2010–2019. Cancers 2025, 17, 3231. https://doi.org/10.3390/cancers17193231
Albayyaa M, Kuo Y-F, Shahinian V, Lopez DS, Digbeu B, Urban R, Baillargeon J. Hormonal Therapy Patterns in Older Men with Prostate Cancer in the United States, 2010–2019. Cancers. 2025; 17(19):3231. https://doi.org/10.3390/cancers17193231
Chicago/Turabian StyleAlbayyaa, Mohanad, Yong-Fang Kuo, Vahakn Shahinian, David S. Lopez, Biai Digbeu, Randall Urban, and Jacques Baillargeon. 2025. "Hormonal Therapy Patterns in Older Men with Prostate Cancer in the United States, 2010–2019" Cancers 17, no. 19: 3231. https://doi.org/10.3390/cancers17193231
APA StyleAlbayyaa, M., Kuo, Y.-F., Shahinian, V., Lopez, D. S., Digbeu, B., Urban, R., & Baillargeon, J. (2025). Hormonal Therapy Patterns in Older Men with Prostate Cancer in the United States, 2010–2019. Cancers, 17(19), 3231. https://doi.org/10.3390/cancers17193231







