Assessment of Early Cardiotoxicity and Cardiac Dysfunction of Radioligand Therapy in Patients with Neuroendocrine Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Tests
2.2. Echocardiography and Blood Pressure Measurement
2.3. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. Clinical Tolerability of RLT
3.3. Therapy-Related Renal, Hepatic, and Bone Marrow Toxicities
3.4. Investigation of the Potential Cardiotoxicity Associated with RLT
3.5. Effect of RLT on Heart Failure and Cardiac Overload
3.6. Impact of Clinical and Biochemical Parameters on RLT-Associated Cardiotoxicity
3.7. Clinically Overt Acute Cardiac Complications During Radioligand Therapy
3.8. Patient with NET Metastasis to the Heart
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| ACE-I | Angiotensin-converting enzyme inhibitor |
| AF | Atrial fibrillation |
| AHA | American Heart Association |
| ARB | Angiotensin II receptor blocker |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| BP | Blood pressure |
| CAPTEM | Capecitabine/temozolomide |
| CCS | Chronic coronary syndrome |
| CHD | Carcinoid heart disease |
| CHT | Chemotherapy |
| CgA | Chromogranin A |
| CK-MB | Creatine Kinase—MB isoenzyme |
| CS | Carcinoid syndrome |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| ELISA | Enzyme-linked immunosorbent assay |
| ESC | European Society of Cardiology |
| GFR | Glomerular filtration rate |
| GLS | Global longitudinal strain |
| HF | Heart failure |
| ICIs | Immune checkpoint inhibitors |
| IC-OS | International Cardio-Oncology Society |
| IQR | Interquartile range |
| LVEF | Left ventricle ejection fraction |
| M | Mean |
| Med | Median |
| MRI | Magnetic resonance imaging |
| mTOR | Mammalian Target of Rapamycin Inhibitors |
| NET | Neuroendocrine tumor |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association |
| PE | Pulmonary embolism |
| RLT | Radioligand therapy |
| RVSP | Right ventricular systolic pressure |
| SBP | Systolic blood pressure |
| SD | Standard deviations |
| STEMI | ST-elevation myocardial infarction |
| TAPSE | Tricuspid annular plane systolic excursion |
| TKI | Tyrosine kinase inhibitors |
| Troponin | Troponin I |
| UPO | Unknown primary origin |
| VIF | Variance inflation factor |
References
- Kennedy, K.R.; Turner, J.H.; MacDonald, W.B.G.; Claringbold, P.G.; Boardman, G.; Ransom, D.T. Long-term survival and toxicity in patients with neuroendocrine tumors treated with 177Lu-octreotate peptide radionuclide therapy. Cancer 2022, 128, 2182–2192. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Nilica, B.; Svirydenka, A.; Fritz, J.; Bayerschmidt, S.; Kroiss, A.; Gruber, L.; Virgolini, I.J. Nephrotoxicity and hematotoxicity one year after four cycles of peptide receptor radionuclide therapy (PRRT) and its impact on future treatment planning—A retrospective analysis. Rev. Española Med. Nucl. E Imagen Mol. 2022, 41, 138–145. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Rao, V.U.; Deswal, A.; Lenihan, D.; Dent, S.; Lopez-Fernandez, T.; Lyon, A.R.; Barac, A.; Palaskas, N.; Chen, M.H.; Villarraga, H.R.; et al. Quality-of-Care Measures for Cardio-Oncology: An IC-OS and ACC Cardio-Oncology Leadership Council Perspective. JACC CardioOncol. 2025, 7, 191–202. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Eberlein, U.; Verburg, F.A. Cardiovascular disease and radiopharmaceutical therapies- an underestimated risk? Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Azizova, T.V.; Richardson, D.B.; Tapio, S.; Bernier, M.-O.; Kreuzer, M.; Cucinotta, F.A.; Bazyka, D.; Chumak, V.; Ivanov, V.K.; et al. Ionising radiation and cardiovascular disease: Systematic review and meta-analysis. BMJ 2023, 380, e072924. [Google Scholar] [CrossRef]
- Narowska, G.; Gandhi, S.; Tzeng, A.; Hamad, E.A. Cardiovascular Toxicities of Radiation Therapy and Recommended Screening and Surveillance. J. Cardiovasc. Dev. Dis. 2023, 10, 447. [Google Scholar] [CrossRef]
- Kolbert, K.S.; Pentlow, K.S.; Pearson, J.R.; Sheikh, A.; Finn, R.D.; Humm, J.L.; Larson, S.M. Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J. Nucl. Med. 2007, 48, 143–149. [Google Scholar]
- Hesselink, E.N.K.; Hesselink, M.S.K.; de Bock, G.H.; Gansevoort, R.T.; Bakker, S.J.; Vredeveld, E.J.; van der Horst-Schrivers, A.N.; van der Horst, I.C.; Kamphuisen, P.W.; Plukker, J.T.; et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: An observational study. J. Clin. Oncol. 2013, 31, 4046–4053. [Google Scholar] [CrossRef]
- Kao, C.-H.; Chung, C.-H.; Chien, W.-C.; Shen, D.H.-Y.; Lin, L.-F.; Chiu, C.-H.; Cheng, C.-Y.; Sun, C.-A.; Chang, P.-Y. Radioactive Iodine Treatment and the risk of Long Term Cardiovascular morbidity and mortality in thyroid Cancer patients: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 4032. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Song, J.E.; Kim, J.Y.; Bae, J.H.; Kim, N.H.; Yoo, H.J.; Kim, H.Y.; Seo, J.A.; Kim, N.H.; Lee, J.; et al. Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: A nationwide cohort study. Ann. Transl. Med. 2020, 8, 1235. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Mittra, E.S.; Kulkarni, H.R.; Deroose, C.M.; Srirajaskanthan, R.; Ramage, J.; Grana, C.M.; Botta, F.; et al. Dosimetry of [177Lu]Lu-DOTATATE in Patients with Advanced Midgut Neuroendocrine Tumors: Results from a Substudy of the Phase III NETTER-1 Trial. J. Nucl. Med. 2025, 66, 449–456. [Google Scholar] [CrossRef]
- Huot Daneault, A.; Desaulniers, M.; Beauregard, J.M.; Beaulieu, A.; Arsenault, F.; April, G.; Turcotte, É.; Buteau, F.A. Highly Symptomatic Progressing Cardiac Paraganglioma With Intracardiac Extension Treated With 177Lu-DOTATATE: A Case Report. Front. Endocrinol. 2021, 12, 705271. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Amini, A.L.; Ahmadzadehfar, H.; Bagheri, D.; Assadi, M. Cardiotoxicity and cardiac monitoring following the use of radiotheranostics agents including 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors. Nuklearmedizin 2021, 60, 99–105. [Google Scholar] [CrossRef]
- Nayernama, A.; Waldron, P.; Salaam, T.; Proestel, S. Postmarketing safety review of everolimus and cardiac failure or left ventricular dysfunction. J. Clin. Oncol. 2016, 34, e18226. [Google Scholar] [CrossRef]
- Altena, R.; Bajalica-Lagercrantz, S.; Papakonstantinou, A. Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies. Cancers 2022, 14, 4665. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Vaage-Nilsen, M.; Schou, M.; Vistisen, K.K.; Lund, C.M.; Kümler, T.; Appel, J.M.; Nielsen, D.L. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020, 59, 475–483. [Google Scholar] [CrossRef]
- Ren, S.; Qian, L.; Daniels, M.J.; Duan, S.; Chen, R.; Wang, Z. Evaluation of contrast-enhanced computed tomography for the differential diagnosis of hypovascular pancreatic neuroendocrine tumors from chronic mass-forming pancreatitis. Eur. J. Radiol. 2020, 133, 109360. [Google Scholar] [CrossRef]
- Sanli, Y.; Garg, I.; Kandathil, A.; Kendi, T.; Zanetti, M.J.B.; Kuyumcu, S.; Subramaniam, R.M. Neuroendocrine Tumor Diagnosis and Management: 68Ga-DOTATATE PET/CT. Am. J. Roentgenol. 2018, 211, 267–277. [Google Scholar] [CrossRef]
- Maxwell, J.E.; Howe, J.R. Imaging in neuroendocrine tumors: An update for the clinician. Int. J. Endocr. Oncol. 2015, 2, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I. Salvage peptide receptor radionuclide therapy in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Nucl. Med. Commun. 2021, 42, 451–458. [Google Scholar] [CrossRef]
- Durma, A.D.; Saracyn, M.; Kołodziej, M.; Jóźwik-Plebanek, K.; Mróz, A.; Kapusta, W.; Dmochowska, B.; Kamiński, G. Re-treatment with [177Lu]Lu-DOTA-TATE or [177Lu]Lu-DOTA-TATE and [90Y]Y-DOTA-TATE of patients with progressive neuroendocrine neoplasm. Nucl. Med. Rev. Cent. East. Eur. 2023, 26, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Choi, J.; Modlin, I.M.; Pisegna, J.R. Persistent cardiomyopathy associated with [177Lu]Lu-DOTATATE therapy for metastatic neuroendocrine tumor. J. Clin. Oncol. 2018, 36 (Suppl. S15), e16178. [Google Scholar] [CrossRef]
- Taïeb, D.; Jha, A.; Treglia, G.; Pacak, K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr. Relat. Cancer 2019, 26, R627–R652. [Google Scholar] [CrossRef]
- Brodowska-Kania, D.A.; Saracyn, M.; Osial, N.; Kołodziej, M.; Kamiński, G. Carcinoid crisis induced by radioligand therapy: A rare but life-threatening complication in patient with neuroendocrine tumor. Nucl. Med. Rev. Cent. East. Eur. 2024, 27, 36–38. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J. Thorac. Dis. 2018, 10 (Suppl. S35), S4282–S4295. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Mincu, R.I.; Mahabadi, A.A.; Settelmeier, S.; Al-Rashid, F.; Rassaf, T.; Totzeck, M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Vejpongsa, P.; Yeh, E.T.; Force, T.; Moslehi, J.J. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ. Res. 2013, 113, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef]
- Verburg, F.A.; vern, M.; Mader, U.; Luster, M.; Reiners, C.; Hänscheid, H. The absorbed dose to the blood is a better predictor of ablation success than the administered 131I activity in thyroid cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 673–680. [Google Scholar] [CrossRef]
- Eberlein, U.; Nowak, C.; Bluemel, C.; Buck, A.K.; Werner, R.A.; Scherthan, H.; Lassmann, M. DNA damage in blood lymphocytes in patients after 177Lu peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1739–1749. [Google Scholar] [CrossRef]
- Schumann, S.; Scherthan, H.; Lapa, C.; Serfling, S.; Muhtadi, R.; Lassmann, M.; Eberlein, U. DNA damage in blood leucocytes of prostate cancer patients during therapy with 177Lu-PSMA. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1723–1732. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Stanciu, M.; Gheorghe, D.C.; Zamfirescu, A. Cardiovascular effects of cumulative doses of radioiodine in differentiated thyroid cancer patients with type 2 diabetes mellitus. Cancers 2022, 14, 2359. [Google Scholar] [CrossRef]
- Fine, R.L.; Gulati, A.P.; Krantz, B.A.; Moss, R.A.; Schreibman, S.; Tsushima, D.A.; Mowatt, K.B.; Dinnen, R.D.; Mao, Y.; Stevens, P.D.; et al. Capecitabine and Temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother. Pharmacol. 2013, 71, 663–670. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.; Helm, J.; Kvols, L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.H.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Witteles, R.M.; Fisher, G.A.; Srinivas, S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol. 2008, 19, 1613–1618. [Google Scholar] [CrossRef]
- Cremonesi, M.; Ferrari, M.; Bodei, L.; Tosi, G.; Paganelli, G. Dosimetry in peptide radionuclide receptor therapy: A review. J. Nucl. Med. 2006, 47, 1467–7145. [Google Scholar] [PubMed]
- Forrer, F.; Waldherr, C.; Maecke, H.R.; Mueller-Brand, J. Targeted radionuclide therapy with 90Y-DOTATOC in patients with neuroendocrine tumors. Anticancer. Res. 2006, 26, 703–707. [Google Scholar]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Mäcke, H.R.; Rochlitz, C.; Müller-Brand, J.; Walter, M.A. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analog [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef]
- Del Prete, M.; Buteau, F.-A.; Arsenault, F.; Saighi, N.; Bouchard, L.-O.; Beaulieu, A.; Beauregard, J.-M. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumors: Initial results from the p-PRRT trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 728–742. [Google Scholar] [CrossRef]
- Kunikowska, J.; Pawlak, D.; Bąk, M.I.; Kos-Kudła, B.; Mikołajczak, R.; Królicki, L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with 90Y/177Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: A 10-year study. Ann. Nucl. Med. 2017, 31, 347–356. [Google Scholar] [CrossRef]
- Kunikowska, J.; Zemczak, A.; Kołodziej, M.; Gut, P.; Łoń, I.; Pawlak, D.; Mikołajczak, R.; Kamiński, G.; Ruchała, M.; Kos-Kudła, B.; et al. Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects - polish multicenter experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 922–933. [Google Scholar] [CrossRef]
- Saracyn, M.; Durma, A.D.; Bober, B.; Kołodziej, M.; Lubas, A.; Kapusta, W.; Niemczyk, S.; Kamiński, G. Long-Term. Complications of Radioligand Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms. Nutrients 2022, 15, 185. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Liu, M.; Armeni, E.; Navalkissoor, S.; Davar, J.; Sullivan, L.; Leigh, C.; O’Mahony, L.F.; Hayes, A.; Mandair, D.; Chen, J.; et al. Cardiac Metastases in Patients with Neuroendocrine Tumours: Clinical Features, Therapy Outcomes, and Prognostic Implications. Neuroendocrinology 2021, 111, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bodei, L.; Chan, J.A.; El-Haddad, G.; Fidelman, N.; Kunz, P.L.; Mailman, J.; Menda, Y.; Metz, D.C.; Mittra, E.S.; et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 222–227. [Google Scholar] [CrossRef] [PubMed]
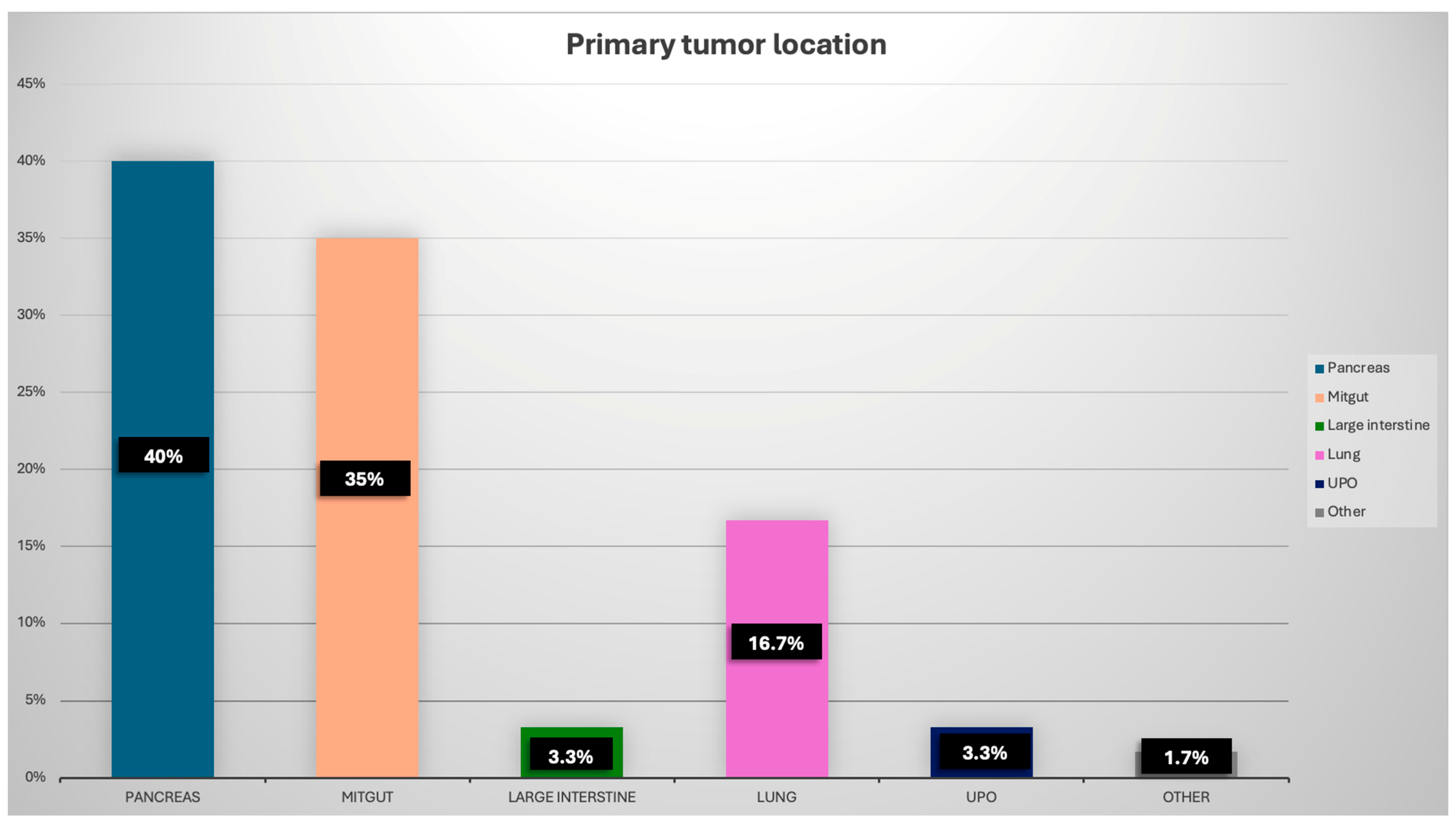
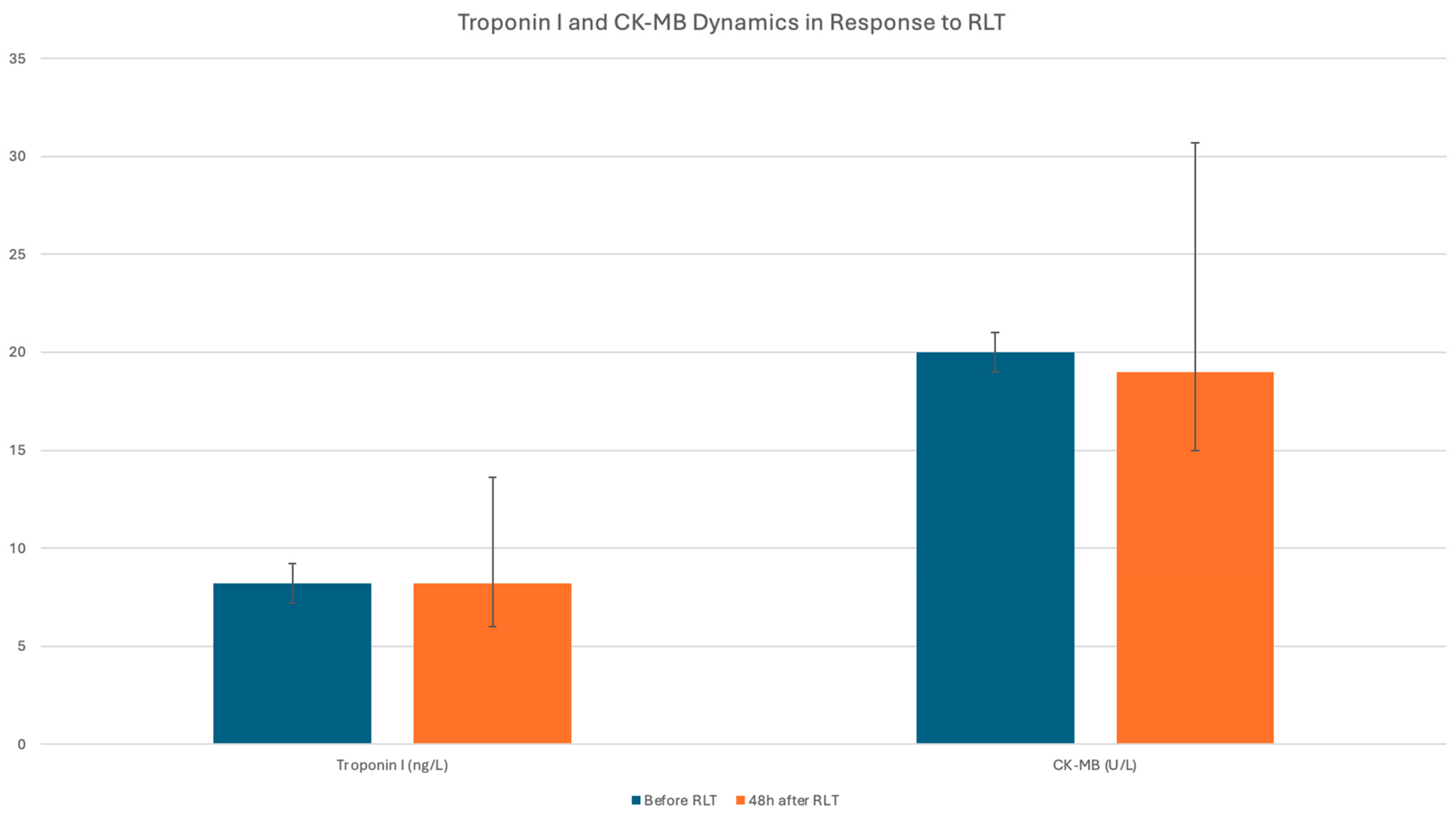

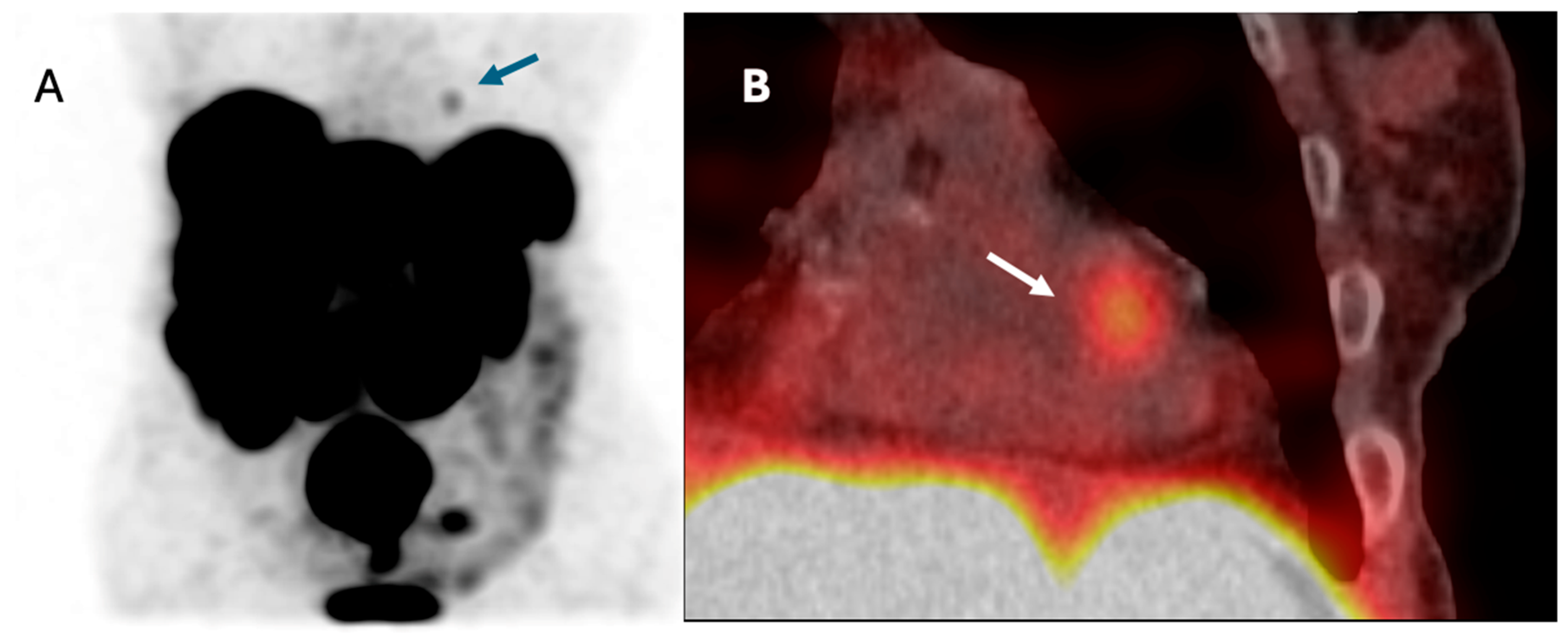
| Parameter | Patients’ Characteristics |
|---|---|
| Age, years median (IQR) | 66.5 (56.7–73.0) |
| Sex: | |
| Male, n (%) | 33 (55.0) |
| Female, n (%) | 27 (45.0) |
| Primary localisation of NET: | |
| Panceras, n (%) | 24 (40) |
| Mitgut, n (%) | 21 (35) |
| Large intestine, n (%) | 2 (3.3) |
| Lung, n (%) | 10 (16.7) |
| UPO, n (%) | 2 (3.3) |
| Other, n (%) | 1 (1.7) |
| Grade: | |
| G1, n (%) | 18 (30.0) |
| G2, n (%) | 40 (66.7) |
| G3, n (%) | 2 (3.3) |
| Ki-67, median (IQR) | 5 (2–10) |
| Prior RLT, n (%) | 9 (15.0) |
| Prior CHT, n (%) | 16 (26.7) |
| Prior treatment with TKI, n (%) | 4 (6.7) |
| BMI, kg/m2, median (IQR) | 25.1 (22.6–27.2) |
| Obesity (BMI ≥ 30), n (%) | 8 (13.3) |
| Hypertension, n (%) | 32 (53.3) |
| Diabetes mellitus, n (%) | 19 (31.7) |
| Hyperlipidemia, n (%) | 14 (23.3) |
| Smoking, n (%) | 10 (16.7) |
| Carcinoid syndrome, n (%) | 22 (36.7) |
| Carcinoid heart disease, n (%) | 4 (6.7) |
| Cardiac metastases, n (%) | 1 (1.7) |
| EF % (IQR) | 61 (56–65) |
| Heart failure, n (%): | 10 (16.7) |
| NYHA 1, n (%) | 5 (8.3) |
| NYHA 2, n (%) | 5 (8.3) |
| Other CVD, n (%): | 14 (23.3) |
| Chronic coronary syndrome, n (%) | 9 (15.0) |
| Atrial fibrillation, n (%) | 4 (6.7) |
| Chronic aortic aneurysm, n (%) | 2 (3.3) |
| History of pulmonary embolism, n (%) | 1 (1.7) |
| Parameter | Before Each RLT Course | 48 h after Each RLT Course | p |
|---|---|---|---|
| Leukocytes, ×109/L | 5.5 [4.3–6.7] | 5.2 [4.0–6.8] | 0.049 |
| Erythrocytes, ×1012/L | 4.0 [3.7–4.3] | 4.0 [3.7–4.5] | 0.18 |
| Blood platelets, ×109/L | 198.0 [162.0–258.0] | 185.0 [138.5–260.0] | <0.001 |
| Neutrophils, ×103/µL | 3.4 [2.6–4.6] | 3.2 [2.4–4.4] | 0.025 |
| Lymphocytes, ×103/µL | 1.1 [0.7–1.4] | 1.0 [0.6–1.4] | 0.11 |
| Creatinine, mg/dL | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 0.37 |
| GFR, mL/min/1.73 m2 | 79.3 [60.4–105.0] | 79.9 [60.4—107.1] | 0.56 |
| AST, U/L | 29.0 [24.0–39.0] | 27.0 [21.0–35.0] | 0.22 |
| Number of Patients | Troponin1 (ng/L) | Troponin2 (ng/L) | ΔTroponin (ng/L) | p | CK-MB1 (U/L) | CK-MB2 (U/L) | ΔCK-MB (U/L) | p | |
|---|---|---|---|---|---|---|---|---|---|
| All Pts | 60 | 8.2 [5.8–13.6] | 8.2 [6.0–13.6] | −0.2 [−1.4–0.3] | 0.007 | 20.0 [16.0–32.7] | 19.0 [15.0–30.7] | 0.0 [−4.0–3.0] | 0.90 |
| Pts treated with [177Lu]Lu-DOTA-TATE | 52 | 8.3 [5.7–13.4] | 8.2 [5.9–13.7] | −0.1 [−1.3–0.3] | 0.008 | 20.0 [16.0–32.7] | 19.0 [15.2–30.7] | 0.0 [−3.5–3.0] | 0.57 |
| Pts treated with tandem therapy | 8 | 11.3 [6.1–16.6] | 10.3 [6.3–15.3] | −0.7 [−1.7–1.3] | 0.68 | 24.0 [15.2–51.0] | 19.0 [14.0–45.7] | −0.5 [−10.7–3.0] | 0.21 |
| Pts with CS | 22 | 8.3 [5.9–13.8] | 8.6 [5.9–13.8] | −0.2 [−1.3–0.3] | 0.10 | 18.0 [15.0–27.0] | 18.0 [14.0–26.0] | 0.0 [−0.4–3.0] | 0.59 |
| Pts with CHD | 4 | 15.4 [5.8–19.1] | 12.8 [5.9–22.6] | 0.2 [−2.2–4.1] | 0.68 | 58.0 [25.5–88.2] | 43.5 [19.0–78.2] | −6.5 [−16.0–3.5] | 0.33 |
| All pts with HF | 10 | 13.5 [11.6–20.7] | 13.3 [10.4–21.7] | −0.7 [−2.5–0.8] | 0.40 | 28.5 [17.7–45.0] | 30.5 [17.7–45.7] | 0.0 [−6.7–13.5] | 0.62 |
| HF NYHA I | 5 | 11.8 [10.7–18.9] | 12.8 [7.9–18.8] | −0.7 [−3.0–0.65] | 0.21 | 21.0 [14.0–20.0] | 19.0 [17.0–43.0] | 1.0 [−4.0–19.5] | 0.67 |
| HF NYHA II | 5 | 16.7 [12.9–21.1] | 16.2 [10.8–25.6] | −0.8 [−1.6–1.8] | 0.65 | 37.0 [21.7–56.0] | 33.5 [21.5–59.7] | −0.5 [−9.7–4.7] | 0.89 |
| Pts with CCS | 9 | 13.8 [11.0–22.5] | 13.7 [9.9–24.0] | −0.45 [−1.5–0.8] | 0.45 | 26.5 [17.0–37.2] | 26.5 [15.7–49.2] | −0.5 [−7.2–3.5] | 0.90 |
| Pts with prior RLT | 9 | 9.0 [7.4–16.0] | 9.8 [6.1–15.6] | −0.8 [−1.7–0.3] | 0.12 | 28.0 [21.0–75.5] | 22.0 [17.0–77.0] | −1.0 [−8.0–34.0] | 0.88 |
| Pts with prior CHT | 16 | 5.7 [3.6–7.2] | 4.5 [3.1–8.1] | −0.1 [−0.6–0.15] | 0.40 | 12.0 [11.0–16.0] | 15.0 [10.0–28.0] | 1.0 [0.0–5.2] | 0.08 |
| Pts with prior TKI | 4 | 7.1 [4.9–9.8] | 6.7 [4.9–7.8] | −0.5 [−3.0–0.3] | 0.11 | 21.0 [18.0–34.5] | 19.5 [18.7–29.2] | 0.5 [−4.5–6.7] | 0.68 |
| Number of Patients | NT-proBNP1 (pg/mL) | NT-proBNP2 (pg/mL) | ΔNT-proBNP (pg/mL) | p | |
|---|---|---|---|---|---|
| All Pts | 60 | 117.5 [40.5–319.7] | 121.5 [37.9–322.2] | −4.0 [−45.6–33.6] | 0.32 |
| Pts treated with [177Lu]Lu-DOTA-TATE | 52 | 106.0 [28.1–316.0] | 127.0 [33.2–350.5] | −4.0 [−46.0–33.6] | 0.53 |
| Pts treated with tandem therapy | 8 | 208.0 [103.0–358.5] | 185.0 [69.7–295.0] | −21.6 [−44.1–16.7] | 0.08 |
| Pts with CS | 22 | 191.1 [58.2–410.0] | 187.0 [55.3–359.0] | −0.5 [−46.0–34.7] | 0.49 |
| Pts with CHD | 4 | 430.0 [224.0–3644.0] | 301.0 [256.0–1925.0] | −7.0 [−290.0–130.0] | 0.79 |
| All pts with HF | 10 | 531.5 [273.2–1166.7] | 659.0 [294.0–1255.0] | −14.8 [−145.7–142.5] | 0.64 |
| Pts with HF NYHA I | 5 | 624.0 [166.0–1039.5] | 615.0 [107.2–906.5] | −92.0 [−317.5–111.7] | 0.26 |
| Pts with HF NYHA II | 5 | 531.5 [296.0–2026.0] | 870.0 [297.0–1333.0] | 18.5 [−83.0–260.0] | 0.51 |
| Pts with CCS | 9 | 306.5 [115.5–609.0] | 274.0 [208.0–569.0] | 0.0 [−214.5–129.5] | 0.76 |
| Pts with prior RLT | 9 | 207.5 [15.8–303.7] | 283.0 [211.0–326.0] | −4.4 [−37.3–116.7] | 0.33 |
| Pts with prior CHT | 16 | 47.4 [17.8–63.3] | 64.4 [30.0–118.0] | 9.9 [−3.6–52.7] | 0.18 |
| Pts with prior Other CVD | 4 | 79.9 [44.3–120.5] | 60.7 [31.7–316.7] | −17.4 [−30.5–216.4] | 0.65 |
| ΔTroponin | ΔCK-MB | ΔNT-proBNP | |
|---|---|---|---|
| Δcreatinine | r = 0.43 p < 0.001 | p = 0.90 | p = 0.83 |
| ΔGFR | p = 0.95 | p = 0.63 | p = 0.73 |
| ΔTroponin | - | p = 0.95 | p = 0.53 |
| ΔCK-MB | p = 0.95 | - | p = 0.23 |
| ΔLeukocytes | p = 0.92 | p= 0.89 | r = 0.39 p <0.001 |
| ΔErythrocytes | p = 0.67 | p = 0.30 | p= 0.96 |
| ΔBlood platelets | p = 0.47 | p = 0.88 | p = 0.59 |
| ΔNeutrophils | p = 0.64 | p = 0.93 | p = 0.87 |
| ΔLymphocytes | p = 0.44 | p = 0.34 | r = 0.20 p = 0.047 |
| ΔAST | p = 0.59 | r = 0.31 p < 0.001 | p = 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwik-Plebanek, K.; Saracyn, M.; Kołodziej, M.; Mądra, W.; Durma, A.D.; Dziuk, M.; Balcerska, Z.; Janiak, K.; Gniadek-Olejniczak, K.; Kamiński, G. Assessment of Early Cardiotoxicity and Cardiac Dysfunction of Radioligand Therapy in Patients with Neuroendocrine Tumors. Cancers 2025, 17, 3219. https://doi.org/10.3390/cancers17193219
Jóźwik-Plebanek K, Saracyn M, Kołodziej M, Mądra W, Durma AD, Dziuk M, Balcerska Z, Janiak K, Gniadek-Olejniczak K, Kamiński G. Assessment of Early Cardiotoxicity and Cardiac Dysfunction of Radioligand Therapy in Patients with Neuroendocrine Tumors. Cancers. 2025; 17(19):3219. https://doi.org/10.3390/cancers17193219
Chicago/Turabian StyleJóźwik-Plebanek, Katarzyna, Marek Saracyn, Maciej Kołodziej, Weronika Mądra, Adam Daniel Durma, Mirosław Dziuk, Zuzanna Balcerska, Katarzyna Janiak, Katarzyna Gniadek-Olejniczak, and Grzegorz Kamiński. 2025. "Assessment of Early Cardiotoxicity and Cardiac Dysfunction of Radioligand Therapy in Patients with Neuroendocrine Tumors" Cancers 17, no. 19: 3219. https://doi.org/10.3390/cancers17193219
APA StyleJóźwik-Plebanek, K., Saracyn, M., Kołodziej, M., Mądra, W., Durma, A. D., Dziuk, M., Balcerska, Z., Janiak, K., Gniadek-Olejniczak, K., & Kamiński, G. (2025). Assessment of Early Cardiotoxicity and Cardiac Dysfunction of Radioligand Therapy in Patients with Neuroendocrine Tumors. Cancers, 17(19), 3219. https://doi.org/10.3390/cancers17193219






